Abstract
Methylene blue (MB) is an effective treatment for methemoglobinemia, ifosfamide-induced encephalopathy, cyanide poisoning, and refractory vasoplegia. However, clinical case reports and preclinical studies indicate potentially neurotoxic activity of MB at certain concentrations. The exact mechanisms of MB neurotoxicity are not known, and while the effects of MB on neuronal tissue from different brain regions and myenteric ganglia have been examined, its effects on primary afferent neurons from dorsal root ganglia (DRG) have not been studied. Mouse DRG were exposed to MB (0.3–10 μM) in vitro to assess neurite outgrowth. Increasing concentrations of MB (0.3–10 μM) were associated with neurotoxicity as shown by a substantial loss of cells with neurite formation, particularly at 10 μM. In parallel experiments, cultured rat DRG neurons were treated with MB (100 μM) to examine how MB affects electrical membrane properties of small-diameter sensory neurons. MB decreased peak inward and outward current densities, decreased action potential amplitude, overshoot, afterhyperpolarization, increased action potential rise time, and decreased action potential firing in response to current stimulation. MB induced dose-dependent toxicity in peripheral neurons, in vitro. These findings are consistent with studies in brain and myenteric ganglion neurons showing increased neuronal loss and altered membrane electrical properties after MB application. Further research is needed to parse out the toxicity profile for MB to minimize damage to neuronal structures and reduce side effects in clinical settings.
Keywords: methylene blue, neurotoxicity, neurite, dorsal root ganglion, dorsal root ganglion, immunocytochemistry
Introduction
The aniline-based dye methylene blue (MB) was originally intended for use in the textile industry, but was later found to be useful in the laboratory as a marker for cell viability and Gram staining of microorganisms and in clinical settings as an antiseptic.1 MB has been widely used for a variety of clinical conditions, including methemoglobinemia, ifosfamide-induced encephalopathy,2 refractory vasoplegia,3 cyanide poisoning,4 and for a variety of surgical procedures such as staining to map sentinel lymph nodes for biopsy in breast cancer,5 epithelial staining prior to biopsy collection during endoscopy for identification of tissue metaplasia or dysplasia in Barrett’s esophagus,6 visualizing abnormalities in bladder mucosa of patients with a history of bladder cancer,7 and visually confirming urine flow after percutaneous kidney stone removal.8 In addition, there are 52 clinical trials registered in the United States (clinicaltrials.gov; National Institute of Health) to investigate the clinical utility of MB for a variety of disorders, including depression,9,10 bipolar disorder,11 Alzheimer’s disease,12 and Parkinson’s disease.13 Indeed, MB has been included in the World Health Organization14’s list of essential medications. MB has been shown to inhibit arterial smooth muscle relaxation in response to vasodilators.15,16 Although some studies suggest that this is mediated through inhibition of guanylate cyclase, its inhibition of nitric oxide synthesis is more potent and direct.15,17-20 MB is also a cholinesterase inhibitor with some affinity for muscarinic binding sites,21 and its metabolite, Azure B, is a potent monoamine oxidase inhibitor.22
Although MB has been generally considered to be a safe drug, there have been several reports of cytotoxicity and neurotoxicity. These include hemolytic anemia,23 phototoxicity,24 and central nervous system apoptosis.25 One case report described the rapid onset of pain and paresthesia, followed by eventual paralysis and loss of vision and smell after lumbar subarachnoid administration of MB, findings which were associated with markers of inflammation in the spinal fluid.26 A neuromuscular assessment in a patient who experienced radiculomyelopathy after lumbar MB indicated the presence of denervation.27 Paralysis and leptomeningeal inflammation following epidural MB in cats were associated with inflammation of myelin sheaths and nerve roots, also suggesting potential for peripheral nervous system damage.28 The precise cellular and molecular mechanisms responsible for MB-induced peripheral neurotoxicity remains unknown. Given the widespread use of this drug, it is important to elucidate mechanisms and conditions underlying neurotoxicity. Therefore, we determined the acute effect of MB on the structure and membrane electrical properties of cultured rodent dorsal root ganglia (DRG) neurons using a wide range of MB concentrations. Cultured rodent DRG neurons have been used previously to assess the toxicity of chemotherapy drugs by quantifying differences in neuronal morphology, including neurite outgrowth, following drug exposure.29,30 By quantifying the proportion of cells that develop neurite outgrowth following exposure to varying concentrations of MB, a dose-response curve can be generated to determine the physiological effects of MB on neuronal health. As neurite outgrowth requires complex coordination between the growth cone and environmental signals, neurite outgrowth was used as an indicator of toxicity following exposure to MB.31, 32 In addition, we performed patch clamp electrophysiology on cultured rat DRG neurons to determine if exposure to MB altered membrane electrical properties.
Materials and methods
Subjects
All experimental protocols were approved by the Institutional Animal Care and Use Committees at the University of Minnesota and at the University of Texas MD Anderson Cancer Center. All studies adhered to the NIH Guide for the Care and Use of Laboratory Animals.
For immunocytochemistry experiments, adult male C3H mice (n = 3) were obtained from Charles River (Kingston, NY) and housed in temperature- and light-controlled (12-h light/dark cycle) conditions with food and water available ad libitum.
For patch clamp electrophysiology studies, adult Sprague-Dawley rats of both sexes (n = 4) were obtained from Harlan (Houston, TX) and housed in temperature- and light-controlled (12-h light/dark cycle) conditions with food and water available ad libitum.
Drugs
A 1 mM stock solution of MB (Sigma Aldrich) was prepared in 50 mL PBS at pH 7.4. After mixing for 1 h, the solution was filtered through a 0.22 μm filter. For experiments using mouse DRG neurons, a 100X solution was made from the 1 mM stock using phosphate-buffered saline (PBS), then further diluted using PBS, before the final dilution in culture media to 0.3 μM, 1 μM, 3 μM, and 10 μM. PBS only was added to the 0 μM control wells. For rat DRG neuron electrophysiology studies, the 1 mM stock was diluted using PBS and before the final dilution in extracellular bath solution to 100 μM.
Cell culture
Cultures of DRGs were prepared as described previously with slight modifications.33 Laminin (Sigma) and Voller’s Carbonate Buffer (1:20) were added to each well of an 8-well slide (Fisher) and incubated at 4°C overnight. The next day, mice (n = 3) were anesthetized with isoflurane then decapitated and sanitized using 70% ethanol. DRGs (10–12) were removed and placed into Puck’s solution (without Ca2+, Mg2+) on ice and nerve roots were removed if necessary. In a flow hood, tissue was transferred to media containing 1:1 Ham’s F-12/DMEM supplemented with 100 mM L-glutamine (Sigma) and 0.41 U/mL Collagenase-D (Roche) and placed in a humidified incubator with 5% CO2 at 37°C for 1 h. The media was decanted, replaced, and incubation continued for another hour. The laminin-coated wells were transferred to the same incubator at this time. The media and DRG tissue were then transferred to a sterile 15 mL tube using a large glass Pasteur pipette. The tissue was gently triturated 5 times, then spun down at 1000 r/min for 5 min at 4°C. The media was removed and replaced by the final media, which contained 1:1 Ham’s F-12/DMEM supplemented with 40 mM glucose (Sigma), 4 mM L-glutamine, 5% Horse Serum (Gibco), 100U/mL penicillin/100 μg/mL streptomycin (Gibco), and a large Pasteur pipette was used to triturate 10 times before a second centrifugation. In a sterile tube, 1.5 mg/mL of DNase 1 (Sigma) was added to final media. The final media was then replaced with the final media + DNAse 1 solution and the DRG tissue triturated 30 times each with the large Pasteur pipette followed by a small Pasteur pipette. Laminin was decanted from the 8-well slides, and between 150 and 300 μL of final media was added to each of the laminin-coated wells along with the dissociated DRG tissue until a confluence of 40–60% was achieved. MB in PBS (pH 7.4) was added for a final concentration of 0 μM, 0.3 μM, 1 μM, 3 μM, or 10 μM. Cells were then incubated at 37°C in 5% CO2 for 16 h. The dose-response experiment was repeated twice with pooled suspension of 10–12 DRGs from 1 or 2 mice.
DRG immunocytochemistry
Following overnight incubation, culture media was removed and cells were fixed in 4% paraformaldehyde for 30 min. The slides were then washed with PBS 3 times for 10 min each time, and 300 μL of block solution (PBS, 0.3% Triton X-100, 5% Normal Donkey Serum) was added and the cells incubated for one hour. The block solution was decanted, and plastic wells removed. Cells were then incubated overnight in a humidifying chamber with 50 μL of primary antibody solution (PBS, 0.003% Triton X-100, 5% normal donkey serum) with anti-PGP 9.5 (AbCam 1:200). The next day, the primary antibody solution was removed, and the wells washed 3 times with PBS for 10 min. Cells were then incubated for 1 h with a secondary antibody solution (1:500 donkey anti-rabbit Cy3, Jackson Laboratories). After the solution was removed, the slides were washed 3 times with PBS for 10 min. The gel around each well was removed and the cells dehydrated by placing them in increasing concentrations of ethanol, starting at 50%, then 70%, 80%, 90%, 95%, and 100%, for 5 min each. The slide was then cleared using xylene before DPX Mountant (Sigma) and a glass cover slip were applied. Dried slides were imaged using a Leica Thunder microscope. The whole area of each well was imaged so that all DRG cells were captured. Cells were counted manually. Following a method similar to that of previous literature, DRG neurons were distinguished from debris based on their size and shape, similar to those described previously.33 Neurons were classified based on the presence or absence of neurites. As multiple cells would occasionally clump together, these groups were always counted as only two cells. Any cells overhanging the edge of a picture were not counted, as neurite outgrowth could have been missed.
Each slide was digitized and split up into a series of images that could then be manually counted while the experimenter was blinded to condition. Initially, multiple blinded researchers counted the DRG neurons in each set of images and the counts were averaged between observers. No significant variations in DRG neuron counts were observed among the researchers, and thus a single individual counted the remaining well slides.
DRG electrophysiology
Glass coverslips (12 mm diameter) were added to the chambers of a six-well plate, coated with poly-L-lysine and placed in an incubator overnight. The plate was washed with distilled water, and the coverslips positioned in the center of each well and the plate placed in an incubator. Adult male (n = 2) and female (n = 2) Sprague-Dawley rats were deeply anesthetized with SomnaSol (pentobarbital 390 mg/mL and phenytoin 50 mg/mL) and perfused with chilled saline on ice. Both pairs of the L4 and L5 DRG were excised and placed in a culture dish containing trypsin (0.0625 mg/mL, Hyclone) and type IA collagenase (1 mg/mL, Sigma-Aldrich) in Dulbecco’s Modified Eagle Medium (DMEM). The dish was shaken in a heated chamber for 50 min at 37°C. The cells were then washed and mechanically dispersed. The suspension was filtered through a 70 μm cell strainer and centrifuged at 180 RCF for 5–7 min at 23°C. The supernatant was removed and the cell pellet resuspended in 600 μL of warm culture media (DMEM with 10% fetal bovine serum). The 6-well plate containing poly-L-lysine-coated wells was removed from the incubator. Glass cylinders (8 mm height, 6 mm inner diameter, 8 mm outer diameter) were placed in the center of each glass coverslip, and 100 μL of cell suspension was added to each. Warmed culture media (500 μL) was then placed into each well outside the glass cylinder. After 40 min, the cylinders were carefully removed, and the plate was incubated overnight.
Whole cell patch recording was performed on rat DRG neurons as described previously.34 Glass coverslips were lifted and transferred to a recording chamber and perfused at 2 mL/min with oxygenated (95% O2 + 5% CO2) extracellular solution containing 117 mM NaCl, 3.6 mM KCl, 1.2 mM NaH2PO4·H2O, 2.5 mM CaCl2, 1.2 mM MgCl2, 25 mM NaHCO3 and 11 mM glucose adjusted to pH 7.4 with NaOH. Glass micropipettes (6–8 MΩ) were filled with an internal solution of 135 mM K-gluconate, 5 mM KCl, 5 mM Mg-ATP, 0.5 mM Na2GTP, 5 mM HEPES, 2 mM MgCl2, 5 mM EGTA, and 0.5 mM CaCl2 adjusted to pH 7.4 with KOH and an osmolarity of 290–300 mOsm. Only small-diameter (≤ 30 μm) neurons with a resting membrane potential of at least −40 mV, stable baseline recordings, and evoked spikes that overshot 0 mV were used for further experiments and analysis. Series resistance (Rs) was compensated to above 70%. All recordings were made at room temperature. Whole cell recordings were completed within 20–28 h after plating. DRG neurons were held at 0 pA to record spontaneous activity for 5 min. Neurons were then held at −60 mV, and activation was evoked with a 15 ms step to potentials ranging from −50 to +90 mV in 10 mV increments. Current density was calculated by normalizing maximal peak currents with cell capacitance. This was followed by a series of 300 ms depolarizing current injections in 10 pA steps, starting from −50 pA until an action potential was evoked. The current that induced the first action potential was defined as the current threshold (1X rheobase). Neurons were then stimulated with 2 s current injections at 1X, 2X, and 3X rheobase. MB (100 μM) was then perfused into the bath solution at 2 mL/min for 10 min. After a 10 min washout period, post-drug maximal peak currents, current threshold, and responses to current stimulation were assessed.
Statistical analysis
All data are expressed as percentages or mean ± standard error of the mean. For DRG immunocytochemistry experiments, the proportion of neurons with neurites present or absent was calculated for each MB concentration (0–10 μM) and expressed as a percentage of the total number of cells. The half maximal effective concentration (EC50), or concentration that produced 50% of neurons with an absence of neurites, was calculated using probit analysis (see https://probitanalysis.wordpress.com/for formulae), from which regression analysis was used to derive EC50 values.35
For DRG neuron patch clamp electrophysiology studies, peak inward current density (pA/pF) and peak outward current density (pA/pF) were compared before and after application of MB using repeated measures analysis of variance (ANOVA), with treatment and stimulus level as within-subjects factors. Bonferroni t-tests were used to identify significant differences between groups. Current thresholds (rheobase, in pA), resting membrane potential (RMP, in mV), and action potential (AP) characteristics at rheobase were compared before and after treatment with MB using dependent t-tests. Responses to current stimulation at 1X, 2X and 3X rheobase were compared using repeated measures ANOVA (followed by Bonferroni t-tests) with treatment and stimulus level (1X, 2X, and 3X) as within-subjects factors. For all analyses, p < 0.05 was considered significant.
Results
MB produced concentration-dependent decrease in neurite outgrowth in DRG neurons
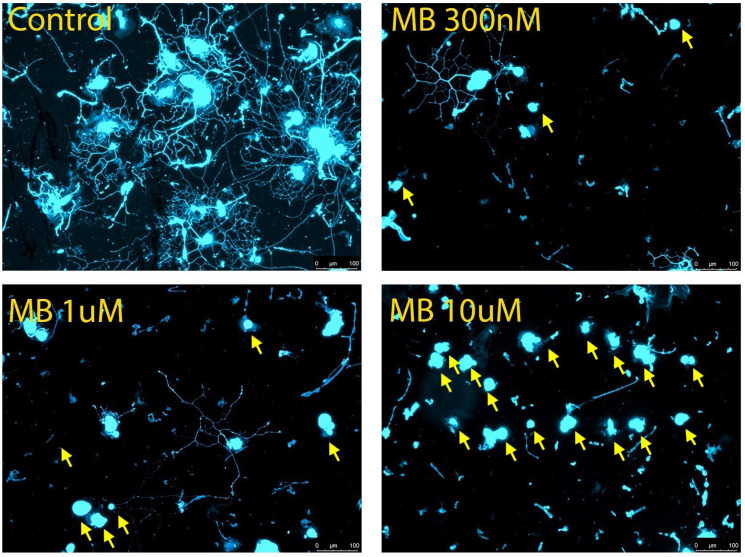
Differences in proportion of neurons with neurite outgrowth was used as the parameter to evaluate neurotoxicity.36 MB was administered at concentrations ranging from 0.3 to 10 μM. The half maximal effective concentration (EC50) for neurite growth inhibition was determined from the concentration response curve for the proportion of cells without neurite extensions. In dissociated mouse DRGs, the concentration of MB that produced the highest level of cytotoxicity was 10 μM, the highest concentration used. At this concentration, nearly all observed cells were found to be devoid of neurite outgrowth (Figure 1). The percentage of DRG neurons with neurites ranged from a high of approximately 50% in the 0 μM control wells to a low of about 2% with the 10 μM MB concentration (Table 1).
Figure 1.
Cytotoxicity of methylene blue in cultured mouse dorsal root ganglion (DRG) neurons. Approximately 1.1 × 103 DRG cells were seeded in each well of an 8 well slide along with methylene blue at different concentrations for 16 h. Immunocytochemistry was performed using anti-PGP 9.5 and neurite outgrowth in each well viewed under fluorescence microscope. Representative images for each concentration except 3 μM are shown: (A) control, 0 μM; (B) MB 0.3 μM; (C) MB 1 μM; (D) MB 10 μM. Neurons without neurite extensions are indicated by yellow arrows. Scale bar represents 100 μm.
Table 1.
Cell counts for cultured mouse DRG neurons after Methylene Blue treatment.
| Methylene blue concentration, μM | Average % with neurites present | Average % with neurites absent |
|---|---|---|
| 0 | 44.625 | 55.375 |
| 0.3 | 42.995 | 56.675 |
| 1 | 25.41 | 74.59 |
| 3 | 13.21 | 86.845 |
| 10 | 2.245 | 97.755 |
The control wells showed similar neurite outgrowth to those seen in previous studies using ex vivo mouse DRGs.33 Although neurite length was not measured quantitatively, a trend was observed that increasing concentrations of MB resulted in generally shorter and less dense neurite outgrowth.
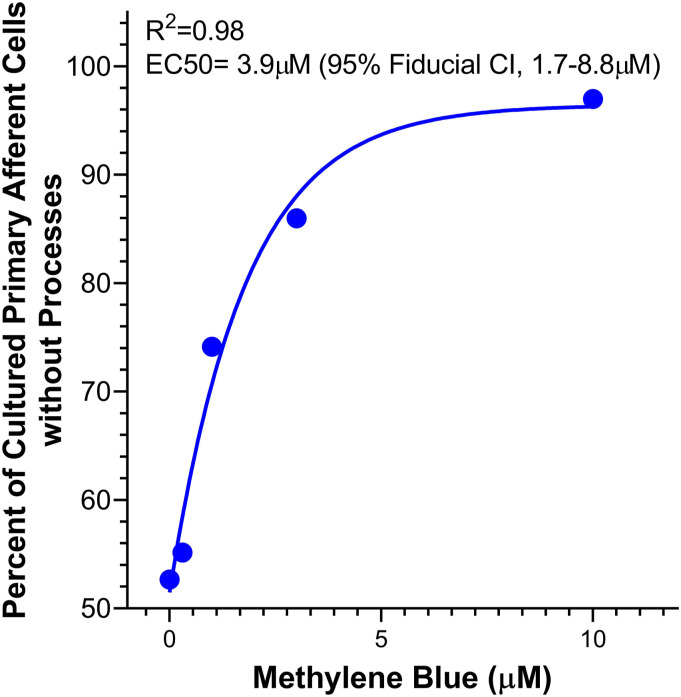
The EC50 of MB was found to be 3.9 μM based on a regression analysis comparing the number of cells devoid of neurite outgrowth to the number of cells without neurite outgrowth in the control wells (Figure 2). This was done by normalizing the number of neurons with neurites absent to that of the control as a baseline survival rate. While in the first trial the number of cells added to each well was 5 × 10,2 and in the second trial approximately 1 × 103 cells were added to each well, there was no statistically difference in the ratio of neurons with neurites present or absent between the two trials.
Figure 2.
Concentration response curve for the proportion of DRG cells without neurite processes used to calculate the EC50 concentration of methylene blue.
MB decreased peak inward and outward current density and altered AP characteristics in cultured rat DRG neurons
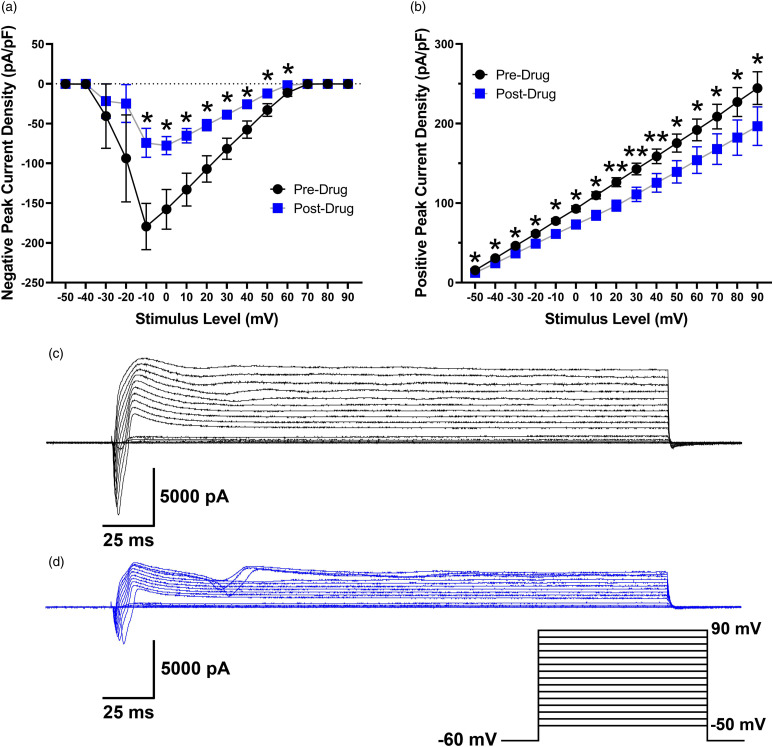
Given the acute nature of the electrophysiology experiments, we used a higher concentration of MB (100 μM for 10 min) compared to that used in culture (0.3 μM–10 μM for 16 h) based on previous reports indicating that acute effects of MB on rat hippocampal neurons were detectable at 10 μM with a stronger impact on sodium currents at 100 μM. Peak inward current density (pA/pF) decreased after bath application of 100 μM MB (F1,6 = 7.8, p = 0.031). Post-hoc tests showed that this difference was significant from −10 mV to 60 mV (Figure 3(a)). Peak outward current density (pA/pF) also significantly decreased following exposure to MB (F1,6 = 13.3, p = 0.011). Significant differences occurred for all step levels (Figure 3(b)). Representative traces of current responses before and after MB application are shown in Figure 3(c) and (d). Whereas outward current density decreased after MB, mean resting membrane potential was not altered (−53.9 ± 1.9 mV to −52.5 ± 2.3 mV; t9 = 0.66, p = 0.529). Action potential characteristics were calculated at rheobase before and after exposure to MB (Table 2).
Figure 3.
Peak negative (A) and positive (B) current density (pA/pF) in rat DRG neurons before and after a 10 min application of MB (n = 7). Traces represent current evoked by command voltage step from −60 mV (holding potential) to test potentials between −50 and 90 mV in 10-mV increments as shown in the inset for a rat DRG neuron before (C) and after (D) application of MB. *p < 0.05, **p < 0.01.
Table 2.
Action potential characteristics of cultured rat DRG neurons before and after a 10 min application of Methylene Blue (100 μM).
| Dorsal root ganglion neuron action potential characteristics | ||||
|---|---|---|---|---|
| Pre-drug | Post-drug | Significance | ||
| Current threshold (pA) | 84.5 ± 26.5 (11) | 74.5 ± 27.9 (11) | n.S. (p = 0.38) | |
| AP amplitude (mV) | 97.4 ± 2.7 (10) | 76.4 ± 5.4 (10)*** | p < 0.005 | |
| AP rise time (ms) | 2.8 ± 0.3 (10) | 4.7 ± 0.4 (10)*** | p < 0.005 | |
| AP fall time (ms) | 14.7 ± 2.3 (10) | 20.9 ± 4.7 (10) | n.S. (p = 0.08) | |
| Width at 0 mV (ms) | 6.2 ± 0.8 (10) | 7.2 ± 1.4 (10) | n.S. (p = 0.22) | |
| AP overshoot (mV) | 52.4 ± 2.9 (10) | 32.4 ± 6.3 (10)** | p < 0.01 | |
| AP afterhyperpolarization (mV) | 11.2 ± 2.9 (10) | 4.5 ± 1.8 (10)** | p < 0.01 | |
| Threshold potential (mV) | −12.6 ± 2.1 (10) | −15.0 ± 4.2 (10) | ns (p = 0.44) | |
Data presented as mean ± SEM, with the number of neurons in parentheses. AP: action potential. **p < 0.01, ***p < 0.005.
MB decreased AP amplitude, AP overshoot, and AP afterhyperpolarization (AHP), and increased AP rise time. Current thresholds, AP fall time, AP width at 0 mV, and AP threshold potential were not altered by MB. Representative examples of individual neurons are shown in Figure 4(a) and (b). Both showed a blunted AP amplitude post-drug, and Figure 4(b) also highlights the decrease in AP afterhyperpolarization.
Figure 4.
Action potential (AP) characteristics in rat DRG neurons (n = 10) before and after a 10 min application of methylene blue (100 μM). Overlapping raw traces from two neurons, one with a shoulder on the falling phase of the action potential (A) and one without (B).
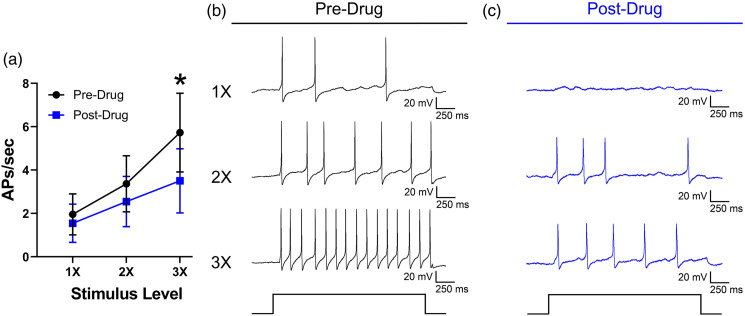
Responses to current stimulation were decreased overall following MB, but the main effect was not significant (F1,10 = 3.8, p = 0.080). However, there was a significant drug x stimulus level interaction (F2,20 = 4.8, p = 0.020). Bonferroni tests indicated that responses following MB application were lower at 3X rheobase (Figure 5(a)). Representative traces of a neuron responding to current stimulation before and after application of MB are shown in Figure 5(b) and (c).
Figure 5.
(A) Mean responses of rat DRG neurons (n = 11) to current stimulation expressed as the number of APs per second at 1X, 2X, and 3X rheobase before and after a 10 min application of methylene blue (100 μM). Traces represent responses to 1X, 2X, and 3X rheobase current stimulation before (B) and after (C) methylene blue application. *p < 0.05.
Discussion
Our results show that exposure to 0.3–10 mM MB produced concentration-dependent absence of neurites in cultured mouse DRG neurons. Although the exact etiology is unclear, our results suggest that clinical application of high concentration MB in peripheral nervous tissue is contraindicated. For most clinical applications, a 1% concentration of MB is used for tissue infiltration, which is equivalent to 31.26 mM. Our results are in agreement with previous studies that showed intravenous administration of MB produced neuronal apoptosis in the cerebral cortex of rats,25 and application of MB in vitro led to cell death in rat hippocampal slice preparations and neurite retraction in cultured neurons at concentrations between 1 and 10 μM after only a 2-h exposure.25 Our results are also consistent with clinical studies showing toxicity following MB administration. For example, MB infusion during parathyroidectomy surgery caused prolonged disorientation in the postoperative period.37-43 Lumbar intrathecal administration of MB was followed by paraplegia and spinal cord necrosis.44 Epidural administration of MB also induced paraplegia and inflammation of the spinal cord and nerve roots in cats28 and quadriplegia associated with spinal inflammation in a patient.26 The intravenous administration of MB caused toxic encephalopathy-like symptoms, including confusion, aphasia, and disorientation.37-43,45 The potential neurotoxic side effects of MB are further supported by our observation that acute treatment with MB impacted membrane electrical properties of cultured rat DRG neurons, with changes in excitability after only 10 min of exposure. MB decreased both positive and negative peak current densities, decreased AP amplitude, AP overshoot, and AP AHP, increased AP rise time, and decreased responses to current stimulation. Notably, while we were able to obtain data 10 min after application of MB, our attempts to assess neuronal activity at 30 min were hindered as the ability to generate an AP was lost or the quality of the patch degraded rapidly, suggesting that acute MB is also neurotoxic at this concentration. We did not assess whether lower concentrations of MB produced a similar effect, and future studies should investigate whether concentrations below the EC50 of 3.9 μM that we observed in cultured DRGs could minimize its effect on membrane electrical properties in an acute setting. Our results are in line with a previously published report using a lower concentration of MB (10 μM), that showed decreased AP amplitude, AP overshoot, and firing frequency in rat hippocampal CA1 pyramidal neurons.46 In addition, the amplitude of INa following MB was reduced following application of 10 and 100 μM, suggesting that our results could be partially explained by inhibition of voltage-gated sodium channels. We observed decreased peak current density in the current study, but interpretation of these results is limited as we did not test for effects on specific ion channel populations. Changes in neuronal excitability following MB were also found in guinea pig small intestinal myenteric ganglion neurons. In those studies, MB decreased hyperpolarizing after-potentials, depolarized resting membrane potential, and increased input resistance, effects which were partially mediated by the inhibition of inward calcium fluxes.47 A study in Xenopus oocytes expressing the α7 subunit of the human nicotinic acetylcholine receptor showed that MB inhibited currents induced by acetylcholine with a half-maximal inhibitory concentration (IC50) of 3.4 ± 0.3 μM without affecting endogenous Ca2+-dependent Cl− channels.48 Nicotinic acetylcholine receptor activity in rat CA1 hippocampal pyramidal neurons was also inhibited by 3 μM MB.48 In the current study, we preferentially studied small-diameter rat DRG neurons, which tend to be nociceptive in nature and exhibit distinct calcium channel function in comparison to medium- and large-diameter neurons in rats, which represent a more heterogenous population of nociceptive and non-nociceptive cells.49-52 Future studies should also explore the effect of MB on the membrane electrical properties of these medium- and large-diameter neurons.
In contrast to our results, neuroprotection has been associated with MB .1,13,53-55 For example, MB was effective as a prophylactic treatment for ifosfamide-induced encephalopathy.56 In a rat model of traumatic brain injury, 1 mg/kg intravenous MB before or after brain injury attenuated deficits in responses to tactile and proprioceptive stimulation of the contralateral fore and hind paws.57 In studies in vitro, MB (0.05–1 μM) increased survival of cerebellar granule neurons in response to mitochondrial toxins.58 Low concentrations of MB (alone or combined with near-infrared light) protected against neuronal degeneration and improved memory function through effects on mitochondrial electron transport chains.53,59 Opposing neurological effects of low and high concentrations complicates the clinical picture for the use of MB.53 As noted above, a generally higher concentration of MB (up to 1%, equivalent to 31.26 mM) is commonly used in most clinical settings.60,61
One limitation in the current study is that our experiments were performed using cultured DRG cells. It has been shown that many mRNAs are expressed in the native tissue, but not in cultured cells.62 We also cannot discount the potential impact of cells that are not present in cultured DRG tissue, such as circulating immune cells that exist in low numbers in naive tissue that can be activated and infiltrate into DRG and peripheral nerves in response to nerve injury or inflammation, such as macrophages.63 In this regard, the presence of non-neuronal cells, which could include satellite glial cells, fibroblasts, and a small amount of immune cells (generally representing less than 1% of cells in naïve mouse DRG tissue), helped mitigate the cytotoxic effects of the MB in the present study, and as the native tissues are much more densely packed in vivo, this could also impact the effective EC50. An EC50 does not describe an absolute value of toxicity, but instead represents acute toxicity given the exact conditions of the experiment.36 As such, it is an inherently variable parameter. Given this, accuracy is not able to be quantified and significant importance should be placed on the precision of the measurement.64 Further studies are needed to determine dose–response relationships for MB in order to mitigate its neurotoxicity in light of its usefulness in clinical settings.
Significance
An overwhelming number of previous studies emphasized neuroprotective effects of MB in contrast to results presented in this study and others.25,26,28,37-47 It is likely that MB is neuroprotective at lower dose and toxic at higher doses. Since clinical application generally uses a MB concentration that is much higher than the highest in vitro concentration used in the present study (10 μM vs 31.26 mM, or 1%), our results are concerning for the safety profile of MB in clinical applications, particularly those in which MB is administered close to peripheral neurons, such as intradiscal,65,66 sacroiliac joint and facet joint injections,67 epidural application after open discectomy,68 and percutaneous injection close to peripheral nerves.69 In pharmacokinetic studies using healthy volunteers, an intravenous bolus of MB at 1.4 mg/kg produced a mean plasma concentration of 5 μM70, and a bolus of 100 mg MB produced blood concentrations between 1 and 10 μM during the first 30 min after administration.71 There are two important limitations in our study, however. First, there may be species differences in MB induced toxicity between rodents and humans. Second, our experiments were done in vitro, and whether a similar concentration of MB will produce neurotoxicity in vivo is not clear. In conclusion, considering the results presented in this study, use of MB in neuronal structures requires caution and should only be used after a risk-benefit evaluation.
Acknowledgements
The authors thank Yan Li, PhD (University of Texas MD Anderson Cancer Center, Houston, TX, USA), and Patrick Dougherty, PhD (University of Texas MD Anderson Cancer Center, Houston, TX, USA) for feedback and guidance.
Footnotes
Author Contributions: Megan L. Uhelski: Conceptualization, Methodology, Formal analysis, Investigation, Writing-Original Draft, Writing-Review & Editing, Review & Editing, Visualization. Malcolm E. Johns: Conceptualization Methodology, Formal analysis, Investigation, Writing- Original Draft, Writing-Review & Editing, Visualization. Alec Horrmann: Investigation, Formal analysis, Sadiq Mohamed: Investigation, Formal analysis, Ayesha Sohail: Investigation, Formal analysis, Iryna A. Khasabova: Formal analysis, Writing-Review & Editing This author helped with interpretation of data and writing the manuscript. Donald A. Simone: Formal analysis, Writing-Review & Editing, Funding acquisition Ratan K. Banik: Conceptualization, Methodology, Formal analysis, Investigation, Writing-Original Draft, Writing-Review & Editing, Visualization, Supervision, Funding acquisition, Project Administration.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Department of Anesthesiology, University of Minnesota and Fairview Medical Center, and by NIH grant CA241627 (DAS).
ORCID iDs
Ayesha Sohail https://orcid.org/0000-0001-8597-6122
Ratan K Banik https://orcid.org/0000-0001-7126-5768
References
- 1.Oz M, Lorke DE, Hasan M, Petroianu GA. Cellular and molecular actions of Methylene Blue in the nervous system. Med Research Reviews 2011; 31: 93–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pelgrims J, De Vos F, Van den Brande J, Schrijvers D, Prové A, Vermorken J. Methylene blue in the treatment and prevention of ifosfamide-induced encephalopathy: report of 12 cases and a review of the literature. Br Journal Cancer 2000; 82: 291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Habib AM, Elsherbeny AG, Almehizia RA. Methylene blue for vasoplegic syndrome postcardiac surgery. Indian J Crit Care Med 2018; 22: 168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanzlik P. Methylene blue as antidote for cyanide poisoning. JAMA: J Am Med Assoc 1933; 100: 357–357. [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Chen X, Qi M, Li Y. Sentinel lymph node biopsy mapped with methylene blue dye alone in patients with breast cancer: a systematic review and meta-analysis. PLoS One 2018; 13: e0204364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dave U, Shousha S, Westaby D. Methylene blue staining: is it really useful in Barrett's esophagus? Gastrointest Endoscopy 2001; 53: 333–335. [DOI] [PubMed] [Google Scholar]
- 7.Mufti G, Shah P, Parkinson M, Riddle P. Diagnosis of clinically occult bladder cancer by in vivo staining with methylene blue. Br Journal Urology 1990; 65: 173–175. [DOI] [PubMed] [Google Scholar]
- 8.Truesdale MD, Elmer-Dewitt M, Sandri M, Schmidt B, Metzler I, Gadzinski A, Stoller ML, Chi T. Methylene blue injection as an alternative to antegrade nephrostography to assess urinary obstruction after percutaneous nephrolithotomy. J Endourology 2016; 30: 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delport A, Harvey BH, Petzer A, Petzer JP. Methylene blue and its analogues as antidepressant compounds. Metab Brain Disease 2017; 32: 1357–1382. [DOI] [PubMed] [Google Scholar]
- 10.Naylor G, Smith A, Connelly P. A controlled trial of methylene blue in severe depressive illness. Biol Psychiatry 1987; 22: 657–659. [DOI] [PubMed] [Google Scholar]
- 11.Narsapur S, Naylor G. Methylene blue. J Affective Disorders 1983; 5: 155–161. [DOI] [PubMed] [Google Scholar]
- 12.Atamna H, Kumar R. Protective role of methylene blue in Alzheimer's disease via mitochondria and cytochrome c oxidase. J Alzheimer's Dis 2010; 20: S439–S452. [DOI] [PubMed] [Google Scholar]
- 13.Smith ES, Clark ME, Hardy GA, Kraan DJ, Biondo E, Gonzalez-Lima F, Cormack LK, Monfils M, Lee HJ. Daily consumption of methylene blue reduces attentional deficits and dopamine reduction in a 6-OHDA model of Parkinson's disease. Neuroscience 2017; 359: 8–16. [DOI] [PubMed] [Google Scholar]
- 14.Organization WH . WHO Model List of Essential Medicines, 20th List, 2017. March 2017, amended August 2017. [Google Scholar]
- 15.Gruetter CA, Kadowitz PJ, Ignarro LJ. Methylene blue inhibits coronary arterial relaxation and guanylate cyclase activation by nitroglycerin, sodium nitrite, and amyl nitrite. Can Journal Physiology Pharmacology 1981; 59: 150–156. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y-X, Cheng X, Pang CCY. Vascular pharmacology of methylene blue in vitro and in vivo: a comparison with NG-nitro-l-arginine and diphenyleneiodonium. Br J Pharmacol 1995; 114: 194–202, DOI: 10.1111/j.1476-5381.1995.tb14925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo D, Das S, Vincent SR. Effects of methylene blue and LY83583 on neuronal nitric oxide synthase and NADPH-diaphorase. Eur J Pharmacol Mol Pharmacol 1995; 290: 247–251. [DOI] [PubMed] [Google Scholar]
- 18.Tesfamariam B, Cohen RA. Inhibition of adrenergic vasoconstriction by endothelial cell shear stress. Circ Research 1988; 63: 720–725. [DOI] [PubMed] [Google Scholar]
- 19.Mayer B, Brunner F, Schmidt K. Inhibition of nitric oxide synthesis by methylene blue. Biochem Pharmacology 1993; 45: 367–374. [DOI] [PubMed] [Google Scholar]
- 20.Mayer B, Brunner F, Schmidt K. Novel actions of methylene blue. Eur Heart Journal 1993; 14: 22–26. [PubMed] [Google Scholar]
- 21.Pfaffendorf M, Bruning T, Batink H, Van Zwieten P. The interaction between methylene blue and the cholinergic system. Br J Pharmacol 1997; 122: 95–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petzer A, Harvey BH, Wegener G, Petzer JP, Azure B. Azure B, a metabolite of methylene blue, is a high-potency, reversible inhibitor of monoamine oxidase. Toxicol Appl Pharmacol 2012; 258: 403–409. DOI: 10.1016/j.taap.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Liao Y-P, Hung D-Z, Yang D-Y. Hemolytic anemia after methylene blue therapy for aniline-induced methemoglobinemia. Vet Human Toxicology 2002; 44: 19–21. [PubMed] [Google Scholar]
- 24.Porat R, Gilbert S, Magilner D. Methylene blue-induced phototoxicity: an unrecognized complication. Pediatrics 1996; 97: 717–721. [PubMed] [Google Scholar]
- 25.Vutskits L, Briner A, Klauser P, Gascon E, Dayer AG, Kiss JZ, Muller D, Licker MJ, Morel DR. Adverse effects of methylene blue on the central nervous system. Anesthesiology 2008; 108: 684–692. [DOI] [PubMed] [Google Scholar]
- 26.Arieff AJ, Pyzik SW. Quadriplegia after intrathecal injection of methylene blue. J Am Med Assoc 1960; 173: 794–796. [DOI] [PubMed] [Google Scholar]
- 27.Schultz P, Schwarz GA. Radiculomyelopathy Following Intrathecal Instillation of Methylene Blue. Arch Neurology 1970; 22: 240–244. [DOI] [PubMed] [Google Scholar]
- 28.Poppers PJ, Mastri AR, Lebeoux M, Covino BG. The effect of methylene blue on neural tissue. Anesthesiology 1970; 33: 335–340. [DOI] [PubMed] [Google Scholar]
- 29.Ustinova EE, Shurin GV, Gutkin DW, Shurin MR. The role of TLR4 in the paclitaxel effects on neuronal growth in vitro. PLoS One 2013; 8: e56886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park SH, Eber MR, Fonseca MM, Patel CM, Cunnane KA, Ding H, Hsu F-C, Peters CM, Ko M-C, Strowd RE, Wilson JA, Hsu W, Romero-Sandoval EA, Shiozawa Y. Usefulness of the measurement of neurite outgrowth of primary sensory neurons to study cancer-related painful complications. Biochem Pharmacol 2021; 188: 114520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lowery LA, Vactor DV. The trip of the tip: understanding the growth cone machinery. Nat Reviews Mol Cell Biology 2009; 10: 332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dickson BJ, Senti K-A. Axon guidance: growth cones make an unexpected turn. Curr Biol 2002; 12: R218–R220. [DOI] [PubMed] [Google Scholar]
- 33.Scott BS. Adult mouse dorsal root ganglia neurons in cell culture. J Neurobiology 1977; 8: 417–427. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, North RY, Rhines LD, Tatsui CE, Rao G, Edwards DD, Cassidy RM, Harrison DS, Johansson CA, Zhang H, Dougherty PM. DRG voltage-gated sodium channel 1.7 is upregulated in paclitaxel-induced neuropathy in rats and in humans with neuropathic pain. J Neurosci 2018; 38: 1124–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akçay A. The Calculation of LD50 Using Probit Analysis. Wiley Online Library, 2013. [Google Scholar]
- 36.Krug AK, Balmer NV, Matt F, Schönenberger F, Merhof D, Leist M. Evaluation of a human neurite growth assay as specific screen for developmental neurotoxicants. Arch Toxicol 2013; 87: 2215–2231. DOI: 10.1007/s00204-013-1072-y. [DOI] [PubMed] [Google Scholar]
- 37.Bach KK, Lindsay FW, Berg LS, Howard RS. Prolonged postoperative disorientation after methylene blue infusion during parathyroidectomy. Anesth Analgesia 2004; 99: 1573–1574. [DOI] [PubMed] [Google Scholar]
- 38.Khan M, North A, Chadwick D. Prolonged postoperative altered mental status after methylene blue infusion during parathyroidectomy: a case report and review of the literature. Ann R Coll Surgeons Engl 2007; 89: W9–W11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kartha SS, Chris CE, Bumpous JM, Fleming M, Lentsch EJ, Flynn MB. Toxic metabolic encephalopathy after parathyroidectomy with methylene blue localization. Otolaryngology-Head Neck Surg 2006; 135: 765–768. [DOI] [PubMed] [Google Scholar]
- 40.Majithia A, Stearns M. Methylene blue toxicity following infusion to localize parathyroid adenoma. The J Laryngol Otology 2006; 120: 138–140. [DOI] [PubMed] [Google Scholar]
- 41.Martindale S, Stedeford J. Neurological sequelae following methylene blue injection for parathyroidectomy. Anaesthesia 2003; 58: 1041–1042. [DOI] [PubMed] [Google Scholar]
- 42.Sweet G, Standiford SB. Methylene-Blue-Associated Encephalopathy. J American College Surgeons 2007; 204: 454–458. [DOI] [PubMed] [Google Scholar]
- 43.Mihai R, Mitchell EW, Warwick J. Dose-response and postoperative confusion following methylene blue infusion during parathyroidectomy. Can J Anesthesia/Journal Canadien D'anesthésie 2007; 54: 79–81. [DOI] [PubMed] [Google Scholar]
- 44.Sharr MM, Weller RO, Brice JG. Spinal cord necrosis after intrathecal injection of methylene blue. J Of Neurol Neurosurg Psychiatry 1978; 41: 384–386. DOI: 10.1136/jnnp.41.4.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nadler JE, Green H, Rosenbaum A. Intravenous injection of methylene blue in man with reference to its toxic symptoms and effect on the electrocardiogram. Am J Med Sci 1934; 188: 15–21 [Google Scholar]
- 46.Zhang Y, Zhao J, Zhang T, Yang Z. In vitro assessment of the effect of methylene blue on voltage-gated sodium channels and action potentials in rat hippocampal CA1 pyramidal neurons. Neurotoxicology 2010; 31: 724–729. [DOI] [PubMed] [Google Scholar]
- 47.Nemeth P, Daly K, Erde S, Wood JD. Effects of methylene blue on electrical behavior of myenteric neurons. Experientia 1985; 41: 259–261. [DOI] [PubMed] [Google Scholar]
- 48.Al Mansouri AS, Lorke DE, Nurulain SM, Ashoor A, Yang Keun-Hang S, Petroianu G, Isaev D, Oz M. Methylene Blue Inhibits the Function of α7-Nicotinic Acetylcholine Receptors. CNS Neurol Disord - Drug Targets 2012; 11: 791–800. DOI: 10.2174/187152712803581010. [DOI] [PubMed] [Google Scholar]
- 49.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature 2001; 413: 203–210. DOI: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 50.Scroggs RS, Fox AP. Calcium current variation between acutely isolated adult rat dorsal root ganglion neurons of different size. J Physiol 1992; 445: 639–658. DOI: 10.1113/jphysiol.1992.sp018944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lawson SN, Waddell PJ. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J Physiol 1991; 435: 41–63. DOI: 10.1113/jphysiol.1991.sp018497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee KH, Chung K, Chung JM, Coggeshall RE. Correlation of cell body size, axon size, and signal conduction velocity for individually labelled dorsal root ganglion cells in the cat. J Comp Neurol 1986; 243: 335–346. DOI: 10.1002/cne.902430305. [DOI] [PubMed] [Google Scholar]
- 53.Rojas JC, Bruchey AK, Gonzalez-Lima F. Neurometabolic mechanisms for memory enhancement and neuroprotection of methylene blue. Prog Neurobiol 2012; 96: 32–45. DOI: 10.1016/j.pneurobio.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rojas JC, Simola N, Kermath BA, Kane JR, Schallert T, Gonzalez-Lima F. Striatal neuroprotection with methylene blue. Neuroscience 2009; 163: 877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rojas JC, John JM, Lee J, Gonzalez-Lima F. Methylene blue provides behavioral and metabolic neuroprotection against optic neuropathy. Neurotoxicity Research 2009; 15: 260–273. [DOI] [PubMed] [Google Scholar]
- 56.Turner AR, Duong CD, Good DJ. Methylene Blue for the Treatment and Prophylaxis of Ifosfamide-induced Encephalopathy. Clin Oncol 2003; 15: 435–439. DOI: 10.1016/S0936-6555(03)00114-6. [DOI] [PubMed] [Google Scholar]
- 57.Stelmashook EV, Genrikhs EE, Mukhaleva EV, Kapkaeva MR, Kondratenko RV, Skrebitsky VG, Isaev NK. Neuroprotective Effects of Methylene Blue In Vivo and In Vitro. Bull Exp Biol Med 2019; 167: 455–459. DOI: 10.1007/s10517-019-04548-3. [DOI] [PubMed] [Google Scholar]
- 58.Abe M, Endoh T, Suzuki T. Angiotensin II-induced ionic currents and signalling pathways in submandibular ganglion neurons. Arch Oral Biol 2003; 48: 401–413. DOI: 10.1016/S0003-9969(03)00041-4. [DOI] [PubMed] [Google Scholar]
- 59.Gonzalez-Lima F, Auchter A. Protection against neurodegeneration with low-dose methylene blue and near-infrared light. Front Cell Neurosci 2015; 9: 179. DOI: 10.3389/fncel.2015.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Özdemir A, Mayir B, Demirbakan K, Oygür N. Efficacy of methylene blue in sentinel lymph node biopsy for early breast cancer. Journal Breast Health 2014; 10: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dumbarton TC, Gorman SK, Minor S, Loubani O, White F, Green R. Local cutaneous necrosis secondary to a prolonged peripheral infusion of methylene blue in vasodilatory shock. Ann Pharmacotherapy 2012; 46: e6. [DOI] [PubMed] [Google Scholar]
- 62.Wangzhou A, McIlvried LA, Paige C, Barragan-Iglesias P, Guzman CA, Dussor G, Ray PR, Gereau RW, Price TJ. Transcriptomic analysis of native versus cultured human and mouse dorsal root ganglia focused on pharmacological targets. BioRxiv 2019; 2019: 766865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 2007; 10: 1361–1368. DOI: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 64.Walum E. Acute oral toxicity. Environ Health Perspect 1998; 106: 497–503. DOI: doi: 10.1289/ehp.98106497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo X, Ding W, Liu L, Yang S. Intradiscal Methylene Blue Injection for Discogenic Low Back Pain: A Meta-Analysis. Pain Pract 2019; 19: 118–129. DOI: 10.1111/papr.12725. [DOI] [PubMed] [Google Scholar]
- 66.Kim SH, Ahn SH, Cho YW, Lee DG. Effect of Intradiscal Methylene Blue Injection for the Chronic Discogenic Low Back Pain: One Year Prospective Follow-up Study. Ann Rehabil Med 2012; 36: 657–664. DOI: 10.5535/arm.2012.36.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parekh J, Eckmann M, Ramamurthy S. Case Series of Methylene Blue Injections for the Treatment of Zygapophysial and Sacroiliac Joint Pain: Results of 5 Cases. Open J Anesthesiology 2013; 03: 301–303. [Google Scholar]
- 68.Farrokhi MR, Lotfi M, Masoudi MS, Gholami M. Effects of methylene blue on postoperative low-back pain and functional outcomes after lumbar open discectomy: a triple-blind, randomized placebo-controlled trial. J Neurosurg Spine 2016; 24: 7–15. DOI: 10.3171/2015.3.Spine141172. [DOI] [PubMed] [Google Scholar]
- 69.Osorio JA, Breshears JD, Arnaout O, Simon NG, Hastings-Robinson AM, Aleshi P, Kliot M. Ultrasound-guided percutaneous injection of methylene blue to identify nerve pathology and guide surgery. Neurosurg Focus 2015; 39: E2. DOI: 10.3171/2015.6.Focus15220. [DOI] [PubMed] [Google Scholar]
- 70.Aeschlimann C, Cerny T, Küpfer A. Inhibition of (mono)amine oxidase activity and prevention of ifosfamide encephalopathy by methylene blue. Drug Metabolism Disposition: The Biological Fate Chemicals 1996; 24: 1336–1339. [PubMed] [Google Scholar]
- 71.Peter C, Hongwan D, Küpfer A, Lauterburg B. Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur Journal Clinical Pharmacology 2000; 56: 247–250. [DOI] [PubMed] [Google Scholar]