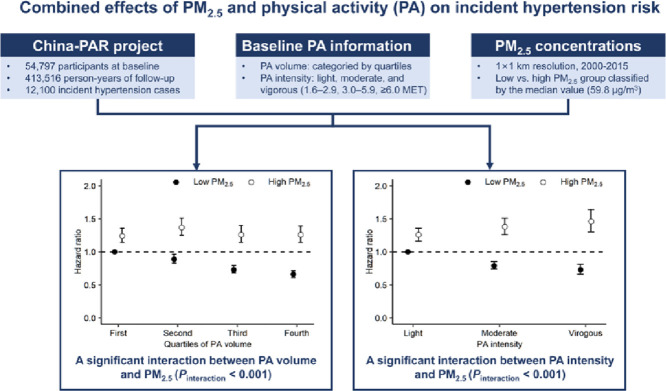
Highlights
-
•
Fine particulate matter (PM2.5) modified the association of physical activity (PA) volume and intensity with incident hypertension.
-
•
Risk of developing hypertension was reduced by increased PA with low PM2.5 exposure.
-
•
The benefits of PA on hypertension prevention were negated or reversed by high PM2.5 exposure.
Keywords: Air pollution, Cohort study, Hypertension, Particulate matter, Physical activity
Abstract
Background
The trade-off between the benefits of regular physical activity (PA) and the potentially detrimental effects of augmented exposure to air pollution in highly polluted regions remains unclear. This study aimed to examine whether ambient fine particulate matter (PM2.5) exposure modified the impacts of PA volume and intensity on hypertension risk.
Methods
We included 54,797 participants without hypertension at baseline in a nationwide cohort of the Prediction for Atherosclerotic Cardiovascular Disease Risk in China (China-PAR) project. PA volume and intensity were assessed by questionnaire, and high-resolution (1 km ×1 km) PM2.5 estimates were generated using a satellite-based model.
Results
During 413,516 person-years of follow-up, 12,100 incident hypertension cases were identified. PM2.5 significantly modified the relationship between PA and hypertension incidence (pinteraction < 0.001). Increased PA volume was negatively associated with incident hypertension in the low PM2.5 stratum (<59.8 μg/m3, ptrend < 0.001), with a hazard ratio of 0.81 (95% confidence interval (95%CI): 0.74–0.88) when comparing the fourth with the first quartile of PA volume. However, the health benefits were not observed in the high PM2.5 stratum (≥59.8 μg/m3, ptrend = 0.370). Moreover, compared with light PA intensity, vigorous intensity was related to a 20% (95%CI: 9%–29%) decreased risk of hypertension for participants exposed to low PM2.5, but a 17% (95%CI: 4%–33%) increased risk for those with high PM2.5 levels.
Conclusion
PA was associated with a reduced risk of hypertension only among participants with low PM2.5 exposure. Our findings recommended regular PA to prevent hypertension in less polluted regions and reinforced the importance of air quality improvement.
Graphical Abstract
1. Introduction
Hypertension is the leading risk factor for cardiovascular disease and the most important contributor to the global disease burden.1 The number of people with hypertension rose by 90% worldwide from 1975 to 2015, with the increase largely occurring in low- and middle-income countries.2 Thus, preventive approaches for hypertension are essential to fight this epidemic.
Physical activity (PA) is beneficial to cardiovascular health,3 whereas air pollution, especially ambient fine particulate matter (PM2.5), elevates the risk of various diseases, including hypertension.4,5 PA markedly increases the inhalation of air pollutants, which might, in turn, reduce or even negate its health benefits.6,7 Globally, 80% of countries have made national policies or plans to promote regular PA,8 meanwhile, more than half of the world's population lives in areas exceeding the least stringent air quality target (annual mean PM2.5 ≤ 35 μg/m3) of the World Health Organization (WHO).9 Therefore, it is a public concern whether or not regular PA should be recommended in highly polluted areas.
Results from recent studies on whether long-term exposure to air pollution modified the protective effects of regular PA remain inconsistent.10, 11, 12, 13, 14, 15 In addition, these studies were generally conducted in areas meeting the WHO's least stringent air quality target (annual mean PM2.5 ≤ 35 μg/m3), thus limiting its generalization to seriously polluted areas, including the mainland of China. Besides, the only study regarding hypertension was further limited by its retrospective design and selection bias, including only participants who were well-educated and had healthy lifestyles.12 Furthermore, previous studies merely pertained to the volume or type of PA but lacked information on PA intensity, which was an evidence gap identified by the 2020 European Society of Cardiology guidelines on cardiology and exercise.16
Using data from satellite-based PM2.5 estimates at high spatial resolution combined with a national scale population-based prospective cohort of the Prediction for Atherosclerotic Cardiovascular Disease Risk in China (China-PAR) project over a 16-year period, we investigated whether long-term exposure to PM2.5 altered the associations between regular PA and incident hypertension.
2. Methods
2.1. Study design and participants
Participants were derived from 3 sub-cohorts in the China-PAR project, including China Multi-Center Collaborative Study of Cardiovascular Epidemiology (China MUCA (1998)), International Collaborative Study of Cardiovascular disease in Asia (InterASIA), and Community Intervention of Metabolic Syndrome in China & Chinese Family Health Study (CIMIC). A detailed description of the study design was published elsewhere.17 Briefly, China MUCA (1998) was established in 1998 and selected participants aged 35–59 years from 15 clusters in China with a cluster random sampling method. InterASIA was initiated in 2000–2001 and selected a nationally representative sample using a 4-stage stratified sampling method based on geographic region (northern vs. southern China, divided by the Yangtze River) and urbanicity (urban vs. rural). CIMIC was set up during 2007–2008, using a cluster random sampling method to recruit participants aged ≥18 years in 4 survey sites from central and eastern China. Together, the 3 sub-cohorts covered 15 Chinese provinces and were last followed up between 2012 and 2015. The China-PAR project was approved by the Institutional Review Board at Fuwai Hospital in Beijing (No. 2018-1061). Written informed consent was obtained from all participants before data collection.
A total of 113,448 adults were enrolled for the baseline examinations, of which 8185 (7.2%) were lost to follow-up. Because the PM2.5 exposure data were available beginning in 2000, the follow-up information after that year was used. We excluded deaths prior to 2000 (n = 67), subjects with cardiovascular disease (n = 2218) or hypertension (n = 35,151) at baseline or before 2000, participants with missing blood pressure (BP) information during follow-up (n = 11,439), and those with missing baseline information for BP (n = 28), PA (n = 1562), or residential address (n = 1). Finally, 54,797 participants were included in the analysis (Supplementary Fig. 1).
2.2. PM2.5 exposure and temperature assessment
The detailed PM2.5 exposure assessment has been published elsewhere and applied in previous environmental epidemiology studies.18,19 In brief, a spatiotemporal model was used to estimate ambient PM2.5 levels at 1 km×1 km spatial resolution across China from 2000 to 2015, based on high-resolution satellite aerosol optical depth data retrieved through the Multi-Angle Implementation of Atmospheric Correction algorithm, land use information, roads, meteorology, and population density data. We validated the model using ground-level PM2.5 measurements from 2013 to 2016 acquired from the China Environmental Monitoring Center (www.cnemc.cn/), with an overall 10-fold cross-validation R2 of 0.95 at the annual level. To assess the prediction accuracy of the period without national ground measurements (before 2013), we compared model predictions with available monitoring data from Hong Kong, China; Taiwan, China; and the US Embassy in China; and the prediction R2 was 0.80 at the annual level. The monthly mean temperature at 2-m height was extracted from the European Center for Medium-Range Weather Forecast Atmospheric Re-analysis dataset Version 5.20
Residential addresses for all participants were collected at baseline and at follow-up visits and were geocoded into latitude and longitude data. Considering the changing residential history over the follow-up period, for each participant time-weighted averages of PM2.5 and temperature from 2000 to 2015 were used as indicators of long-term exposure, with weights defined as the duration spent at each residence. We divided participants into 2 groups according to the median of PM2.5 exposure: low (31.2 to <59.8 μg/m3) and high (59.8 to 88.8 μg/m3).
2.3. PA assessment
For InterASIA and CIMIC sub-cohorts, we collected the daily duration of light, moderate, and vigorous PA on weekdays and weekends over the previous year. China MUCA (1998) covered more detailed PA information, collecting the daily time spent on specific types of PA in occupation, household, transportation, and leisure-time domains on weekdays and weekends over the previous year. The intensity of each activity was expressed in metabolic equivalent (MET), which was equivalent to 1 kcal/kg of body weight per hour. For InterASIA and CIMIC, we assigned 2 MET, 4 MET, and 8 MET values to activities with light PA, moderate PA, and vigorous PA, respectively; we assigned MET values to each activity in China MUCA (1998) according to the 2011 Compendium of Physical Activities.21 For every individual, time spent on each activity was multiplied by its MET value, and the sum of all activities was used as the measurement of daily PA volume (MET-h/day). We classified participants into 4 groups (≤18.0 MET-h/day, >18.0–32.0 MET-h/day, >32.0–54.5 MET-h/day, and >54.5 MET-h/day) according to the quartiles of PA volume. The average PA intensity for each subject was created by dividing the daily PA volume by the total daily hours spent on PA.22 Participants were categorized by average PA intensity as follows: light (1.6–<3.0 MET), moderate (3.0–<6.0 MET), and vigorous (≥6.0 MET).
2.4. Outcome measurements
Blood pressure (BP) measurements were obtained by trained and certified staff according to the protocol recommended by the American Heart Association.23 BP (mmHg) was read with a standardized mercury sphygmomanometer (XJ11D; Yutu, Shanghai, China) for China MUCA (1998) and InterASIA, and an electronic sphygmomanometer (HEM-770A; Omron, Kyoto, Japan) for CIMIC. We required participants to rest for 5 min in a sitting position and to avoid alcohol, cigarette smoking, coffee/tea, and exercising for at least 30 min before their BP measurements. The average of 3 right-arm BP measurements obtained within 30 s intervals was used in the analysis. We used the unified protocol to collect information on antihypertensive drugs at each survey for all sub-cohorts. Hypertension was defined as systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg or self-reported taking of antihypertensive medication within the past 2 weeks. The incident date of hypertension was identified as the date of first diagnosis or initial use of antihypertensive agents.
2.5. Covariates
Information on demographics, residential addresses, lifestyle risk factors, and medical history was obtained using similar questionnaires at baseline for all sub-cohorts. Smoking was defined as having smoked more than 400 cigarettes or at least 1 cigarette per day for 1 year or more. Smokers were further categorized as current and former smokers by asking whether the smoker had quit smoking by the time of survey. Alcohol drinking was defined as alcohol consumption at least once per week during the previous year. Height and weight were measured with light indoor clothing and without shoes, using a standardized soft tape measure and platform scale, respectively. Body mass index was calculated as weight divided by squared height (kg/m2). Blood samples after overnight fasting of at least 10 h were drawn to measure serum glucose and lipids levels. Diabetes mellitus was defined as fasting glucose level of ≥7.0 mmol/L and/or the use of insulin or oral hypoglycemic agents and/or diagnosed medical history of diabetes.
2.6. Statistical analysis
The baseline characteristics are presented as mean ± SD for continuous variables or as numbers (percentages) for categorical variables. Person-years of follow-up were calculated from baseline date or from January 1, 2000 (if baseline date was earlier than 2000), to the date of incident hypertension, death, or the last follow-up, whichever came first.
We used Cox proportional hazards models stratified by sub-cohorts to assess the hazard ratios (HRs) and 95% confidence intervals (95%CIs) of incident hypertension with PA volume, average PA intensity, and PM2.5. The proportional hazards assumption was tested by evaluating the weighted Schoenfeld residuals and no violations were observed (p > 0.05). Covariates in the Cox model included age, sex, education level (less than high school, or high school or above), urbanization (urban or rural), geographic region (north, east, north eastern, south, central, south western, or north western), smoking status (never, former, or current), alcohol drinking (yes or no), body mass index, systolic BP, diabetes mellitus, total cholesterol, temperature, and PM2.5 (for the association with PA volume), PA volume (for the association with PM2.5), or both PM2.5 and PA volume (for the association with PA intensity). To account for the nonlinear association of temperature with hypertension, we used a regression spline with 3 degrees of freedom. Stratified analyses were conducted to examine the associations between PA and the risk of hypertension in each PM2.5 stratum. Tests for trend were performed by including the median of each PA category as a continuous variable in the models. Interaction effects of PM2.5 and PA (volume or intensity) were detected by including both the main effects and the interaction terms of PM2.5 groups and PA (volume or intensity) categories in the model. The effects of 8 joint categories of PM2.5 (low and high) and PA volume (first to fourth quartiles) were calculated by comparing each group with reference to the participants who were in the first quartile of PA volume and exposed to low PM2.5. Similarly, subjects were cross-classified into 6 groups to estimate the combined effects of PM2.5 (low and high) and PA intensity (light, moderate, and vigorous), with the group of light PA intensity and low PM2.5 as reference.
Three sensitivity analyses were performed. First, we excluded hypertension cases occurring within the first year of follow-up. Second, we used county-level averaged years of education (demographic census of China, 2000) as a surrogate for socioeconomic status and further adjusted for it in the Cox model. Third, PM2.5 exposure was dichotomized by the 75th percentile of the exposure range (77.7 μg/m3) in order to explore whether different cutoff points would differentiate the main findings.
Statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA) software. All statistical tests were two-sided, with a p value of <0.05 considered to be significant.
3. Results
3.1. Baseline characteristics
The average annual PM2.5 concentration from 2000 to 2015 at participants’ residences was 65.4 μg/m3, ranging from 31.2 μg/m3 to 88.8 μg/m3. Table 1 presents the baseline characteristics overall and according to quartiles of PA volume. At baseline, the mean age of participants was 48.8 years and 38.5% were men. Participants with higher PA volume were more likely to smoke and drink alcohol, to have a lower education level and lower prevalence of diabetes, and to do PA with greater intensity.
Table 1.
Baseline characteristics of participants overall and according to quartiles of PA volume.
| All | Quartiles of PA volume (MET-h/day) |
||||
|---|---|---|---|---|---|
| First (≤18.0) | Second (>18.0–32.0) | Third (>32.0–54.5) | Fourth (>54.5) | ||
| No. of participants | 54,797 | 14,594 | 12,938 | 13,566 | 13,699 |
| Age (year) | 48.8 ± 11.5 | 49.3 ± 12.8 | 47.0 ± 11.3 | 48.6 ± 11.0 | 50.2 ± 10.5 |
| Male | 21,099 (38.5) | 5334 (36.5) | 4621 (35.7) | 5099 (37.6) | 6045 (44.1) |
| Urban | 5914 (10.8) | 3654 (25.0) | 1765 (13.6) | 421 (3.1) | 74 (0.5) |
| Education level>high school | 8530 (15.6) | 3828 (26.2) | 2543 (19.7) | 1249 (9.2) | 910 (6.6) |
| Smokinga | |||||
| Never smoker | 40,478 (73.9) | 10,953 (75.1) | 9682 (74.8) | 10,134 (74.7) | 9709 (70.9) |
| Former smoker | 1748 (3.2) | 553 (3.8) | 421 (3.3) | 361 (2.7) | 413 (3.0) |
| Current smoker | 12,375 (22.6) | 3026 (20.7) | 2762 (21.3) | 3030 (22.3) | 3557 (26.0) |
| Alcohol consumption | 9534 (17.4) | 2233 (15.3) | 2049 (15.8) | 2370 (17.5) | 2882 (21.0) |
| Body mass index (kg/m2) | 23.1 ± 3.3 | 23.3 ± 3.4 | 23.1 ± 3.3 | 23.1 ± 3.3 | 23.0 ± 3.3 |
| Systolic blood pressure (mmHg) | 117.0 ± 11.5 | 116.9 ± 11.8 | 116.6 ± 11.5 | 117.0 ± 11.2 | 117.5 ± 11.3 |
| Diastolic blood pressure (mmHg) | 73.6 ± 8.0 | 74.0 ± 7.8 | 73.9 ± 7.9 | 73.3 ± 8.0 | 73.3 ± 8.0 |
| Diabetes mellitus | 2252 (4.1) | 816 (5.6) | 534 (4.1) | 473 (3.5) | 429 (3.1) |
| Total cholesterol (mg/dL) | 171.5 ± 34.3 | 175.2 ± 35.9 | 170.2 ± 34.5 | 169.4 ± 33.6 | 170.9 ± 32.6 |
| PA volume (MET-h/day) | 37.1 ± 23.3 | 11.2 ± 5.3 | 25.1 ± 4.4 | 43.2 ± 6.0 | 70.1 ± 10.8 |
| PA intensity (MET) | 4.2 ± 2.0 | 2.3 ± 0.8 | 3.2 ± 1.4 | 4.7 ± 1.3 | 6.6 ± 1.1 |
| PM2.5 exposure (μg/m3) | 65.4 ± 13.6 | 66.4 ± 14.5 | 64.5 ± 12.7 | 64.5 ± 12.8 | 66.0 ± 14.0 |
Note: Values are presented as mean ± SD for continuous variables or as n (%) for categorical variables.
A total of 196 participants had missing information on smoking status.
Abbreviations: MET = metabolic equivalent; PA = physical activity; PM2.5 = fine particulate matter.
3.2. Associations of PA or PM2.5 with hypertension
During 413,516 person-years, 12,100 incident hypertension events were identified. Table 2 shows the independent associations of long-term exposure to PM2.5 and PA with incident hypertension. High PM2.5 was associated with a 53% (95%CI: 43%–64%) elevated risk of developing hypertension after adjusting for multiple covariates, including regular PA volume. By contrast, higher volume and intensity of habitual PA were related to a reduced risk after adjusting for a wide range of covariates, including PM2.5. With the first quartile of PA volume as reference, the adjusted HRs (95%CI) were 1.01 (0.96–1.06), 0.87 (0.82–0.92), and 0.82 (0.77–0.87) in the second to fourth quartiles of PA volume, respectively. Compared with light intensity, the multivariable-adjusted HRs (95%CIs) were 0.93 (0.88–0.99) for moderate intensity PA and 0.93 (0.85–1.01) for vigorous intensity PA.
Table 2.
HR and 95%CI of hypertension associated with the volume and intensity of PA and long-term exposure to PM2.5.
| HR (95%CI)a | |
|---|---|
| PA volume (MET-h/day) | |
| Quartile 1 (≤18.0) | 1.00 |
| Quartile 2 (>18.0–32.0) | 1.01 (0.96–1.06) |
| Quartile 3 (>32.0–54.5) | 0.87 (0.82–0.92) |
| Quartile 4 (>54.5) | 0.82 (0.77–0.87) |
| Average PA intensity (MET) | |
| Light (1.6–<3.0) | 1.00 |
| Moderate (3.0–<6.0) | 0.93 (0.88–0.99) |
| Vigorous (≥6.0) | 0.93 (0.85–1.01) |
| PM2.5 level (μg/m3) | |
| Low (<59.8) | 1.00 |
| High (≥59.8) | 1.53 (1.43–1.64) |
Cox proportional hazard model, stratified by cohort and adjusted for age, sex, education level, urbanicity, geographic region, temperature, smoking status, alcohol drinking, body mass index, systolic blood pressure, diabetes mellitus, total cholesterol, and PM2.5 (for the association with PA volume) or PA volume (for the association with PM2.5) or both PM2.5 and PA volume (for the association with PA intensity).
Abbreviations: 95%CI = 95% confidence interval; HR = hazard ratio; MET = metabolic equivalent; PA = physical activity; PM2.5 = fine particulate matter.
3.3. Associations of PA with hypertension stratified by PM2.5 level
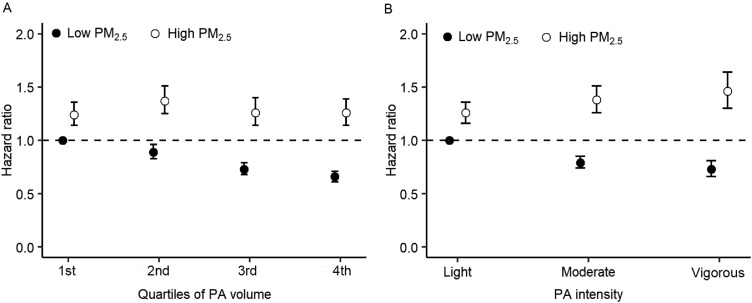
There was a significant interaction between PM2.5 and PA volume in association with incident hypertension (pinteraction < 0.001, Table 3). Among participants exposed to low PM2.5, the adjusted HRs (95%CIs) were 1.00 (reference), 0.93 (0.86–1.00), 0.86 (0.80–0.94), and 0.81 (0.74–0.88) for the first to fourth quartile of PA volume, respectively (ptrend < 0.001). Among participants exposed to high PM2.5, corresponding HRs (95%CIs) were 1.00 (reference), 1.10 (1.03–1.18), 1.04 (0.96–1.13), and 1.06 (0.97–1.15) (ptrend = 0.370). We also observed a significant interaction between PA intensity and PM2.5 exposure (pinteraction < 0.001, Table 4). Compared with light PA intensity, vigorous PA intensity was related to a 20% (95%CI: 9%–29%) decreased risk of hypertension for participants exposed to low PM2.5, but a 17% (95%CI: 4%–33%) increased risk for those with high PM2.5 levels.
Table 3.
HR and 95%CI for hypertension associated with the volume of PA stratified by long-term exposure to PM2.5.
| Quartiles of PA volume (MET-h/day) |
ptrend | ||||
|---|---|---|---|---|---|
| First (≤18.0) | Second (>18.0–32.0) | Third (>32.0–54.5) | Fourth (>54.5) | ||
| Low PM2.5 (<59.8 μg/m3) | |||||
| Cases number | 1741 | 1477 | 1522 | 1535 | |
| Person-years | 46,967 | 46,685 | 52,313 | 50,095 | |
| HR (95%CI)a | 1.00 | 0.93 (0.86–1.00) | 0.86 (0.80–0.94) | 0.81 (0.74–0.88) | <0.001 |
| High PM2.5 (≥59.8 μg/m3) | |||||
| Cases number | 2049 | 1487 | 1150 | 1139 | |
| Person-years | 62,409 | 45,653 | 39,541 | 41,492 | |
| HR (95%CI) | 1.00 | 1.10 (1.03–1.18) | 1.04 (0.96–1.13) | 1.06 (0.97–1.15) | 0.370 |
Cox proportional hazard model, stratified by cohort and adjusted for age, sex, education level, urbanicity, geographic region, temperature, smoking status, alcohol drinking, body mass index, systolic blood pressure, diabetes mellitus, and total cholesterol.
Abbreviations: 95%CI = 95% confidence interval; HR = hazard ratio; MET = metabolic equivalent; PA = physical activity; PM2.5 = fine particulate matter.
Table 4.
HR and 95%CI for hypertension associated with the intensity of PA stratified by long-term exposure of PM2.5.
| Average PA intensity (MET) |
ptrend | |||
|---|---|---|---|---|
| Light (1.6–<3.0) | Moderate (3.0–<6.0) | Vigorous (≥6.0) | ||
| Low PM2.5 (<59.8 μg/m3) | ||||
| Cases number | 2503 | 2291 | 1403 | |
| Person-years | 71,973 | 77,185 | 44,905 | |
| HR (95%CI)a | 1.00 | 0.87 (0.80–0.94) | 0.80 (0.71–0.91) | <0.001 |
| High PM2.5 (≥59.8 μg/m3) | ||||
| Cases number | 2450 | 1992 | 1255 | |
| Person-years | 75,239 | 65,618 | 44,784 | |
| HR (95%CI) | 1.00 | 1.11 (1.02–1.20) | 1.17 (1.04–1.33) | 0.016 |
Cox proportional hazard model, stratified by cohort and adjusted for age, sex, education level, urbanicity, geographic region, temperature, smoking status, alcohol drinking, body mass index, systolic blood pressure, diabetes mellitus, total cholesterol, and PA volume in MET-h/day.
Abbreviations: 95%CI = 95% confidence interval; HR = hazard ratio; MET = metabolic equivalent; PA = physical activity; PM2.5 = fine particulate matter.
3.4. Joint effects of PA and PM2.5 on hypertension
The joint effects of PA (volume and intensity) and PM2.5 exposure are presented in Fig. 1. Generally, participants exposed to high PM2.5 levels had a consistently higher risk of hypertension compared with those exposed to low PM2.5 levels, irrespective of PA volume or intensity. Furthermore, participants with low PM2.5 exposure combined with the highest volume or vigorous intensity of PA had the lowest hypertension risk.
Fig. 1.
Joint effects of PM2.5 and (A) PA volume or (B) PA intensity on incident hypertension. Cox proportional hazard model was stratified by cohort and adjusted for age, sex, urbanicity, geographic region, temperature, education level, smoking status, alcohol drinking, body mass index, systolic blood pressure, diabetes mellitus, and total cholesterol (further adjusted for PA volume when analyzing the joint effects of PM2.5 and PA intensity). PA = physical activity; PM2.5 = fine particulate matter.
3.5. Sensitivity analyses
Sensitivity analyses yielded similar results after excluding those who developed hypertension during the first follow-up year (Supplementary Table 1), or considering the potential confounding effects of socioeconomic status (Supplementary Table 2), or grouping participants into low and high PM2.5 categories by the 75th percentile of PM2.5 concentration (77.7 μg/m3, Supplementary Table 3).
4. Discussion
To our knowledge, this is the first prospective cohort study to examine the joint effects of long-term PM2.5 exposure with both PA volume and intensity on the development of hypertension in highly polluted regions. Among participants with low PM2.5 exposure, habitual PA volume and intensity were inversely associated with the risk of incident hypertension. In contrast, among those exposed to high PM2.5, insignificant or even positive associations between PA and hypertension risk were observed. The associations of PA volume and intensity with hypertension incidence were significantly modified by ambient PM2.5 levels.
Previous cohort studies, typically conducted in settings with relatively good air quality, have found protective health effects from higher PA volume across PM2.5 levels.10, 11, 12,24 For instance, a cohort in Taiwan, China found a reduced risk of developing hypertension associated with habitual PA volumes in people exposed to PM2.5 levels ranging from 6 μg/m3 to 50 μg/m3.12 In line with the existing evidence, our study demonstrated that individuals could benefit from higher PA volume when the PM2.5 concentration was lower than approximately 60 μg/m3. Therefore, we recommended higher PA volume for people residing in regions with low PM2.5 concentrations.
High levels of air pollution prevent people from active outdoor exercise despite the clinical importance of PA,25,26 thus it is very important to determine the optimal PA behaviors in highly polluted regions. Existing cohort studies suggested no evidence of interaction between regular PA volume and long-term PM2.5 exposure.10, 11, 12,24 However, the average PM2.5 concentration for previous cohort studies was generally below the WHO's Interim Target 1 of 35 μg/m3. Globally, the population-weighted PM2.5 levels were 44.2 μg/m3 in 2015, and 4 of the 10 most populous countries had estimated concentrations above the global level (e.g., 58.4 μg/m3 for China).27 Thus, findings from previous cohort studies had low generalization to a large proportion of the world's population in high PM2.5 pollution settings, and our study filled this gap. This prospective cohort study, with a median PM2.5 of approximately 60 μg/m3, found that higher PA volume had no protective effects on incident hypertension among participants exposed to high PM2.5. Similarly, a modeling study (PM2.5 ranging from 5 to 200 μg/m3)28 and a cross-sectional study from Henan, China (PM2.5 concentrations from 68 to 85 μg/m3)29 also found that a high level of ambient air pollution attenuated or negated the benefits of PA volume.
Cumulative literature demonstrated that light PA intensity was beneficial to cardiovascular health and that higher intensity conferred greater benefits.3,22,30 Current PA guidelines generally recommended moderate or vigorous PA intensity for adults.31,32 An experimental study found that greater PA intensity played a protective role with respect to the pulmonary and metabolic responses to exercise during short-term diesel exhaust exposure.33 However, to our knowledge, previous studies have not examined the interactive effects of regular PA intensity and long-term air pollution. In this study, we found that greater PA intensity was related to a decreased risk of hypertension among participants with long-term low PM2.5 exposure but to an increased risk among those exposed to long-term high PM2.5. Our findings implied that people living in regions with high ambient PM2.5 concentrations did not benefit from outdoor vigorous PA and that engaging in indoor PA while reducing air pollution with air filtration systems might be a good option for them. The difference between our study and previous short-term study results might be the findings of a delayed but cumulative effect. This suggests the need for further research to confirm our findings.
The health effects of PM2.5 and PA had some mechanisms in common, such as changes in systemic inflammation and the autonomic nervous system.4,34 PA augmented the intake of air pollutants due to increased ventilation rate, higher deposition fraction in airways, and switching from nasal breathing to oral,35 and because of their shared pathways, the risks caused by extra PM2.5 inhalation may nullify the benefits of PA. Moreover, PA intensity contributed to the extra inhalation of air pollutants. For example, previous research reported a 3- to 4.5-fold increase in the number of particles deposited in the airway during light-intensity exercise and a 6- to 10-fold increase during vigorous intensity exercise.7,36 This might partly explain our results, which showed that greater intensity was related to a higher risk of developing hypertension among participants in highly polluted settings.
Our study had several strengths. First, because it covered a broader PM2.5 concentration range (31.2–88.8 μg/m3), our study filled the gap in research relative to serious ambient PM2.5 levels, which means our findings are generalizable to other countries with similar PM2.5 levels to China. Second, this study was based on a prospective cohort with a large sample size, high rate of follow-up (92.8%), long follow-up period, and stringent quality control procedures. Third, a satellite-based spatiotemporal model with high accuracy (1 km×1 km resolution) was used to estimate the concentration of ambient PM2.5, which enabled us to capture the fine-scale PM2.5 variability and assign the PM2.5 concentrations prior to 2013.
Still, several limitations should be noted. First, we did not collect information to distinguish outdoor PA from indoor PA, hence we could not exclusively examine outdoor PA. However, Chinese people commonly exercise outdoors.37 Second, we used the PM2.5 at residence as a proxy of PM2.5 levels encountered during PA, assuming that participants’ daily activity mainly took place in proximity to their residences; this might lead to misclassification of PM2.5 exposure. Third, the effects of gaseous pollutants, including sulfur dioxide, nitrogen dioxide, and ozone, were not examined due to lack of information.
5. Conclusion
This study provided the first prospective evidence from highly polluted settings that severe PM2.5 exposure negates the benefits of regular PA against hypertension. Our study implies that regular outdoor PA, especially vigorous PA, should not be recommended for individuals in seriously polluted areas, and it reinforces the importance of air quality improvement for the prevention of hypertension.
Acknowledgments
Acknowledgments
This study was supported by the National Key Research and Development Program of China (2018YFE0115300 and 2017YFC0211703), the National Natural Science Foundation of China (91643208, 82073658, and 91843302), the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2021-I2M-1-010, 2017-I2M-1-004, and 2019-I2M-2-003), Research Unit of Prospective Cohort of Cardiovascular Diseases and Cancers, Chinese Academy of Medical Sciences (2019RU038), and the China Medical Board (15-220). The work of Y Liu was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health (Award #1R01ES032140). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank the staff and participants of the China-PAR project for their important participation and contribution.
Authors’ contributions
QL performed the formal analysis, drafted the original manuscript, and revised the manuscript; KH, FLiang, and XY performed data acquisition, contributed to the methodology, and revised the manuscript. JL, JChen, XLiu, JCao, CS, LY, YZ, YD, YLi, DH, and XLu collected data and revised the manuscript; YLiu contributed to the PM2.5 exposure assessment protocol and methodology; DG implemented the study and revised the manuscript critically; FLiu and JH conceived the study, participated in its design and coordination, and revised the manuscript critically. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials associated with this article can be found in the online version at doi:10.1016/j.jshs.2022.01.004.
Contributor Information
Fangchao Liu, Email: fangchaoliu@126.com.
Jianfeng Huang, Email: jianfhuang@sina.com.
Supplementary materials
References
- 1.Stanaway JD, Afshin A, Gakidou E, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the global burden of disease study 2017. The Lancet. 2018;392:1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou B, Bentham J, Di Cesare M, et al. Worldwide trends in blood pressure from 1975 to 2015: A pooled analysis of 1479 population-based measurement studies with 19·1 million participants. The Lancet. 2017;389:37–55. doi: 10.1016/S0140-6736(16)31919-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.2018 Physical Activity Guidelines Advisory Committee . Department of Health and Human Services; Washington, DC: U.S: 2018. 2018 physical activity guidelines advisory committee scientific report. [Google Scholar]
- 4.Rajagopalan S, Al-Kindi SG, Brook RD. Air pollution and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72:2054–2070. doi: 10.1016/j.jacc.2018.07.099. [DOI] [PubMed] [Google Scholar]
- 5.Huang K, Yang X, Liang F, et al. Long-term exposure to fine particulate matter and hypertension incidence in China. Hypertension. 2019;73:1195–1201. doi: 10.1161/HYPERTENSIONAHA.119.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Int Panis L, de Geus B, Vandenbulcke G, et al. Exposure to particulate matter in traffic: A comparison of cyclists and car passengers. Atmos Environ. 2010;44:2263–2270. [Google Scholar]
- 7.Daigle CC, Chalupa DC, Gibb FR, et al. Ultrafine particle deposition in humans during rest and exercise. Inhal Toxicol. 2003;15:539–552. doi: 10.1080/08958370304468. [DOI] [PubMed] [Google Scholar]
- 8.Sallis JF, Bull F, Guthold R, et al. Progress in physical activity over the Olympic Quadrennium. The Lancet. 2016;388:1325–1336. doi: 10.1016/S0140-6736(16)30581-5. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . World Health Organization; Geneva: 2021. WHO global air quality guidelines: particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide. [PubMed] [Google Scholar]
- 10.Sun S, Cao W, Qiu H, et al. Benefits of physical activity not affected by air pollution: A prospective cohort study. Int J Epidemiol. 2020;49:142–152. doi: 10.1093/ije/dyz184. [DOI] [PubMed] [Google Scholar]
- 11.Kim SR, Choi S, Keum N, Park SM. Combined effects of physical activity and air pollution on cardiovascular disease: A population-based study. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.013611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo C, Zeng Y, Chang LY, et al. Independent and opposing associations of habitual exercise and chronic PM2.5 exposures on hypertension incidence. Circulation. 2020;142:645–656. doi: 10.1161/CIRCULATIONAHA.120.045915. [DOI] [PubMed] [Google Scholar]
- 13.Fisher JE, Loft S, Ulrik CS, et al. Physical activity, air pollution, and the risk of asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;194:855–865. doi: 10.1164/rccm.201510-2036OC. [DOI] [PubMed] [Google Scholar]
- 14.Andersen ZJ, de Nazelle A, Mendez MA, et al. A study of the combined effects of physical activity and air pollution on mortality in elderly urban residents: The Danish Diet, Cancer, and Health Cohort. Environ Health Perspect. 2015;123:557–563. doi: 10.1289/ehp.1408698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SR, Choi S, Kim K, et al. Association of the combined effects of air pollution and changes in physical activity with cardiovascular disease in young adults. Eur Heart J. 2021;42:2487–2497. doi: 10.1093/eurheartj/ehab139. [DOI] [PubMed] [Google Scholar]
- 16.Pelliccia A, Sharma S, Gati S, et al. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2021;42:17–96. doi: 10.1093/eurheartj/ehaa735. [DOI] [PubMed] [Google Scholar]
- 17.Yang X, Li J, Hu D, et al. Predicting the 10-year risks of atherosclerotic cardiovascular disease in Chinese population: The China-PAR project (prediction for ASCVD risk in China) Circulation. 2016;134:1430–1440. doi: 10.1161/CIRCULATIONAHA.116.022367. [DOI] [PubMed] [Google Scholar]
- 18.Liang F, Xiao Q, Huang K, et al. The 17-y spatiotemporal trend of PM2.5 and its mortality burden in China. Proc Natl Acad Sci U S A. 2020;117:25601–25608. doi: 10.1073/pnas.1919641117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang F, Liu F, Huang K, et al. Long-term exposure to fine particulate matter and cardiovascular disease in China. J Am Coll Cardiol. 2020;75:707–717. doi: 10.1016/j.jacc.2019.12.031. [DOI] [PubMed] [Google Scholar]
- 20.Hersbach H, Bell B, Berrisford P, et al. The ERA5 global reanalysis. Q J Roy Meteor Soc. 2020;146:1999–2049. [Google Scholar]
- 21.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 22.Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. JAMA. 2002;288:1994–2000. doi: 10.1001/jama.288.16.1994. [DOI] [PubMed] [Google Scholar]
- 23.Perloff D, Grim C, Flack J, et al. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460–2470. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Hoek G, Chang LY, et al. Particulate matter air pollution, physical activity and systemic inflammation in Taiwanese adults. Int J Hyg Environ Health. 2018;221:41–47. doi: 10.1016/j.ijheh.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Tainio M, Jovanovic Andersen Z, Nieuwenhuijsen MJ, et al. Air pollution, physical activity and health: A mapping review of the evidence. Environ Int. 2021;147 doi: 10.1016/j.envint.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.An R, Zhang S, Ji M, Guan C. Impact of ambient air pollution on physical activity among adults: A systematic review and meta-analysis. Perspect Public Health. 2018;138:111–121. doi: 10.1177/1757913917726567. [DOI] [PubMed] [Google Scholar]
- 27.Cohen AJ, Brauer M, Burnett R, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the global burden of diseases study 2015. The Lancet. 2017;389:1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tainio M, de Nazelle AJ, Götschi T, et al. Can air pollution negate the health benefits of cycling and walking? Prev Med. 2016;87:233–236. doi: 10.1016/j.ypmed.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hou J, Liu X, Tu R, et al. Long-term exposure to ambient air pollution attenuated the association of physical activity with metabolic syndrome in rural Chinese adults: A cross-sectional study. Environ Int. 2020;136 doi: 10.1016/j.envint.2020.105459. [DOI] [PubMed] [Google Scholar]
- 30.Swain DP, Franklin BA. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol. 2006;97:141–147. doi: 10.1016/j.amjcard.2005.07.130. [DOI] [PubMed] [Google Scholar]
- 31.Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320:2020–2028. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization . World Health Organization; Geneva: 2020. WHO guidelines on physical activity and sedentary behaviour. [PubMed] [Google Scholar]
- 33.Giles LV, Brandenburg JP, Carlsten C, Koehle MS. Physiological responses to diesel exhaust exposure are modified by cycling intensity. Med Sci Sports Exerc. 2014;46:1999–2006. doi: 10.1249/MSS.0000000000000309. [DOI] [PubMed] [Google Scholar]
- 34.Fiuza-Luces C, Santos-Lozano A, Joyner M, et al. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat Rev Cardiol. 2018;15:731–743. doi: 10.1038/s41569-018-0065-1. [DOI] [PubMed] [Google Scholar]
- 35.Giles LV, Koehle MS. The health effects of exercising in air pollution. Sports Med. 2014;44:223–249. doi: 10.1007/s40279-013-0108-z. [DOI] [PubMed] [Google Scholar]
- 36.Oravisjärvi K, Pietikäinen M, Ruuskanen J, Rautio A, Voutilainen A, Keiski RL. Effects of physical activity on the deposition of traffic-related particles into the human lungs in silico. Sci Total Environ. 2011;409:4511–4518. doi: 10.1016/j.scitotenv.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 37.General Administration of Sport of China. Survey report of exercise activities for the general public. 2014. Available at: Http://www.Sport.Gov.Cn/n16/n1077/n1422/7300210.Html?From=singlemessage&isappinstalled=0. [accessed 28.12.2020] [in Chinese].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.