Abstract
To assess the quality of Indian clinical practice guidelines (CPG)s for the management of cardiovascular conditions, MEDLINE, Embase, Google Scholar and websites of relevant medical associations and government organisations were searched, from inception until August 2020, to identify Indian CPGs for the management of cardiovascular disease (CVD) conditions, produced in or between 2010 and 2019. Excluded were CPGs that were not specific to India, focused on alternative systems of medicine, of non-CVD conditions (even if they included a component of CVD), and those related to the electronic devices, cardiac biomarkers, or diagnostic procedures. Quality of the each included CPG was assessed using the AGREE II tool by four reviewers in duplicate, independently. Each AGREE II domain score and overall quality score was considered low (≤40%), moderate (40.1%-59.9%), and high (≥60%). Of the 23 CPGs included, six (26%) were reported to be adapted from other CPGs. Fourteen (61%) CPGs were produced by medical associations, six (26%) by individual authors and three (13%) by government agencies. Based on the AGREE II overall quality score, two (9%) CPGs were of high quality, four (17%) and seventeen (74%) CPGs were of moderate and low quality, respectively. Except for scope and purpose, and clarity of presentation all other domains were rated low. The quality of most Indian CPGs for managing CVD conditions assessed using the AGREE II tool was moderate-to-low. Combined efforts from different stakeholders are needed to develop, disseminate and implement high-quality CPGs while identifying and addressing barriers to their uptake to optimize patient care and improve outcomes.
Keywords: Clinical, evidence based practice, guidelines, cardiovascular medicine, cardiology and cardiovascular medicine
Introduction
Cardiovascular diseases (CVD) are the leading cause of mortality and rising health care expenditure globally, owing to the increasing prevalence of CVD and CVD-related risk factors. The prevalence of CVD has drastically increased from 271 million in 1990 to 523 million in 2019, and CVD-related mortality has gradually increased from 12.1 million in 1990 to 18.6 million in 2019. Low- and middle-income countries account for about 80% of all CVD deaths.1 In India, CVD is the main cause of death, and accounted for over 28% of all deaths and 14% of disability-adjusted life years in 2016.2
Clinical practice guidelines (CPG)s are defined by the National Academy of Medicine (NAM) as, “statements that include recommendations intended to optimize the patient care that are informed by a systematic review of evidence and an assessment of the benefits and harms of alternative care options”.3 Clinical consensus statements (CCS)s or documents (CCD)s are, “statements based on expert opinion and the best available research evidence for which consensus is sought using an explicit a priori methodology to identify areas of agreement and disagreement.4 The resulting statements for which consensus is achieved identify opportunities to improve patient care and clinical outcomes. A CCS/CCD is most applicable to situations where the evidence base is insufficient for a clinical practice guideline but for which significant practice variations and quality improvement opportunities exist”.4 CPGs and CCSs/CCDs (henceforth collectively referred as CPGs) are intended to reduce the gap between research evidence and practice and to provide health professionals with explicit recommendations to make informed decisions, optimize outcomes, minimize risks, and promote cost-effective practice.
Previous studies have identified several issues in the development and reporting of the Indian CPGs.5,6 We assessed the quality of the Indian CPGs for the management of CVD conditions.
Methods
Literature search
We conducted a systematic search of MEDLINE and Embase, from January 2010 to August 2020, to identify Indian CPGs for the management of CVD conditions,7 including hypertension, coronary artery disease (CAD), stroke, heart failure, peripheral heart disease, cardiomyopathies, myocardial infarction, congenital heart disease, and rheumatic heart disease. The search strategy included relevant medical subject headings and key words (full search strategy is reported in Table 1). Secondary searches were conducted by reviewing the websites of the Ministry of Health, relevant professional medical associations in India (listed in Supplementary Table 3), World Health Organization, and on Google Scholar. We also discussed the eligibility criteria and list of included CPGs with one senior cardiologist to identify additional eligible CPGs that we may have missed.
Table 1.
Search strategy.
| Database(s): Embase 1974 to 2019 January 28 | ||
|---|---|---|
| # | Searches | Results |
| 1 | exp GUIDELINE/ | 0 |
| 2 | exp PRACTICE GUIDELINE/ | 485,941 |
| 3 | exp Practice Guidelines as Topic/ | 485,941 |
| 4 | exp guidelines as topic/ | 485,941 |
| 5 | exp Consensus/ | 59,224 |
| 6 | clinical protocols/ | 87,502 |
| 7 | (guideline$ or guide-line$).ti. | 92,250 |
| 8 | recommendation$.ti. | 43,253 |
| 9 | statement$.ti. | 16,915 |
| 10 | (clinical adj standard$).ti. | 229 |
| 11 | (treatment adj algorithm$).ti. | 1163 |
| 12 | consensus.ti. | 25,978 |
| 13 | or/1–12 | 612,783 |
| 14 | exp india/ | 130,228 |
| 15 | india.tw. | 118,655 |
| 16 | or/14–15 | 167,580 |
| 17 | 13 and 16 | 4391 |
| 18 | limit 17 to human | 3865 |
| 19 | limit 18 to yr = "2010–2020" | 3410 |
| Database(s): Ovid MEDLINE(R) 1946 to August week 1 2020 | ||
| # | Searches | Results |
| 1 | exp GUIDELINE/ | 30,828 |
| 2 | exp PRACTICE GUIDELINE/ | 24,109 |
| 3 | exp Practice Guidelines as Topic/ | 107,703 |
| 4 | exp guidelines as topic/ | 148,105 |
| 5 | exp Consensus/ | 9834 |
| 6 | clinical protocols/ | 26,289 |
| 7 | (guideline$ or guide-line$).ti. | 60,444 |
| 8 | recommendation$.ti. | 29,956 |
| 9 | statement$.ti. | 13,367 |
| 10 | (clinical adj standard$).ti. | 173 |
| 11 | (treatment adj algorithm$).ti. | 664 |
| 12 | consensus.ti. | 18,254 |
| 13 | or/1–12 | 257,710 |
| 14 | exp india/ | 95,129 |
| 15 | india.tw. | 65,147 |
| 16 | or/14–15 | 112,430 |
| 17 | 13 and 16 | 1188 |
| 18 | limit 17 to human | 1108 |
| 19 | limit 18 to yr = "2010–2019" | 770 |
Eligibility criteria for selection of CPGs
We included CPGs for the clinical management of CVD conditions in Indians that were published between 01 January 2010 and 31 December 2019. We also included CCSs, as the approach to developing and reporting them is similar to CPGs, and previous studies have used AGREE II to assess the quality of CCSs.4,8–11 We included CPGs with a broad scope and that covered diagnosis and/or treatment. We excluded CPGs that were not specific to India, focused on alternative systems of medicine, covered non-CVD conditions (even though they included a component of CVD), and those related to electronic devices, cardiac biomarkers, or diagnostic procedures only.
Selection of CPGs and data collection
After excluding duplicate records, the title and abstract of each retrieved record was screened against the eligibility criteria. Of these, full texts of the potentially eligible records were retrieved and reviewed in detail for determining eligibility for inclusion. The process of screening references and inclusion was undertaken independently by two reviewers in duplicate. Any conflicts between the two reviewers were resolved by discussion or by involving a third reviewer.
From each included CPG the following data were collected independently by two reviewers in duplicate: year of publication, number of authors, type of the document (guideline or consensus statement), CVD condition, guideline developing body, guideline development approach (de novo, adapted, or adopted), scope of the guideline, number of references cited, and funding source.
Appraisal of the quality of CPGs
Quality of each included CPG was assessed using the AGREE II tool12 which consists of 25 questions (items). Twenty-three items to be rated on a seven-point scale from 1 (strongly disagree) to 7 (strongly agree) are grouped in six domains: scope and purpose, stakeholder involvement, rigour of development, clarity of presentation, applicability, and editorial independence. One global item on overall quality, to be rated on a seven-point scale from 1 (lowest possible quality) to 7 (highest possible quality), and one global item on whether the guideline can be recommended for use, to be rated as, “Yes”, or “Yes with modifications”, or “No”.“My AGREE PLUS”,13 which is an online AGREE II assessment platform, was used independently by four reviewers in duplicate to conduct the AGREE II assessment online for each CPG following the AGREE II user's manual.14 To ensure consistency between the reviewers, rules were developed and followed for rating the two items (2, 13) that were considered to be subjective by the study team. First, for the scope and purpose item 2, in the absence of specifically described questions, we also considered the CPG contents and section headings. Second, for item 13, peer review facilitated by journals before publishing was not considered as external review by experts. For adapted CPG, items 7, 8, 9, 11, and 12 (List of items are reported in the Supplementary Table 4) were considered not applicable. However, if information for these items was provided in the CPG, they were rated high, and if not, they were rated low.
Data management and analysis
The score for each domain and overall quality score (for the global item) were automatically calculated online by My AGREE PLUS13 using the following formula:
Domain and overall quality scores are reported descriptively as median of percentage along with the inter quartile range (IQR), with higher scores suggesting higher quality. The AGREE II tool does not provide criteria to classify CPGs as low or high quality based on domain or overall quality scores. For this study, CPGs with AGREE II median overall quality score ≤40%, 40.1%–59.9%,, and ≥60% were arbitrarily classified as of low, moderate, and high quality, respectively. These thresholds were adopted from a previous study.15 In a sensitivity analysis based on domain scores, CPGs were considered to be of high quality if 5 or more domains were rated >60%, of moderate quality if 3 or 4 domains were rated >60%, and low if ≤ 2 domains were rated >60%.16,17 Whether a CPG is recommended for use was based on the reviewer's opinion, classified as “Yes”, “Yes, with modifications” and “No”.
Inter-rater (reviewer) reliability, for each CPG, was assessed by intra class correlation coefficient (ICC), using the model two-way mixed for consistency in SPSS. An ICC score of ICC <0.20 was considered to be slight agreement; 0.21–0.40 as fair agreement; 0.41–0.60 as moderate agreement; 0.61–0.80 as substantial agreement; and 0.81–1.00 as very high agreement.18 A two-sided P value of <0.05 was considered as agreement not due to chance.
Results
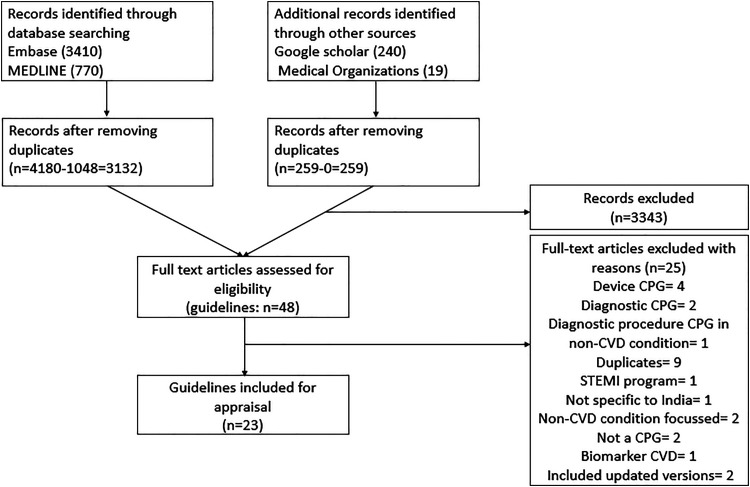
A total of 4439 records were identified through databases search. After removing duplicates, titles and abstracts of 3391 records were screened, of which 48 records were eligible for full text review, and of these, 23 eligible CPGs were included. PRISMA flow diagram (Figure 1) presenting the selection process is shown in Figure 1. A list of excluded CPGs along with reasons for exclusion is reported in Supplementary Table 1.
Figure 1.
PRISMA flowchart of selection of CPGs/CCSs/CCDs.
STEMI = ST-elevation in myocardial infarction, CPG = clinical practice guideline, CVD = cardiovascular disease, CCS = clinical consensus statements, CCD = clinical consensus documents.
Characteristics of included CPGs
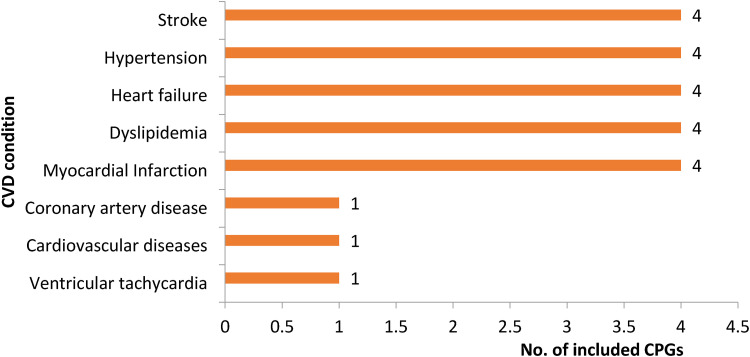
Table 1 presents a summary of characteristics of the included CPGs. Four (17%) CPGs each were available for the management of each of the following five conditions: stroke, hypertension, heart failure, myocardial infarction, and dyslipidaemia. One CPG was available for each of the following conditions: coronary artery disease, CVD (congenital heart disease, acute coronary syndrome/Non-ST segment Elevation MI, ST elevation myocardial infarction (STEMI)), and ventricular tachycardia (Figure 2). Fourteen (61%) were developed as CCSs or CCDs, eighteen (78%) were new (not revisions of previous versions), six (26%) were adapted from other CPGs, and nineteen (83%) had a broad scope (i.e. covered aspects of screening, diagnosis, prevention and treatment). Number of authors ranged from 1 to 230, and number of references cited in the CPGs ranged from 23 to 582. Fifteen (65%) CGPs did not report information on funding for developing CPGs, and 19 (83%) were published in journals and the rest of them were found on the websites of Ministry of Health, medical associations, and WHO.
Figure 2.
Distribution of CPGs by CVD conditions.
Quality of CPGs
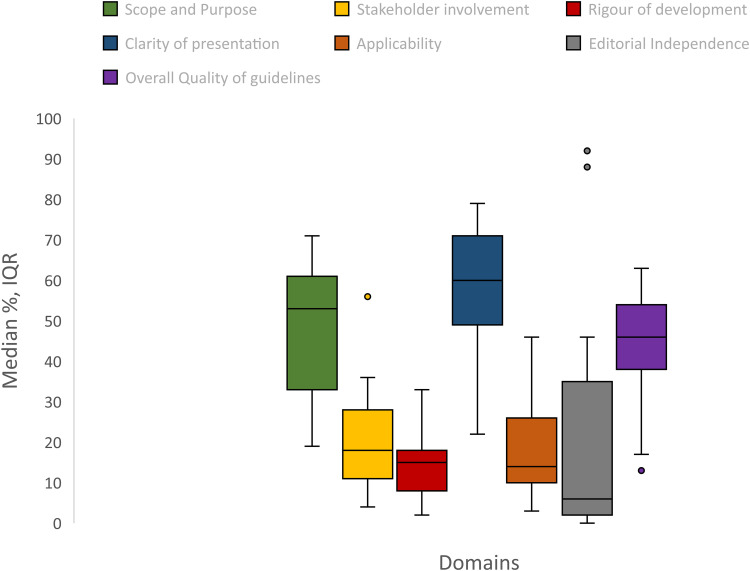
AGREE II median scores for domains and overall quality for all CPGs are reported in Figure 3 and are stratified by key characteristics in Table 2. AGREE II median score for individual CPGs are reported in Supplementary Table 1.
Figure 3.
AGREE II domain scores and overall quality score.
Table 2.
Summary of characteristics of Indian CPG/CCS/CCD related to cardiovascular diseases.
| Year | CVD condition | CPG/CCS/ CCD | New/ Update | Guideline developing body/individuals | Scope of the guideline | No. of Authors | No. of references cited | Funding Source (Government / Non-Profit Organization / Pharmaceutical) | Published in a Journal |
|---|---|---|---|---|---|---|---|---|---|
| 2010 | Hypertension | CPG | New | Anish19 | Broad | 1 | 33 | Not reported | Yes |
| 2010 | Cardiovascular diseases | CPG | New | Government (Ministry of Health & Family Welfare)20 | Broad | 16 | 35 | Not reported | No |
| 2010 | Ventricular tachycardia | CPG | New | Ajay21 | Narrow (treatment) | 2 | 29 | None | Yes |
| 2011 | Acute MI | CPG | New | Kumar Banerjee et al.22 | Broad | 2 | 23 | Not reported | Yes |
| 2014 | Myocardial infarction (STEMI) | CCD | New | Professional Society (in collaboration with STEMI India)23 | Narrow (diagnosis and treatment) | 152 | 35 | Pharmaceutical | Yes |
| 2014 | Dyslipidemia | CCD | New | Professional Society (Cardiological society of India)24 | Broad | 20 | 339 | Not reported | Yes |
| 2016 | Stable CAD | CPG | New | Professional Society (Cardiological society of India)25 | Broad | 8 | 275 | Not reported | Yes |
| 2018 | Heart failure | CCD | New | Vijay Kumar Chopra et al.26 | Broad | 16 | 37 | Concept development, compilation of data and document authoring: pharmaceutical | Yes |
| 2018 | Heart failure | CCD | New | Professional Society (Cardiological society of India)27 | Broad | 17 | 78 | Logistic support: pharmaceutical | Yes |
| 2018 | Ischemic stroke | CCS | Update | Professional Society (Indian Stroke Association)28 | Broad | 13 | 192 | Not reported | Yes |
| 2018 | Ischemic stroke | CCS | New | Professional Society (Indian Stroke Association)29 | Narrow (treatment) | Not mentioned | 24 | Not reported | No |
| 2016 | Hypertension | CPG | New | Government (Ministry of Health & Family Welfare)30 | Broad | 12 | 92 | Not reported | No |
| 2016 | Dyslipidemia | CCS (part 1) | New | Professional Society (Lipid Association of India)31 | Broad | 34 | 365 | Not reported | Yes |
| 2017 | Dyslipidemia | CCS (part 2) | New | Professional Society (Lipid Association of India)32 | Broad | 45 | 442 | Not reported | Yes |
| 2017 | Heart failure | CPG | Update | Seth et al.33 | Broad | 6 | 26 | None | Yes |
| 2017 | Myocardial infarction (STEMI) | CCD | New | Professional Society34 | Broad | 64 | 224 | Not reported | Yes |
| 2017 | Hypertension in CKD | CCD | Update | Professional Society (Expert Panel of Indian Nephrologists)35 | Broad | 15 | 59 | Not reported | Yes |
| 2018 | Stroke Prevention in Atrial Fibrillation | CCD | Update | Professional Society (Stroke Prevention in Atrial Fibrillation India experts)36 | Narrow (Treatment) | 15 + SPAF Academy India experts | 161 | Scientific support: pharmaceutical | Yes |
| 2018 | Dyslipidemia | CCD | New | Professional Society (Cardiological society of India)37 | Broad | 7 | 63 | Concept development, compilation of data, document authoring: pharmaceutical | Yes |
| 2018 | Heart failure | CCS | New | Professional Society (Cardiological society of India)38 | Broad | 5 | 582 | Not reported | Yes |
| 2018 | STEMI | CCS | New | Tiny Nair et al.39 | Broad | 6 (expert panel: 19) | 36 | None | Yes |
| 2019 | Stroke | CPG | New | Government Of India40 | Broad | 16 | NA | Not reported | No |
| 2019 | Hypertension | CPG | Update | Professional society (The Association of Physicians of India)41 | Broad | 230 | 215 | Not reported | Yes |
CAD = coronary artery disease, CKD = chronic kidney disease, CCD = clinical consensus document, CCS = clinical consensus statement, CPG = clinical practice guideline, CVD = cardiovascular, MI = myocardial infarction, NA = not available, SPAF = stroke prevention academy India experts, STEMI = ST segment elevated myocardial infarction.
Scope and purpose
The median for this domain was 53% (IQR 33%-61%). Sixteen (70%) CPGs were rated moderate-to-high range for this domain. For CPGs that were rated low for this domain, although they had a clearly defined objective of the CPG, they did not adequately explain the health questions addressed in the CPG and the population to whom the CPG is intended to apply.
Stakeholder involvement
The median score for this domain was 10% (IQR:11%–-28%). Twenty- two (96%) CPGs were rated low for this domain. None of the CPG were rated high for this domain. Irrespective of the source of funding, the developers of the CPG, and the publication status, the CPGs were rated low for this domain. Common reasons for low rating for most CPGs was lack of involvement of systematic review expert, epidemiologist, statistician, or library scientist in the CPG development group, and lack of information on whether the views and preferences of the population were taken into consideration when developing the CPGs.
Rigour of development
The median score for this domain was 15% (IQR: 8%–18%) with almost all the CPGs rating low for this domain. Systematic methods employed to search for relevant evidence were reported by 2(9%) CPGs. Details on criteria for selecting evidence and explicit links between the recommendations and supporting evidence were not adequately mentioned. Potential benefits, harms, and risks were not considered by most CPGs. Among all the included CPGs, external review prior to the publication was reported by four (17%) CPGs. Only two (9%) CPGs reported that the CPG would be updated, but the procedures for updating the CPG was not reported. The CPGs that were not funded or did not report the source of funding, or developed by individuals, were rated lower for this domain compared with other CPGs.
Clarity of presentation
The median score for this domain was 60% (IQR: 49%–71%), which was highest of all the domains. Twenty-two (96%) CPGs were rated moderate to high for this domain. Most CPGs included specific and unambiguous therapies for managing the condition as well as key recommendations that were easily identifiable. CPGs with pharmaceutical funding support were rated low for this domain.
Applicability
The median score for this domain was 14% (IQR: 10%–26%). Twenty-one (88%) CPGs were rated low for this domain while only two CPGs were rated high. The CPGs that were not funded or have not reported the source of funding, and those developed de novo were rated low for this domain.
Editorial independence
The median for this domain was 6% (IQR2%–35%), which was lowest of the all the domains. Twenty-one (91%) CPGs were rated low-to-moderate for this domain. The conflicts of interest that may have influenced CPG development and reporting was adequately reported by nine (39%) CPGs. Only 4 (17%) CPGs reported the information on funding source and information to assess if the CPG was editorially independent from the funding body was lacking.
Overall quality
Overall median score was 46% (IQR38% to 54%). Two CPGs were found to be of high quality (≥60%), fifteen (65%) and six (26%) CPGs were found to be of moderate (40.9%–59.9%) and low (≤40%) quality. Based on the domain scores, all the included CPGs were found to be of low quality (≤2 domains were rated >60%). CPGs that received funding from pharmaceutical company, developed by a group of individuals, and those that were not published in the journals were rated low. Median scores were similar for CPGs published and not published in journals (44% vs 46%), for CPGs and CCSs (42% vs 46%), and for adapted and de novo CPGs (46% vs 42%) (Table 3).
Table 3.
Median percentage of maximum possible score for each domain and overall, and by characteristics of CPGs.
| No. of CPGs | Scope and purpose | Stakeholder involvement | Rigour of development | Clarity of presentation | Applicability | Editorial independence | Overall quality score | |
|---|---|---|---|---|---|---|---|---|
| CVD condition | ||||||||
| Stroke | 4 | 51.5 | 18.5 | 12.5 | 61.5 | 22.5 | 3 | 44 |
| Hypertension | 4 | 57 | 15.5 | 18 | 57 | 10 | 15.5 | 50 |
| Heart Failure | 4 | 54.5 | 22.5 | 13 | 64.5 | 25 | 19.5 | 37.5 |
| Dyslipidaemia | 4 | 60.5 | 20.5 | 16 | 56 | 25.5 | 22.5 | 46 |
| Myocardial Infarction | 4 | 57 | 21 | 12.5 | 63 | 13.7 | 25 | 48 |
| Coronary Artery Disease | 1 | 53 | 36 | 24 | 68 | 10 | 35 | 58 |
| Cardiovascular Diseases | 1 | 33 | 11 | 12 | 43 | 10 | 2 | 38 |
| Ventricular Tachycardia | 1 | 19 | 6 | 4 | 22 | 8 | 2 | 13 |
| Publication | ||||||||
| Published in journal | 20 | 57 | 16.5 | 15 | 63 | 13.5 | 7 | 44 |
| Not published in Journal | 3 | 53 | 26 | 12 | 54 | 19 | 4 | 46 |
| Source of funding | ||||||||
| Pharmaceutical | 7 | 46 | 32 | 44 | 44 | 26 | 20 | 35 |
| Not reported | 13 | 53 | 18 | 15 | 60 | 16 | 4 | 46 |
| None | 3 | 31 | 6 | 6 | 44 | 8 | 88 | 33 |
| Developers | ||||||||
| Professional society | 14 | 58.5 | 19.5 | 16.5 | 65 | 15 | 9 | 46 |
| Individual | 6 | 45 | 10.5 | 6 | 50 | 10.5 | 3 | 31 |
| Government | 3 | 33 | 26 | 12 | 54 | 19 | 4 | 46 |
| Type of the document | ||||||||
| Guideline | 9 | 53 | 13 | 8 | 54 | 10 | 4 | 42 |
| Consensus statement/document | 14 | 57 | 23.5 | 17 | 65 | 16 | 31 | 46 |
| Development process | ||||||||
| Adapted | 6 | 59 | 27.5 | 16 | 56 | 22.5 | 20.5 | 46 |
| de novo | 17 | 53 | 13 | 14 | 63 | 13 | 4 | 42 |
| All | 23 | 53 | 10 | 15 | 60 | 14 | 6 | 46 |
Recommending CPG for use
None of the CPGs were recommended for use in their current form by any of the four reviewers. Twenty (87%) of CPGs were recommended for use with some modifications by three or more reviewers (Supplementary Table 1). One (4%) CPG was not recommended for use by three reviewers. Two (9%) CPGs were not recommended by two reviewers each with the other two reviewers recommending with modifications.
Inter-rater reliability
The ICC values for inter-rater reliability for individual CPGs ranged from 0.91 (95% confidence intervals [CI]: 0.84–0.95) to 0.98(95% CI: 0.97–0.99), with a p value of <0.05 for all showing that the degree of agreement between the reviewers was very high.
Discussion
Summary of findings
We aimed to assess the quality of Indian CPGs for managing CVD conditions. Based on overall quality score, more than half of the CPGs were found to be of low to moderate quality. Similar results were observed based on the domain scores. Our study found that there is evidence of duplication of CPGs across CVD conditions and that the quality of most CPGs was generally low to moderate. Two CPGs were found to be of high quality (≥60%) while all the other included CPGs were found to be of low quality, based on the domain scores. Two CPGs were found to be of high quality (≥60%). Seventeen (74%) and six (26%) CPGs were found to be of low (≤40%) and moderate (40.9%–59.9%) quality. Based on the domain scores, all the included CPGs were found to be of low quality. The median scores obtained from the AGREE II evaluation were low for most domains except for scope and purpose and clarity of presentation. Most CPGs may be recommended for use if they are revised to improve their methodological and reporting quality.
Strengths and limitations
This is the first study to assess the quality of Indian CPGs for managing CVD conditions. Our search for CPGs was comprehensive, and quality assessments of each included CPG were independently performed by four reviewers using a standard Agree II tool and its user manual,12 which increases the reliability of our findings. However, as Agree-II tool itself has some limitations, our study cannot be regarded as an absolute test of the quality of the CPGs. Although AGREE II covers important aspects (domains) of CPG development and reporting, it does not consider relative importance of the domains. If rigour of development is poor, then high scores on other domains may be of relatively lower value. Another important limitation of AGREE II is the subjective assessment of overall quality of a CPG and whether reviewer would recommend the CPG for use, as separate to the assessment of six domains comprising 23 items. Classification of CPGs based on arbitrary cut-offs for overall quality or domain scores, as used in our study, should be interpreted with caution. Also, two (9%) CPGs included in our study were authored by one individual; therefore, including such CPGs might have led to underestimation of quality of CPGs overall, but given the small number of such CPGs it is unlikely that their influence on our findings could be significant.
Findings in the context of previous research
Bhaumik et al. reported in 2018 the assessment of the quality of 11 CPGs of four leading causes of disability adjusted life years in India using AGREE II. In this study, consistent with our study findings, domains of scope and purpose and clarity of presentation were rated higher than other domains. The authors also identified, through interviews with CPG developers, barriers to the development and implementation of quality CPGs, including lack of methodological expertize, governance structure, and funding. Further, CPG development has previously been undertaken as an academic activity by elite institutions rather than including varied stakeholders. A poor understanding of conflict of interest by the CPG authors has also been previously reported, which is similar to our study results.5
AGREE II was used by Radhika et al.42 in 2019 and Sonawne et al.6 in 2015 to assess the quality of CPGs in obstetrics and gynecology, and maternity management and family planning respectively. Except for scope and purpose, and clarity of presentation, the other domains were rated low in both studies, which is consistent with the current study findings.
Implications
Access to healthcare has been improving in India, but quality of health care remains suboptimal and uneven. This could be due, at least in part, to sub-optimal uptake of CPG recommendations in practice. Development of high-quality CPGs that are relevant to local contexts, along with efforts to implement and monitor their uptake, is needed in India. Developing CPGs de novo is challenging and resource intensive. However, adapting CPGs, which is defined as the systematic approach to modify and contextualize evidence-based CPGs to suit implementation in the local healthcare system, is a more efficient and appropriate approach,43,44 particularly in resource-limited settings like India.45 Adaptation of CPGs provides the opportunity to consider applicability of recommendations to the target settings, including consideration of values, costs, and resources.
In India, since 2014, efforts have been made by the central government to develop “standard treatment guidelines” by adapting existing CPGs to tailor to the Indian context. So far, 62 standard treatment CPGs have been produced by a task force following a simple CPG development/adaptation handbook based on the US National Academy of Medicine's principles and standards for a trustworthy CPG.46,47 Two standard treatment CPGs were included in our study. Although their quality was better compared to the other included CPGs, there was still room for improvement. In particular, one CPG lacked in rigour of development, applicability and editorial independence, while the other CPG was rated low in almost all the domains. While the initiative to develop standard treatment CPGs relevant to India is a significant step in the right direction, there is little known about efforts to implement these CPGs and monitoring of implementation and adherence to them, which are pivotal for optimizing patient care and improving outcomes.
While developers should be guided by the principles and standard of developing and reporting CPGs (e.g. NAM standards,47 GRADE-ADOLOPMENT,48 ADAPTE,49 AGREE II,12 RIGHT checklist50), editors may encourage writing groups to clarify added value from potentially duplicate CPGs and scrutinize CPGs for adherence to principles and standards of developing and reporting CPGs before publishing them.
Research is needed to identify barriers to implementation of CPGs in India, and to develop and test implementation strategies of CPGs, while increasing awareness of availability of CPGs and benefits of following them for optimizing patient care and improving outcomes.
Conclusion
Most Indian CPGs for CVD conditions are of low-to moderate quality as assessed by the AGREE II tool. The quality of CPGs in India needs to be improved, which can be achieved by following governance structures, and developing, adopting, and adapting, CPGs following principles and standards for developing, reporting, and disseminating them.
Supplemental Material
Supplemental material, sj-docx-1-shr-10.1177_20542704221127178 for Quality of the Indian clinical practice guidelines for the management of cardiovascular conditions by Rupasvi Dhurjati, Vidya Sagar, Raju Kanukula, Nusrath Rehana, Padinhare P Mohanan, Mark D. Huffman, Soumyadeep Bhaumik and Abdul Salam in JRSM Open
Acknowledgements
We thank Dr. Josyula Lakshmi and Dr. Gautam Sateesh for reviewing the manuscript.
Footnotes
Contributors: AS, SB, RD, VK: conceived and designed the study. RD, VK were involved in study selection. AS, SB, RD, RK, NR: carried out Agree II assessments and drafted the manuscript. MH, PPM have provided substantial input to the analysis and edits to the manuscript.
Competing interests: In the last 3 years, MDH has received support from the American Heart Association, Verily, and AstraZeneca for work unrelated to this research. MDH has planned patents for combination therapy for the treatment of heart failure. The George Institute for Global Health has a patent, license, and has received investment funding with intent to commercialize fixed-dose combination therapy through its social enterprise business, George Medicines. SB has previously consulted on evidence syntheses for WHO guidelines. Other authors declare no conflict of interest.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article
Ethics approval and patient consent: Not applicable.
Guarantor: Rupasvi Dhurjati is the guarantor of the study.
ORCID iD: Rupasvi Dhurjati https://orcid.org/0000-0002-8579-3972
Supplemental material: Supplemental material for this article is available online.
References
- 1.Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol 2020; 76: 2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prabhakaran D, Jeemon P, Sharma M, et al. The changing patterns of cardiovascular diseases and their risk factors in the states of India: the Global Burden of Disease Study 1990–2016. Lancet Global Health 2018;6(12): e1339–e1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.IOM (Institute of Medicine). Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press, 2011, p. 290. [Google Scholar]
- 4.Rosenfeld RM, Nnacheta LC, Corrigan MD. Clinical consensus statement development manual. Otolaryngol Head Neck Surg 2015;153(2_suppl): S1–S4. [DOI] [PubMed] [Google Scholar]
- 5.Bhaumik S, Jagadesh S, Ellatar M, Kohli N, Riedha M, Moi M. Clinical practice guidelines in India: quality appraisal and the use of evidence in their development. J Evid Based Med 2018;11(1): 26–39. [DOI] [PubMed] [Google Scholar]
- 6.Sonawane DB, Karvande SS, Cluzeau FA, Chavan SA, Mistry NF. Appraisal of maternity management and family planning guidelines using the agree II instrument in India. Indian J Public Health 2015;59(4): 264. [DOI] [PubMed] [Google Scholar]
- 7.https://www.who.int/cardiovascular_diseases/about_cvd/en/
- 8.The IDSA Handbook for Clinical Practice Guidelines Development (Updated January 2021).
- 9.Guidelines International Network, http://www.g-i-n.net/ (accessed 26 May 2014).
- 10.Jacobs C, Graham ID, Makarski J, et al. Clinical practice guidelines and consensus statements in oncology–an assessment of their methodological quality. PLoS One 2014;9(10): e110469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White P, Finch C. An assessment of the 2008 Zurich consensus statement on concussion in sport using the appraisal of guidelines for research and evaluation II (AGREE II). J Sci Med Sport 2013;16: e22. [Google Scholar]
- 12.https://www.agreetrust.org/agree-ii/
- 13.https://www.agreetrust.org/login/?redirect_to=https%3A%2F%2Fwww.agreetrust.org%2Fmy-agree%2F
- 14.https://www.agreetrust.org/wp-content/uploads/2017/12/AGREE-II-Users-Manual-and-23-item-Instrument-2009-Update-2017.pdf
- 15.Romeo V, Stanzione A, Ugga L, et al. A critical appraisal of the quality of glioma imaging guidelines using the AGREE II tool: a EuroAIM initiative. Front Oncol 2019;9: 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chorath K, Garza L, Tarriela A, Luu N, Rajasekaran K, Moreira A. Clinical practice guidelines on newborn hearing screening: a systematic quality appraisal using the AGREE II instrument. Int J Pediatr Otorhinolaryngol 2021;141: 110504. [DOI] [PubMed] [Google Scholar]
- 17.Romeo V, Stanzione A, Cocozza S, et al. A critical appraisal of the quality of head and neck cancer imaging guidelines using the AGREE II tool: a EuroAIM initiative. Cancer Med 2019;8(1): 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu Y, Yang H, Yu X, et al. Critical appraisal of the quality and content of clinical practice CPG for pneumocystis jiroveci pneumonia (PJP) prophylaxis using the AGREE II instrument. J Clin Pharm Ther 2020;45(6): 1325–1333. [DOI] [PubMed] [Google Scholar]
- 19.Anish C. Hypertension guidelines. Gujarat Med J 2010;65(2): 27–35. [Google Scholar]
- 20.http://clinicalestablishments.gov.in/WriteReadData/149.pdf
- 21.Naik A. Management of ventricular tachycardia: identification and therapy guide for multidisciplinary doctors. Gujarat Med J 2010;65(2): 36–42. [Google Scholar]
- 22.Banerjee AK, Kumar S. Guidelines for management of acute myocardial infarction. J Assoc Physicians India 2011;59(Suppl): 37–42. [PubMed] [Google Scholar]
- 23.Dalal JJ, Alexander T, Banerjee PS, et al. 2013 Consensus statement for early reperfusion and pharmaco-invasive approach in patients presenting with chest pain diagnosed as STEMI (ST elevation myocardial infarction) in an Indian setting. J Assoc Physicians India 2014;62: 13. [PubMed] [Google Scholar]
- 24.Chandra KS, Bansal M, Nair T, et al. Consensus statement on management of dyslipidemia in Indian subjects. Indian Heart J 2014;66(Suppl 3): S1–S51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mishra S, Ray S, Dalal JJ, et al. Management standards for stable coronary artery disease in India. Indian Heart J 2016;68: S31–S49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chopra VK, Kumar S, Dasbiswas A, et al. ESC 2016 Heart Failure Guidelines: a consensus document from Indian experts for adaptation in India. J Clin Diagn Res 2018;12(4): OE01–OE07. [Google Scholar]
- 27.Mishra S, Mohan JC, Nair T, et al. Management protocols for chronic heart failure in India. Indian Heart J 2018;70(1): 105–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khurana D, Padma MV, Bhatia R, et al. Recommendations for the early management of acute ischemic stroke: a consensus statement for healthcare professionals from the Indian Stroke Association. J Stroke Med 2018;1(2): 79–113. [Google Scholar]
- 29.ISA consensus statement: recommendations for the early management of acute ischemic stroke with endovascular treatment, http://www.stroke-india.org/downloads/endovascular.pdf
- 30.Ministry of Health and Family Welfare. Screening, diagnosis, assessment, and management of primary hypertension in adults in India.
- 31.Iyengar S, Puri R, Narasingan S. Lipid association of India expert consensus statement on management of dyslipidemia in Indians 2016-part 1. J Pract Cardiovasc Sci 2016;2(2): 134. [PubMed] [Google Scholar]
- 32.Iyengar SS, Puri R, Narasingan SN, et al. Lipid Association of India (LAI) expert consensus statement on management of dyslipidaemia in Indians 2017: part 2. Clin Lipidol 2017;12(1): 56–109. [Google Scholar]
- 33.Seth S, Ramakrishnan S, Parekh N, Karthikeyan G, Singh S, Sharma G. Heart failure guidelines for India: update 2017. J Pract Cardiovasc Sci 2017;3(3): 133. [Google Scholar]
- 34.Guha S, Sethi R, Ray S, et al. Cardiological Society of India: position statement for the management of ST elevation myocardial infarction in India. Indian Heart J 2017;69(Suppl 1): S63–S97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abraham G, Arun KN, Gopalakrishnan N, et al. Management of hypertension in chronic kidney disease: consensus statement by an expert panel of Indian nephrologists. J Assoc Physicians India 2017;65(2 Suppl): 6–22. [PubMed] [Google Scholar]
- 36.Dalal J, Bhave A, Oomman A, et al. The Indian consensus guidance on stroke prevention in atrial fibrillation: an emphasis on practical use of nonvitamin K oral anticoagulants. Indian Heart J 2015;67: S13–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ray S, Sawhney JP, Das MK, et al. Adaptation of 2016 European Society of Cardiology/European Atherosclerosis Society guideline for lipid management to Indian patients–A consensus document. Indian Heart J. 2018;70(5): 736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guha S, Harikrishnan S, Ray S, et al. CSI Position statement on management of heart failure in India. Indian Heart J 2018;70(Suppl 1): S1–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nair T, Agrawal R, Bansal S, Dutta A, Ray R, Ray S. Expert consensus document on management of ST-elevation myocardial infarction: adaptation of 2012 ESC guidelines. Indian J Crit Care Med 2018;22(4): 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.https://main.mohfw.gov.in/sites/default/files/Guidelines%20for%20Prevention%20and%20Managment%20of%20Stroke.pdf
- 41.Shah SN, Munjal YP, Kamath SA, et al. Indian guidelines on hypertension-IV (2019). J Hum Hypertens 2020;34(11):745–758. [DOI] [PubMed] [Google Scholar]
- 42.Radhika AG, John D. Quality appraisal of clinical guidelines in obstetrics and gynecology in India. Open J Obstet Gynecol 2019;9(10): 1419–1428. [Google Scholar]
- 43.Fervers B, Burgers JS, Haugh MC, et al. Adaptation of clinical guidelines: literature review and proposition for a framework and procedure. Int J Qual Health Care 2006;18: 167–176. [DOI] [PubMed] [Google Scholar]
- 44.Schünemann HJ, Fretheim A, Oxman AD. Improving the use of research evidence in guideline development: 13. Applicability, transferability and adaptation. Health Res Policy Syst 2006;4: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehndiratta A, Sharma S, Gupta NP, Sankar MJ, Cluzeau F. Adapting clinical guidelines in India—a pragmatic approach. Br Med J 2017;17: 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.http://clinicalestablishments.gov.in/En/1068-standard-treatment-guidelines.aspx
- 47.IOM (Institute of Medicine). Clinical practice guidelines we can trust. Washington, DC: The National Academies Press, 2011. [Google Scholar]
- 48.Schünemann HJ, Wiercioch W, Brozek J, et al. GRADE Evidence to Decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT. J Clin Epidemiol 2017;81: 101–110. [DOI] [PubMed] [Google Scholar]
- 49.https://g-i-n.net/wp-content/uploads/2021/03/ADAPTE-Resource-toolkit-March-2010.pdf
- 50.http://www.right-statement.org/right-statement/checklist
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-shr-10.1177_20542704221127178 for Quality of the Indian clinical practice guidelines for the management of cardiovascular conditions by Rupasvi Dhurjati, Vidya Sagar, Raju Kanukula, Nusrath Rehana, Padinhare P Mohanan, Mark D. Huffman, Soumyadeep Bhaumik and Abdul Salam in JRSM Open