Abstract
Antegrade in situ laser fenestration allows for incorporation of visceral and renal arteries during endovascular repair of complex abdominal and thoracoabdominal aortic aneurysms. This technique can be particularly useful for urgent and emergent cases and for centers without access to manufactured fenestrated-branched endovascular aneurysm repair devices. In the present report, we have described two techniques of antegrade in situ fenestration, the common pitfalls, and the anatomic considerations for each technique.
Keywords: Endovascular aneurysm repair, In situ laser fenestration, Ruptured aortic aneurysm, Technique, Thoracoabdominal aortic aneurysm
In situ fenestration of aortic endografts was first described for incorporation of the left subclavian artery during thoracic endovascular aortic repair (EVAR).1 The feasibility of antegrade in situ laser fenestration in the thoracoabdominal aorta was first demonstrated by Le Houérou et al2 in 16 patients who were not candidates for custom-manufactured fenestrated-branched devices. In situ fenestrated EVAR (IS-FEVAR) can be advantageous for ruptured complex abdominal and thoracoabdominal aortic aneurysms because it can achieve rapid control of hemorrhage by deployment of the aortic endograft before branch vessel incorporation.3 In the present report, we have summarized two different techniques of IS-FEVAR: the total transfemoral approach and the caudally directed approach.
Technique
Preoperative imaging and anatomic considerations
The evaluation begins by identifying the healthy proximal and distal landing zones to repair the aneurysm. A minimum of 2 cm of parallel vessel wall should be chosen for the proximal and distal seal zones. The location and diameter of the seal zones will dictate the aortic device configuration (ie, straight, tapered, or bifurcated). Next, we assess the angulation of the pararenal and paravisceral aortic segments. In general, straight aortas with a tangential takeoff of the branch vessels will be ideally suited for the total transfemoral approach, and angulated aortic segments and target vessels tortuous at their origin will be more suited to transbranchial caudally directed IS-FEVAR. One should also note the presence of ostial stenosis, direction of the takeoff, any aberrant anatomy, and spacing between the target visceral renal vessels. Similar to any fenestrated-branched EVAR (FB-EVAR), target vessel seal zones are assessed in terms of the diameter and length before the major branch points. Finally, one should note the diameters, tortuosity, and disease extent in the iliofemoral, brachial, axillary, and subclavian arteries and aortic arch. However, the clock position and arc length calculations for the target vessels are not necessary and no back table modification will be needed because the custom modification is performed within the aorta after device deployment.
Operative planning and setup
A hybrid operating room with a physician and staff who are familiar with FB-EVAR and the laser system are necessary. We use the Gen 4 CVX-300 laser system with a 2.3-mm Turbo Elite laser atherectomy catheter (Spectranetics, Colorado Springs, CO). The operating room must have a compatible outlet for the laser system. We operate the laser at a fluency of 60 mJ/mm2 as described during ex vivo analysis of laser fenestration of aortic stent grafts.4 The laser system should be set up, verified to be functioning, and calibrated before starting the procedure.
Our preference is to start all ruptured cases with the patient local anesthesia to avoid hemodynamic collapse on induction. Percutaneous access to the femoral arteries is established using ultrasound guidance. Although percutaneous closure devices can be loaded in hemodynamically stable patients, we will not hesitate to upsize the sheaths without such devices in unstable patients. Access cutdown and repair can be performed over the sheath after completion of the aortic repair.
Total transfemoral IS-FEVAR
Target vessel catheterization and stenting
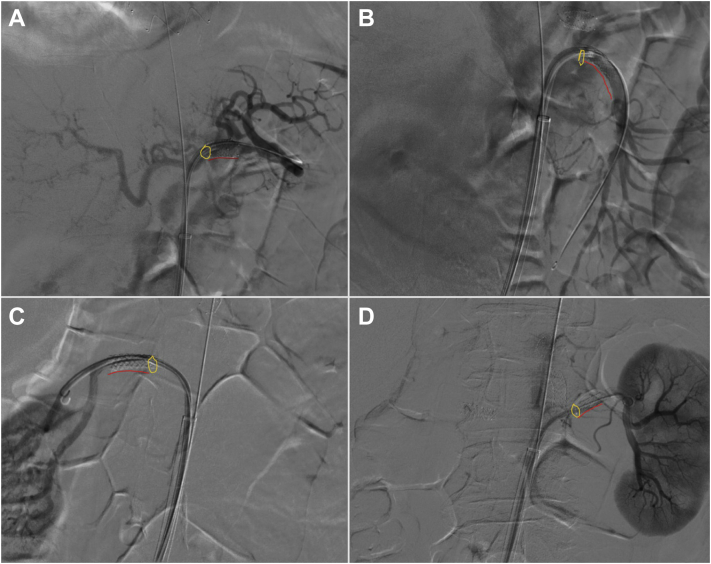
Using a 7F × 55-cm TourGuide steerable sheath (Medtronic, Minneapolis, MN), the visceral renal target vessels are catheterized and short balloon expandable stents deployed. These stents are used to guide the position of the laser probe during in situ fenestration. We have preferred covered balloon expandable stents because they are easier targets by preventing the guidewire from traversing between the stent struts. Typically, a stent length of ≤22 mm will be used, with the stent sized 1:1 to the target vessel diameter, consistent with elective repairs. Care is required to ensure deployment of the stents entirely in the branch vessel, with no protrusion into the aorta to avoid crushing during aortic endograft deployment (Fig 1). For patients with a ruptured aneurysm, limited time should be spent in attempting to cannulate small or highly stenotic vessels. Vessels for which cannulation does not appear feasible from the preoperative imaging findings can intentionally not be targeted. We believe that each vessel should not take >10 minutes to stent.
Fig 1.
Prestenting completed using a steerable sheath to cannulate each target artery, and a balloon expandable stent is placed with no protrusion into the aorta in the celiac artery (A), superior mesenteric artery (SMA; B), right renal artery (C), and left renal artery (D). Yellow rings and red lines highlight the origin and contour of the stents, respectively.
Aortic component implantation
For the aortic components, any polyester endograft with wide stent spacing will be suitable for IS-FEVAR. Commonly used endografts have included Zenith Alpha Thoracic (Cook Medical Inc, Bloomington, IN), Valiant Captiva (Medtronic, Minneapolis, MN), Endurant (Medtronic), RelayPro (Terumo Medical Corp, Somerset, NJ), and Treo (Terumo Medical Corp) endografts. Aortic endografts constructed with expanded polytetrafluoroethylene (ePTFE) are generally not suitable for in situ fenestration because it will be more difficult to penetrate ePTFE, and toxic hydrogen chloride and trifluoroacetate compounds are known to be released from the thermolysis of ePTFE.5 The aortic endograft of choice is introduced into the aorta and fully deployed across the target vessels. At this point, the ischemia time to the target vessel begins, and, in ruptured cases, the aortic seal is achieved. If a bifurcated endograft is needed distally to the modified endograft to achieve a complete seal, we will typically use an Excluder AAA endoprosthesis (W.L. Gore & Associates, Flagstaff, AZ) because of the lack of suprarenal stents and the ease of deployment. Balloon molding of the aortic seal zones and device overlap zones should be performed at this stage. Once the aortic seal has been completed, general anesthesia can be induced, and liberal resuscitation initiated as hemorrhage is controlled.
In situ laser fenestration and bridging stents
The 2.3-mm, 0.035-in. laser probe is introduced through the steerable sheath from the femoral access. Alternatively, others have reported the use of a 2-mm laser probe. Although the 2-mm laser probe will result in a smaller fenestration, making it more tolerant to misalignment, it requires a 0.018-in. system and additional catheter exchanges. Guidewire support is optional during the creation of the in situ fenestrations. When using the wire with the laser probe, the wire must be kept inside the probe to avoid disrupting the contact between the laser probe and fabric. The probe should be positioned at the center of the first prestented target vessel. Alignment of the laser probe should be confirmed with two views of the target vessel stent—down the barrel and a profile view to improve the accuracy of the in situ fenestration (Fig 2). Next, with provision of enough forward support to ensure laser probe apposition on the fabric, the laser probe is activated. When performed properly, the probe will be seen to pop through the fabric into the target branch stent within ∼2 to 3 seconds of energy delivery. Next, angiography is performed through the laser probe to confirm target vessel cannulation. After confirmation, a 0.035-in. guidewire is placed through the laser probe and then exchanged over a catheter for a support wire (Fig 3, A). The laser fenestration should be predilated with a 4-mm noncutting balloon (Fig 3, B and C), which will reduce the risk of fabric tearing and facilitate bridging stent delivery.4
Fig 2.
Use of the down-the-barrel view (A) and profile view (B) of the target vessel stent during in situ laser fenestration to improve fenestration accuracy.
Fig 3.
A, Illustration of in situ fenestration and placement of a guidewire into the target vessel. B, Balloon angioplasty of the fenestration showing the waist (white arrow) that is still present in the fenestration. C, View showing the fenestration has been expanded to the size of the balloon.
Next, the bridging balloon expandable stents are introduced into the target branch stent and flared across the fenestration with a 10-mm balloon. These steps should be repeated for each target vessel (Fig 4). Early in our experience, our approach was to target the superior mesenteric artery (SMA) first, followed by the renal arteries and then the celiac artery. However, as our experience increased, we observed that the SMA will usually be the easiest target vessel to incorporate and more tolerant to warm ischemia than the renal arteries.3 Therefore, we now target the renal arteries first, followed by the SMA. The celiac artery might not need to be revascularized in ruptured cases if angiography of the SMA demonstrates retrograde filling of the celiac artery without a type II endoleak.
Fig 4.
Process of transfemoral in situ fenestration. A, Bridging stents placed from the in situ fenestration to the target vessel. B, In situ fenestration and bridging stents should be repeated for each vessel one at a time. C, Completed in situ fenestrated endovascular aortic repair (IS-FEVAR).
Caudally directed IS-FEVAR
The following are the key differences in technique during caudally directed IS-FEVAR. First, the target vessels should not be prestented. With the patient under local anesthesia from the femoral access site, the aortic endograft is partially deployed in the descending thoracic aorta until at least the first two stent rows are opposing the aortic wall, and the next stent row is partially unsheathed ∼2 to 3 cm above the celiac artery (Fig 5, A). This will create a seal similar to that of an occlusion balloon. General anesthesia can be induced at this time because the hemorrhage is controlled. Steady forward support on the endograft must be applied to avoid the windsock effect and caudal migration of the endograft. For patients who do not need aortic occlusion, an additional sheath or balloon can be introduced alongside the aortic endograft from the contralateral femoral artery to create a temporary gutter leak that will prevent visceral renal ischemia and limit the windsock effect.
Fig 5.
During transbrachial caudally directed in situ fenestrated endovascular aortic repair (IS-FEVAR), the aortic graft is partially deployed to achieve a seal, and then in situ fenestrations are created (A). B, An antegrade in situ branch portal is created. C, The aortic graft is deployed down to the next row of stents, and in situ fenestration and stenting are repeated for the remaining target vessels. D, The partially deployed aortic graft (green outline) and an antegrade branch portal with a completed bridging stent (blue outline) can be seen under fluoroscopy.
Second, upper extremity access is performed. We have routinely used the left high brachial artery as the preferred access site. This can be accessed percutaneously or with quick surgical exposure, and a long steerable sheath is delivered into the descending thoracic aorta. We prefer the use of an 8.8F × 91-cm Nagare steerable sheath (Terumo Medical Corp) for this approach because of its ability to deflect bidirectionally.
Third, a vertical in situ fenestration is created at the partially deployed stent row. The fenestration should be predilated with a 4-mm noncutting balloon, and a 6 × 22-mm balloon expandable covered stent (iCAST, Atrium Medical Corp, Merrimack, NH) is deployed across the fenestration creating an antegrade in situ branch portal (Fig 5, B). The target vessel is then catheterized using a ≥125-cm guiding catheter (Kumpe catheter; Cook Medical), and bridging balloon expandable stents are placed. Once the first two branches have been incorporated, the aortic stent is deployed further down to the next stent row, and the same steps are repeated (Fig 5, C and D). During our initial experience, the order for branch incorporation was the top-down approach, starting with the celiac artery, followed by the SMA and then the renal arteries to minimize the length of the bridging stents. Similar to the transfemoral approach, our approach has evolved to incorporating the higher renal artery first, followed by the celiac artery, SMA, and, finally, the lower renal artery. Unlike the transfemoral approach, precise in situ fenestration is not necessary with the caudally directed in situ fenestration technique. However, it is important to create the in situ fenestration ∼2 cm higher than the target vessel to preserve the working space and on the same laterality as the target vessel to prevent the bridging stents from pulling out during full deployment of the aortic component. After deployment of all bridging stents, the aortic component can be fully deployed. If a distal bifurcated component is needed, the typical approach for endovascular repair of an infrarenal EVAR would be conducted at this time. Finally, an aortic cuff can be placed alongside the in situ fenestrations to stabilize these branches (Fig 6).
Fig 6.
Aortic cuffs (yellow dashed lines) placed to support the in situ fenestrations (red circles) and stabilize the bridging stents (blue outline).
Discussion
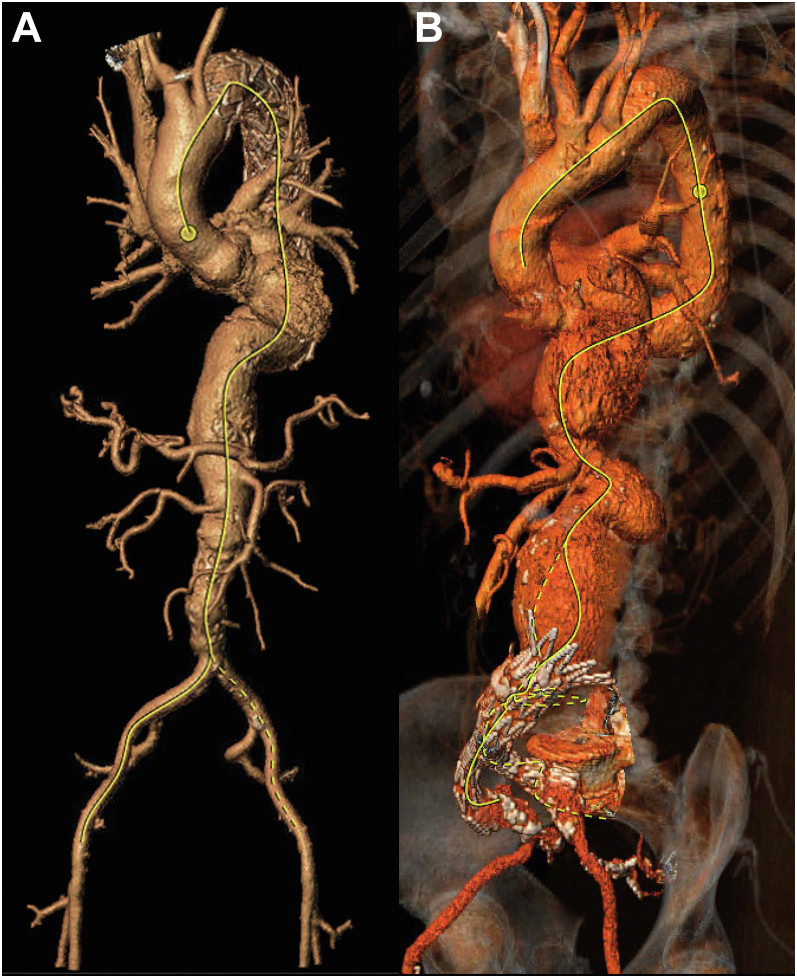
The decision between the choice of the total transfemoral or caudally directed IS-FEVAR should be determined by the aortic anatomy and angulation of the target vessels. The transfemoral approach is more suitable for a straight aorta with little angulation. Highly angulated pararenal and/or paravisceral segments will make precise prestenting of the target vessels difficult and subsequent precise alignment of the transfemoral steerable sheath difficult (Fig 7). In such cases, caudally directed IS-FEVAR will be more suitable because the proximal seal will be achieved above the angulated pararenal and/or paravisceral segments and the in situ fenestration will be created above the target vessels. This approach separates the location of the in situ fenestration and the target vessel, allowing for treatment of a greater range of anatomic variability. Nevertheless, if the patient anatomy is suitable, our preference has been to complete the procedure using a total transfemoral approach.
Fig 7.
A, Anatomy suitable for total transfemoral in situ fenestrated endovascular aortic repair (IS-FEVAR), based on a minimal angulation of the paravisceral aorta and good proximal landing zone for the aortic endograft. B, Anatomy more suitable for caudally directed IS-FEVAR because of the pararenal aortic angulation and caudal renal orientation.
Fusion overlay will not be necessary during total transfemoral IS-FEVAR because the prestents will provide image guidance for the target vessels. Use of the fusion overlay can be useful for caudally directed IS-FEVAR. This technology typically requires additional time to register before the procedure, which could make it unsuitable for unstable patients with a ruptured aneurysm and challenging for awake patients who have received local anesthesia.
The common pitfalls of the two techniques include misalignment of the fenestrations with the target vessel, a prolonged ischemia time, and the occurrence of endoleaks.
Misalignment of fenestrations with target vessel
Misalignment of the laser probe with the intended target vessel can occur with an angulated vessel takeoff. In our experience, the renal arteries have the highest risk owing to their smaller caliber and the presence, more often, of angulated anatomy. Laser fenestration outside the renal artery will result in retroperitoneal perforation (Fig 8). When this occurs, the in situ fenestration can be covered with an aortic cuff. Subsequent attempts at laser fenestration will be more difficult because penetration of two layers of endografts will be required. Precatheterizing these challenging renal arteries from above and buddy ballooning can create a working space and provide a bailout snorkel branch option from the transfemoral approach. However, such maneuvers will result in an incomplete proximal aortic seal and, therefore, is not suited for ruptured cases. The use of the caudally directed IS-FEVAR can help avoid this complication.
Fig 8.
Perforation into the retroperitoneum during in situ laser fenestration of a highly angulated renal artery. A, Angiogram demonstrating perforation (white arrow). B, Illustration of the misalignment due to an angulated target artery.
Ischemia time
Although the visceral renal ischemia time is an inherent part of IS-FEVAR, it is important to be completely prepared for in situ fenestration and placement of bridging stents before deployment of the aortic endograft, because, although the endograft can quickly seal the rupture, it will also stop flow through the visceral renal arteries. As stated, fenestration of each target vessel should be timed, and it could be necessary to move on from a difficult target artery to the next target artery if >10 minutes have elapsed. Creation of a temporary gutter by introducing a buddy sheath or balloon across the proximal seal zone can reduce the ischemia but might not be suitable during ruptured cases (Fig 9).
Fig 9.

A temporary gutter is created by placing a buddy sheath (black arrow) alongside the partially deployed aortic graft (white arrow).
Endoleaks
Type Ic endoleaks after IS-FEVAR have been observed.3,6 The occurrence of type Ic endoleaks is especially true for patients who present with hypotension because the vessels will have spasmed at the time of preoperative computed tomography angiography. A 1:1 ratio should still be used for stent sizing because the degree of vasospasm cannot be accurately determined. Thus, no evidence of an endoleak might be present at the end of the case; however, after the patient has been resuscitated and the arteries vasodilate, a type Ic endoleak can develop. These endoleaks will not have caused a repeat of bleeding and can be repaired in an elective setting in our experience.
The occurrence of type IIIc endoleaks is an obvious concern after in situ laser fenestration because no reinforcement is present around the broken fibers. Material fatigue over time at the unreinforced in situ fenestration is intuitive. In vivo accelerated testing has revealed inconsistent results regarding an increasing fenestration size over time.7,8 The effect of the off-set distance between the fenestration and aortic wall in this situation remains poorly understood. The concern with endograft fabric durability is the reason we do not use this technique in elective cases.
Conclusions
In situ laser fenestration represents an alternative strategy for branch incorporation during endovascular repair of complex abdominal and thoracoabdominal aortic aneurysms. Total transfemoral IS-FEVAR with prestenting and caudally directed IS-FEVAR are two different techniques that can complement each other. Familiarity with FB-EVAR is necessary for technical success. Although these techniques can be advantageous for patients with ruptured aneurysms, the long-term durability remains unknown.
Footnotes
W.L. Gore & Associates provided research support to the institution.
Author conflict of interest: S.M.H. is a consultant for W.L. Gore & Associates, Cook Medical Inc, and Medtronic and is on the scientific advisory board for W.L. Gore & Associates and Vestek. A.D.D. has no conflicts of interest.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Murphy E.H., Dimaio J.M., Dean W., Jessen M.E., Arko F.R. Endovascular repair of acute traumatic thoracic aortic transection with laser-assisted in-situ fenestration of a stent-graft covering the left subclavian artery. J Endovasc Ther. 2009;16:457–463. doi: 10.1583/09-2746.1. [DOI] [PubMed] [Google Scholar]
- 2.Le Houérou T., Fabre D., Alonso C.G., Brenot P., Bourkaib R., Angel C., et al. In situ antegrade laser fenestrations during endovascular aortic repair. Eur J Vasc Endovasc Surg. 2018;56:356–362. doi: 10.1016/j.ejvs.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Pyun A.J., Potter H.A., Magee G.A., Manzur M., Weaver F., Ziegler K., et al. Comparative early results of in situ fenestrated endovascular aortic repair and other emergent complex endovascular aortic repair techniques for ruptured suprarenal and thoracoabdominal aortic aneurysms at a regional aortic center. J Vasc Surg. 2022;76:875–883. doi: 10.1016/j.jvs.2022.04.036. [DOI] [PubMed] [Google Scholar]
- 4.Lin J., Rodriguez L.E., Nutley M., Jun L., Mao Y., Parikh N., et al. Optimal in situ fenestration technique with laser perforation and balloon dilation for aortic stent-grafts. J Endovasc Ther. 2021;28:300–308. doi: 10.1177/1526602820981980. [DOI] [PubMed] [Google Scholar]
- 5.Ellis D.A., Mabury S.A., Martin J.W., Muir D.C. Thermolysis of fluoropolymers as a potential source of halogenated organic acids in the environment. Nature. 2001;412:321–324. doi: 10.1038/35085548. [DOI] [PubMed] [Google Scholar]
- 6.Sonesson B., Dias N., Abdulrasak M., Resch T. Midterm results of laser generated in situ fenestration of the left subclavian artery during thoracic endovascular aneurysm repair. J Vasc Surg. 2019;69:1664–1669. doi: 10.1016/j.jvs.2018.09.052. [DOI] [PubMed] [Google Scholar]
- 7.Eadie L.A., Soulez G., King M.W., Tse L.W. Graft durability and fatigue after in situ fenestration of endovascular stent grafts using radiofrequency puncture and balloon dilatation. Eur J Vasc Endovasc Surg. 2014;47:501–508. doi: 10.1016/j.ejvs.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Ruthrauff A.A., King M.W., Soulez G., Tan K.T., Crawford S.A., Roche-Nagle G., et al. Effects of pulsatile fatigue on in situ antegrade fenestrated polyester stent grafts deployed in a patient-specific phantom model of juxtarenal aortic aneurysm. J Vasc Interv Radiol. 2015;26:1551–1558. doi: 10.1016/j.jvir.2015.06.038. [DOI] [PubMed] [Google Scholar]