Abstract
Objective: To explore the clinical efficacy and safety of betahistine hydrochloride tablets in patients with vertebrobasilar insufficiency vertigo (VBIV). Methods: A total of 133 patients with vertigo caused by vertebrobasilar insufficiency treated in The First People’s Hospital of Shangqiu City from March 2019 to August 2021 were selected and analyzed retrospectively. Among them, 63 patients treated with flunarizine tablets were seen as the control group (CG), and 70 with flunarizine tablets combined with betastine hydrochloride tablets were considered as the observation group (OG). The treatment efficacy and adverse reactions were compared, and the vertigo symptom score, quality of life and vertebrobasilar artery hemodynamics were observed before and after treatment. Results: The effective rate in the OG was higher than that of the CG (P<0.05), but there was no obvious difference in adverse reactions (P>0.05). Compared with the CG, the arterial hemodynamics and cerebral blood perfusion as well as SF-36 score were higher, while the scores of dizziness assessment rating scale (DARS) and dizziness handicap inventory (DHI) scale were lower in the OG (all P<0.05). Conclusion: Betahistine hydrochloride can effectively improve arterial hemodynamics and cerebral blood flow perfusion in vertebrobasilar insufficiency patients and enhance the clinical efficacy with high safety profile, which is worthy of wide application.
Keywords: Betahistine hydrochloride tablets, vertebrobasilar insufficiency vertigo, clinical efficacy, flunarizine tablets
Introduction
The vertebrobasilar artery is an essential blood supply artery in the brain. The left and right vertebral arteries pass through the transverse process foramen on both sides of the cervical vertebra and move upward into the skull. The two blood vessels merge into one basilar artery in the brain [1]. When the blood flow of vertebrobasilar artery system decreases to a certain extent by various causes, there will be corresponding symptoms of cerebral ischemia, usually lasting no more than 24 h, but can occur repeatedly, which is called vertebrobasilar insufficiency (VBI) in medicine [2]. VBI causes vertebrobasilar artery stenosis for various reasons, which leads to a series of intermittent and recurrent neurological disorders. Vertebrobasilar insufficiency vertigo (VBIV) is a familiar disease, which is more common in middle-aged and elderly people [3,4]. The main symptoms are vertigo, motor sensory disturbance, nausea, vomiting and so on, which pose a serious threat to the quality of life of patients [5]. The symptoms of VBI are various and complex, so a series of comprehensive examinations are needed before a firm diagnosis [6].
At the moment, most VBI patients are treated with drugs to dilate blood vessels and improve cerebral blood flow to alleviate their clinical symptoms [7]. However, due to the wide variety of clinical drugs, the research report on the efficacy and prognosis of drug treatment is not comprehensive enough. Betahistine hydrochloride tablets are histamine drugs. It is found that betahistine hydrochloride tablets have obvious efficacy on acute ischemic cerebrovascular diseases. In addition, betahistine hydrochloride tablets also have a certain advantage in improving patients’ symptoms such as vertigo and tinnitus [8,9]. There are few clinical reports about the role and significance of betahistine hydrochloride tablets in VBIV treatment. To further clarify the efficacy and safety of betahistine hydrochloride tablets in VBIV patients, we investigated the clinical significance of betahistine hydrochloride tablets in VBIV, to provide a reliable theoretical basis for clinical practice.
Materials and methods
Research subjects
A total of 133 patients with vertigo caused by vertebrobasilar insufficiency treated in the First People’s Hospital of Shangqiu City from March 2019 to August 2021 were selected and analyzed retrospectively. Among them, 63 patients treated with flunarizine tablets were seen as the control group (CG), and 70 with flunarizine tablets combined with betastine hydrochloride tablets were considered as the observation group (OG). This research had been approved by the First People’s Hospital of Shangqiu [2018 (Review) 073a].
Inclusion and exclusion criteria
Inclusion criteria
Patients who were diagnosed as having VBI [10]; Patients who had obvious symptoms of vertigo and were diagnosed with VBIV by clinicians; Patients who were examined and treated for the first time; Patients who agreed and cooperated with the experimental research; and Patients who had complete clinical data.
Exclusion criteria
Those with drug contraindications; those with serious infection; those with other major diseases; those with severe abnormal heart, liver and kidney function; those with immune diseases; or pregnant or lactating women. This research was approved by the Ethics Committee of the First People’s Hospital of Shangqiu City, and informed consent forms were obtained.
Methods
The CG was treated with flunarizine tablets (Xinchang Pharmaceutical Factory of Zhejiang Medicine Co., Ltd., SFDA Approval No. H10950200), 2 tablets per/day, before sleep. On the basis of the CG, the OG was treated with betahistine hydrochloride tablets (Heilongjiang Ruige Pharmaceutical Co., Ltd., SFDA Approval No. H23020159), orally 2 tablets each time, 3 times a day. The patients kept a reasonable diet and exercised properly every day, without smoking or alcohol consuming. Both groups were treated continuously for two weeks.
Outcome measures
(1) The treatment efficacy of both groups after treatment was compared (Evaluation criteria: Markedly effective: Imaging examination revealed that the blood flow index was normal, and the symptoms such as vertigo disappeared. Effective: Imaging ultrasonography revealed that the blood flow index was improved compared with that before treatment, and the symptoms such as vertigo disappeared. Ineffective: The blood flow index examined by ultrasound showed no obvious change compared with that before treatment, and the vertigo still existed). Total effective rate = (markedly effective cases + effective cases)/total number ×100%. (2) The incidence of adverse reactions was analyzed. (3) The mean blood flow velocity of the left vertebral artery, right vertebral artery and basilar artery was measured by Transcranial Doppler (TCD) (Kejin transcranial Doppler blood flow analyzer KJ2V1M). (4) The erythrocyte aggregation index, hematocrit and whole blood viscosity of both groups were measured by automatic hemorheometer. (5) The vertigo symptoms before and after treatment were assessed via dizziness assessment rating scale (DARS) [11] and dizziness handicap inventory (DHI) [12] scale. The DARS consists of 6 entries, each of which includes 7 levels of asymptomatic, very mild, mild, mild to moderate, moderate, moderate to severe, and severe, with each level scores 0, 1, 2, 3, 4, 5, and 6, respectively. The total score is 0 to 36, the higher the score, the more severe the vertigo. The DHI-S consists of 10 entries, each of which includes 3 levels of: yes (4), sometimes (2), and no (0). The total score is 0-40, the higher the score, the more severe the vertigo. (6) The MOS 36-Item Short Form Health Survey (SF-36) [13] was conducted to evaluate the changes of quality of life after treatment; The scoring system includes a total of 8 items, including physiological function (PF), role-physical (RP), body pain (BP), general health (GH), vitality (VT), social function (SF), role-emotional (RE) and mental health (MH). The higher the score, the better the quality of life.
Statistical methods
SPSS 22.0 statistical method was employed to analyze the data. All figures were drawn by GraphPad Prism 9 software. The measurement data were recorded in the form of mean ± standard deviation. Independent sample t-test was used for comparison between groups, and paired t-test used for that before and after treatment. The counting data were marked as percentage, and compared through chi-square test. P<0.05 was considered to have statistical difference.
Results
Comparison of clinical baseline data
There was no marked difference in the general data between groups, such as age, gender, course of disease, living environment, and history of smoking, drinking and hypertension (P>0.05) (Table 1).
Table 1.
Clinical baseline data
| Observation group (n=70) | Control group (n=63) | t or χ2 | P | |
|---|---|---|---|---|
| Age (year) | 54.26±5.12 | 53.40±4.34 | 1.039 | 0.301 |
| Gender | 0.648 | 0.421 | ||
| Male | 36 (51.43) | 28 (44.44) | ||
| Female | 34 (48.57) | 35 (55.56) | ||
| Course of disease (year) | 2.13±0.61 | 1.94±0.66 | 1.725 | 0.087 |
| Living environment | 1.009 | 0.315 | ||
| Countryside | 23 (32.86) | 26 (41.27) | ||
| Cities and towns | 47 (67.14) | 37 (58.73) | ||
| Smoking | 0.904 | 0.342 | ||
| Yes | 38 (54.29) | 29 (46.03) | ||
| No | 32 (45.71) | 34 (53.97) | ||
| Drinking | 0.757 | 0.384 | ||
| Yes | 43 (61.43) | 34 (53.97) | ||
| No | 27 (38.57) | 29 (46.03) | ||
| History of hypertension | 0.246 | 0.620 | ||
| Yes | 9 (12.86) | 10 (15.87) | ||
| No | 61 (87.14) | 53 (84.13) | ||
| Degree of stenosis | 0.074 | 0.964 | ||
| Mild | 24 (34.29) | 21 (33.33) | ||
| Moderate | 34 (48.57) | 32 (50.79) | ||
| Severe | 12 (17.14) | 10 (15.87) |
Comparison of clinical efficacy
As shown in Table 2, the total effective rate of the OG was 94.29%, while that of the CG was 82.54% (P<0.05).
Table 2.
Clinical efficacy
| Observation group (n=70) | Control group (n=63) | χ2 | P | |
|---|---|---|---|---|
| Markedly effective | 28 (40.00) | 20 (31.75) | ||
| Effective | 38 (54.29) | 32 (50.79) | ||
| Ineffective | 4 (5.71) | 11 (17.46) | ||
| Total effective rate (%) | 66 (94.29) | 52 (82.54) | 4.572 | 0.033 |
Comparison of incidence of adverse reactions
As shown in Table 3, adverse reactions such as dry mouth, loss of appetite, skin itching and stomach discomfort occurred in both groups. This denoted that the total incidence of adverse reactions in the OG was 7.14%, while that in the CG was 14.29%. However, there was no marked difference between the two groups (P>0.05).
Table 3.
Incidence of adverse reactions
| Adverse reactions | Observation group (n=70) | Control group (n=63) | χ2 | P |
|---|---|---|---|---|
| Dry mouth | 2 (2.86) | 3 (4.76) | ||
| Loss of appetite | 1 (1.43) | 2 (3.17) | ||
| Skin itching | 1 (1.43) | 2 (3.17) | ||
| Stomach discomfort | 1 (1.43) | 2 (3.17) | ||
| Total incidence of adverse reactions (%) | 5 (7.14) | 9 (14.29) | 1.796 | 0.180 |
Comparison of hemodynamics
There was no marked difference in average blood flow velocity of the left vertebral artery (LVE), right vertebral artery (RVE) and basilar artery, erythrocyte aggregation index, hematocrit and whole blood viscosity between the two groups before treatment (all P>0.05) (Figure 1). After treatment, the average blood flow velocity of LVE, RVE and basilar artery increased, and the OG was higher than the CG (all P<0.05). The erythrocyte aggregation index, hematocrit and whole blood viscosity all decreased, and the OG was lower than the CG (all P<0.05).
Figure 1.
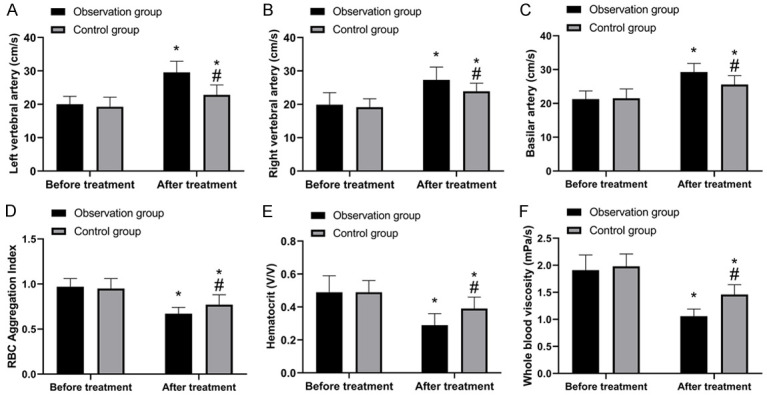
Comparison of hemodynamics. A. Comparison of blood flow velocity of left vertebral artery before and after treatment. B. Comparison of blood flow velocity of right vertebral artery before and after treatment. C. Comparison of blood flow velocity of basilar artery before and after treatment. D. Comparison of erythrocyte aggregation index before and after treatment. E. Comparison of hematocrit before and after treatment. F. Comparison of whole blood viscosity before and after treatment. *, compared with before treatment (P<0.05), and #, compared with observation group (P<0.05).
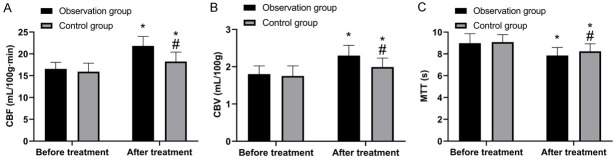
Comparison of cerebral blood flow perfusion
As shown in Figure 2, there was no marked difference in cerebral blood flow perfusion between groups before treatment (P>0.05). After treatment, the CBF and CBV levels in the OG were higher than those in the CG, while the MTT level in the former was lower (P<0.05).
Figure 2.
Comparison of cerebral blood flow perfusion. A. Comparison of CBF before and after treatment. B. Comparison of CBV before and after treatment. C. Comparison of MTT before and after treatment. *, compared with before treatment (P<0.05); #, compared with observation group (P<0.05).
Comparison of DARS and DHI scores
As shown in Figure 3, there was no marked difference in the scores of DARS and DHI before treatment (P>0.05). But the scores decreased after treatment with significantly lower scores in the OG than the CG (P<0.05).
Figure 3.
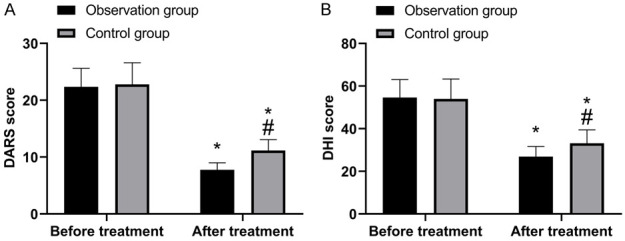
Comparison of DARS and DHI scores. A. DARS scores before and after treatment. B. DHI scores before and after treatment. *, compared with before treatment (P<0.05); #, compared with observation group (P<0.05).
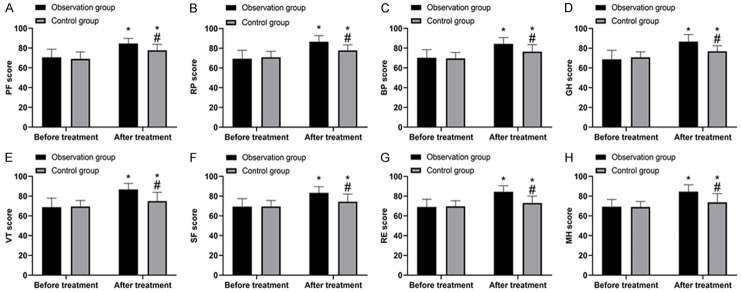
Comparison of quality of life
As shown in Figure 4, there was no marked difference in SF-36 scores of PF, RP, BP, GH, VT, SF, RE and MH between groups before treatment (all P>0.05). After treatment, the scores of both groups increased (P<0.05), and the quality of life of the OG was significantly better than that of the CG (P<0.05).
Figure 4.
Comparison of quality of life. A. APF scores before and after treatment. B. RP scores before and after treatment. C. BP scores before and after treatment. D. GH scores before and after treatment. E. VT scores before and after treatment. F. SF scores before and after treatment. G. RE scores before and after treatment. H. MH scores before and after treatment. *, compared with before treatment (P<0.05); #, compared with the observation group (P<0.05).
Discussion
Modern medicine found that the vertebrobasilar artery not only supplies blood to 2/5ths of the area of the cerebral hemisphere and part of the diencephalon, but also supplies blood to the brainstem, the inner ear of the cerebellum and the high cervical spinal cord, with a wide blood supply range [14]. Therefore, when the vertebrobasilar artery is affected by cerebral arteriosclerosis, vertebral artery compression or cervical sympathetic nerve stimulation, there will be lumen stenosis and spasms, thus affecting blood flow velocity. In addition, vertebrobasilar artery blood supply insufficiency also brings about vertigo, nausea, vomiting, etc. Although infarctions are rarely formed in the early stage, VBI vertigo easily occurs repeatedly. If not effectively treated, it can lead to brainstem, cerebellar and occipital lobe infarction, and nervous system dysfunction, thereby affecting the normal life of patients and endangers their life [15]. Therefore, timely and effective treatment is vital for VBI. Betahistine hydrochloride tablets have been proved to have excellent effects in vasodilation [16,17], but there is a lack of reliable guiding research in VBI. Thus, this research has important reference significance for clinical practice.
In this research, we first compared the clinical efficacy of both groups of patients. It turned out that the clinical efficacy of the OG was better than the CG, indicating that the use of betahistine hydrochloride tablets can improve the efficacy of VBI. In previous studies, we also found that betahistine hydrochloride tablets had excellent efficacy on diseases such as Meniere’s disease and vestibular vertigo [18,19], which can also support the results of this experiment. Besides, we also found that there was no obvious difference in the clinical safety of both groups, which also demonstrates that the use of betahistine hydrochloride tablets has high safety and is worth popularizing in clinical use. Subsequently, to further understand the effect of betahistine hydrochloride tablets on VBI, we compared the hemodynamics and cerebral blood flow perfusion before and after treatment. Results revealed that both groups were improved after treatment, but the hemodynamics and cerebral blood flow perfusion was more improved in the OG. This suggests that the efficacy of betahistine hydrochloride tablets on VBI is better by more effectively improving the hemodynamics and cerebral blood perfusion of patients. It is well known that betahistine hydrochloride is a partial agonist of H2 receptor, which has obvious dilation effect on cerebral vessels, coronary arteries and peripheral vessels, especially on vertebral artery system, and can increase brain, heart and peripheral blood flow, but does not increase capillary permeability [20,21]. What’s more, betahistine hydrochloride also has mild anticoagulant, antiplatelet aggregation and diuretic effects, and is an effective drug for VBIV treatment [22,23]. In modern pharmacological studies, it has been found that betahistine hydrochloride can exert its anti-vertigo effect by relaxing the blood vessels of the brain and the inner ear, and can also affect the vestibular nucleus in a dose-dependent manner. It also has non-specific effects on the electrophysiology of the cerebral cortex and cochlear nerves [24]. Betastine hydrochloride has an anticoagulant effect similar to that of low molecular weight dextran, which can inhibit plasma coagulation, interfere with ADP-induced platelet aggregation, and reduce blood viscosity and blood lipids [25]. Hence, in VBI patients, betahistine hydrochloride can dilate blood vessels, promote hemodynamics, and inhibit the process of coagulation and impulsive nerve conduction. On the one hand, it improves the pathological manifestations of blood insufficiency; on the other hand, it relieves the neurological sensation of vertigo. We also found that the DARS and DHI scores of the OG were lower than those of the CG after treatment, which also supports the above point of view. In modern clinical medicine, the quality of life of patients is also one of the key focuses. We finally compared the quality of life after treatment, and verified that patients in the OG had a better quality of life after treatment, again emphasizing the excellent application value of betahistine hydrochloride in VBI.
Nevertheless, there are still many limitations. For example, there are many clinical treatments for VBI, but this research only compared betahistine with flunarizine alone. We also need to compare with more treatment options to further understand the effect of betahistine hydrochloride. In addition, we also need to include more research subjects, prolong the experimental cycle, and obtain more comprehensive results for clinical reference. In the follow-up study, we will conduct a more in-depth and comprehensive analysis of the application of betahistine hydrochloride in VBI, so as to provide more reliable protection for patients.
To sum up, betahistine hydrochloride can effectively improve arterial hemodynamics and cerebral blood flow perfusion in VBI patients, and enhance the clinical efficacy. It has high safety and can be used widely.
Disclosure of conflict of interest
None.
References
- 1.Han SM, Lee HS, Chae HS, Seo YJ. Usefulness of vertebrobasilar artery radiological finding as a predictive and prognostic factor for sudden sensorineural hearing loss. Auris Nasus Larynx. 2021;48:823–829. doi: 10.1016/j.anl.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Thomas L, Treleaven J. Should we abandon positional testing for vertebrobasilar insufficiency? Musculoskelet Sci Pract. 2020;46:102095. doi: 10.1016/j.msksp.2019.102095. [DOI] [PubMed] [Google Scholar]
- 3.Rennert RC, Steinberg JA, Strickland BA, Ravina K, Bakhsheshian J, Fredrickson V, Pannell JS, Khalessi AA, Russin JJ. Extracranial-to-intracranial bypass for refractory vertebrobasilar insufficiency. World Neurosurg. 2019;126:552–559. doi: 10.1016/j.wneu.2019.03.184. [DOI] [PubMed] [Google Scholar]
- 4.Guo Z, Su Z, Wang Z, Luo X, Lai R. The effect of Chinese herbal medicine Banxia Baizhu Tianma Decoction for the treatment of vertebrobasilar insufficiency vertigo: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. 2017;31:27–38. doi: 10.1016/j.ctim.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Ke C, Zheng CN, Wang J, Yao D, Fang X, Luo Y, Wu J, Zheng X, Wang P. Evaluation on the application of transcranial Doppler (TCD) and electroencephalography (EEG) in patients with vertebrobasilar insufficiency. J Orthop Surg Res. 2020;15:470. doi: 10.1186/s13018-020-01915-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Liu M, Zhang Y, Li Z, Wang D, Yan X. Acupuncture for vertebrobasilar insufficiency vertigo: protocol for a systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e9261. doi: 10.1097/MD.0000000000009261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shulev YA, Gordienko KS, Trashin AV, Pechiborshch DA. Microvascular decompression in trigeminal neuralgia following vertebrobasilar dolichoectasia. Zh Vopr Neirokhir Im N N Burdenko. 2020;84:50–63. doi: 10.17116/neiro20208405150. [DOI] [PubMed] [Google Scholar]
- 8.Ramos Alcocer R, Ledezma Rodriguez JG, Navas Romero A, Cardenas Nunez JL, Rodriguez Montoya V, Deschamps JJ, Liviac Ticse JA. Use of betahistine in the treatment of peripheral vertigo. Acta Otolaryngol. 2015;135:1205–1211. doi: 10.3109/00016489.2015.1072873. [DOI] [PubMed] [Google Scholar]
- 9.Adrion C, Fischer CS, Wagner J, Gurkov R, Mansmann U, Strupp M, Group BS. Efficacy and safety of betahistine treatment in patients with Meniere’s disease: primary results of a long term, multicentre, double blind, randomised, placebo controlled, dose defining trial (BEMED trial) BMJ. 2016;352:h6816. doi: 10.1136/bmj.h6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutting N, Verhagen AP, Vijverman V, Keesenberg MD, Dixon G, Scholten-Peeters GG. Diagnostic accuracy of premanipulative vertebrobasilar insufficiency tests: a systematic review. Man Ther. 2013;18:177–182. doi: 10.1016/j.math.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Felisati G, Pignataro O, Di Girolamo A, Bruno E, Alessandrini M, Guidetti G, Monzani D, Beldi AM, Mira E, Benazzo M, Pallestrini E, Caligo G, Casani A, Battaglia A. Nicergoline in the treatment of dizziness in elderly patients. A review. Arch Gerontol Geriatr Suppl. 2004:163–170. doi: 10.1016/j.archger.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson GP, Calder JH. A screening version of the Dizziness Handicap Inventory (DHI-S) Am J Otol. 1998;19:804–808. [PubMed] [Google Scholar]
- 13.Lins L, Carvalho FM. SF-36 total score as a single measure of health-related quality of life: scoping review. SAGE Open Med. 2016;4:2050312116671725. doi: 10.1177/2050312116671725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang FC, Chen SY, Yin JH, Lin CC, Sung YF, Chou CH, Chung CH, Chien WC, Tsai CK, Tsai CL, Lin GY, Lee JT. The association between vertebrobasilar insufficiency and the risk of dementia: a nationwide register-based retrospective cohort study in Taiwan. BMJ Open. 2017;7:e017001. doi: 10.1136/bmjopen-2017-017001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diprose WK, Diprose JP, Tarr GP, Sutcliffe J, McFetridge A, Brew S, Caldwell J, McGuinness B, Wang MTM, Barber PA. Vertebrobasilar artery calcification and outcomes in posterior circulation large vessel occlusion thrombectomy. Stroke. 2020;51:1301–1304. doi: 10.1161/STROKEAHA.119.027958. [DOI] [PubMed] [Google Scholar]
- 16.Murdin L, Hussain K, Schilder AG. Betahistine for symptoms of vertigo. Cochrane Database Syst Rev. 2016;2016:CD010696. doi: 10.1002/14651858.CD010696.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zamergrad MV, Kunelskaya NL, Guseva AL, Amelin AV, Lilenko SV, Samartcev IN, Zaytseva OV, Melnikov OA, Voronov VA, Lyapin AV. Betahistine in vestibular disorders: current concepts and perspectives. Vestn Otorinolaringol. 2021;86:73–81. doi: 10.17116/otorino20218602173. [DOI] [PubMed] [Google Scholar]
- 18.Casani AP, Guidetti G, Schoenhuber R Consensus Conference Group. Report from a consensus conference on the treatment of Meniere’s disease with betahistine: rationale, methodology and results. Acta Otorhinolaryngol Ital. 2018;38:460–467. doi: 10.14639/0392-100X-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirtane MV, Biswas A. Efficacy of betahistine by patient-reported outcomes and its tolerability profile in indian patients with vestibular vertigo. J Assoc Physicians India. 2017;65:18–24. [PubMed] [Google Scholar]
- 20.Smith RC, Maayan L, Wu R, Youssef M, Jing Z, Sershen H, Szabo V, Meyers J, Jin H, Zhao J, Davis JM. Betahistine effects on weight-related measures in patients treated with antipsychotic medications: a double-blind placebo-controlled study. Psychopharmacology (Berl) 2018;235:3545–3558. doi: 10.1007/s00213-018-5079-1. [DOI] [PubMed] [Google Scholar]
- 21.Gananca MM, Caovilla HH, Gazzola JM, Gananca CF, Gananca FF. Betahistine in the treatment of tinnitus in patients with vestibular disorders. Braz J Otorhinolaryngol. 2011;77:499–503. doi: 10.1590/S1808-86942011000400014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Djelilovic-Vranic J, Alajbegovic A, Tiric-Campara M, Volic A, Sarajlic Z, Osmanagic E, Todorovic L, Beslagic O. Betahistine or cinnarizine for treatment of Meniere’s disease. Med Arch. 2012;66:396–398. doi: 10.5455/medarh.2012.66.396-398. [DOI] [PubMed] [Google Scholar]
- 23.Tang KT, Chao YH, Chen DY, Lim YP, Chen YM, Li YR, Yang DH, Lin CC. Betahistine attenuates murine collagen-induced arthritis by suppressing both inflammatory and Th17 cell responses. Int Immunopharmacol. 2016;39:236–245. doi: 10.1016/j.intimp.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 24.Kang D, Jing Z, Li R, Hei G, Shao T, Li L, Sun M, Yang Y, Wang Y, Wang X, Long Y, Huang X, Wu R. Effect of betahistine and metformin on antipsychotic-induced weight gain: an analysis of two clinical trials. Front Psychiatry. 2018;9:620. doi: 10.3389/fpsyt.2018.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiao YL, Xiang WQ, Liu F, Jin S. Clinical therapeutic effects of gastrodin in combination with betahistine on vertigo: a protocol for systematic review and meta-analysis. Medicine (Baltimore) 2021;100:e23825. doi: 10.1097/MD.0000000000023825. [DOI] [PMC free article] [PubMed] [Google Scholar]