Abstract
Several complications have been reported in rotational atherectomy, and these complications are closely associated with cardiac tamponade, emergent surgery, and death. Here we describe a case of left main coronary artery, bullet-like perforation treated with a novel approach—transvascular balloon occlusion. (Level of Difficulty: Advanced.)
Key Words: complex high-risk indicated procedure, covered stents, left main coronary artery perforation, ping pong technique, rotablator burr, transvascular sealing
Abbreviations and Acronyms: CABG, coronary artery bypass graft; CHIP, complex high-risk and indicated procedure; LAD, left anterior descending artery; LCx, left circumflex artery; LIMA, left internal mammary artery; LM, left main; NC, noncompliant; RA, rotational atherectomy; SVG, saphenous vein graft; TTE, transthoracic echocardiogram
Central Illustration

The left main (LM) perforation is a rare but severe complication of rotational atherectomy (RA) described in 0.2%-0.6% of patients, with a mortality rate shown by some series of >20%.1 A high-risk group of patients that deserves a special mention are the complex high-risk and indicated procedure (CHIP) patients. Herein we describe a novel bailout technique for the treatment of large LM perforation—the transvascular inflation of a noncompliant (NC) balloon.
Learning Objectives
-
•
To illustrate a novel bailout technique using a transvascular balloon inflation for sealing a complete perforation of the LM.
-
•
To recognize rotawire fatigue and kinking as a major potential mechanism of the perforations during RA.
-
•
To acknowledge that patients after CABG who are experiencing a coronary perforation may develop effusions in unusual locations such as pleural effusion and loculated pericardial effusion.
History of Presentation
A 71-year-old man reported a recurrence of Canadian Cardiovascular Society grading of angina class II angina 6 months after his coronary artery bypass graft (CABG) surgery.
Medical History
The patient had history of hypertension, hyperlipidemia, type 2 diabetes, and previous CABG surgery (left internal mammary artery [LIMA] to left anterior descending artery [LAD], saphenous vein graft [SVG] to left circumflex [LCx], and SVG to right coronary artery [RCA]).
Investigations
The analysis revealed a low-density lipoprotein cholesterol level of 122 mg/dL and glycosylated hemoglobin of 7.4; the renal function and cardiac enzymes were both normal. The electrocardiogram showed negative T waves in the inferior and lateral leads, the transthoracic echocardiogram (TTE) was normal, and a cardiac single-photon emission computed tomography SPECT showed inducible ischemia in the LCx territory. His cardiac catheterization revealed a critical and calcified stenosis affecting the distal LM and LCx ostium (Figure 1B), a suboccluded LAD from its ostium (Figure 1C) with a patent LIMA-LAD bypass graft (Figure 1D), the 2 SVGs were occluded, and the RCA had a proximal stenosis with a negative nonhyperemic index (diastolic hyperemia-free ratio of 1.0). After a heart team discussion, percutaneous coronary intervention to distal LM-LCx, using RA as a plaque modification technique, was scheduled.
Figure 1.
Baseline Cardiac Imaging
(A) Cardiac single-photon emission computed tomography with inducible ischemia in the LCx territory. (B) Calcified stenosis of LM-LCx ostium. (C) Suboccluded LAD from the ostium. (D) Patent LIMA-LAD bypass graft. LAD = left anterior descending; LCx = left circumflex; LIMA = left internal mammary artery; LM = left main.
Management
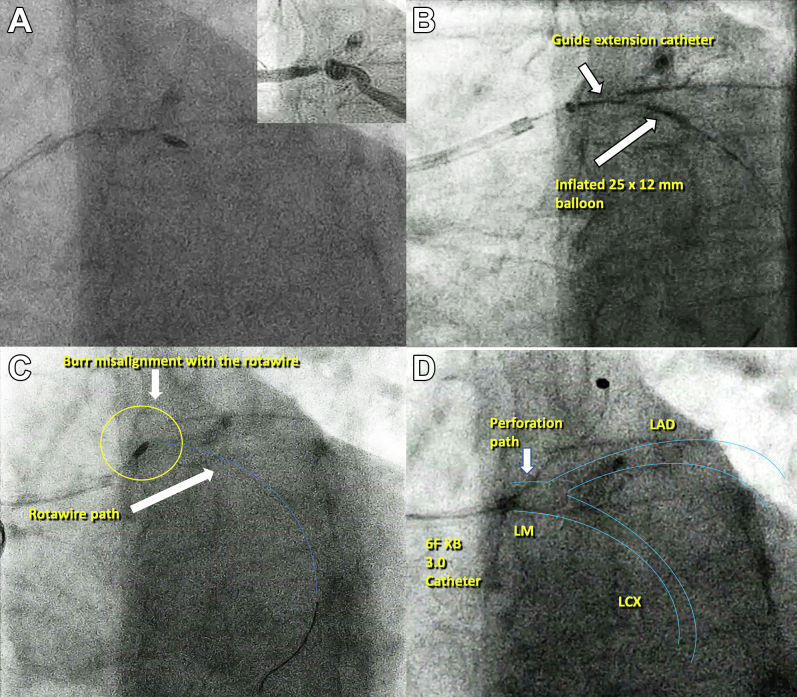
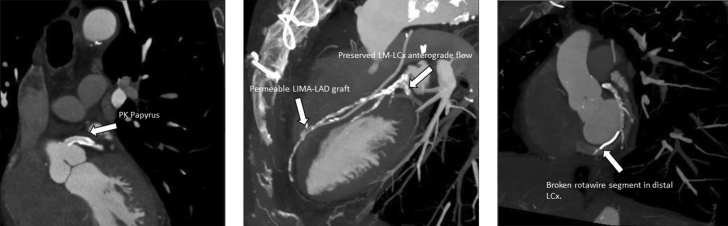
Via right radial access, we cannulated the LM using a 6-F XB 3.0 guide catheter. Through a rotawire floppy (Boston Scientific) we advanced the rotablator burr. We performed RA using the Rotapro System (Boston Scientific) with a 1.5 burr at 160,000 rpm (Video 1). The burr crossed the LM stenosis, but not the proximal LCx (Figure 2A). We also failed to advance a microcatheter due to the proximal bend and severity of the stenosis of LCx. We performed RA a second time at 18,000 rpm without success, therefore, we left the rotawire in the distal LCx, and with the aid of a guide extension catheter, we advanced a parallel SION BLUE Extra Support (Asahi Intecc) wire to the distal LCx. After this, we successfully advanced a 1.25 × 10 mm NC balloon and a 2.5 × 15 mm NC balloon and predilated the critical stenosis (Figure 2B). However, this last balloon did not cross the lesion entirely and remained underexpanded. We also failed to advance the intravascular ultrasound catheter and further balloons. We considered the lesion to be uncrossable and decided to perform RA one last time. This time, however, the burr stopped in the LM with an unusual orientation (Figure 2C). And during our next attempt, the burr unexpectedly transected the rotawire and completely perforated the LM (Figure 2D, Video 2). Angiography confirmed the perforation (Figure 3A, Video 3), and the patient experienced a nonshockable cardiac arrest. Advanced cardiopulmonary resuscitation maneuvers were initiated. We attempted to advance a SION wire (Asahi Intecc), however, we were unable to cross to distal LCx and the wire kept advancing through the perforation site to the pericardial space. We decided to leave the wire in the pericardial space, advanced a 5 × 10 mm NC balloon through the perforation, inflated it in the pericardial space, and then pulled it back occluding the perforation (Figure 3B, Video 4). The patient was intubated, ventilated, and a pericardiocentesis was performed, which obtained 100 mL of blood. With the patient stabilized, a new 6-F XB 3.5 guide catheter was advanced via the right femoral artery to the LM, maintaining the previously inflated balloon occluding the perforation (ping pong technique) (Figure 3C, Video 5). We then deflated the LM balloon and rapidly advanced a new SION wire to distal LCx and a 3.5 × 20 mm PK Papyrus (Biotronik) stent was implanted from the ostium of LM to the bifurcation and postdilated with a 4.0 NC balloon obtaining a complete sealing of the perforation (Figure 3D, Video 6). A TTE and transesophageal echocardiogram showed no pericardial effusion, however, a massive pleural effusion was noted (no images available). We performed an injection through the pericardiocentesis catheter and confirmed that the pericardial catheter had perforated the right ventricle wall into the right ventricle outflow tract (Video 7). The pericardial drainage catheter was removed in the operating room and surgical pericardial and pleural drainages were inserted. The patient was transferred to the intensive care unit, where his condition remained stable. A cardiac computed tomography scan confirmed the correct sealing of the perforation (Figures 4A and 4B). We also observed a segment of the broken rotawire in the distal LCx. The patient was discharged from the hospital 10 days after the event.
Figure 2.
Percutaneous Coronary Intervention
(A) 1.5 burr stopped in the proximal LCx. (B) Inflated 2.5 × 15 mm NC balloon. (C) 1.5 burr stops in the LM with a strange orientation, the blue line shows the rotawire path. (D) Complete perforation of the LM. NC = noncompliant; other abbreviations as in Figure 1.
Figure 3.
Left Main Perforation and Complication Management
(A) Massive contrast extravasation to the pericardial space. (B) Transvascular sealing of the perforation with a 5.0-mm balloon. (C) Ping-pong technique. (D) Covered stent sealing the LM perforation. Abbreviation as in Figure 1.
Figure 4.
Cardiac CT
(A) Covered stent covering the LM. (B) Anterograde (LM-LCx) and retrograde (LIMA-LAD) flow preserved. (C) CT with a fractured rotawire segment in distal LCx. CT = computed tomography; other abbreviations as in Figure 1.
Discussion
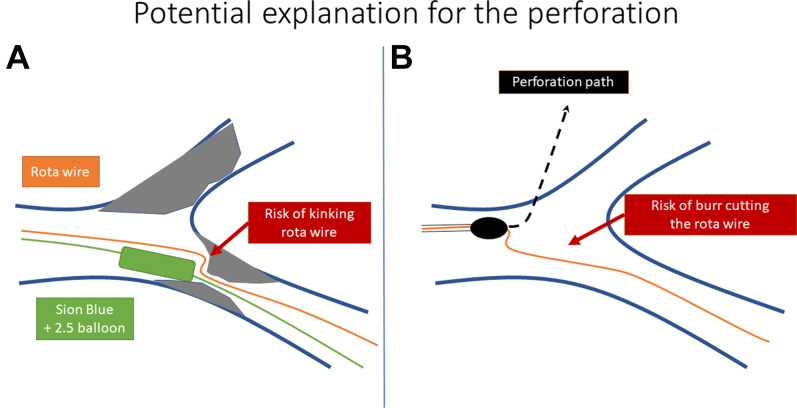
Predictors of coronary artery perforations during RA include type-C lesions, previous CABG, older patient age, and distal LM stenosis affecting the proximal LCx with significant bend.2 A total of 4 mechanisms were identified, including: 1) rotawire tip injury; 2) using an inappropriately sized burr; 3) wire damage with subsequent transection; and 4) unintended and unnoticed bias cutting into noncalcified plaque or through a calcified vessel wall.3 In our case, the fundamental reason for the perforation was wire damage due to repeated balloon inflation, which caused kinking of the rotawire and friction during the burr advancement triggering a LM perforation (Figure 5 shows this potential mechanism). For this reason, it is highly recommended to use a new rotawire that in the event of needing further rotablation runs after extensive balloon dilatation or manipulation. With current resolution of fluoroscopy, kinking of the 0.09-inch part is poorly visualized. To our knowledge this is the first case describing the transvascular sealing with a NC balloon through the pericardial space for the treatment of a LM perforation. Although this unconventional technique could have the risk of exacerbating the perforation, in our case we inflated the balloon in the pericardial space and then we pulled it back as if “corking a wine bottle”; we believe that this maneuver reduced the risk of worsening the perforation and in combination with the ping pong technique allowed us to maximize the occlusion time, preventing further hemodynamic deterioration until a covered stent could be implanted. The second-generation covered stents have become an alternative to surgery when other conservative approaches fail, becoming an indispensable tool in the cardiac catheterization laboratory.4 Finally, it is relevant to comment that, in patients after CABG who undergo complex percutaneous coronary intervention with a resultant coronary perforation, these patients may develop effusions in unusual locations such as loculated pericardial effusions and or pleural effusions as shown in our case. Therefore, imaging scans in this context should go beyond the pericardial space and include an evaluation of the pleural space using ultrasound or computed tomography.
Figure 5.
Potential Mechanism
(A) Kinking of the rotawire (green) due to the inflation of the balloon (blue). (B) The burr stopped at the kinked zone of the rotawire, and when the RA started the burr perforated the LM. RA = rotational atherectomy; other abbreviation as Figure 1.
Follow-up
At the 3-month follow-up, he was asymptomatic for angina and his repeat TTE was normal.
Conclusions
This case highlights a life-threatening complication of RA, a LM perforation, and a novel bailout treatment. The transvascular inflation of a NC balloon occluding the perforation, which, in conjunction with the ping-pong technique, allowed us to implant a covered stent in a safer manner and with a good final result.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
First Rotablator Run With a 1.5 Burr at 160,000 rpm
Complete Perforation of the LM With the 1.5 Burr
Massive Extravasation of Contrast Through the Perforation Site
Transvascular Sealing: NC Balloon Pulling From Pericardial Space Occluding the Perforation
Ping-Pong Technique
Complete Sealing of the Perforation With a 3.5 × 20 mm Covered Stent
Contrast Injected Through the Pericardial Drain Confirming the RV Perforation
References
- 1.Sakakura K., Inohara T., Kohsaka S., et al. S-i, Incidence and determinants of complications in rotational atherectomy. Circ Cardiovasc Interv. 2016;9 doi: 10.1161/CIRCINTERVENTIONS.116.004278. [DOI] [PubMed] [Google Scholar]
- 2.Sakakura K., Ito Y., Shibata Y., et al. Clinical expert consensus document on rotational atherectomy from the Japanese association of cardiovascular intervention and therapeutics. Cardiovasc Interv Ther. 2021;36:1–18. doi: 10.1007/s12928-020-00715-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y.H., Chen W.J., Chen Y.W., et al. Incidence and mechanisms of coronary perforations during rotational atherectomy in modern practice. J Interv Cardiol. 2020;2020:1894389. doi: 10.1155/2020/1894389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kandzari D.E., Birkemeyer R. PK papyrus covered stent: device description and early experience for the treatment of coronary artery perforations. Catheter Cardiovasc Interv. 2019;94:564–568. doi: 10.1002/ccd.28306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
First Rotablator Run With a 1.5 Burr at 160,000 rpm
Complete Perforation of the LM With the 1.5 Burr
Massive Extravasation of Contrast Through the Perforation Site
Transvascular Sealing: NC Balloon Pulling From Pericardial Space Occluding the Perforation
Ping-Pong Technique
Complete Sealing of the Perforation With a 3.5 × 20 mm Covered Stent
Contrast Injected Through the Pericardial Drain Confirming the RV Perforation