Abstract
Objective: To determine the effects of mesalazine combined with probiotics on inflammation and immune function of patients with inflammatory bowel disease (IBD). Methods: In this retrospective study, a total of 116 patients with IBD treated in Renmin Hospital of Wuhan University from September 2018 to September 2021 were enrolled and divided into a control group (n=55, treated with mesalazine alone) and a research group (n=61, treated with mesalazine combined with probiotics) according to the treatment regimen. The two groups were compared in the levels of inflammatory factors, immune factors, adverse reactions, clinical efficacy and improvement of patients’ disease condition before and after treatment. Logistic regression was used to analyze the independent risk factors of infection in patients with IBD at 6 months after admission. Results: The research group showed a significantly higher the total effective rate than the control group (P<0.05), and there was no notable difference between the two groups in the incidence of adverse reactions (P>0.05). In addition, compared with the control group, the research group showed significantly lower levels of immunoglobulin A (IgA), immunoglobulin G (IgG), immunoglobulin M (IgM), Tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), C-reactive protein (CRP), and had significantly lower scores of clinical activity index (CAI) and endoscopic activity index (EAI) after treatment (all P<0.05). Higher IgG, IgM, IL-6, CRP and EAI levels at admission were independent risk factors for infection in patients with IBD. Conclusion: Mesalazine combined with probiotics can substantially improve the disease condition of patients with IBD, improve their immune ability and reduce their inflammation level, with a good safety profile.
Keywords: Mesalazine, probiotics, inflammatory function, immune function, inflammatory bowel disease
Introduction
Inflammatory bowel disease (IBD) is a chronic intestinal inflammatory disease, which includes two types, namely, Crohn’s disease and ulcerative colitis [1]. Crohn’s disease and ulcerative colitis have highly similar pathogenesis, both of which can give rise to chronic intestinal inflammation [2]. IBD mainly occurs among young people, with diverse clinical manifestations and long course of disease, and IBD patients often suffer complications [3]. Patients with long-term IBD face a significantly increased risk of colorectal dysplasia and cancer [4]. The specific pathogenesis of IBD is still unclear, but the intestinal immune disorder requires attention [5]. Macrophages play a crucial role in the pathogenesis of IBD. They can produce inflammatory factors such as interleukin-1β (IL-1β), interleukin-6 (IL-6), IL-23 and tumor necrosis factor-α (TNF-α) and thus activate other immune cells and cause immune disorders. Under the action of IFN-γ and TNF-α, macrophages polarize into M1 macrophages which can efficiently present antigens, secrete proinflammatory cytokines such as IL-1 and IL-6 at high levels, activate Th1 immune response and promote inflammation [6].
Currently, aminosalicylate, corticosteroids, immunosuppressants and biological agents are mainly used for the treatment of IBD [7]. Mesalazine, the effective component of which is 5-aminosalicylic acid, can be released in the colon and ileum, and is thus used as a common drug for ulcerative enteritis [8]. Peroxidase in colonic mucosa can form leukotrienes and prostaglandins, and mesalazine can inhibit the formation of prostaglandins and leukotrienes, thus inhibiting the development of intestinal inflammation [9]. According to studies, mesalazine can relieve symptoms, but also carries a risk of infection, hepatitis, leukopenia and pancreatitis [10].
Inflammation can also be alleviated by regulating the intestinal bacterial microenvironment in IBD imbalance, so as to achieve the therapeutic effect [11]. Probiotics are living microorganisms, which have been used in the treatment of diarrhea [12]. Probiotics can inhibit the overgrowth of potentially pathogenic bacteria to regulate the composition and inflammatory level of the intestinal tract. Khan et al. [13] pointed out that probiotics could lower the level of inflammation in mice with colitis and alleviate colitis through intestinal microflora and immune response. In addition, according to prior research, mesalazine combined with probiotics can deliver higher efficacy by shortening the course of disease and preventing recurrence [14].
Accordingly, we compared the treatment efficacy of mesalazine alone and its combination with probiotics in IBD patients, with the purpose of exploring the effect of combined treatment on the inflammation and immune function of patients with IBD.
Materials and methods
Patient information
A total of 116 patients with IBD treated in Renmin Hospital of Wuhan University from September 2018 to September 2021 were retrospectively selected and grouped into two groups according to the treatment regimen. A total of 55 patients treated with mesalazine alone were assigned to the control group, and the other 61 patients treated with mesalazine combined with probiotics were assigned to the research group. This study was approved by the Medical Ethics Committee of Renmin Hospital of Wuhan University (IRB-20180403), and all patients signed informed consent forms after understanding the purpose and contents of the study.
Inclusion and exclusion criteria
Inclusive criteria: Patients diagnosed with IBD by colonoscopy, imaging examination or pathological examination according to the diagnostic criteria in the diagnostic guidelines issued by the Asian Organization of Crohn’s and Colitis (AOCC) [15]; patients ≥18 years old; and patients with complete clinical data.
Exclusion criteria: Patients allergic to therapeutic drugs used in this study; patients with poor compliance with treatment; patients with comorbid infection or autoimmune disease; patients with other comorbid digestive diseases, or comorbid malignant tumour; patients with incomplete detection results of relevant indicators; or pregnant/lactating woman.
Treatment mode
Patients in the control group were given Mesalazine Slow-Release Tablets orally (Sunflower Pharmaceutical Group Jiamusi Luling Pharmaceutical Co., Ltd; State Food and Drug Administration (SFDA) approval number: H19980148; 0.25 g*24 s), 1 g a day 3 times for 2 months. Patients in the research group were given probiotics, Enteric-coated Bifida triple viable capsules (Bifidobacterium longum, Lactobacillus acidophilus and Enterococcus faecalis) (Shanghai Pharmaceuticals Sine, SFDA approval number: S10950032; 0.21 g × 24 s) in addition to treatment in the control group. Specifically, each patient was required to take 2 capsules each time, three times a day.
Outcome measures
The clinical efficacy of the two groups was compared. Markedly effective: After therapy, the clinical symptoms of the patient disappeared completely, and the colonoscopy showed that the mucosa of the patient was normal; Effective: After therapy, the patient’s clinical symptoms were alleviated to a certain extent, and the colonoscopy results showed that the patient’s mucosa had polyps and slight congestion; Ineffective: After therapy, the patient’s clinical symptoms and the colonoscopy results were not changed. Total effective treatment rate = [(number of cases with markedly effective + cases of effective)/the total number of patients] × 100%.
The incidence of adverse reactions in the treatment process of the two groups was counted.
Before and after therapy, 5 mL venous blood was extracted from each patient and placed in coagulation promoting tubes, followed by immediate centrifugation (3000 × g at 4°C for 10 min). The levels of IgG, IgM and IgA in serum were analyzed using an automatic immune analyzer (Beckman Image 800), and the levels of serum CRP, IL-6, and TNF-α were measured by enzyme-linked immunosorbent assay (ELISA) kits (Thermo Fisher Scientific, USA; KHA0031, KHC0061, and KHC3011).
The clinical activity index (CAI) and endoscopic activity index (EAI) of all patients were determined before and after treatment. The evaluation of CAI covered seven items: defecation frequency, stool blood volume, abdominal pain symptoms, fever, anemia, ESR changes and systemic symptoms. Evaluation of EAI covered reflective granules, vascular morphology, mucosal fragility and mucosal injury degree under colonoscopy. A higher score suggested a more severe situation.
The number of patients with infection within half a year after admission to hospital in the two groups was counted. The immune and inflammatory indexes of infected and uninfected patients were compared, and multivariate analysis was conducted to explore the independent risk factors of infection.
Statistical analyses
SPSS 20.0 software (Chicago SPSS Co., Ltd.) was used for statistical analysis. Continuous variables were expressed as mean ± standard deviation. The independent t-test was used for comparison between the two groups, and the paired t-test was used for comparison of the same group at different time periods, and the results were expressed by t. For classification variables, the data were expressed as the number or percentages, and analyzed using the chi-square analysis, and the results were expressed using X2. Logistic multivariate regression was used to analyze the independent risk factors of infection in patients with IBD 6 months after admission. P<0.05 indicated a statistical difference.
Results
Baseline data
There was no significant difference in age, gender, course of disease, body mass index (BMI), type, lesion location, smoking history, alcoholism history and place of residence between the two groups (P>0.05, Table 1).
Table 1.
Baseline data
| Control group (n=55) | Research group (n=61) | t/X2 | P-value | |
|---|---|---|---|---|
| Age (years) | 36.78±4.94 | 36.10±4.32 | 0.791 | 0.431 |
| Gender | 0.693 | 0.405 | ||
| Male | 31 (56.36) | 39 (63.93) | ||
| Female | 24 (43.64) | 22 (36.07) | ||
| Course of disease (years) | 3.68±0.54 | 3.8±0.63 | 1.096 | 0.276 |
| BMI (kg/m2) | 21.47±2.42 | 20.76±2.2 | 1.655 | 0.101 |
| Type | 0.307 | 0.579 | ||
| Ulcerative colitis | 43 (78.18) | 45 (73.77) | ||
| Crohn’s disease | 12 (21.82) | 16 (26.23) | ||
| Lesion site | 0.696 | 0.706 | ||
| Rectum | 18 (32.73) | 15 (24.59) | ||
| Left colon | 12 (21.82) | 15 (24.59) | ||
| Extensive colon | 25 (45.45) | 31 (50.82) | ||
| Smoking history | 17 (30.91) | 15 (24.59) | 0.578 | 0.447 |
| Alcoholism history | 15 (27.27) | 13 (21.31) | 0.561 | 0.454 |
| Place of residence | 0.261 | 0.610 | ||
| Rural area | 43 (78.18) | 50 (81.97) | ||
| Cities and towns | 12 (21.82) | 11 (18.03) |
BMI, Body Mass Index.
Comparison of efficacy between the two groups
According to comparison of efficacy between the two groups, the total effective rate of the research group was significantly higher than that of the control group (88.52% vs. 70.91%, P<0.05, Table 2).
Table 2.
Therapeutic efficacy
| Control group (n=55) | Research group (n=61) | X2 | P-value | |
|---|---|---|---|---|
| Remarkably effective | 21 (38.18) | 27 (44.26) | ||
| Effective | 18 (32.73) | 27 (44.26) | ||
| Ineffective | 16 (29.09) | 7 (11.48) | ||
| Total effective rate | 39 (70.91) | 54 (88.52) | 5.646 | 0.018 |
Incidence of adverse reactions in the two groups
During the treatment, patients in the control group suffered from rash, nausea and constipation, showing a total incidence of adverse reactions of 7.27%, while patients in the research group suffered rash, nausea, diarrhea and constipation, showing a total incidence of adverse reactions of 11.48%. The total incidence of adverse reactions between the two groups was not significantly different (P>0.05, Table 3).
Table 3.
Adverse reactions
| Control group (n=55) | Research group (n=61) | t/X2 | P | |
|---|---|---|---|---|
| Rash | 1 (1.82) | 2 (3.28) | ||
| Nausea | 1 (1.82) | 3 (4.92) | ||
| Diarrhea | 0 (0.00) | 1 (1.64) | ||
| Constipation | 2 (3.64) | 1 (1.64) | ||
| Total adverse reactions | 4 (7.27) | 7 (11.48) | 0.595 | 0.440 |
Improvement of patients’ immune function
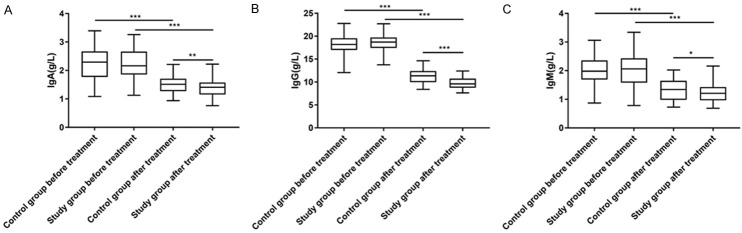
According to comparison of the changes in immune function between the two groups, IgA, IgG and IgM in both groups decreased significantly after treatment (P<0.05), with significantly lower levels in the research group than those in the control group (all P<0.05, Figure 1).
Figure 1.
Improvement of patients’ immune function. A. There was no significant difference in IgA between the two groups before treatment (P>0.05). After treatment, IgA of the two groups decreased significantly (P<0.001), with a significantly lower level in the research group than that in the control group (P<0.001). B. There was no significant difference in IgG between the two groups before treatment (P>0.05). After treatment, IgG of the two groups decreased significantly (P<0.001), with a significantly lower level in the research group than that in the control group (P<0.001). C. There was no significant difference in IgM between the two groups before treatment (P>0.05). After treatment, IgM of the two groups decreased significantly (P<0.001), with a significantly lower level in the research group than that in the control group (P<0.001). Notes: *P<0.05, **P<0.01, and ***P<0.001. IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M.
Changes of inflammatory levels in patients
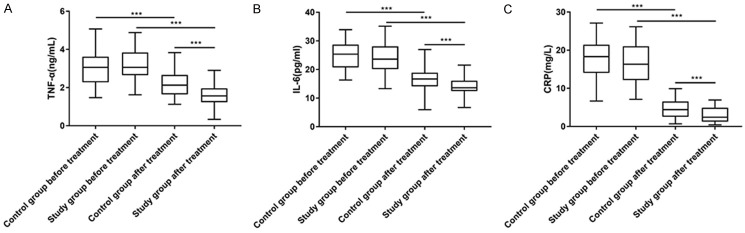
After treatment, TNF-α, IL-6 and CRP in both groups decreased significantly (P<0.05), and the levels in the research group were notably lower than those in the control group (P<0.05, Figure 2).
Figure 2.
Changes of the inflammation level before and after treatment. A. There was no significant difference in TNF-α between the two groups before treatment (P>0.05). After treatment, TNF-α in both groups decreased significantly (P<0.001), with a significantly lower level in the research group than that in the control group (P<0.001). B. There was no significant difference in IL-6 level between the two groups before treatment (P>0.05). After treatment, IL-6 of the two groups decreased significantly (P<0.001), with a significantly lower level in the research group than that in the control group (P<0.001). C. There was no significant difference in CRP between the two groups before treatment (P>0.05). After treatment, CRP of the two groups decreased significantly (P<0.001), with a significantly lower level in the research group than that in the control group (P<0.001). Note: ***P<0.001. CRP, C-reactive protein; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6.
Improvement of patients’ disease condition
After treatment, the CAI and EAI scores of both groups decreased significantly (P<0.05), with significantly lower CAI and EAI scores in the research group than the control group (P<0.05, Figure 3).
Figure 3.
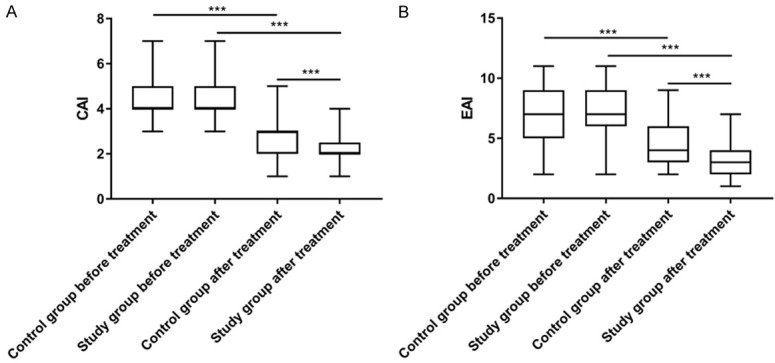
Improvement of the patient’s condition. A. There was no significant difference in clinical activity index (CAI) scores between the two groups before treatment (P>0.05). After treatment, CAI scores of the two groups decreased significantly (P<0.001), with significantly lower scores in the research group than those of the control group (P<0.001). B. There was no significant difference in endoscopic activity index (EAI) scores between the two groups before treatment (P>0.05). After treatment, EAI scores of the two groups decreased significantly (P<0.001), with significantly lower scores in the research group than those of the control group (P<0.001).
Relationship between immune and inflammatory markers and infection in patients
After 6 months of treatment, there were 11 cases of infection in the control group (20.00%) and 8 cases in the study group (13.11%), and the incidence of infection was not significantly different between the two groups (X2=1.001, P=0.317). The patients were further divided into the infection group and non-infection group, and there were significant differences in the course of disease, levels of IgA, IgG, IgM, TNF-α, IL-6 and CRP, CAI score and EAI score at admission between the two groups (P<0.05, Table 4).
Table 4.
Univariate analysis
| Infection group (n=19) | Non-infection group (n=97) | t/X2 | P | |
|---|---|---|---|---|
| Age (years) | 36.47±4.27 | 36.15±5.45 | 0.242 | 0.810 |
| Gender | 1.690 | 0.194 | ||
| Male | 14 (73.68) | 56 (57.73) | ||
| Female | 5 (26.32) | 41 (42.27) | ||
| Course of disease (years) | 4.11±0.67 | 3.67±0.55 | 3.074 | 0.003 |
| BMI (kg/m2) | 20.74±2.10 | 21.16±2.37 | 0.719 | 0.474 |
| Type | 2.003 | 0.157 | ||
| Ulcerative colitis | 12 (63.16) | 76 (78.35) | ||
| Crohn’s disease | 7 (36.84) | 21 (21.65) | ||
| Lesion site | 0.790 | 0.674 | ||
| Rectum | 7 (36.84) | 26 (26.80) | ||
| Left colon | 4 (21.05) | 23 (23.71) | ||
| Extensive colon | 8 (42.11) | 48 (49.48) | ||
| History of smoking | 7 (36.84) | 25 (25.77) | 0.975 | 0.324 |
| History of alcoholism | 7 (63.16) | 21 (74.23) | ||
| Place of residence | 0.602 | 0.438 | ||
| Rural area | 14 (73.68) | 79 (81.44) | ||
| Urban area | 5 (26.32) | 18 (18.56) | ||
| IgA at admission | 2.45±0.47 | 2.15±0.57 | 2.222 | 0.028 |
| IgG at admission | 19.62±1.89 | 18.21±1.95 | 2.896 | 0.005 |
| IgM at admission | 2.24±0.42 | 1.97±0.54 | 2.058 | 0.042 |
| TNF-α at admission | 3.53±0.97 | 3.04±0.78 | 2.402 | 0.018 |
| IL-6 at admission | 26.80±5.28 | 23.98±4.42 | 2.461 | 0.015 |
| CRP at admission | 21.91±4.53 | 16.15±4.95 | 4.699 | <0.001 |
| CAI score at admission | 4.95±1.22 | 4.38±0.97 | 2.242 | 0.027 |
| EAI score at admission | 8.53±1.90 | 6.86±2.23 | 3.052 | 0.003 |
| Treatment mode | 1.001 | 0.317 | ||
| Mesalazine alone | 11 (57.89) | 44 (45.36) | ||
| Salazine combined with probiotics | 8 (42.11) | 53 (54.64) |
BMI, Body Mass Index; EAI, Endoscopic Activity Index; CRP, C-reactive Protein; IgA, Immunoglobulin A; IgG, Immunoglobulin G; IgM, Immunoglobulin M; TNF-α, Tumor Necrosis Factor-α; IL-6, Interleukin-6.
Multivariate analysis
Univariate analysis showed that course of disease, IgA, IgG, IgM, TNF-α, IL-6, CRP, CAI score and EAI score at admission were the influencing factors of infection in patients with IBD. Binary logistics analysis further showed that relatively high IgG, IgM, IL-6, and CRP levels at admission and relatively high EAI score at admission were independent risk factors for infection events in patients with IBD (Table 5).
Table 5.
Multivariate analysis
| Factors | B | S.E | Wals | Sig. | Exp (B) | 95% C.I. of EXP (B) | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Lower limit | Upper limit | ||||||
| IgG at admission | 0.536 | 0.266 | 4.058 | 0.044 | 1.709 | 1.015 | 2.877 |
| IgM at admission | 2.179 | 1.046 | 4.339 | 0.037 | 8.834 | 1.137 | 68.614 |
| IL-6 at admission | 0.266 | 0.096 | 7.732 | 0.005 | 1.305 | 1.082 | 1.575 |
| CRP at admission | 0.178 | 0.084 | 4.479 | 0.024 | 1.829 | 1.084 | 3.085 |
| EAI score at admission | 0.604 | 0.267 | 5.118 | 0.001 | 6.784 | 2.265 | 20.324 |
EAI, Endoscopic Activity Index; CRP, C-reactive Protein; IgG, Immunoglobulin G; IgM, Immunoglobulin M; IL-6, Interleukin-6.
Discussion
As a typical chronic autoimmune disease, IBD is closely related to many factors, including imbalance of anti-inflammatory and pro-inflammatory factors, diet, genetic factors, infection, environment and imbalance of intestinal flora [16]. Mesalazine containing 5-aminosalicylic acid can inhibit the synthesis of activated inflammatory mediators such as prostaglandin E2, leukotriene B4 and platelets in the serum of patients with IBD, which can help inhibit inflammatory reaction and thus achieve the purpose of treatment [17]. According to prior research [18], corticosteroids and immunosuppressants are effective for severe IBD patients, but long-term use of corticosteroids may give rise to acne, weight gain, infection, osteoporosis and cataracts, aggravating the disease [18]. Probiotics stand out, because they can improve the function of immune system and intestinal mucosal barrier, correct intestinal microecological disorder, promote the secretion of anti-inflammatory factors and inhibit the growth of harmful bacteria [19].
In the present study, mesalazine combined with probiotics showed more effective curative outcome than mesalazine alone in the treatment of IBD, and the two methods did not bring significantly different adverse reactions. However, in the combined treatment group, some patients suffered diarrhea, possibly because probiotics can increase gastrointestinal motility, which may induce diarrhea in IBD patients, but the patients were self-healed later. Dang et al. [20] pointed out that compared with mesalazine alone, the patients who received the combination treatment of the probiotic mixture had better improvement, which was consistent with our research.
In terms of the inflammation and immune function of patients after treatment, we found that mesalazine combined with probiotics can improve the immune function of patients more effectively and reduce the inflammatory level of patients more significantly. The initial damage of mucosal barrier and intestinal epithelium causes the increase of intestinal permeability, which exposes intestinal bacteria, pathogens and antigens to immune cells, and then results in intestinal inflammation and inappropriate immune response [21]. However, the immune regulation of probiotics takes effect through the interaction with intestinal cells and dendritic cells, which leads to the regulation of innate and adaptive immune system [22]. Liu et al. [23] induced chronic colitis in mice with dextran sodium sulfate, and found that the levels of IgM, IgG and IgA in the colonic mucus of chronic colitis mice increased, while a mixture of probiotics significantly reduced the levels of IgM, IgG and IgA.
As a living microorganism that produces health benefits to the host, probiotics have many specific mechanisms: first, they can bind to intestinal mucosal epithelial cells to strengthen the mucosal defence barrier; secondly, they can also promote the growth of normal microorganisms, inhibit the reproduction of pathogenic microorganisms, and regulate intestinal microecology. Additionally, probiotics can also regulate the mucosal immune response and promote intestinal lymphocytes to differentiate into regulatory T cells. Some studies have also confirmed that probiotics can effectively alleviate intestinal inflammation [24,25]. Therefore, the combination of probiotics can further strengthen the treatment effect on inflammation, mucosal immunity and intestinal motility, and jointly promote a good outcome from disease.
Finally, we also found that mesalazine combined with probiotics significantly reduced CAI and EAI scores, which indicated that the combined treatment could improve the intestinal barrier function of patients to a great extent, as well as reduce the damage caused by diseases, and thus accelerate the recovery of patients. CAI is a scoring method based on clinical symptoms and laboratory results, while EAI is a scoring method specifically used to reflect endoscopic mucosal lesions [26]. The significant improvement of the two scores indicates that combined therapy is effective for disease relief in patients with IBD. Palumbo et al. [27] also revealed that mesalazine combined with probiotics could replace corticosteroids in the treatment of mild to moderate ulcerative colitis. Through multivariate analysis, we found that relatively high IgG, IgM, IL-6 and CRP levels on admission and relatively high EAI score on admission were independent risk factors for infection in IBD patients. Analyzing the reasons, when patients have high inflammation and immune disorders, their intestinal homeostasis is disrupted, and the destruction of inate resistance makes the intestinal tract susceptible to many bacteria and pathogens, resulting in infection.
This study still has some limitations. Some studies have shown that mesalazine with probiotics can contribute to clinical remission for most patients with mild to moderate IBD [28]. However, in this study, due to the limited samples, we were unable to find the difference between the efficacy in severe cases and moderate to mild cases. We hope to conduct further research with a larger sample size in the future. Secondly, the risk factors of adverse reactions caused by treatment have not been discussed, which also requires a larger sampled study.
To sum up, mesalazine combined with probiotics can substantially alleviate IBD symptoms by improving patients’ immune ability and reducing inflammation level, with a good safety profile.
Disclosure of conflict of interest
None.
References
- 1.Wang CJ, Byun MJ, Kim SN, Park W, Park HH, Kim TH, Lee JS, Park CG. Biomaterials as therapeutic drug carriers for inflammatory bowel disease treatment. J Control Release. 2022;345:1–19. doi: 10.1016/j.jconrel.2022.02.028. [DOI] [PubMed] [Google Scholar]
- 2.Schneider AM, Ozsoy M, Zimmermann FA, Brunner SM, Feichtinger RG, Mayr JA, Kofler B, Neureiter D, Klieser E, Aigner E, Schutz S, Stummer N, Sperl W, Weghuber D. Expression of oxidative phosphorylation complexes and mitochondrial mass in pediatric and adult inflammatory bowel disease. Oxid Med Cell Longev. 2022;2022:9151169. doi: 10.1155/2022/9151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caban M. Glucocorticosteroid induced cataract - drug-induced damage of vision organ which is worth paying attention in the treatment of inflammatory bowel diseases. Postepy Biochem. 2019;65:227–230. doi: 10.18388/pb.2019_277. [DOI] [PubMed] [Google Scholar]
- 4.Ngamruengphong S, Aihara H, Friedland S, Nishimura M, Faleck D, Benias P, Yang D, Draganov PV, Kumta NA, Borman ZA, Dixon RE, Marion JF, D’Souza LS, Tomizawa Y, Jit S, Mohapatra S, Charabaty A, Parian A, Lazarev M, Figueroa EJ, Hanada Y, Wang AY, Wong Kee Song LM. Endoscopic submucosal dissection for colorectal dysplasia in inflammatory bowel disease: a US multicenter study. Endosc Int Open. 2022;10:E354–E360. doi: 10.1055/a-1783-8756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langer V, Vivi E, Regensburger D, Winkler TH, Waldner MJ, Rath T, Schmid B, Skottke L, Lee S, Jeon NL, Wohlfahrt T, Kramer V, Tripal P, Schumann M, Kersting S, Handtrack C, Geppert CI, Suchowski K, Adams RH, Becker C, Ramming A, Naschberger E, Britzen-Laurent N, Sturzl M. IFN-gamma drives inflammatory bowel disease pathogenesis through VE-cadherin-directed vascular barrier disruption. J Clin Invest. 2019;129:4691–4707. doi: 10.1172/JCI124884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neurath MF. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat Immunol. 2019;20:970–979. doi: 10.1038/s41590-019-0415-0. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Shen J. Core indicators of an evaluation and guidance system for quality of care in inflammatory bowel disease centers: a critical review. EClinicalMedicine. 2022;46:101382. doi: 10.1016/j.eclinm.2022.101382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smatlik N, Mortada N, Rocken M, Yazdi AS, Zierhut M. Mesalazine suppresses proinflammatory cytokines in patients with acute anterior uveitis independently of HLA-B27. Ocul Immunol Inflamm. 2021:1–9. doi: 10.1080/09273948.2021.1873396. [DOI] [PubMed] [Google Scholar]
- 9.Tang P, Sun Q, Zhao L, Pu H, Yang H, Zhang S, Gan R, Gan N, Li H. Mesalazine/hydroxypropyl-beta-cyclodextrin/chitosan nanoparticles with sustained release and enhanced anti-inflammation activity. Carbohydr Polym. 2018;198:418–425. doi: 10.1016/j.carbpol.2018.06.106. [DOI] [PubMed] [Google Scholar]
- 10.Gisbert JP, Gomollon F, Mate J, Pajares JM. Role of 5-aminosalicylic acid (5-ASA) in treatment of inflammatory bowel disease: a systematic review. Dig Dis Sci. 2002;47:471–488. doi: 10.1023/a:1017987229718. [DOI] [PubMed] [Google Scholar]
- 11.Zaidi D, Huynh HQ, Carroll MW, Mandal R, Wishart DS, Wine E. Gut microenvironment and bacterial invasion in paediatric inflammatory bowel diseases. J Pediatr Gastroenterol Nutr. 2020;71:624–632. doi: 10.1097/MPG.0000000000002848. [DOI] [PubMed] [Google Scholar]
- 12.Drago L, Meroni G, Chiaretti A, Laforgia N, Cucchiara S, Baldassarre ME On Behalf Of The Surveyflor Group. Effect of limosilactobacillus reuteri LRE02-lacticaseibacillus rhamnosus LR04 combination on antibiotic-associated diarrhea in a pediatric population: a national survey. J Clin Med. 2020;9:3080. doi: 10.3390/jcm9103080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan I, Wei J, Li A, Liu Z, Yang P, Jing Y, Chen X, Zhao T, Bai Y, Zha L, Li C, Ullah N, Che T, Zhang C. Lactobacillus plantarum strains attenuated DSS-induced colitis in mice by modulating the gut microbiota and immune response. Int Microbiol. 2022;25:587–603. doi: 10.1007/s10123-022-00243-y. [DOI] [PubMed] [Google Scholar]
- 14.Tursi A. Diverticular disease: a therapeutic overview. World J Gastrointest Pharmacol Ther. 2010;1:27–35. doi: 10.4292/wjgpt.v1.i1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim ES, Chen M, Lee J, Lee CK, Kim YS. Diagnosis of inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 2(nd) Asian Organization for Crohn’s and Colitis (AOCC) meeting in Seoul. Intest Res. 2016;14:224–230. doi: 10.5217/ir.2016.14.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarris G, de Rougemont A, Charkaoui M, Michiels C, Martin L, Belliot G. Enteric viruses and inflammatory bowel disease. Viruses. 2021;13:104. doi: 10.3390/v13010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bossuyt P, Baert F, Coenegrachts JL, De Vos M, Dewit O, Ferrante M, Fontaine F, Mana F, Vandervoort J, Moreels T. Ulcerative colitis treatment: an insight into daily clinical practice. Acta Gastroenterol Belg. 2019;82:365–372. [PubMed] [Google Scholar]
- 18.Sullivan PW, Ghushchyan VH, Globe G, Schatz M. Oral corticosteroid exposure and adverse effects in asthmatic patients. J Allergy Clin Immunol. 2018;141:110–116. e117. doi: 10.1016/j.jaci.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Shen ZH, Zhu CX, Quan YS, Yang ZY, Wu S, Luo WW, Tan B, Wang XY. Relationship between intestinal microbiota and ulcerative colitis: mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J Gastroenterol. 2018;24:5–14. doi: 10.3748/wjg.v24.i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dang X, Xu M, Liu D, Zhou D, Yang W. Assessing the efficacy and safety of fecal microbiota transplantation and probiotic VSL#3 for active ulcerative colitis: a systematic review and meta-analysis. PLoS One. 2020;15:e0228846. doi: 10.1371/journal.pone.0228846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JH, Peyrin-Biroulet L, Eisenhut M, Shin JI. IBD immunopathogenesis: a comprehensive review of inflammatory molecules. Autoimmun Rev. 2017;16:416–426. doi: 10.1016/j.autrev.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Foligne B, Dewulf J, Breton J, Claisse O, Lonvaud-Funel A, Pot B. Probiotic properties of non-conventional lactic acid bacteria: immunomodulation by Oenococcus oeni. Int J Food Microbiol. 2010;140:136–145. doi: 10.1016/j.ijfoodmicro.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Liu XJ, Yu R, Zou KF. Probiotic mixture VSL#3 alleviates dextran sulfate sodium-induced colitis in mice by downregulating T follicular helper cells. Curr Med Sci. 2019;39:371–378. doi: 10.1007/s11596-019-2045-z. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Yang H, Li Y. The triple interactions between gut microbiota, mycobiota and host immunity. Crit Rev Food Sci Nutr. 2022:1–21. doi: 10.1080/10408398.2022.2094888. [DOI] [PubMed] [Google Scholar]
- 25.Li A, Wang Y, Li Z, Qamar H, Mehmood K, Zhang L, Liu J, Zhang H, Li J. Probiotics isolated from yaks improves the growth performance, antioxidant activity, and cytokines related to immunity and inflammation in mice. Microb Cell Fact. 2019;18:112. doi: 10.1186/s12934-019-1161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplan M, Yuksel M, Ates I, Kilic ZM, Kilic H, Kuzu UB, Kayacetin E. Is ischemia modified albumin a disease activity marker for inflammatory bowel diseases? J Gastroenterol Hepatol. 2016;31:1120–1125. doi: 10.1111/jgh.13254. [DOI] [PubMed] [Google Scholar]
- 27.Palumbo VD, Romeo M, Marino Gammazza A, Carini F, Damiani P, Damiano G, Buscemi S, Lo Monte AI, Gerges-Geagea A, Jurjus A, Tomasello G. The long-term effects of probiotics in the therapy of ulcerative colitis: a clinical study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016;160:372–377. doi: 10.5507/bp.2016.044. [DOI] [PubMed] [Google Scholar]
- 28.Kaur L, Gordon M, Baines PA, Iheozor-Ejiofor Z, Sinopoulou V, Akobeng AK. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2020;3:CD005573. doi: 10.1002/14651858.CD005573.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]