Abstract
Objective: This study aimed to evaluate the intrinsic cerebral activity alternations in experimental odontogenic pain with resting-state functional magnetic resonance imaging (fMRI). Materials and Methods: Forty-nine participants in an odontogenic pain group and 49 participants in control group underwent imaging using fMRI in this prospective study. Odontogenic pain was induced by experimental tooth movement. We calculated the fractional amplitude of low-frequency fluctuation (fALFF) value to evaluate regional cerebral function and compared it between the two groups utilizing a voxel-based two-sample t-test. Results: In comparison with the healthy controls, the participants in odontogenic pain group showed increased fALFF value in the left cerebellum, right posterior cingulate gyrus, and bilateral inferior temporal gyrus, as well as decreased fALFF in the medial prefrontal cortex, the left anterior cingulate cortex, bilateral angular gyrus, left inferior parietal cortex, middle temporal gyrus, and miscellaneous cerebral regions (P < 0.001 familywise error-corrected VOXEL > 100). Conclusion: The present study showed abnormal cerebral activity in odontogenic pain, and reveled that the aberrant regional functional activities were mainly located within the default mode network. The finding could provide insight into the underlying neural mechanism of odontogenic pain. Registry of clinical trials (Trial number ChiCTR1800018589) - http://www.chictr.org.cn/showproj.aspx?proj=31424.
Keywords: Magnetic resonance imaging, odontogenic pain, brain, low-frequency fluctuations, cortex
Introduction
Odontogenic pain, initiating from the dental pulp or periodontium and resulting from dental or non-dental diseases, can severely affect patients’ quality of life and daily activities [1]. Odontogenic pain is very common in the population [2,3]. Peripheral mechanisms associated with odontogenic pain have been well documented: the nociception is transmitted from sensory nerve endings of first-order neurons in trigeminal ganglia to second-order neurons, which send this information to the thalamus, and then the information is relayed to the cortex by the thalamus [4]. We previously reported that different subregions of thalamus are involved in the perception of orofacial pain [5]. While it is known that the cortex undertakes the final process of nociception, which is to determine the location, intensity, and duration of pain, limited studies have explored cerebral structure or functional alteration in odontogenic pain [6,7].
Functional magnetic resonance imaging (fMRI) measures cerebral activity non-invasively by detecting blood oxygen level-dependent signals [8]. Recently, fMRI has been used in oral neuroscience in a broad range to explore the central neural mechanisms underlying orofacial pain, particularly non-odontogenetic pain. For example, several studies showed neural activity changes in the middle temporal gyrus, insula, thalamus, and primary somatosensory cortex in the patients with idiopathic trigeminal neuralgia [9,10]. And a previous study in patients with burning mouth syndrome showed functional differences in the hippocampus and medial prefrontal cortex (mPFC) [11]. Altered neural activities in middle frontal gyrus and insula were also observed in patients with temporomandibular disorders [12]. As for odontogenic pain, several fMRI studies have revealed cortical representation of pain induced by electrical stimulation, thermal stimulation and mechanical stimulation [13-15]. However, as far as we know, most previous studies used tasking-state fMRI, during which subjects were under a tasking state, and only a few researches have investigated alterations of spontaneous cerebral activity in subjects with odontogenic pain by resting-state fMRI [16]. In recent years, the use of resting-state fMRI to study the central neural mechanisms behind chronic pain has become increasingly common, allowing a shorter time of scanning and providing various results with multiple analytic methods and it has been considered a sensitive and specialized technology to study the changes in brain function [17,18]. The amplitude of low-frequency fluctuations (ALFF) is an index used to detect regional properties of spontaneous cerebral activity by resting-state fMRI [19], and the fractional ALFF (fALFF) value is calculated as the ratio of regional power spectrum of low frequency to power spectrum of the whole frequency range, improving sensitivity and specificity of detecting spontaneous neural activity. Thus, our aim in this study was to explore the intrinsic cerebral activity alternations in experimental odontogenic pain using fALFF by resting-state fMRI.
Materials and methods
Participants
Each participant was informed about the protocol and inherent risks prior to providing written informed consent. The present study had formal approval by the local ethics committee and all the research procedures of the current study followed the Declaration of Helsinki principle.
The minimal required sample size was calculated using G*Power software (ver. 3.1; Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany) [1], based on the mean pain intensity of 10.9 and the standard deviation of 16.9 estimated according to a previous study [2]. Utilizing a power of 0.8 and an alpha of 0.05, 39 participants were calculated for each group. Assuming a small attrition rate, 49 consecutive participants were included in the odontogenic pain group in this prospective study, and 49 healthy controls were included and matched to the pain group for age and gender. Inclusion criteria of participants were as follows: (I) right-handed; (II) aged from 18 to 45 years; (III) capable of undergoing MRI examination. The exclusion criteria were as follows: (I) participants with existing maxillofacial diseases that cause orofacial pain, such as gingivitis, periodontitis, or pulpitis; (II) with history of neurologic dysfunction; (III) with history of drug or alcohol abuse; (IV) contraindicated for MRI; (V) having severe malocclusion (particularly those having abnormal/open interproximal contacts between the lower left second premolar and the first molar); (VI) with a history of prior orthodontic treatment. Five participants were excluded due to significant head motion (exceeding 2 mm or 2°) during scan. Finally, 44 participants with odontogenic pain and 49 healthy controls were enrolled in the present study.
Study design
A 4 mm elastic separator (Morelli-Sorocaba-SP, Brazil) was placed between the second bicuspid and the first molar of the lower jaw in the odontogenic pain group by a trained orthodontist, which could result in a non-physiologically reversible load on the periodontium and pain after a period of time, and fMRI scans were taken 24 h after the placement of the separators [20-23]. In the healthy control group, participants were scanned with no separator. The pain of the participants was quantified using a visual analogue scale (VAS) - where 0 indicated “no pain” and 100 indicated “the most intense pain imaginable”. The Symptom Checklist-90-Revision (SCL-90-R) was used for psychological evaluation. Pain and psychological evaluation were obtained twice in pain group: once before placement of the separator and then, 24 h later, before performing the scan, while at baseline in control group.
MRI data acquisition
Functional MRI data were obtained with a Siemens 3-Tesla MR scanner (Trio; Siemens, Erlangen, Germany). Participants were scanned with a standard eight-channel phased-array head coil, and foam pads were fixed on both sides of the head. During imaging, all the participants were told to remain still and keep their eyes closed but stay awake, not to engage in active thinking. The scanning parameters were as follows: repetition time (ms)/echo time (ms), 2000/30; matrix size, 64 × 64; field of view, 240 × 240 mm2; voxel size, 3.75 × 3.75 × 5 mm3; slice thickness, 5 mm; and flip angle, 90°. Further, each fMRI sequence contained 205 image volumes.
MRI data processing and analysis
The fMRI data were preprocessed by using SPM8 package (SPM8, http://www.fil.ion.ucl.ac.uk/spm/software/spm8). To enable magnetization equilibrium, the first 10 time points for each participant were excluded. The remaining 195 scans were used for further analysis and were subjected to slice timing and realigned for head movement correction. The resulting functional images were spatially normalized to the Montreal Neurological Institute space and smoothing were performed with a 4 mm full-width at half-maximum Gaussian kernel; each voxel was resampled to 3 × 3 × 3 mm3.
We calculated the fALFF with Resting-State fMRI Data Analysis Toolkit (REST) (http://www.restfmri.net/forum/REST, version 1.8) [24]. The time series of the whole-brain signal were converted to the frequency domain power spectrum by a fast Fourier transform. We calculated the mean square root of the power spectrum at each frequency at the range of 0.01-0.1 Hz, which was regarded as ALFF. The fALFF value was calculated as the ratio of the power spectrum of low-frequency (0.01-0.1 Hz) to that of the entire frequency range.
Statistical analyses
Demographic and clinical variables were assessed using two-sample t-tests or chi-square tests with IBM SPSS software (version 20, Chicago, USA). Differences in clinical variables recorded before and after the placement were assessed using the paired t-tests. The fALFF distinctions between the two groups were statistically analyzed using two-sample, two-sided t-tests, with framewise displacement, age, and sex as covariates. The voxel-based statistical tests were corrected for multiple comparisons with familywise error (FWE) correction. The significance level was set to be equal to 0.05. Pearson correlation analyses were performed to reveal the relationships between the fALFF values in the entire brain and VAS score. P < 0.05 (AlphaSim corrected) was set as significance level.
Results
Demographic and clinical characteristics
Forty-four participants with odontogenic pain and 49 healthy controls were finally included in the present study. Demographic information for the participants is summarized in Table 1, and the results indicated non-significant differences between odontogenic pain group and healthy control group in age distribution, sex ratio, VAS or SCL-90-R at base line. Paired t-tests showed that the VAS score in the odontogenic pain group increased significantly 24 h after placement of the elastic separators (6.8±16.7, t=-2.7, P=0.01), and there were no statistically significant differences in SCL-90-R before and after the placement of separators (1.6±8.2, t=-1.3, P=0.206).
Table 1.
Participant demographics and evaluation of pain
| Odontogenic pain group (n=44) | Control group (n=49) | P value | |
|---|---|---|---|
| Age | 21.0±0.9 | 21.6±0.9 | 0.991 |
| Sex ratio | 24/20 | 27/22 | 0.957 |
| VAS | 14.7±17.0 | 13.7±16.4 | 0.768 |
| SCL-90-R | 27.7±11.0 | 26.4±11.1 | 0.573 |
VAS, Visual Analogue Scale; SCL-90-R, Symptom Checklist-90-Revision. All values are mean ± standard deviation (SD).
FALFF differences
In comparison to the healthy controls, the odontogenic pain group exhibited increased fALFF in the left cerebellum, right posterior cingulate cortex (PCC), and bilateral inferior temporal gyrus, while exhibiting widespread decreased fALFF in the mPFC, the left anterior cingulate cortex (ACC), bilateral angular gyrus, left inferior parietal cortex, middle temporal gyrus, and miscellaneous cerebral regions (P < 0.001 FWE corrected VOXEL > 100) (Figures 1 and 2).
Figure 1.
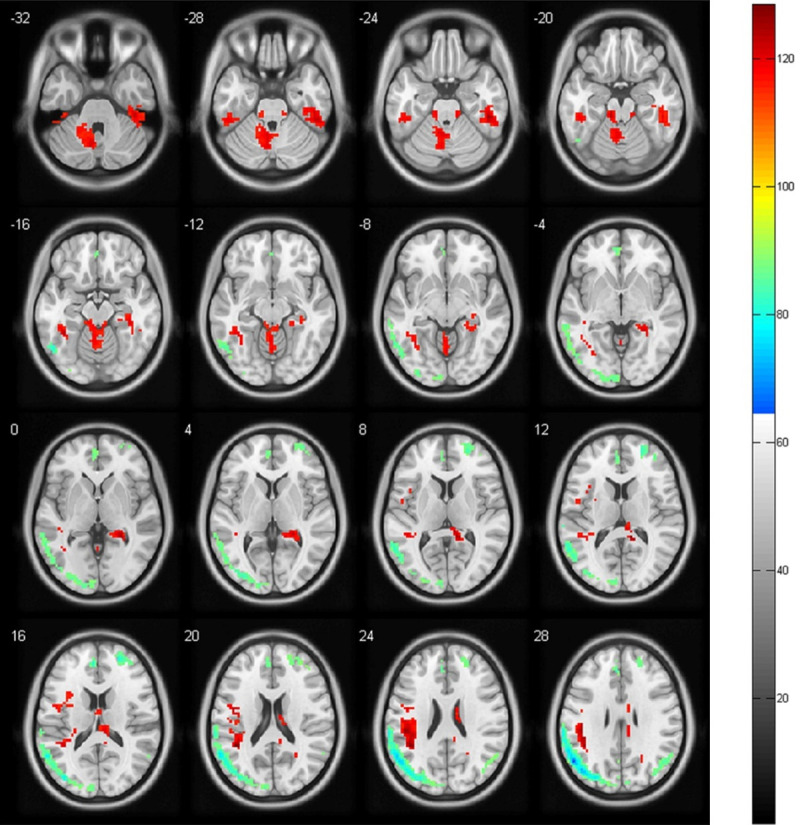
Transverse plane. Images showing the results of the fractional amplitude of the low-frequency fluctuation (fALFF) analysis. Compared with the controls, the odontogenic patients show an increased fALFF (red) in the left cerebellum, bilateral inferior temporal gyrus, and a decreased fALFF (blue) in the medial prefrontal cortex, the left anterior cingulate cortex, bilateral angular gyrus, left inferior parietal cortex, middle temporal gyrus, and miscellaneous cerebral regions (P < 0.001, familywise error corrected VOXEL > 100).
Figure 2.
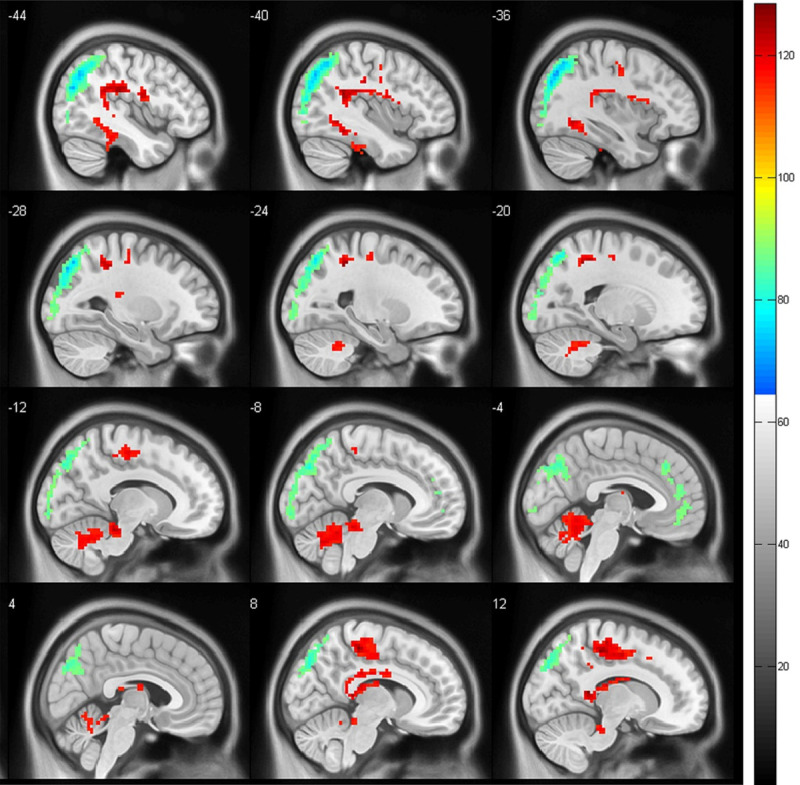
Sagittal plane. Images showing the results of the fractional amplitude of the low-frequency fluctuation (fALFF) analysis. Compared with the controls, the odontogenic patients show an increased fALFF (red) in the left cerebellum and right posterior cingulate cortex and a decreased fALFF (blue) in the medial prefrontal cortex, the left anterior cingulate cortex, bilateral angular gyrus, left inferior parietal cortex, middle temporal gyrus, and miscellaneous cerebral regions (P < 0.001, familywise error corrected VOXEL > 100).
Correlation analysis
The correlation analysis showed there was no statistically significant relationship between the fALFF values and the VAS scores.
Discussion
In the present study, we used non-invasive functional MRI to examine the cerebral regional activity in participants with experimental odontogenic pain by measuring fALFF value of the whole brain. In comparison to the healthy controls, the odontogenic pain group exhibited abnormal patterns of brain activity.
ACC
We found significantly decreased fALFF in the left ACC in odontogenic pain group, which is consist with other brain imaging studies on chronic facial pain. ACC, considered as the cortical area exceedingly related to the experience of pain, participates in signaling the unpleasantness in acute pain, and contributes to the affective component of chronic pain [25]. Pain is an experience integrated sensory and emotional aspects, and ACC has been shown to play a crucial role in the emotional aspects of pain [26-29]. The ACC receives inputs from both the medial thalamus and primary somatosensory cortex, integrates the nociceptive information and regulates the aversive response to pain [30]. Moreover, an animal experiment revealed that microinjections of oxytocin into the ACC could reduce chronic-pain-induced anxiety [31]. In addition, it has been well documented that odontogenic pain is accompanied with negative affect, such as anxiety [32-35]. Thus, the decreased fALFF in the ACC in our results may suggest that there might be functional abnormality in ACC, which may partly underlie neural mechanism of negative affect related to odontogenic pain.
MPFC
We also found decreased fALFF in the mPFC. And previous human MRI data have also shown that the mPFC activity correlates negatively with pain intensity [36]. The mPFC was reported to play dual and opposing roles in pain processing, including regulating antinociceptive effects and inducing pain chronification [37]. In addition, increased activation of mPFC is also associated with placebo analgesia [38]. A study in hyperalgesia showed that the higher the activation in the mPFC, the smaller the hyperalgesic area [39]. Thus, decreased function of mPFC may be related to modulation of odontogenic pain. However, the specific relationship between activation of mPFC and odontogenic pain needs to be further researched.
The default mode network (DMN)
Interestingly, we found that most of the regions that displayed notable changes in activity were the key nodes of the DMN, such as the PCC, mPFC, inferior parietal cortex, and angular gyrus. The DMN is the most stable network at rest and has been reported to be more active in passive tasks than in goal-directed tasks, and it may participate in internally directed cognitive activity and external environment monitoring as a sentinel [40,41]. Some investigations have reported significant changes in the DMN’s functions in different pain conditions [42-44]. Consistent with these previous studies, we observed DMN functional changes in participants with odontogenic pain. According to a recent publication [43], pain is a stimulus that demands attention and competes for cognition. Functional changes in the DMN may reflect the presence of pain per se and may form the basis of some attention and cognitive changes that occur during pain. Therefore, DMN involved in the process of odontogenic pain may cause some attention and cognitive changes leading to attention and mental flexibility deficits, which need to be paid attention to during odontogenic pain treatments. Additionally, alterations in functional connectivity between the nodes within the DMN need to be examined. Moreover, dysfunction of the DMN is involved in many psychiatric disorders, including depression and anxiety [40], so it is also essential to pay attention to mental health in odontogenic pain, especially chronic pain. More importantly, training for attentional focus, with DMN nodes as the therapeutic targets, using short-term mindfulness-based interventions and long-term meditative practices, can be used to relieve some unexplained odontogenic pain or odontogenic pain caused by treatment with almost no side effects [45,46].
Medial temporal lobe (MTL)
We also found decreased fALFF in the MTL. The MTL is known to play an essential role in memory [47] and provides information from previous experiences in the form of memory and association, forming the basis of mental simulation [40]. It’s reported dental patients tend to recall more pain than they originally reported during the dental procedures, and this phenomenon is more pronounced in patients with dental fear [48]. Therefore, the reduced functional activity in the MTL may be due to its involvement in memory encoding during odontogenic pain, however further investigations are required for its evaluation.
Cerebellum
It’s interesting that we found increased fALFF in the cerebellum. The fundamental function of cerebellum has long been thought to be postural balance maintaining. Recently, the role of the cerebellum in pain management has gathered increasing interest [49,50]. It was demonstrated that the cerebellum played a previously underestimated role in pain perception and control [51]. A previous study revealed altered function in cerebellum of idiopathic trigeminal neuralgia patients [9]. And in earlier studies [52,53], researchers found that the cerebellum is highly active during trigeminal nociception and is involved in both sensory and cognitive perception of pain. Thus, the cerebellum may be important in perception and modulation of odontogenic pain.
This study also has its limitations. First, we did not analyze the functional connectivity between these brain regions, which could provide comprehensive information about brain networks, such as DMN. Further analysis of functional connectivity may elucidate the underlying neurobiological mechanisms of odontogenic pain. Second, the participants in the present study were relatively young, and we should recruit some older participants in future studies.
Conclusions
This study provides insight into the central neural mechanism of experimental odontogenic pain. Our results revealed that the participants with experimental odontogenic pain have abnormal cerebral activity in the cerebellum, PCC, mPFC, ACC, angular gyrus, MTL and miscellaneous brain regions, which are mainly associated with pain modulation and emotional regulation. And DMN is likely to play a key role in the processing of odontogenic pain. A greater awareness of the roles of various cortical regions in pain perception may contribute to the development of new treatments to ameliorate odontogenic pain in the future.
Acknowledgements
We are grateful to all the participants for their support and willingness to participate. This research was funded by National Natural Science Foundation, grant number 81571004.
Disclosure of conflict of interest
None.
References
- 1.Oghli I, List T, Su N, Häggman-Henrikson B. The impact of oro-facial pain conditions on oral health-related quality of life: a systematic review. J Oral Rehabil. 2020;47:1052–1064. doi: 10.1111/joor.12994. [DOI] [PubMed] [Google Scholar]
- 2.Thayer T. Pain part 4: odontogenic pain. Dent Update. 2015;42:622–624. 627–630. doi: 10.12968/denu.2015.42.7.622. [DOI] [PubMed] [Google Scholar]
- 3.Renton T. Tooth-related pain or not? Headache. 2020;60:235–246. doi: 10.1111/head.13689. [DOI] [PubMed] [Google Scholar]
- 4.Sacerdote P, Levrini L. Peripheral mechanisms of dental pain: the role of substance P. Mediators Inflamm. 2012;2012:951920. doi: 10.1155/2012/951920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin Y, Yang H, Zhang F, Wang J, Liu H, Yang X, Long H, Li F, Gong Q, Lai W. The medial thalamus plays an important role in the cognitive and emotional modulation of orofacial pain: a functional magnetic resonance imaging-based study. Front Neurol. 2021;11:589125. doi: 10.3389/fneur.2020.589125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novaes AP, da Rocha MJ, Leite-Panissi CR. Tooth movement activates the central amygdala and the lateral hypothalamus by the magnitude of the force applied. Angle Orthod. 2010;80:111–115. doi: 10.2319/100708-522.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horinuki E, Shinoda M, Shimizu N, Koshikawa N, Kobayashi M. Orthodontic force facilitates cortical responses to periodontal stimulation. J Dent Res. 2015;94:1158–1166. doi: 10.1177/0022034515586543. [DOI] [PubMed] [Google Scholar]
- 8.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 9.Yuan J, Cao S, Huang Y, Zhang Y, Xie P, Zhang Y, Fu B, Zhang T, Song G, Yu T, Zhang M. Altered spontaneous brain activity in patients with idiopathic trigeminal neuralgia: a resting-state functional MRI study. Clin J Pain. 2018;34:600–609. doi: 10.1097/AJP.0000000000000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li M, Yan J, Li S, Wang T, Zhan W, Wen H, Ma X, Zhang Y, Tian J, Jiang G. Reduced volume of gray matter in patients with trigeminal neuralgia. Brain Imaging Behav. 2017;11:486–492. doi: 10.1007/s11682-016-9529-2. [DOI] [PubMed] [Google Scholar]
- 11.Khan SA, Keaser ML, Meiller TF, Seminowicz DA. Altered structure and function in the hippocampus and medial prefrontal cortex in patients with burning mouth syndrome. Pain. 2014;155:1472–1480. doi: 10.1016/j.pain.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 12.He SS, Li F, Song F, Wu S, Chen JY, He N, Zou SJ, Huang XQ, Lui S, Gong QY, Chen S. Spontaneous neural activity alterations in temporomandibular disorders: a cross-sectional and longitudinal resting-state functional magnetic resonance imaging study. Neuroscience. 2014;278:1–10. doi: 10.1016/j.neuroscience.2014.07.067. [DOI] [PubMed] [Google Scholar]
- 13.Jantsch HHF, Kemppainen P, Ringler R, Handwerker HO, Forster C. Cortical representation of experimental tooth pain in humans. Pain. 2005;118:390–399. doi: 10.1016/j.pain.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Upadhyay J, Granitzka J, Bauermann T, Baumgärtner U, Breimhorst M, Treede RD, Birklein F. Detection of central circuits implicated in the formation of novel pain memories. J Pain Res. 2016;9:671–681. doi: 10.2147/JPR.S113436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meier ML, Brügger M, Ettlin DA, Luechinger R, Barlow A, Jäncke L, Lutz K. Brain activation induced by dentine hypersensitivity pain-an fMRI study. J Clin Periodontol. 2012;39:441–447. doi: 10.1111/j.1600-051X.2012.01863.x. [DOI] [PubMed] [Google Scholar]
- 16.Yang J, Li B, Yu QY, Ye L, Zhu PW, Shi WQ, Yuan Q, Min YL, He YL, Shao Y. Altered intrinsic brain activity in patients with toothaches using the amplitude of low-frequency fluctuations: a resting-state fMRI study. Neuropsychiatr Dis Treat. 2019;15:283–291. doi: 10.2147/NDT.S189962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee MH, Smyser CD, Shimony JS. Resting-state fMRI: a review of methods and clinical applications. AJNR Am J Neuroradiol. 2013;34:1866–1872. doi: 10.3174/ajnr.A3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai W, Qiu E, Chen Y, Xing X, Xi W, Zhang M, Li K, Tian L, Dong Z, Yu S. Enhanced functional connectivity between habenula and salience network in medication-overuse headache complicating chronic migraine positions it within the addiction disorders: an ICA-based resting-state fMRI study. J Headache Pain. 2021;22:107. doi: 10.1186/s10194-021-01318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu H, Zuo Y, Gu H, Waltz JA, Zhan W, Scholl CA, Rea W, Yang Y, Stein EA. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proc Natl Acad Sci U S A. 2007;104:18265–18269. doi: 10.1073/pnas.0705791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Melh MA, Andersson L. The effect of a lidocaine/prilocaine topical anesthetic on pain and discomfort associated with orthodontic elastomeric separator placement. Prog Orthod. 2017;18:1. doi: 10.1186/s40510-016-0156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marini I, Bartolucci ML, Bortolotti F, Innocenti G, Gatto MR, Alessandri Bonetti G. The effect of diode superpulsed low-level laser therapy on experimental orthodontic pain caused by elastomeric separators: a randomized controlled clinical trial. Lasers Med Sci. 2015;30:35–41. doi: 10.1007/s10103-013-1345-y. [DOI] [PubMed] [Google Scholar]
- 22.Michelotti A, Farella M, Martina R. Sensory and motor changes of the human jaw muscles during induced orthodontic pain. Eur J Orthod. 1999;21:397–404. doi: 10.1093/ejo/21.4.397. [DOI] [PubMed] [Google Scholar]
- 23.Cioffi I, Michelotti A, Perrotta S, Chiodini P, Ohrbach R. Effect of somatosensory amplification and trait anxiety on experimentally induced orthodontic pain. Eur J Oral Sci. 2016;124:127–134. doi: 10.1111/eos.12258. [DOI] [PubMed] [Google Scholar]
- 24.Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Bliss TV, Collingridge GL, Kaang BK, Zhuo M. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci. 2016;17:485–496. doi: 10.1038/nrn.2016.68. [DOI] [PubMed] [Google Scholar]
- 26.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 27.Meda KS, Patel T, Braz JM, Malik R, Turner ML, Seifikar H, Basbaum AI, Sohal VS. Microcircuit mechanisms through which mediodorsal thalamic input to anterior cingulate cortex exacerbates pain-related aversion. Neuron. 2019;102:944–959. e3. doi: 10.1016/j.neuron.2019.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Turnbull IM. Bilateral cingulumotomy combined with thalamotomy or mesencephalic tractotomy for pain. Surg Gynecol Obstet. 1972;134:958–962. [PubMed] [Google Scholar]
- 30.Singh A, Patel D, Li A, Hu L, Zhang Q, Liu Y, Guo X, Robinson E, Martinez E, Doan L, Rudy B, Chen ZS, Wang J. Mapping cortical integration of sensory and affective pain pathways. Curr Biol. 2020;30:1703–1715. e5. doi: 10.1016/j.cub.2020.02.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li XH, Matsuura T, Xue M, Chen QY, Liu RH, Lu JS, Shi W, Fan K, Zhou Z, Miao Z, Yang J, Wei S, Wei F, Chen T, Zhuo M. Oxytocin in the anterior cingulate cortex attenuates neuropathic pain and emotional anxiety by inhibiting presynaptic long-term potentiation. Cell Rep. 2021;36:109411. doi: 10.1016/j.celrep.2021.109411. [DOI] [PubMed] [Google Scholar]
- 32.Dou L, Vanschaayk M, Zhang Y, Fu X, Ji P, Yang D. The prevalence of dental anxiety and its association with pain and other variables among adult patients with irreversible pulpitis. BMC Oral Health. 2018;18:101. doi: 10.1186/s12903-018-0563-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao M, Yan X, Zhao R, Shan Y, Chen Y, Jian F, Long H, Lai W. Comparison of pain perception, anxiety, and impacts on oral health-related quality of life between patients receiving clear aligners and fixed appliances during the initial stage of orthodontic treatment. Eur J Orthod. 2021;43:353–359. doi: 10.1093/ejo/cjaa037. [DOI] [PubMed] [Google Scholar]
- 34.Long H, Gao M, Zhu Y, Liu H, Zhou Y, Liao L, Lai W. The effects of menstrual phase on orthodontic pain following initial archwire engagement. Oral Dis. 2017;23:331–336. doi: 10.1111/odi.12612. [DOI] [PubMed] [Google Scholar]
- 35.Lin CS, Wu SY, Yi CA. Association between anxiety and pain in dental treatment: a systematic review and meta-analysis. J Dent Res. 2017;96:153–162. doi: 10.1177/0022034516678168. [DOI] [PubMed] [Google Scholar]
- 36.Derbyshire SWG, Jones AKP, Gyulai F, Clark S, Townsend D, Firestone LL. Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain. 1997;73:431–445. doi: 10.1016/S0304-3959(97)00138-3. [DOI] [PubMed] [Google Scholar]
- 37.Ong WY, Stohler CS, Herr DR. Role of the prefrontal cortex in pain processing. Mol Neurobiol. 2019;56:1137–1166. doi: 10.1007/s12035-018-1130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 39.Seifert F, Bschorer K, De Col R, Filitz J, Peltz E, Koppert W, Maihöfner C. Medial prefrontal cortex activity is predictive for hyperalgesia and pharmacological antihyperalgesia. J Neurosci. 2009;29:6167–6175. doi: 10.1523/JNEUROSCI.4654-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 41.Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- 42.Baliki MN, Mansour AR, Baria AT, Apkarian AV. Functional reorganization of the default mode network across chronic pain conditions. PLoS One. 2014;9:e106133. doi: 10.1371/journal.pone.0106133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alshelh Z, Marciszewski KK, Akhter R, Di Pietro F, Mills EP, Vickers ER, Peck CC, Murray GM, Henderson LA. Disruption of default mode network dynamics in acute and chronic pain states. Neuroimage Clin. 2017;17:222–231. doi: 10.1016/j.nicl.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alshelh Z, Di Pietro F, Youssef AM, Reeves JM, Macey PM, Vickers ER, Peck CC, Murray GM, Henderson LA. Chronic neuropathic pain: it’s about the rhythm. J Neurosci. 2016;36:1008–1018. doi: 10.1523/JNEUROSCI.2768-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harrison R, Zeidan F, Kitsaras G, Ozcelik D, Salomons TV. Trait mindfulness is associated with lower pain reactivity and connectivity of the default mode network. J Pain. 2019;20:645–654. doi: 10.1016/j.jpain.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 46.Dickenson J, Berkman ET, Arch J, Lieberman MD. Neural correlates of focused attention during a brief mindfulness induction. Soc Cogn Affect Neurosci. 2013;8:40–47. doi: 10.1093/scan/nss030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yassa MA, Stark CE. Multiple signals of recognition memory in the medial temporal lobe. Hippocampus. 2008;18:945–954. doi: 10.1002/hipo.20452. [DOI] [PubMed] [Google Scholar]
- 48.Kyle BN, McNeil DW, Weaver B, Wilson T. Recall of dental pain and anxiety in a cohort of oral surgery patients. J Dent Res. 2016;95:629–634. doi: 10.1177/0022034516631977. [DOI] [PubMed] [Google Scholar]
- 49.Coombes SA, Misra G. Pain and motor processing in the human cerebellum. Pain. 2016;157:117–127. doi: 10.1097/j.pain.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 50.Michelle Welman FHS, Smit AE, Jongen JLM, Tibboel D, van der Geest JN, Holstege JC. Pain experience is somatotopically organized and overlaps with pain anticipation in the human cerebellum. Cerebellum. 2018;17:447–460. doi: 10.1007/s12311-018-0930-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruscheweyh R, Kühnel M, Filippopulos F, Blum B, Eggert T, Straube A. Altered experimental pain perception after cerebellar infarction. Pain. 2014;155:1303–1312. doi: 10.1016/j.pain.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Diano M, D’Agata F, Cauda F, Costa T, Geda E, Sacco K, Duca S, Torta DM, Geminiani GC. Cerebellar clustering and functional connectivity during pain processing. Cerebellum. 2016;15:343–356. doi: 10.1007/s12311-015-0706-4. [DOI] [PubMed] [Google Scholar]
- 53.Mehnert J, Schulte L, Timmann D, May A. Activity and connectivity of the cerebellum in trigeminal nociception. Neuroimage. 2017;150:112–118. doi: 10.1016/j.neuroimage.2017.02.023. [DOI] [PubMed] [Google Scholar]


