Abstract
Objectives: Liver cancer stem cells (LCSCs) are regarded as the frequent cause of hepatocellular carcinoma (HCC) relapse and therapeutic resistance. The epithelial cell adhesion molecule (EpCAM) is one of the key biomarkers for LCSCs. EpCAM+ cells from HCC have been reported to display cancer stem cell-like (CSC-like) properties. Therefore, we aimed to verify the effect of MASM, a novel derivative of matrine, on CSC-like properties of EpCAM+ HCC cells. Methods: EpCAM+ cells were isolated from Hep3B and Huh7 cells using the magnetic-activated cell sorting. The capacity for self-renewal and proliferation of EpCAM+ HCC cells was determined by the sphere-formation and cell counting kit 8 assays. After these cell populations were exposed to increasing concentrations of MASM, sphere formation, cell proliferation, apoptosis, resistance to chemotherapy and colony formation were evaluated, respectively. Moreover, the stemness-associated gene expression and underlying mechanisms were evaluated by quantitative real-time polymerase chain reaction and sphere-forming assay. Results: MASM significantly inhibited proliferation without inducing apoptosis, down-regulated the expression of stemness-related genes, decreased the percentage of EpCAM+ HCC cells and up-regulated mature hepatocyte-related genes. Moreover, MASM suppressed the formation and reduced the size of not only primary spheroids but also subsequent spheroids. Additionally, our results showed that MASM inhibited the AKT/GSK3β/β-catenin signaling pathway. Conclusion: MASM treatment is effective against EpCAM+ cells and may be considered as a novel drug candidate in HCC therapy.
Keywords: Matrine derivative, hepatocellular carcinoma, cancer stem cells, AKT/GSK3β/β-catenin signaling
Introduction
Hepatocellular carcinoma (HCC) is the third most prevalent type of cancer worldwide. Despite the use of aggressive surgical resection and chemotherapy, the overall five-year mortality of advanced HCC patients with recurrent tumors still remains unsatisfactory [1-5]. Hence, it is imperative to establish unique treatment approaches for HCC.
Cancer stem cells (CSCs) play an important role in the recurrence and metastasis of HCC [6-9]. The epithelial cell adhesion molecule (EpCAM) has been used as a CSC marker in HCC [10,11]. High expression of EpCAM in HCC cells were significantly correlated with a poor prognosis [12,13]. Moreover, EpCAM+ HCC cells exhibited CSC-like properties such as self-renewal, differentiation, tumorigenesis and chemoresistance [14-16]. Thus, liver cancer cells expressing CSC marker EpCAM might be recognized as a therapeutic target for HCC [17].
Matrine, a primary active alkaloid of the Chinese herbaceous drug Sophora flavescens Ait, has anti-inflammation, antivirus and anti-cancer features [18-22]. However, the potency of sophora alkaloids is restricted owing to their reasonably reduced actions and limited half-lives [23]. To enhance their curative impacts, we have semi-manufactured a number of secondary matrines by transforming the carbonyl oxygen atom with a sulfur atom and inserting different amino groups to the keto-beta location. MASM ((6aS, 10S, 11aR, 11bR, 11cS)-10-methylamino-dodecahydro-3a, 7a-diazabenzo (de) anthracene-8-thione; Figure 1A) is one of the unique matrine derivatives with developed pharmacological actions compared with matrine and sophocarpine [24]. We have previously shown that MASM suppressed HCC cells, sphere cells and HCC xenograft growth via decreasing the AKT/GSK3β/β-catenin signaling pathway. In this study, we examined MASM for its effect on EpCAM+ HCC cells, as well as the associated mechanisms.
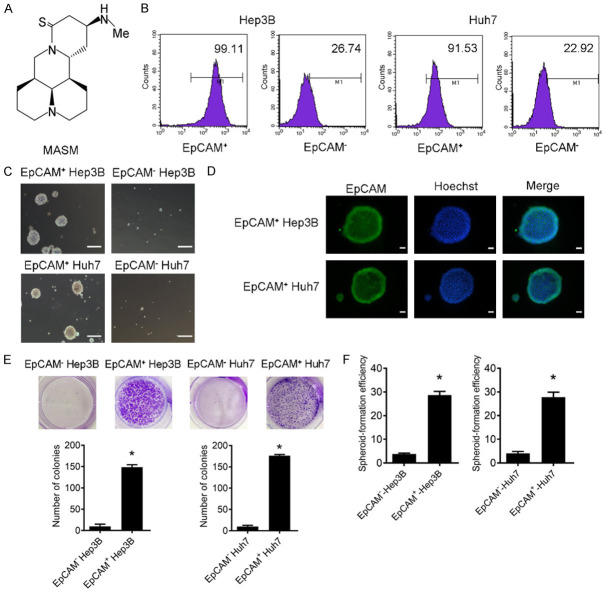
Figure 1.
EpCAM+ Hep3B and Huh7 cells exhibit CSC-like properties. A. Chemical structure of MASM. B. Purity of the sorted EpCAM+ and EpCAM- cells from Hep3B and Huh7 was analyzed by flow cytometry. C. Representative image of spheres formed by EpCAM+ and EpCAM- cells isolated from Hep3B and Huh7 (scale bar = 200 μm). D. Isolated EpCAM+ and EpCAM- cells were immunostained with antibodies against EpCAM and counterstained with hoechst. Fluorescence images were captured with fluorescence microscope. Hoechst dye (blue) is for nuclear acid (nuclear) staining. Scale bar = 50 µm. E. Representative images of the plates including colonies obtained from EpCAM+ and EpCAM- cells. Colony experiments were performed in triplicate (mean ± SD). *P < 0.05 vs. EpCAM- cells. F. Sphere-forming efficiency of isolated EpCAM+ and EpCAM- cells cultured in serum-free conditions for 3-4 weeks, and spheres with diameter > 50 µm were counted. *P < 0.05 vs. EpCAM- cells.
Materials and methods
Cell lines and reagents
The human HCC cell lines Hep3B and Huh7 were generously donated by the Department of Gastroenterology, Shanghai Changzheng Hospital, Naval Medical University. In a 37°C medium with 5% CO2, the cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (ThermoFisher Scientific, MA, USA), 1% penicillin and streptomycin (Beyotime, Shanghai, China).
Michael addition was employed to create MASM maleate (> 98% purity), and the procedures were as described in a previous report [24]. AKT inhibitor MK2206 and GSK3β inhibitor CHIR99021 were purchased from Selleck Chemicals (Shanghai, China).
Isolation of EpCAM+ and EpCAM- HCC cells
EpCAM antibodies combined with magnetic beads (Miltenyi Biotec, North Rhine-Westphalia, Germany) were mixed with individual cells. A magnetic column (Miltenyi Biotec, North Rhine-Westphalia, Germany) was employed to isolate the EpCAM+ cells. Flow cytometry was employed to examine the isolated cells.
Spheroids formation assay
After cell isolating, EpCAM+ cells were placed in 24-well ultralow dishes (Corning, NY, USA) and cultivated in DMEM/F-12 supplemented with recombinant human basic fibroblast growth factor, recombinant human epidermal growth factor, B27 (1 ×), ITS (1 ×) and L-glutamine (1 ×; Invitrogen, CA, USA). Sphere cells were trypsinized and resuspended at 1 × 103 cells in ultra-low adherent 96-well plates (Corning, NY, USA), then subjected to different doses of MASM for one week. The second and third passages of the cells were cultured for 7 days without MASM. Under a phase contrast microscope, spheroids with diameters more than 50 μm were identified and captured on camera (Olympus, Tokyo, Japan).
Cell proliferation and colony formation assay
Isolated cells were placed in 96-well plates and exposed for 3 days to MASM at various doses (0-40 μmol/L). A cell counting kit 8 (CCK-8, Beyotime, Shanghai, China) was employed to measure cell proliferation. EpCAM+ or EpCAM- were cultured at 1 × 103 cells per well in 6-well plates for the colony formation experiment, and the cells were cultivated for 17 days to three weeks. Every three days, the culture media was replaced. Crystal violet (Beyotime, Shanghai, China) was employed to dye the colonies before being imaged.
Anti-EpCAM-PE antibody was kept with the cells in PBS including 1% bovine serum albumin. Isotype-matched mouse anti-IgG1-PE (Miltenyi Biotec, North Rhine-Westphalia, Germany) was used for controls. Samples were analyzed with a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA).
Immunofluorescence
Cells were stained with rabbit anti-EpCAM as primary antibody. FITC-conjugated anti-rabbit IgG was used as secondary antibody (Miltenyi Biotec, North Rhine-Westphalia, Germany). Then, cells were counterstained with Hoechst 33358 (Beyotime, Shanghai, China) and photographed under a phase contrast microscope (Olympus, Tokyo, Japan).
Apoptosis
The treated cells were resuspended in 0.5 mL (1 × 106 cells/mL) PBS, rinsed once more, and colored using the Annexin V-FITC and propidium iodide (PI) double-labeled flow cytometry kit (KeyGen, Nanjing, China). Cells were examined by a FACSCalibur flow cytometer.
Ammonia metabolism
Isolated cells were placed in 24-well plates and subjected to MASM for one week. Using the Ammonium Assay Kit (Hayward, CA, USA), ammonia concentration of the media was measured at 340 nm by using a microplate reader (BioTek Instruments, GA, USA).
Quantitative real-time polymerase chain reaction (qRT-PCR)
EpCAM+ cells were treated with MASM for the indicated times. Total RNA was extracted using the RNAfast200 kit (Fastagen Biotech Company, Shanghai, China). Then, 1 μg of RNA was transformed in cDNA using the PrimeScript RT reagent Kit (Takara, Dalian, China), and the PCR amplification was performed in triplicate on a StepOnePlusTM realtime PCR system (Applied Biosystems, USA) using the SYBR Premix Ex TaqTM PCR Kit (Takara, Dalian, China). The primer sequences for the genes are listed in Table 1.
Table 1.
Sequence of primers
| Gene | Sequence |
|---|---|
| β-actin | F: 5’-ACCCACACTGTGCCCATCTATG-3’ |
| R: 5’-AGAGTACTTGCGCTCAGGAGGA-3’ | |
| EpCAM | F: 5’-GCTCTGAGCGAGTGAGAACCT-3’ |
| R: 5’-GACCAGGATCCAGATCCAGTTG-3’ | |
| CD133 | F: 5’-ACATGAAAAGACCTGGGGG-3’ |
| R: 5’-GATCTGGTGTCCCAGCATG-3’ | |
| Oct3/4 | F: 5’-CGACCATCTGCCGCTTTGAG-3’ |
| R: 5’-CCCCCTGTCCCCCATTCCTA-3’ | |
| CK18 | F: 5’-TCAACTTCCTCAGGCAGCTATATG-3’ |
| R: 5’-TGCTTCTGCTGGCTTAATG-3’ | |
| CK19 | F: 5’-CGAAGCCAATATGAGGTC-3’ |
| R: 5’-CGGTTCAATTCTTCAGTCC-3’ | |
| CK8 | F: 5’-TCAACTTCCTCAGGCAGCTATATG-3’ |
| R: 5’-GGTTGGCAATATCCTCGTACTGT-3’ | |
| AFP | F: 5’-TACGTCCCTCCACCATTTC-3’ |
| R: 5’-ATCCTGGTCTTTGCAGCACT-3’ | |
| ALB | F: 5’-AGCCTAAGGCAGCTTGACTT-3’ |
| R: 5’-CTCGATGAACTTCGGGATGA-3’ |
Statistical analysis
All data were represented as the mean ± standard deviation (SD) and obtained from at least three independent experiments. Student’s t-tests were used to assess statistical differences between two groups. The significance of differences among multiple groups was analyzed using two-way analysis of variance followed by Bonferroni’s post hoc test via GraphPad Prism 8.0.1 software (GraphPad Software, La Jolla, CA, USA). P < 0.05 was considered as the minimum level of significance.
Results
Isolation and characterization of EpCAM+ cells in HCC
EpCAM+ and EpCAM- subpopulations were separated from Hep3B and Huh7 cells using magnetic-activated cell sorting, and a purity of 99.11% was found in sorted EpCAM+ Hep3B cells, and 91.53% in EpCAM+ Huh7 cells (Figure 1B). In a serum-free environment, the EpCAM+ population proliferated as floating spheroids, whereas most of the EpCAM- population died (Figure 1C). Spheroids obtained from EpCAM+ fraction were positive for EpCAM (Figure 1D). The colony formation assay showed that EpCAM+ cells from Hep3B and Huh7 cells were able to induce more and bigger colonies than EpCAM- cells (Figure 1E). Furthermore, EpCAM+ cells displayed higher spheroid-forming efficiency in spheroid-forming assays in comparison with the EpCAM- cells (Figure 1F).
CSCs have been reported to be more resistant to chemotherapy than other tumor cells [25]. We tested whether EpCAM+ and EpCAM- cells have different sensitivity to the therapeutic agents, doxorubicin (DOX) and 5-fluorouracil (5-FU). Isolated EpCAM+ and EpCAM- cells were subjected to different concentrations of DOX (0, 1, 2, and 5 µM) and 5-FU (0, 50, 100, and 200 µM) for 72 hours, and our data demonstrated that EpCAM+ cells were more resistant to DOX and 5-FU than EpCAM- cells (Figure 2A, 2B).
Figure 2.
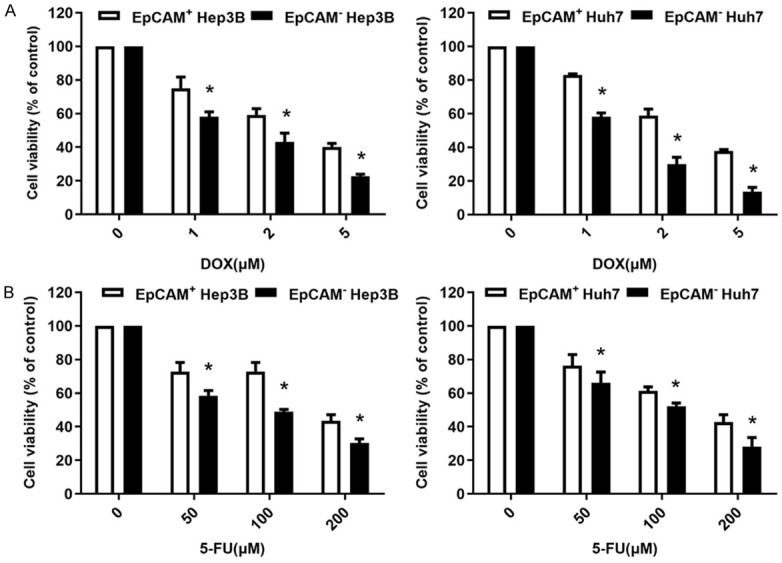
Chemoresistance of EpCAM+ cells. (A, B) Isolated EpCAM+ and EpCAM- cells were treated with 0, 1, 2, and 5 µM of DOX (A) or 0, 50, 100, and 200 µM of 5-FU (B) for 72 hours. Cell viability was determined by CCK-8 (white bar, EpCAM+ cells; black bar, EpCAM- cells). Data presented as mean ± SD were derived from 3 independent trials with triplicate wells per situation. *P < 0.05 vs. EpCAM+ cells.
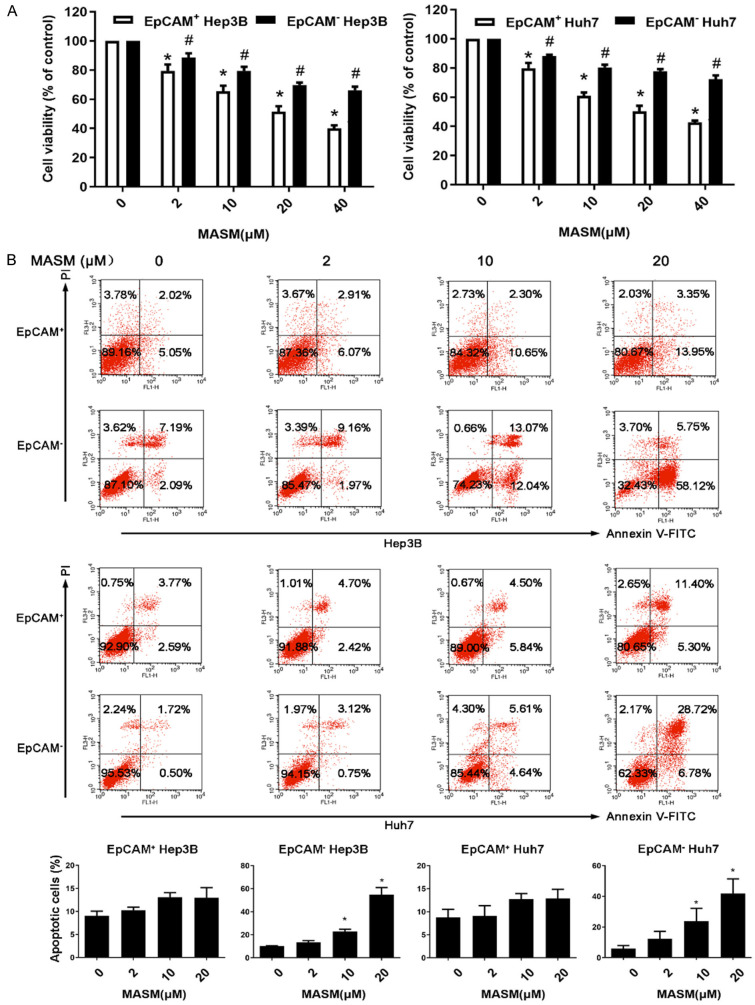
Impacts of MASM on cell proliferation and apoptosis in EpCAM+ and EpCAM- cells
We compared the impact of MASM on the viability of isolated EpCAM+ and EpCAM- cells. The results revealed that MASM preferentially inhibited the EpCAM+ cells as compared with the EpCAM- cells (Figure 3A). To determine whether the inhibition of cell viability was through the induction of apoptosis in sorted cells, EpCAM+ and EpCAM- cells were detected with Annexin V-FITC and PI double staining. The results showed that MASM induced apoptosis of the EpCAM- cells in a dose-dependent manner, while it had a mild effect on the EpCAM+ cells (Figure 3B).
Figure 3.
Effect of MASM on the proliferation and apoptosis of EpCAM+ and EpCAM- cells. A. EpCAM+ and EpCAM- cells were isolated from Hep3B and Huh7, and after being exposed to different doses of MASM (0, 2, 10, 20, and 40 μmol/L) for 72 hours, cell viability was determined by CCK-8. The results presented as the mean ± SD were from 3 independent experiments. *P < 0.05 vs. control cells, #P < 0.05 vs. EpCAM+ cells. B. Different doses of MASM (0, 2, 10, and 20 μmol/L) were added into the 6-well culture plates and incubated for 72 hours. Apoptotic cells were determined by flow cytometry. Graph of percentages of apoptotic cells are shown. n = 3. Mean ± SD. *P < 0.05 vs. control.
Effects of MASM on CSC-like properties of EpCAM+ HCC cells
We performed sphere formation assays to investigate the effect of MASM on the self-renewing capacity of EpCAM+ HCC cells. The results showed that MASM decreased the numbers and dimensions of primary spheroids (Figure 4A, 4B). Furthermore, the number of spherical colonies significantly decreased when MASM-treated primary spheroids were cultured for the subsequent two passages in the absence of drug (Figure 4C). In EpCAM+ cells, MASM reduced the expression of stem cell biomarkers and raised the expression of the liver cell biomarkers CK18, ALB and AFP (Figure 4D, 4E). We further assessed the ammonia concentration of the differentiated cells to see whether the induced cells possess liver cell functions. The outcomes demonstrated that after being exposed to MASM for 7 days, the ammonia concentration was reduced in the supernatant of cultures of EpCAM+ cells in a dose-dependent way (Figure 4F).
Figure 4.
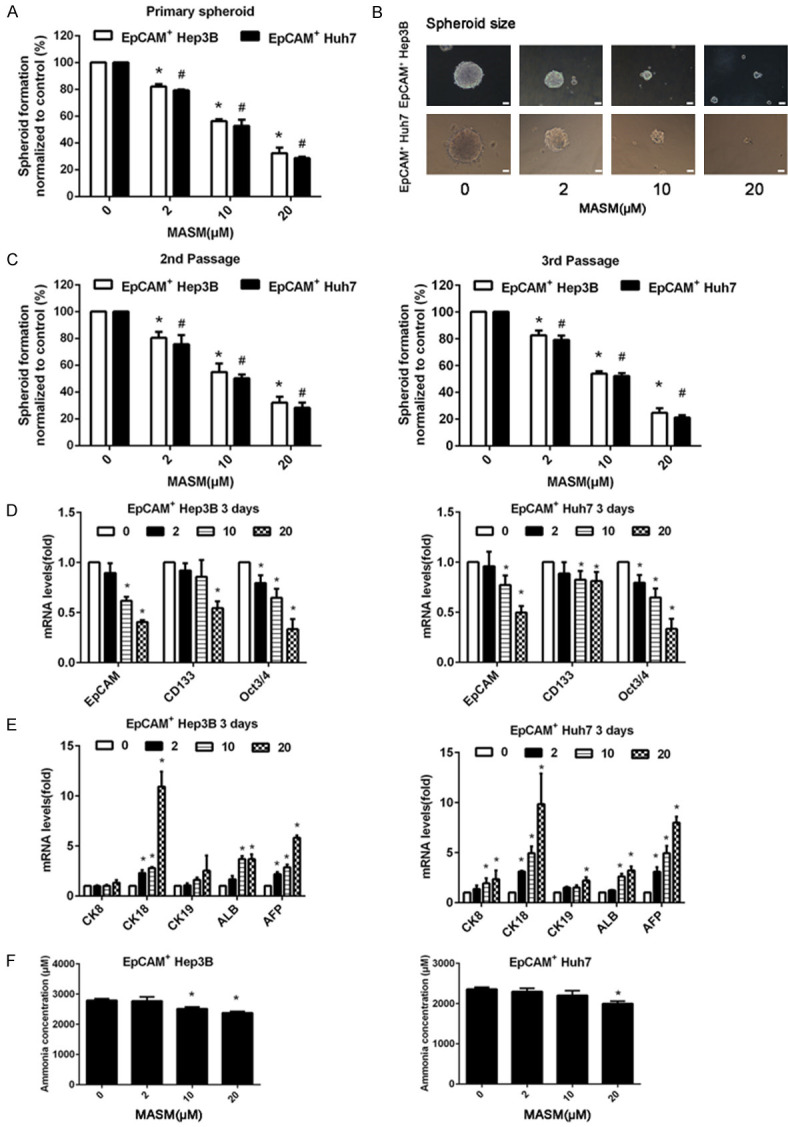
MASM inhibits CSC-like properties of EpCAM+ cells in vitro. A. MASM suppressed primary spheroid formation. n = 3. Mean ± SD. B. MASM decreased the dimensions of main spheroids (Scale bar = 100 µm). C. MASM-treated spheroids exhibited reduced self-generation abilities. In the lack of MASM, MASM-treated primary spheroids created less spheroids in the subsequent two pathways compared with the control-treated primary spheroids. n = 3. Mean ± SD. *P < 0.05 vs. untreated EpCAM+ Hep3B cells, #P < 0.05 vs. untreated EpCAM+ Huh7 cells. D, E. qRT-PCR analysis were used to determine the effect of MASM on the expressions of stem cell markers (EpCAM, CD133, and Oct3/4) and hepatocyte markers (CK18, ALB, and AFP). The mRNA levels were normalized to β-actin and relative to the control. n = 3. Mean ± SD. *P < 0.05 vs. control. F. Ammonia concentration of EpCAM+ cells was decreased by MASM in a concentration-dependent manner. n = 3. Mean ± SD. *P < 0.05 vs. control.
MASM suppresses the AKT/GSK3β/β-catenin pathway in EpCAM+ cells
In HCC cells, MASM inhibited the AKT/GSK3β/β-catenin pathway, as we had previously reported. To investigate if the inhibition of the AKT/GSK3β/β-catenin pathway contributed to the inhibitory actions of MASM on EpCAM+ cells, we treated EpCAM+ cells with series concentrations of MASM and specific AKT inhibitor MK2206 (0.5 μM) or GSK3β inhibitor CHIR99021 (2.5 μM). The results showed that treatment with MK2206 resulted in slight inhibition of cell proliferation and spheroid formation, and co-treatment of EpCAM+ cells with MASM and MK2206 resulted in a greater inhibition than that observed with MASM alone (Figure 5A, 5B). In contrast, CHIR99021 increased cell proliferation and spheroid formation, and antagonized effects of MASM on inhibiting the growth and spheroid formation of EpCAM+ cells.
Figure 5.
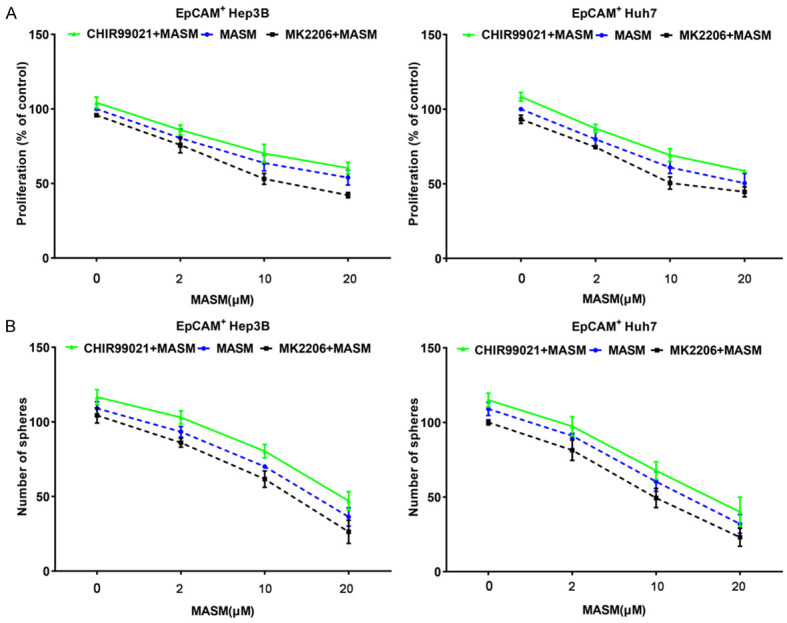
MASM suppresses the AKT/GSK3β/β-catenin pathway in vitro. A. Proliferation of EpCAM+ cells treated with MASM, MASM and MK2206 (0.5 μM), or MASM and CHIR99021 (2.5 μM). n = 3. B. Spheroid formation of EpCAM+ cells treated with MASM, MASM and MK2206 (0.5 μM), or MASM and CHIR99021 (2.5 μM). n = 3.
Discussion
CSCs are one driving force of liver tumorigenesis and malignancy, and the therapies for HCC require the excision of CSCs [26]. Our earlier research has shown that MASM suppresses HCC cells, sphere cells and HCC xenograft growth via inhibiting the AKT/GSK3β/β-catenin signaling pathway [17]. However, the impact of MASM on CSCs is not yet understood.
In this study, we successfully isolated EpCAM+ cells from Hep3B and Huh7. EpCAM+ cells had significantly higher colony-formation ability and stronger proliferative capacity in vitro than EpCAM- cells. In addition, EpCAM+ cells were more resistant to cytotoxic chemotherapy such as DOX and 5-FU, in comparison with the EpCAM- population. These findings suggest that EpCAM+ cells possess CSC-like properties. Using these CSC-like cells, we found that MASM treatment significantly inhibited the proliferation of EpCAM+ cells, whereas it did not significantly induce the apoptosis of EpCAM+ cells as assessed by FACS. In addition, MASM caused an obvious reduction not only for primary spheroids, but also for spheroids in the subsequent two passages without MASM. RT-PCR analysis showed that treatment of MASM reduced the expressions of stemness genes CD133, EpCAM and Oct3/4, demonstrating that MASM affected the stemness of EpCAM+ cells. To further study the hepatocyte differentiation potential of isolated EpCAM+ cells, we evaluated the gene expression of hepatocytic and cholangiocytic biomarkers using RT-PCR. MASM elevated the expression of the liver-related genes (CK18, ALB, and AFP) in a dose-dependent manner. However, isolated EpCAM+ cells were expressing CK-19, an indicator for bile epithelial cells, at low levels after treatment of MASM, demonstrating that MASM induces CSC-like cells to differentiate into hepatocytes. Hepatocyte differentiation was further examined by functional tests using ammonia metabolism analysis, a typical test for the detection of hepatocyte function [27,28]. Our data showed that the MASM-treated EpCAM+ cells not only expressed hepatocyte-specific biomarkers but also possessed the features of functional hepatocytes. In summary, the MASM-induced reduction of CSC-like properties was related to the inhibition of self-regeneration and the support of differentiation from CSC-like cells to functional hepatocytes.
Wnt/β-catenin signaling is one of the important pathways thought to be involved in CSCs self-renewal [29]. A previous study showed that stimulation of Wnt-β-catenin signaling could induce EpCAM expression [30]. Our preliminary results showed that MASM inhibited hepatoma cell proliferation, led to cell death and growth arrest, inhibited sphere cells and suppressed the AKT/GSK3β/β-catenin signaling pathways [17]. Recently, our group has reported that WM130, another matrine analog, substantially suppressed CSC-like cells, and this impact may be through the reduction of the AKT/GSK3β/β-catenin pathway [31]. Thus, we speculated that the inhibition of MASM on CSC-like cells may be correlated with the suppression of the AKT/GSK3β/β-catenin pathway. Data presented in this study showed that MK2206 suppressed, whereas CHIR99021 elevated EpCAM+ cell proliferation and spheroid production. Notably, co-treatment of EpCAM+ cells with MASM and MK2206 had an additive impact, while CHIR99021 antagonized MASM’s impact. These findings revealed that the suppression impact of MASM on CSC-like cells was modulated by suppressing the AKT/GSK3β/β-catenin signaling pathway.
Conclusions
In conclusion, our data revealed that MASM suppressed the self-renewal of EpCAM+ cells and promoted the differentiation from CSC-like cells to hepatocytes. These effects were accompanied with the down-regulation of the AKT/GSK3β/β-catenin pathway. The findings may shed light on the potential clinical utility of MASM in treating HCC.
Acknowledgements
The study was funded by the Shanghai Nature Science Foundation (grant No. 19ZR1419600) and the project of Shanghai University Young Talents Launch (grant.N.13-G210-22-432).
Disclosure of conflict of interest
None.
References
- 1.Piñero F, Dirchwolf M, Pessôa MG. Biomarkers in hepatocellular carcinoma: diagnosis, prognosis and treatment response assessment. Cells. 2020;9:1370. doi: 10.3390/cells9061370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 4.Sahu SK, Chawla YK, Dhiman RK, Singh V, Duseja A, Taneja S, Kalra N, Gorsi U. Rupture of hepatocellular carcinoma: a review of literature. J Clin Exp Hepatol. 2019;9:245–256. doi: 10.1016/j.jceh.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartke J, Johnson M, Ghabril M. The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn Pathol. 2017;34:153–159. doi: 10.1053/j.semdp.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Liu YC, Yeh CT, Lin KH. Cancer stem cell functions in hepatocellular carcinoma and comprehensive therapeutic strategies. Cells. 2020;9:1331. doi: 10.3390/cells9061331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown HK, Tellez-Gabriel M, Heymann D. Cancer stem cells in osteosarcoma. Cancer Lett. 2017;386:189–195. doi: 10.1016/j.canlet.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Butti R, Gunasekaran VP, Kumar TVS, Banerjee P, Kundu GC. Breast cancer stem cells: biology and therapeutic implications. Int J Biochem Cell Biol. 2019;107:38–52. doi: 10.1016/j.biocel.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Atashzar MR, Baharlou R, Karami J, Abdollahi H, Rezaei R, Pourramezan F, Zoljalali Moghaddam SH. Cancer stem cells: a review from origin to therapeutic implications. J Cell Physiol. 2020;235:790–803. doi: 10.1002/jcp.29044. [DOI] [PubMed] [Google Scholar]
- 10.Haraguchi N, Utsunomiya T, Inoue H, Tanaka F, Mimori K, Barnard GF, Mori M. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006;24:506–513. doi: 10.1634/stemcells.2005-0282. [DOI] [PubMed] [Google Scholar]
- 11.Hu C, Li H, Li J, Zhu Z, Yin S, Hao X, Yao M, Zheng S, Gu J. Analysis of ABCG2 expression and side population identifies intrinsic drug efflux in the HCC cell line MHCC-97L and its modulation by Akt signaling. Carcinogenesis. 2008;29:2289–2297. doi: 10.1093/carcin/bgn223. [DOI] [PubMed] [Google Scholar]
- 12.Pandit H, Li Y, Zheng Q, Guo W, Yu Y, Li S, Martin RCG. Carcinogenetic initiation contributed by EpCAM+ cancer cells in orthotopic HCC models of immunocompetent and athymic mice. Oncotarget. 2020;11:2047–2060. doi: 10.18632/oncotarget.27454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou L, Zhu Y. The EpCAM overexpression is associated with clinicopathological significance and prognosis in hepatocellular carcinoma patients: a systematic review and meta-analysis. Int J Surg. 2018;56:274–280. doi: 10.1016/j.ijsu.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Ji J, Yamashita T, Budhu A, Forgues M, Jia HL, Li C, Deng C, Wauthier E, Reid LM, Ye QH, Qin LX, Yang W, Wang HY, Tang ZY, Croce CM, Wang XW. Identification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology. 2009;50:472–480. doi: 10.1002/hep.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogawa K, Tanaka S, Matsumura S, Murakata A, Ban D, Ochiai T, Irie T, Kudo A, Nakamura N, Tanabe M, Arii S. EpCAM-targeted therapy for human hepatocellular carcinoma. Ann Surg Oncol. 2014;21:1314–1322. doi: 10.1245/s10434-013-3430-7. [DOI] [PubMed] [Google Scholar]
- 16.Park DJ, Sung PS, Kim JH, Lee GW, Jang JW, Jung ES, Bae SH, Choi JY, Yoon SK. EpCAM-high liver cancer stem cells resist natural killer cell-mediated cytotoxicity by upregulating CEACAM1. J Immunother Cancer. 2020;8:e000301. doi: 10.1136/jitc-2019-000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Qi Y, Bai ZH, Ni CX, Ren QH, Xu WH, Xu J, Hu HG, Qiu L, Li JZ, He ZG, Zhang JP. A novel matrine derivate inhibits differentiated human hepatoma cells and hepatic cancer stem-like cells by suppressing PI3K/AKT signaling pathways. Acta Pharmacol Sin. 2017;38:120–132. doi: 10.1038/aps.2016.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Tang Z, Wen L, Jiang C, Feng Q. Matrine: a review of its pharmacology, pharmacokinetics, toxicity, clinical application and preparation researches. J Ethnopharmacol. 2021;269:113682. doi: 10.1016/j.jep.2020.113682. [DOI] [PubMed] [Google Scholar]
- 19.Sun N, Zhang H, Sun P, Khan A, Guo J, Zheng X, Sun Y, Fan K, Yin W, Li H. Matrine exhibits antiviral activity in a PRRSV/PCV2 co-infected mouse model. Phytomedicine. 2020;77:153289. doi: 10.1016/j.phymed.2020.153289. [DOI] [PubMed] [Google Scholar]
- 20.Chen MH, Gu YY, Zhang AL, Sze DM, Mo SL, May BH. Biological effects and mechanisms of matrine and other constituents of Sophora flavescens in colorectal cancer. Pharmacol Res. 2021;171:105778. doi: 10.1016/j.phrs.2021.105778. [DOI] [PubMed] [Google Scholar]
- 21.Sun XY, Jia LY, Rong Z, Zhou X, Cao LQ, Li AH, Guo M, Jin J, Wang YD, Huang L, Li YH, He ZJ, Li L, Ma RK, Lv YF, Shao KK, Zhang J, Cao HL. Research advances on matrine. Front Chem. 2022;10:867318. doi: 10.3389/fchem.2022.867318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin Y, He F, Wu L, Xu Y, Du Q. Matrine exerts pharmacological effects through multiple signaling pathways: a comprehensive review. Drug Des Devel Ther. 2022;16:533–569. doi: 10.2147/DDDT.S349678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Liu W, Zhang R, Wang Z, Shen Z, Chen X, Bi K. Pharmacokinetic study of matrine, oxymatrine and oxysophocarpine in rat plasma after oral administration of Sophora flavescens Ait. extract by liquid chromatography tandem mass spectrometry. J Pharm Biomed Anal. 2008;47:892–898. doi: 10.1016/j.jpba.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 24.Hu H, Wang S, Zhang C, Wang L, Ding L, Zhang J, Wu Q. Synthesis and in vitro inhibitory activity of matrine derivatives towards pro-inflammatory cytokines. Bioorg Med Chem Lett. 2010;20:7537–7539. doi: 10.1016/j.bmcl.2010.09.075. [DOI] [PubMed] [Google Scholar]
- 25.Hasan S, Taha R, Omri HE. Current opinions on chemoresistance: an overview. Bioinformation. 2018;14:80–85. doi: 10.6026/97320630014080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee TK, Guan XY, Ma S. Cancer stem cells in hepatocellular carcinoma - from origin to clinical implications. Nat Rev Gastroenterol Hepatol. 2022;19:26–44. doi: 10.1038/s41575-021-00508-3. [DOI] [PubMed] [Google Scholar]
- 27.Yin C, Lin Y, Zhang X, Chen YX, Zeng X, Yue HY, Hou JL, Deng X, Zhang JP, Han ZG, Xie WF. Differentiation therapy of hepatocellular carcinoma in mice with recombinant adenovirus carrying hepatocyte nuclear factor-4alpha gene. Hepatology. 2008;48:1528–1539. doi: 10.1002/hep.22510. [DOI] [PubMed] [Google Scholar]
- 28.Jung KH, Zhang J, Zhou C, Shen H, Gagea M, Rodriguez-Aguayo C, Lopez-Berestein G, Sood AK, Beretta L. Differentiation therapy for hepatocellular carcinoma: multifaceted effects of miR-148a on tumor growth and phenotype and liver fibrosis. Hepatology. 2016;63:864–879. doi: 10.1002/hep.28367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiménez-Guerrero R, Belmonte-Fernández A, Flores ML, González-Moreno M, Pérez-Valderrama B, Romero F, Japón MÁ, Sáez C. Wnt/β-catenin signaling contributes to paclitaxel resistance in bladder cancer cells with cancer stem cell-like properties. Int J Mol Sci. 2021;23:450. doi: 10.3390/ijms23010450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamashita T, Budhu A, Forgues M, Wang XW. Activation of hepatic stem cell marker EpCAM by Wnt-beta-catenin signaling in hepatocellular carcinoma. Cancer Res. 2007;67:10831–10839. doi: 10.1158/0008-5472.CAN-07-0908. [DOI] [PubMed] [Google Scholar]
- 31.Ni CX, Qi Y, Zhang J, Liu Y, Xu WH, Xu J, Hu HG, Wu QY, Wang Y, Zhang JP. WM130 preferentially inhibits hepatic cancer stem-like cells by suppressing AKT/GSK3β/β-catenin signaling pathway. Oncotarget. 2016;7:79544–79556. doi: 10.18632/oncotarget.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]