Abstract
Background:
Gait recovery is one of the primary goals of stroke rehabilitation. Gait independence is a key functional component of independent activities in daily living and social participation. Therefore, early prediction of gait independence is essential for stroke rehabilitation. Trunk function is important for recovery of gait, balance, and lower extremity function. The Trunk Impairment Scale (TIS) was developed to assess trunk impairment in patients with stroke.
Objective:
To evaluate the predictive validity of the TIS for gait independence in patients with acute stroke.
Methods:
A total of 102 patients with acute stroke participated in this study. Every participant was assessed using the TIS, Stroke Impairment Assessment Set (SIAS), and Functional Independence Measure (FIM) within 48 h of stroke onset and at discharge. Gait independence was defined as FIM gait scores of 6 and 7. Multiple regression analysis was used to predict the FIM gait score, and multiple logistic regression analysis was used to predict gait independence. Cut-off values were determined using receiver operating characteristic (ROC) curves for variables considered significant in the multiple logistic regression analysis. In addition, the area under the curve (AUC), sensitivity, and specificity were calculated.
Results:
For the prediction of the FIM gait score at discharge, the TIS at admission showed a good-fitting adjusted coefficient of determination (R2 = 0.672, p < 0.001). The TIS and age were selected as predictors of gait independence. The ROC curve had a TIS cut-off value of 12 points (sensitivity: 81.4%, specificity: 79.7%) and an AUC of 0.911. The cut-off value for age was 75 years (sensitivity: 74.6%, specificity: 65.1%), and the AUC was 0.709.
Conclusion:
The TIS is a useful early predictor of gait ability in patients with acute stroke.
Keywords: acute stroke, gait, predictive validity, prognostic prediction, Trunk Impairment Scale (TIS)
Introduction
Gait recovery is a primary goal of stroke rehabilitation. Therefore, early prediction of gait independence is important for rehabilitation. Using neuroimaging, clinical studies have shown that the size of the brain lesion in stroke affects gait recovery.1,2 Studies on the prognostic value of gait have reported effects on lower limb muscle strength, balance, and trunk function.3–5 Gait disturbances in patients with stroke are caused by weakness (paresis or paralysis), abnormal tone in the limbs or trunk, or by disturbances in the sensory-motor system or central control mechanisms.6 Trunk control is an essential component of functional gait.7
In stroke rehabilitation, trunk control is a crucial element of motor activity for performing many functional tasks.8 A role for compensatory activation of noncrossing pathways in the recovery of trunk function has been suggested.9,10 Clinical assessment tools to evaluate trunk function after stroke have been the subject of several systematic reviews.11,12 Fujiwara et al.8 developed their Trunk Impairment Scale (TIS) to assess trunk function from a functional perspective and evaluated its psychometric properties. Many previous reports on trunk dysfunction after stroke have analyzed patient outcomes several weeks after stroke onset,13,14 and not from the acute early onset. This may be due to the lack of an established method for acutely assessing the functional aspects of trunk dysfunction in patients with stroke.
Early inpatient rehabilitation can improve mortality and lessen the severity of disability.15,16 A study on the length of hospital stays and outcomes of patients with stroke using the Uniform Data System for Medical Rehabilitation database reported that the hospital stay length decreased from an average of 19.6 days (±12.8 days) to 16.5 days (±9.8 days) over an 8-year study period.17 In the future, early rehabilitation interventions will become more important as the length of hospital stay is further reduced. Hence, early prediction of prognosis is necessary.
Our research question was: what is the predictive validity of the TIS for gait independence at hospital discharge when performed within 48 h of acute stroke onset? Thus, this study aimed to evaluate the predictive validity of the TIS for gait independence in patients with acute stroke.
Materials and methods
Participants
Patients who were admitted to the hospital for cerebral infarction or hemorrhage between April 2020 and December 2021 were recruited. Before the illness, the patient was able to walk and take care of himself independently. Severity of illness at admission was assessed by the National Institutes of Health Stroke Scale (NIHSS).18 The inclusion criteria were as follows: (1) the initial diagnosis of unilateral stroke was based on confirmation by computed tomography or magnetic resonance imaging of the brain, (2) rehabilitation began within 48 h of stroke onset, and (3) the level of consciousness was ‘awake without stimulation’. The exclusion criteria were impaired consciousness, obvious bone deformity, previous surgery, worsening stroke, or death. A worsening stroke was defined as an increase of ≧4 from the after emergency transport NIHSS score.19
The purpose of the study was explained to the participants, and written informed consent was obtained. For patients who were physically unable to sign, written informed consent was provided by a family member or an authorized representative. This study was approved by the Ethical Review Committee of SHIODA Medical Corporation (Chiba, Japan) (approval number: 201911). All procedures associated with this study were conducted in accordance with the principles of the Declaration of Helsinki.
Methods
The main characteristics of the participants are shown in Table 1. Basic demographic information, such as age, sex, and length of stay (LOS), was collected from electronic medical records. For stroke severity, the NIHSS18 was used. The TIS (Fujiwara version),8 which consists of seven items, was used to assess trunk function. Abdominal muscle strength and verticality items were derived from the Stroke Impairment Assessment Set (SIAS). The other five items, which were originally developed for the TIS, consist of the perception of trunk verticality, trunk rotation muscle strength on both the affected and unaffected sides, and righting reflexes on both the affected and unaffected sides. The Fujiwara version has been shown to have high reliability and validity. The scores ranged from a minimum of 0 to a maximum of 21. Motor function on the affected side was assessed using the Stroke Impairment Assessment Set Motor Items (SIAS-M).20 The SIAS-M is used to evaluate the proximal and distal motor functions of the upper and lower limbs (5 items, 25 points in total), with a high score indicating high motor function. The Functional Independence Measure (FIM)21 was used to evaluate gait function. Mobility is generally scored by the most frequently used mode of mobility, walking or wheelchair. The criterion was based on which mode would be used primarily at the time of hospital discharge. Independent gait was defined as an FIM gait item score of 6 or 7. For this study, scoring of the FIM gait items was performed on admission and at discharge. The same examiner performed the initial (within 48 h of stroke onset) and final evaluations (the day before discharge).
Table 1.
Participant characteristics.
| Number of participants (male/female) | 102 (57/45) | |
| Age (years old, median, IQR) | 78 (70.0–83.0) | |
| Length of stay (median days, IQR) | 20 (16.0–27.0) | |
| Cerebral infarction | 75 | |
| Cerebral hemorrhage | 27 | |
| Paralyzed side (Rt/Lt) | 51/51 | |
| Admission | Discharge | |
|---|---|---|
| NIHSS (median, IQR) | 6.5 (4–13) | 4 (3–7.8) |
| TIS (median, IQR) | 12 (6–14) | 17 (14–18.8) |
| SIAS-M (median, IQR) | 14 (7.4–18) | 18 (13–21) |
| FIM gait item score (median, IQR) | 1 (1–2) | 5 (2–6) |
FIM, Functional Independence Measure; IQR, interquartile range; Lt, left side; NIHSS, National Institutes of Health Stroke Scale; Rt, right side; SIAS-M, Stroke Impairment Assessment Set Motor Item; TIS, Trunk Impairment Scale.
Statistical analysis
The Shapiro–Wilk test was performed before each test to determine whether all variables followed a normal distribution. Correlation analysis using Spearman’s rank correlation coefficient was performed to investigate the presence or absence of variables with an absolute value ⩾0.9 for the correlation coefficient r, considering the problem of multicollinearity. We investigated the presence or absence of variables with a variance inflation factor ⩾10.
Stepwise multiple regression analysis was performed to investigate the predictive validity of the TIS for independent gait. The dependent variable was the FIM gait score at discharge, and explanatory variables were significantly correlated with age, LOS, FIM gait score, NIHSS, TIS, and SIAS-M at admission.
The Mann–Whitney U test was then performed for age, LOS, FIM gait item scores, NIHSS, TIS, and SIAS-M at admission, and χ2 for Disease type, Sex, and Paralyzed side to compare the results between the independent and nonindependent gait groups. To predict gait independence at discharge, we performed multiple logistic regression analysis using the method of variable increase by the likelihood ratio, with independent gait and nonindependent gait (independent, 0; nonindependent, 1) as dependent variables, and using the items that showed a significant difference in the univariate analysis as independent variables. The area under the curve (AUC), sensitivity, and specificity were calculated for the variables selected as significant in the multiple logistic regression analysis using receiver operating characteristic (ROC) curves, and the cut-off values were determined. IBM SPSS Statistics for Windows, version 28 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses. The significance level was set at 5%.
Results
Relationship between the FIM gait score and each item
The correlation coefficients between the FIM gait item score and each item are listed in Table 2. The correlation between the FIM gait item score at discharge and the TIS at admission was quite strong.
Table 2.
Relationship between FIM gait score and each item.
| Age | LOS | NIHSS score at admission | FIM gait item score at admission | TIS at admission | SIAS-M at admission | NIHSS score at discharge | FIM gait item score at discharge | TIS at discharge | SIAS-M at discharge | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | – | |||||||||
| LOS | 0.18 | – | ||||||||
| NIHSS score at admission | −0.038 | 0.311** | – | |||||||
| FIM gait item score at admission | 0.083 | −0.095 | −0.522** | – | ||||||
| TIS at admission | −0.220* | −0.418** | −0.788** | 0.379** | – | |||||
| SIAS-M at admission | −0.005 | −0.415** | −0.802** | 0.393** | 0.851** | – | ||||
| NIHSS score at discharge | −0.181 | −0.266** | 0.900** | −0.556** | −0.770** | −0.752** | – | |||
| FIM gait item score at discharge | −0.252* | −0.248* | −0.653** | 0.343** | 0.800** | 0.691** | −0.678** | – | ||
| TIS at discharge | −0.377** | −0.382** | −0.685** | 0.244** | 0.838** | 0.736** | −0.665** | 0.774** | – | |
| SIAS-M at discharge | −0.097 | −0.376** | −0.761** | 0.328** | 0.836** | 0.921** | −0.744** | 0.752** | 0.804** | – |
FIM, Functional Independence Measure; LOS, length of stay; NIHSS, National Institutes of Health Stroke Scale; SIAS-M, Stroke Impairment Assessment Set Motor Item; TIS, Trunk Impairment Scale.
p < 0.01; *p < 0.05.
Predictive validity of the TIS for gait independence
We performed multiple regression analysis with the discharge FIM gait score as the dependent variable, and age, LOS, NIHSS at admission, TIS, and SIAS-M as the independent variables. We found that only the TIS on admission was extracted, with a good-fitting adjusted coefficient of determination (Table 3).
Table 3.
Results of the stepwise multiple regression analysis.
| Dependent variable | Independent variables analyzed | Nonstandard coefficient | Standard coefficient | t value | p value | Adjusted R2 | |
|---|---|---|---|---|---|---|---|
| B | SE | β | |||||
| Discharge FIM gait item score | Constant | 0.649 | 0.275 | 2.356 | 0.02 | 0.672 | |
| TIS at admission | 0.345 | 0.024 | 0.822 | 5.127 | <0.001 | ||
FIM, Functional Independence Measure; SE, standard error; TIS, Trunk Impairment Scale.
Comparison of gait independent and gait nonindependent groups
Of the 102 participants, 43 were in the independent gait group and 59 were in the nonindependent gait group. The results of the univariate analysis are presented in Table 4 showing significant differences were found in age, sex, and the NIHSS, TIS, and SIAS-M at hospital admission.
Table 4.
Comparison between gait independent and nonindependent groups.
| Survey item | Gait independent group (n = 43) | Gait nonindependent group (n = 59) | p value |
|---|---|---|---|
| Cerebral hemorrhage/cerebral infarction | 9/34 | 18/41 | 0.279 |
| Age (years old, median, IQR) | 71.0 (66.0–80.5) | 80.0 (75.5–84.0) | <0.001 |
| Sex (male/female) | 29/14 | 28/31 | 0.045 |
| Length of stay (median, IQR) | 19.0 (16.0–25.0) | 21 (17.0–30.0) | 0.068 |
| Paralyzed side (Rt/Lt) | 24/19 | 27/32 | 0.316 |
| FIM gait item score at admission (median, IQR) | 1.0 (1.0–2.0) | 1.0 (1.0–1.0) | 0.103 |
| NIHSS at admission (median, IQR) | 5.0 (4.0–6.0) | 11.0 (5.0–15.5) | <0.001 |
| TIS at admission (median, IQR) | 15.0 (13.0–16.0) | 7.0 (3.0–12.0) | <0.001 |
| SIAS-M at admission (median, IQR) | 18.0 (14.5–20) | 8.0 (4.0–15.0) | <0.001 |
FIM, Functional Independence Measure; IQR, interquartile range; Lt, left side; NIHSS, National Institutes of Health Stroke Scale; Rt, right side; SIAS-M, Stroke Impairment Assessment Set Motor Item; TIS, Trunk Impairment Scale.
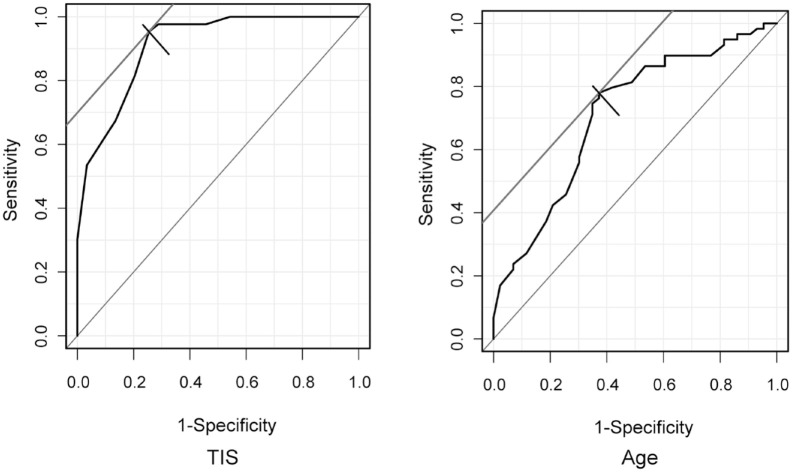
The results of the multiple logistic regression analysis are shown in Table 5. For the results, TIS and age were selected. TIS had a greater impact on gait independence/nonindependence than age. The results of the Hosmer–Lemeshow test showed good predictive accuracy. It was also correctly predicted by discriminant analysis. The results of the ROC analysis of the two items selected for multiple logistic regression analysis showed that TIS was the evaluation method with the highest sensitivity and specificity, which also showed high accuracy in AUC (Table 6). ROC curves for TIS and age are shown in Figure 1.
Table 5.
Results of logistic regression analysis.
| Partial regression coefficient | p value | Odds ratio | 95% confidence interval | |
|---|---|---|---|---|
| TIS | −0.677 | <0.001 | 0.508 | 0.376–0.687 |
| Age | 0.120 | 0.004 | 1.127 | 1.039–1.222 |
Model χ2 test p < 0.01, Hosmer–Lemeshow p = 0.928, median of discrimination 82.4%.
TIS, Trunk Impairment Scale.
Table 6.
Cut-off values and results of sensitivity, specificity, and the AUC to discriminate between independent and nonindependent gait.
| Cut-off value | Sensitivity (%) | Specificity (%) | AUC | |
|---|---|---|---|---|
| TIS | 12.0 | 81.4 | 79.7 | 0.911 |
| Age | 75.0 | 74.6 | 65.1 | 0.709 |
AUC, area under the curve; TIS, Trunk Impairment Scale.
Figure 1.
ROC curves for TIS and age. ROC curves for the TIS and age in discriminating gait independence. The cut-off value for TIS was 12.0 points (sensitivity, 81.4%; specificity, 79.7%), and AUC was 0.911. The cut-off value for age was 75.0 years (sensitivity, 74.6%; specificity, 65.1%), and the AUC was 0.709.
AUC, area under the curve; ROC, receiver operating characteristic; TIS, Trunk Impairment Scale.
Discussion
In this study, we evaluated the predictive validity of the TIS for gait independence in patients with acute stroke. For the prediction of the FIM gait score at discharge, the TIS at admission was evaluated by multiple regression analysis. Furthermore, the TIS at admission and age was selected as predictors of gait independence using multiple logistic regression analysis. The TIS was found to be a useful early predictor of gait ability in patients with acute stroke.
Poststroke dysfunction is significantly affected by poor gait ability. Most patients with stroke state that improving gait function is a priority goal. Rehabilitation can help maximize functional independence.22 However, LOS in acute care hospitals is limited, decreasing the time available for acute rehabilitation. The Organisation for Economic Cooperation and Development reports that the reduction in average LOS is due to the use of less invasive surgical procedures and the expansion of early discharge programs that allow patients to return home for postdischarge care.23 In the future, the LOS in acute care hospitals is expected to be further shortened. We believe that early prognosis determination will become important in the appropriate selection of a hospital/facility for post-acute care.
Early rehabilitation interventions have a significant effect on prognosis.24–26 Previous reports on the prognosis of gait have studied other predictors including the Berg Balance Scale, which assesses combined balance function; Fugl–Meyer scale, which assesses lower limb motor paralysis; and the TIS (Verheyden version), which assesses trunk function.3,5,27 Trunk performance is significantly associated with balance and gait, clearly demonstrating that trunk function is impaired in patients with non-acute and chronic stroke.5 However, in several previous studies, assessments were performed 2 weeks after stroke onset, with results revealing a prognosis of 3 or 6 months.13,14 To the best of our knowledge, no study has examined the relationship between variables assessed within 48 h of onset and gait ability at approximately 3 weeks after onset, as in this study.
In this study, the TIS version developed by Fujiwara et al.8 was used to assess trunk function within 48 h of stroke onset. A portion of this TIS includes the recognition of trunk vertical perception, righting reflexes, and trunk rotation force. The verticality of the trunk is important for maintaining vertical posture, the ability to induce a righting reflex is required for maintaining dynamic sitting balance, and abdominal strength is essential for the transfer from the supine to sitting position.8 A previous study using the TIS in patients within 48 h of stroke onset showed a high adjusted coefficient of determination for predicting FIM-M at discharge.28
Decreased trunk function is a major functional impairment resulting in abnormal gait parameters after stroke.29,30 Correlations have been found between trunk function, FIM scores, and gait speed.10 In this study, TIS and FIM gait item scores were significantly correlated at discharge, but not at admission. This may be due to the patient’s symptoms not stabilizing within 48 h of admission, resulting in a limitation of gait activities due to safety. Multiple regression analysis with the FIM gait item scores at discharge as the dependent variable showed that the TIS was a good predictor in patients with acute stroke.
The results of the two-group comparison between the independent and nonindependent gait groups showed significant differences in age, sex, TIS, and SIAS-M. Age and level of motor impairment have been shown to be significant predictors of gait outcomes.31 Knee extensor muscle strength has been found to be closely related to gait ability in patients with stroke.32,33 AUC values for patients with stroke using knee extensor muscle strength have been shown to have moderate accuracy in identifying gait independence.34 The proximal part of the trunk must be stabilized to allow the lower limbs to move well. Moreover, the trunk is an important segment of the body for control and mobility. Good trunk movement control is a prerequisite for controlling finger and foot movements and balance. In addition, good trunk control facilitates functional mobility and gait after stroke.6,35
In our study, the results of the multiple logistic regression analysis showed that two items, age and TIS, were selected, and that the TIS had a greater influence than age. We developed ROC curves representing the relationship between sensitivity and specificity with respect to the TIS and age, determined the cut-off values, and further calculated the AUCs. Results showed that the TIS was the most accurate outcome predictor between the independent and nonindependent gait groups and that it demonstrated high accuracy in discriminating gait independence. Our results suggest that the TIS, which can be easily assessed in an acute care setting, is a good predictor of gait ability and is of high clinical significance in rehabilitation. Moreover, the TIS assessment of trunk function in patients with stroke within 48 h of onset is sufficient to predict gait independence. Safely assessing trunk function at the bedside of patients with acute stroke is possible, even if they have difficulty maintaining a sitting position.
This study has several limitations. First, results may differ regarding the improvement of physical function due to differences in idle patient time outside of training (rehabilitation), duration of training, and content. Second, this study was conducted in patients with acute stroke at the same institution. Third, we did not consider the effects of changing assessors. In the future, external criterion-related validity and cross-validity should be examined regarding the validity of the TIS for the evaluation of gait ability in patients with stroke after discharge from acute care hospitals and after returning home.
Conclusion
The results suggest that TIS, which assesses trunk function in acute stroke patients, is sufficient in predicting gait independence. This study is novel because there is currently no established method to evaluate trunk dysfunction in patients with acute stroke from a functional perspective. The TIS, which can be easily evaluated at the bedside of patients with acute stroke without the use of special equipment, is considered to be of high clinical significance in rehabilitation. In addition, early rehabilitation can effectively improve mortality and disability levels in these patients.
Acknowledgments
We would like to express sincere gratitude to the members of the Laboratory of Rehabilitation Medicine, Juntendo University Graduate School of Medicine, for their guidance and encouragement. We would also like to thank the Shioda Medical Corporation for their support in conducting this study.
Footnotes
ORCID iD: Masahiro Ishiwatari  https://orcid.org/0000-0001-8564-4793
https://orcid.org/0000-0001-8564-4793
Contributor Information
Masahiro Ishiwatari, Department of Rehabilitation Medicine, Graduate School of Medicine, Juntendo University, 2-1-1 Hongo, Bunkyo, Tokyo 113-8421, Japan; Department of Rehabilitation, Kiminomori Rehabilitation Hospital, Chiba, Japan.
Mami Tani, Department of Rehabilitation Medicine, Graduate School of Medicine, Juntendo University, Tokyo, Japan.
Reina Isayama, Department of Rehabilitation Medicine, Graduate School of Medicine, Juntendo University, Tokyo, Japan.
Kaoru Honaga, Department of Rehabilitation Medicine, Graduate School of Medicine, Juntendo University, Tokyo, Japan.
Masato Hayakawa, Shioda Medical Corporation, Chiba, Japan.
Tomokazu Takakura, Department of Rehabilitation Medicine, Graduate School of Medicine, Juntendo University, Tokyo, Japan.
Akira Tanuma, Department of Rehabilitation Medicine, Graduate School of Medicine, Juntendo University, Tokyo, Japan.
Akihiro Kurosu, Department of Rehabilitation Medicine, Graduate School of Medicine, Juntendo University, Tokyo, Japan.
Kozo Hatori, Department of Rehabilitation Medicine, Graduate School of Medicine, Juntendo University, Tokyo, Japan.
Futoshi Wada, Department of Rehabilitation Medicine, Graduate School of Medicine, Juntendo University, Tokyo, Japan.
Toshiyuki Fujiwara, Department of Rehabilitation Medicine, Graduate School of Medicine, Juntendo University, Tokyo, Japan; Department of Physical Therapy, Faculty of Health Science, Juntendo University, Tokyo, Japan.
Declarations
Ethics approval and consent to participate: The purpose of the study was explained to the participants and written informed consent was obtained. For patients who were physically unable to sign, written informed consent was provided by a family member or an authorized representative. This study was approved by the Ethical Review Committee of SHIODA Medical Corporation (Chiba, Japan; approval number: 201911). All procedures associated with this study were conducted in accordance with the principles of the Declaration of Helsinki.
Consent for publication: Not applicable.
Author contributions: Masahiro Ishiwatari: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Resources; Writing – original draft; Writing – review & editing.
Mami Tani: Conceptualization; Formal analysis; Investigation; Methodology; Software; Validation.
Reina Isayama: Conceptualization; Formal analysis; Investigation; Methodology; Software; Validation.
Kaoru Honaga: Conceptualization; Formal analysis; Investigation; Methodology; Software; Validation.
Masato Hayakawa: Conceptualization; Investigation; Validation.
Tomokazu Takakura: Conceptualization; Investigation; Methodology; Validation.
Akira Tanuma: Conceptualization; Formal analysis; Methodology; Software; Validation.
Akihiro Kurosu: Conceptualization; Methodology; Validation.
Kozo Hatori: Conceptualization; Methodology; Validation.
Futoshi Wada: Conceptualization; Formal analysis; Investigation; Methodology; Software; Validation.
Toshiyuki Fujiwara: Conceptualization; Formal analysis; Methodology; Project administration; Software; Supervision; Visualization; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: The data that support the findings of this study are available from the corresponding author, [M.I], upon reasonable request.
References
- 1. Alexander LD, Black SE, Patterson KK, et al. Association between gait asymmetry and brain lesion location in stroke patients. Stroke 2009; 40: 537–544. [DOI] [PubMed] [Google Scholar]
- 2. Lee KB, Kim JS, Hong BY, et al. Brain lesions affecting gait recovery in stroke patients. Brain Behav 2017; 7: e00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verheyden G, Nieuwboer A, De Wit L, et al. Trunk performance after stroke: an eye catching predictor of functional outcome. J Neurol Neurosurg Psychiatry 2007; 78: 694–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Masiero S, Avesani R, Armani M, et al. Predictive factors for ambulation in stroke patients in the rehabilitation setting: a multivariate analysis. Clin Neurol Neurosurg 2007; 109: 763–769. [DOI] [PubMed] [Google Scholar]
- 5. Verheyden G, Vereeck L, Truijen S, et al. Trunk performance after stroke and the relationship with balance, gait and functional ability. Clin Rehabil 2006; 20: 451–458. [DOI] [PubMed] [Google Scholar]
- 6. Karthikbabu S, Nayak A, Vijayakumar K, et al. Comparison of physio ball and plinth trunk exercises regimens on trunk control and functional balance in patients with acute stroke: a pilot randomized controlled trial. Clin Rehabil 2011; 25: 709–719. [DOI] [PubMed] [Google Scholar]
- 7. Kim TJ, Seo KM, Kim DK, et al. The relationship between initial trunk performances and functional prognosis in patients with stroke. Ann Rehabil Med 2015; 39: 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fujiwara T, Liu M, Tsuji T, et al. Development of a new measure to assess trunk impairment after stroke (Trunk Impairment Scale): its psychometric properties. Am J Phys Med Rehabil 2004; 83: 681–688. [DOI] [PubMed] [Google Scholar]
- 9. Fujiwara T, Sonoda S, Okajima Y, et al. The relationships between trunk function and the findings of transcranial magnetic stimulation among patients with stroke. J Rehabil Med 2001; 33: 249–255. [DOI] [PubMed] [Google Scholar]
- 10. Tsuji T, Liu M, Hase K, et al. Trunk muscles in persons with hemiparetic stroke evaluated with computed tomography. J Rehabil Med 2003; 35: 184–188. [DOI] [PubMed] [Google Scholar]
- 11. Sorrentino G, Sale P, Solaro C, et al. Clinical measurement tools to assess trunk performance after stroke: a systematic review. Eur J Phys Rehabil Med 2018; 54: 772–784. [DOI] [PubMed] [Google Scholar]
- 12. Lima E, Teixeira-Salmela LF, Simões L, et al. Assessment of the measurement properties of the post stroke motor function instruments available in Brazil: a systematic review. Braz J Phys Ther 2016; 20: 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petrilli S, Durufle A, Nicolas B, et al. Prognostic factors in the recovery of the ability to walk after stroke. J Stroke Cerebrovasc Dis 2002; 11: 330–335. [DOI] [PubMed] [Google Scholar]
- 14. Duarte E, Marco E, Muniesa JM, et al. Trunk control test as a functional predictor in stroke patients. J Rehabil Med 2002; 34: 267–272. [DOI] [PubMed] [Google Scholar]
- 15. Kalra L, Langhorne P. Facilitating recovery: evidence for organized stroke care. J Rehabil Med 2007; 39: 97–102. [DOI] [PubMed] [Google Scholar]
- 16. Lin IH, Tsai HT, Wang CY, et al. Effectiveness and superiority of rehabilitative treatments in enhancing motor recovery within 6 months poststroke: a systemic review. Arch Phys Med Rehabil 2019; 100: 366–378. [DOI] [PubMed] [Google Scholar]
- 17. Granger CV, Markello SJ, Graham JE, et al. The Uniform Data System for Medical Rehabilitation: report of patients with stroke discharged from Comprehensive Medical Programs in 2000–2007. Am J Phys Med Rehabil 2009; 88: 961–972. [DOI] [PubMed] [Google Scholar]
- 18. NIH Stroke Scale. National Institute of Neurological Disorders and Stroke (NINDS), Bethesda, MD, https://www.stroke.nih.gov/documents/NIH_Stroke_Scale_508C.pdf
- 19. Kim YD, Choi HY, Jung YH, et al. The ischemic stroke predictive risk score predicts early neurological deterioration. J Stroke Cerebrovasc Dis 2016; 25: 819–824. [DOI] [PubMed] [Google Scholar]
- 20. Chino N, Sonoda S, Domen K, et al. Stroke Impairment Assessment Set (SIAS). In: Chino N, Melvin JL. (eds) Functional evaluation of stroke patients. Tokyo, Japan: Springer-Verlag, 1996, pp. 19–31. [Google Scholar]
- 21. Fiedler RC, Granger CV. The Functional Independence Measure: a measurement of disability and medical rehabilitation. In: Chino N, Melvin JL. (eds) Functional evaluation of stroke patients. Tokyo, Japan: Springer-Verlag, 1996, pp. 75–92. [Google Scholar]
- 22. Müller M, Grill E, Stier-Jarmer M, et al. Validation of the comprehensive ICF Core Sets for patients receiving rehabilitation interventions in the acute care setting. J Rehabil Med 2011; 43: 92–101. [DOI] [PubMed] [Google Scholar]
- 23. Organisation for Economic Co-operation and Development (OECD). Health at a glance 2019: OECD indicators, OECD Publishing, Paris, 2019. [Google Scholar]
- 24. Bernhardt J, Dewey H, Thrift A, et al. A very early rehabilitation trial for stroke (AVERT): phase II safety and feasibility. Stroke 2008; 39: 390–396. [DOI] [PubMed] [Google Scholar]
- 25. Diserens K, Moreira T, Hirt L, et al. Early mobilization out of bed after ischaemic stroke reduces severe complications but not cerebral blood flow: a randomized controlled pilot trial. Clin Rehabil 2012; 26: 451–459. [DOI] [PubMed] [Google Scholar]
- 26. Luft AR, Kesselring J. Critique of A Very Early Rehabilitation Trial (AVERT). Stroke 2016; 47: 291–292. [DOI] [PubMed] [Google Scholar]
- 27. Gianella MG, Gath CF, Bonamico L, et al. Prediction of gait without physical assistance after inpatient rehabilitation in severe subacute stroke subjects. J Stroke Cerebrovasc Dis 2019; 28: 104367. [DOI] [PubMed] [Google Scholar]
- 28. Ishiwatari M, Honaga K, Tanuma A, et al. Trunk impairment as a predictor of activities of daily living in acute stroke. Front Neurol 2021; 12: 665592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dickstein R, Shefi S, Marcovitz E, et al. Electromyographic activity of voluntarily activated trunk flexor and extensor muscles in post-stroke hemiparetic subjects. Clin Neurophysiol 2004; 115: 790–796. [DOI] [PubMed] [Google Scholar]
- 30. Saeys W, Vereeck L, Truijen S, et al. Randomized controlled trial of truncal exercises early after stroke to improve balance and mobility. Neurorehabil Neural Repair 2012; 26: 231–238. [DOI] [PubMed] [Google Scholar]
- 31. Sánchez-Blanco I, Ochoa-Sangrador C, López-Munaín L, et al. Predictive model of functional independence in stroke patients admitted to a rehabilitation programme. Clin Rehabil 1999; 13: 464–475. [DOI] [PubMed] [Google Scholar]
- 32. Suzuki K, Imada G, Iwaya T, et al. Determinants and predictors of the maximum walking speed during computer-assisted gait training in hemiparetic stroke patients. Arch Phys Med Rehabil 1999; 80: 179–182. [DOI] [PubMed] [Google Scholar]
- 33. Bohannon RW. Knee extension power, velocity and torque: relative deficits and relation to walking performance in stroke patients. Clin Rehabil 1992; 6: 125–131. [Google Scholar]
- 34. Akazawa N, Okawa N, Tamura K, et al. Determining the cut-off value for knee extensor strength for identifying independence in gait in chronic stroke survivors. J Rehabil Med 2017; 49: 765–767. [DOI] [PubMed] [Google Scholar]
- 35. Jijimol G, Fayaz RK, Vijesh PV. Correlation of trunk impairment with balance in patients with chronic stroke. Neurorehabilitation 2013; 32: 323–325. [DOI] [PubMed] [Google Scholar]