Abstract
The practice of physical exercise induces a series of physiological changes in the body at different levels, either acutely or chronically. During exercise, the increase in oxygen consumption promotes the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), which are necessary to maintain homeostasis. ROS/RNS activate cellular signaling pathways, such as the antioxidant cytoprotective systems, inflammation, and cell proliferation, which are crucial for cell survival. However, in exhaustive-extended physical exercise, workloads can exceed the endogenous antioxidant defenses, which may be related to impairment of muscle contraction, fatigue, and a decrease in athletic performance. This review addresses the role of some antioxidants from plant-derived extracts called phytochemicals that can mediate the response to oxidative stress induced by physical exercise by activating signaling pathways, such as Nrf2/Keap1/ARE, responsible for the endogenous antioxidant response and possibly having an impact on sports performance.
Keywords: Oxidative stress, physical exercise, muscle contraction, performance, phytochemicals, antioxidants, Nrf2
Introduction
Physical exercise induces an increase in oxygen consumption to cover the metabolic demands. As a result, it leads to several changes at the organic and subcellular levels. Depending on the type, frequency, and intensity of training, some metabolic pathways predominate over others; for example, in the case of long-term aerobic endurance exercises, in which there is a predominance of the oxidative pathway, there must be a constant supply of oxygen in type-I contractile muscle fibers [1]. Indeed, the contractile skeletal muscle itself demands a large amount of oxygen-rich blood. The supply of oxygen and nutrients to the cells is in turn accompanied by a series of vascular, respiratory, and neuroendocrine adaptations, among many others [2]. When the oxidative pathway is predominantly active, the cellular avidity to metabolize a large amount of oxygen generates a greater number of products, such as reactive oxygen species (ROS), among which are the superoxide anion (O2 •-), the radical hydroxyl (•OH), and hydrogen peroxide (H2O2) [3,4]. In the same manner, reactive nitrogen species (RNS) are molecular entities deriving from nitrogen metabolism; among these, nitric oxide (NO) peroxynitrite (ONOO-) is prominent [3,4]. These reactive species have been described with radical activity, which, from the chemical point of view, are highly molecularly unstable and therefore tend to destabilize cellular structures, such as membrane lipids, proteins, and nucleic acids. Thus, in the literature, it has been widely described that when they reach high cellular and systemic concentrations, they are responsible for generating damage and chronic inflammation, giving rise to diseases such as diabetes or cancer [5,6]. However, adequate physiological amounts of ROS/RNS are also necessary to regulate cellular processes in order to preserve homeostasis, such as the activation of cell signaling pathways, division, proliferation, regeneration, and apoptosis. In this sense, it is pertinent to assume that there must be a balance between the amount of ROS/RNS present in biological systems and the ability to neutralize them. This redox balance is linked to the defense capacity of cells mediated by the coordination of various cytoprotective systems; among these is the transcriptionally regulated antioxidant defense system by the nuclear factor erythroid 2-related factor 2 (Nrf2), which will be discussed in greater depth in this work [7].
Based on the preceding data, it is known that the practice of physical exercise induces the production of ROS/RNS, which already generates an increase in cellular oxidative stress, and it has been previously described that only the practice of physical exercise is a sufficient stimulus to induce the antioxidant response and thus mediate the stressor event [7]. However, exercise is not alone in being responsible for increasing oxidative stress; there is also the presence of mechanical, pressure and thermal stressors, which contribute to and influence the development of defense and adaptive responses [8,9]. The present work attempted to give a general overview regarding the impact of exercise in oxidative stress which is more evident in those highly demanding sport activities. Current available data indicate that some biologically active phytochemicals can induce certain molecular mechanisms in cells to defend them from damage including antioxidant response. If so, that might represent a novel area to explore that would be beneficial in physical performance and recovery.
Exercise-induced oxidative stress
The practice of physical exercise increases oxygen consumption up to 20 times depending on the intensity and duration, due to the demands of contracting muscles, leading to an increase in the production of O2 •-, which is the main ROS formed within the cells from the reduction of a single electron of molecular oxygen. O2 •- is an entity with radical activity, which is negatively charged, with a relatively high half-life, and it is impermeable to the cell membrane [3,10]. The dismutation of O2 •- is carried out by a family of superoxide dismutases (SOD) that produce H2O2 and O2. In turn, H2O2 is a non-free radical molecule, relatively stable, with a long half-life, and that can reach a high intra- and extracellular concentrations due to its high diffusion capacity and membrane permeability. Due to these characteristics, it works as an activator of intracellular signaling pathways but also, when reaching high concentrations, it can induce cytotoxicity [11]. Therefore, there is a broad enzymatic and non-enzymatic system capable of biotransforming H2O2, in which the reduced form of glutathione (GSH) and the families of enzymes responsible for its synthesis and metabolism are prominent, such as glutathione reductases (GRx), glutathione peroxidases (GPx), thioredoxins (Trx), and glutaredoxins (Grx), in addition to catalase (CAT) and peroxiredoxins (Prx) [7]. Notwithstanding this, H2O2, when transformed together with O2 •-, gives rise to the formation of •OH radicals that possess a high oxidizing power but do not permeate the cell membrane, causing localized damage at the site where they are produced [11,12].
Increased oxidative stress in muscle fibers is largely the result of O2 •- production, which is in turn a result of increased oxidative phosphorylation. To date, it is known that mitochondria are one of the main O2 •--producing organelles that can even produce more ROS in a baseline 4-state of respiration compared to an active 3-state of respiration [13]. Furthermore, it has even been reported that active and healthy contractile fibers produce fewer ROS during the electron transport chain (ETC). Other cellular sites where ROS are synthetized include the T-tubules, the sarcoplasmic reticulum, and the sarcolemma [14,15]. In these, adenine dinucleotide phosphate oxidase (NADPH oxidase), nicotinamide, xanthine oxidase, monoamine oxidases (MAO), and phospholipase A2 (PLA2) are the main producers [16]. In particular, the latter PLA2 has the ability to activate the calcium-dependent isoforms of NADPH oxidase in contracting-stretching fibers during exercise. The increase in ROS through the PLA2 pathway is related to the increase in calcium uptake in the mitochondria, which activates the PLA2, promoting the synthesis of arachidonic acid in the ETC, which in turn is a substrate for lipoxygenases, an important group of ROS generators derived from membrane phospholipids [17,18]. On the other hand, NOX2 is an isoform of NADPH oxidases that is highly ROS-producing and it is in the T-tubules and the sarcolemma of active muscles [19,20]. Additionally, certain cells of the immune system have been observed as potential producers of ROS in contractile muscles during exercise, such as macrophages, eosinophils, neutrophils, and monocytes [3].
Cell antioxidant defenses against ROS
Antioxidant defenses include complex systems that are responsible for preserving the redox balance to maintain cellular integrity. In this respect, although it is not an objective of this work to review these systems, this will be mentioned briefly with the purpose of supporting the bases for the analysis regarding the effect that phytochemicals can exert on some of the elements that compose them.
It can be said that antioxidants are substances or elements that prevent or reverse cellular oxidative processes. For this, there are antioxidants of various enzymatic and non-enzymatic origins, both acting synergistically to mediate the oxidative events triggered by ROS [7] (Table 1). Among enzymatic antioxidants, the aforementioned O2 •- SOD dismutation enzymes are highlighted, with three identified isoforms, SOD1 (Cu/ZnSOD) located in the mitochondrial intermembrane space, and cytosol, using copper and zinc as cofactors; the SOD2 (MnSOD) site in the mitochondrial matrix uses manganese as cofactor and SOD3 found outside the cell [21]. SOD is one of the most active antioxidant enzymes in type-I oxidative myofibers in comparison with less oxidative type-IIX myofibers [22,23].
Table 1.
ROS cell removal antioxidant systems
| Antioxidant system | Location | Activity |
|---|---|---|
| Enzymatic | ||
| SOD | Dismutation of O2 •- | |
| -SOD1 (Cu/ZnSOD) | -Mitochondrial intermembrane space/cytosol | |
| -SOD2 (MnSOD) | -Mitochondrial matrix | |
| -SOD3 | -Outside cell | |
| CAT | Peroxisomes, mitochondria and cytosol | Remotion of H2O2 does not require electron donor |
| GPx | Reduction of H2O2 uses GSH as electron donor | |
| -GPx1 | -Cytosol and mitochondria | |
| -GPx2 | -Cytosol | |
| -GPx3 | -Cytosol and extracellular space | |
| -GPx4 | -Phospholipid environment | |
| -GPx5 | -Mammalian epididymis | |
| -GPx6 | -Epithelial cells | |
| -Gpx7 | -Endoplasmic reticulum | |
| -Gpx8 | -Endoplasmic reticulum | |
| GR | Cytosol and mitochondrial matrix | Reduce GSSH requires NADPH |
| Pxr | Reduction of H2O2, peroxynitrite and alkyl peroxides | |
| -Pxr1, Pxr2 and Pxr4 | Cytosol | |
| -Pxr3 | Mitochondria | |
| -Pxr5 | Mitochondria and cytosol | |
| -Pxr6 | Extracellular space | |
| Trx | Remotion of hydroperoxides, vitamin C recycling | |
| -Trx1 | Cytosol | |
| Trx2 | Mitochondria | |
| Gxr | Elimination of hydroperoxides | |
| -Gxr1 | Cytosol | |
| -Gxr2 and Gxr3 | Mitochondria | |
| Non-ezymatic | ||
| GSH | Ubiquitous | Electron donor for electrophiles such H2O2 and hydroperoxides |
| Vitamin C, E, Se, Zn, Cu, β-caroteno, polyphenols | Dietary sources and plant derived phytochemicals | Electron donors, GHS recycling, enzymatic cofactors |
SOD: Superoxide Dismutase; Cu: Copper; Zn: Zinc; Mn: Manganese; O2 •-: Anion Superoxide; CAT: Catalase; GPx: Glutathione Peroxidase; H2O2: Hydrogen Peroxide; GSH: Reduced Glutathione; GSSG: Glutathione Disulfide; GR: Glutathione Reductase; Pxr: Peroxiredoxins; Trx: Thioredoxins; Gxr: Glutaredoxins; NADPH: Reduced Nicotinamide Adenine Dinucleotide Phosphate.
The removal of H2O2 is performed by enzymatic and non-enzymatic elements, all of which work together synergistically. Basically, CAT is one of the most relevant removers located in peroxisomes, cytosol, and mitochondria. It does not require an electron donor to remove H2O2, and it is highly active in oxidative-type-I myofibers. GPx enzymes comprise a family of peroxidases that use GSH as electron donor; a variety of isoforms are located in different cell compartments [24]. The process of GSH peroxidation results in the oxidized form of glutathione (GSSG) as disulfide, but it is reduced by the GR using NADPH from NADPH-specific isocitrate dehydrogenase and by glyceraldehyde-3-phosphate dehydrogenase [24]. Trx are enzymes that are able to transfer electrons, forming a disulfide bond to maintain target proteins in a reduced state [25]. Pxr participates in the reduction of H2O2, peroxynitrite and alkyl peroxides [26], as well as Gxr, which transfer electrons from NADPH to the disulfide substrates and the protein and non-protein thiols, favoring the elimination of hydroperoxides [27].
Redox sensitive antioxidant pathways in exercise
ROS play a fundamental role in the activation of several cell signalings; for instance, H2O2 regulates a number of transcriptional factors, such as Nrf2, HSF-1, NF-κB, SP1, NOTCH, HIF-1, AP-1, T953, and SCREB-1, which are crucial for signaling with regard to inflammation, apoptosis, proliferation, cell growth, aging, muscle contraction, the circadian cycle, and many others [28]. Certain doses of H2O2 are required to trigger signals; for example, it is necessary to reach an intracellular concentration of 0.1 and 0.01 µM, where angiogenesis, cell migration, and proliferation take place. The master regulator of the antioxidant response, Nrf2, coordinates a complex cytoprotective system responsible for stress and the adaptive response; it is active at a concentration ranging from 0.1-10 µM. More aggressive conditions, such as inflammation, cell death, fibrogenesis, tumor growth, and metastasis, are performed at 1-10 µM intracellularly and at 10-1,000 µM in the extracellular space [29].
Available data indicate that exercise-induced ROS production is required to enhance oxidative capacity and the antioxidant cell response in skeletal muscle [4]. As part of adaptative responses, redox-sensitive pathways drive a constant crosstalk dialogue between diverse transcriptional factors such as NF-κB, peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α), and Nrf2, which upregulate or downregulate the transcription of target genes, promoting, for instance, mitochondrial biogenesis and the antioxidant response [4,30]. In endurance exercise, the redox-sensitive PGC-1α is considered mainly responsible for adaptations; it is active in the presence of H2O2, interacting with other transcription factors, such as Nrf1, Nrf2, and estrogen-related receptor α (ERRα), for mitochondrial biogenesis and angiogenesis [12,31,32]. Simultaneously, hypertrophy in resistance training is mediated by the presence of ROS as well, because they stimulate the phosphatidylinositol 3-kinase (PI3K)/serine threonine kinase (Akt) pathway by driving Nrf2 to form a complex with actin as a result of actin-myofilament reorganization; thus, Nrf2 is translocated into the nucleus. In addition, the gene transactivation of the CCAAT/enhancer binding protein-beta (C/EBPβ), the peroxisome proliferator-activated receptor-gamma (PPARγ), and the retinoid X receptor (RXR) heterodimer contribute to the induction of phase-II antioxidant enzymes [33]. The PI3K/Akt promotes the target of the rapamycin pathway in mammals (mTOR), and denominated PI3K/Akt/mTOR pathway is intrinsically involved in the contractile protein synthesis of muscle fibers [34]. Protein kinase B, which stimulates mTOR and protein synthesis, is also redox-sensitive, and it appears to be activated by H2O2 [35]. Of note, myofibrillar protein synthesis is also influenced by the release of myokines (LIF, IL-4, IL-6, IL-7, and IL-15), which can act as signaling molecules for stimulation of the mTOR pathway [36].
It is recognized that several of the processes of adult myogenesis undergone by satellite cells during muscle regeneration from injury are redox-regulated; however, the role of ROS/RNS in muscle satellite-cell activation, especially during exercise-induced muscle damage (EIMD), remains unclear [37].
Nrf2 signaling and antioxidant response
Nrf2 is a member of the basic leucine zipper (bZIP) transcription factor family, and it is ubiquitously encoded by the NFE2L2 gene responsible for the regulation of around 250 genes intrinsically involved in redox-sensitive pathways. Therefore, it is known as the master regulator of stress responses [38]. During exercise, the intracellular levels of ROS rise, promoting the dissociation of Nrf2 from its negative regulator, the Kelch-like ECH-associated protein (Keap1), which is bound by the Neh2 domain in the two motifs, ETGE and DLG, forming a dimer link with Keap1 [39]. Under homeostatic conditions, Keap1 directs the ubiquitination of seven lysins at the two binding motifs sites and the consequent degradation of Nrf2 by the 26S proteosome [40]. However, Keap1 is susceptible to the oxidation of its cysteine residues in the presence of electrophilic compounds, ROS, or other activators; the exercise-induced ROS elevation promotes the oxidation of the rich-cysteine protein Keap1, making a conformational change [41]. Therefore, Nrf2 is released from Keap1 and translocated into the nucleus, where it is heterodimerized with the musculo-aponeurotic fibrosarcoma proteins (sMaf) in the antioxidant response element (ARE), a specific DNA sequence (5’-puGTGACNNNGC-3’) [42,43]. ARE is a central DNA site for the upregulation of cytoprotector and antioxidant response genes including phase-II detoxifying enzymes, enzymes involved in the synthesis and metabolism of glutathione, energy metabolism, and cell survival [44,45]. Data from studies in animals indicate that impairment in the activation of Nrf2/Keap1/ARE pathway is associated with resistance to counteracting high levels of oxidative stress and a greater risk of developing chronic diseases; thus, Nrf2 has become a potential target in pharmacology and genetics in order to elucidate the mechanisms of activation and/or disruption of Nrf2 [46].
The effect of both acute and chronic physical exercise on the activation of the Nrf2 pathway through increased ROS production has been previously described in literature. In different studies, the stimulus of a single exhaustive training bout has been sufficient to activate the Nrf2 pathway. When the stimulus is repeated, as in regular exercise, sustained activation promotes the regulation of antioxidant defenses and cell protection. This has given rise to the notion that factors such as training intensity, duration, and frequency play an important role in the way exercise-induced oxidative stress modulates antioxidant pathways [47,48]. Regular exercise promotes a wide variety of physiological and metabolic changes that allow the organism to withstand increasingly heavy physical workloads as a result of close interaction between regulatory elements and signaling [49]. Cell remodeling and plasticity can be some of the most notorious modifications. Therefore, exercise training is a potent stimulus for the promotion of the strengthening of cell defenses [30].
There are many discrepancies among the studies concerning whether a single bout is sufficient to activate the complete defense system. It has been observed that acute exercise increases Nrf2 gene expression and protein in skeletal muscle; however, this does not necessarily mean that complete antioxidant defenses are activated. Previously, it was demonstrated that ROS, particularly the H2O2 produced during exercise, can cause modifications in cell signaling and gene expression, turning on redox-sensitive pathways [50]. A single bout of exercise stimulates the mitochondrial production of H2O2 that is able to promote the expression of p(66Shc) and FOXO3a in a time-dependent manner in correlation with H2O2 content in ICR/C-1 mouse skeletal muscle [51]. Furthermore, H2O2 released by acute exercise upregulated the Ref1/Nrf2 pathway by increasing the expression of the redox effector factor 1 (Ref1) and Nrf2 genes, as well as protein content, along with the enzymes CuZnSOD and MnSOD and glutathione content [48].
Different models of acute physical exercise show the close relationship that activation of the Nrf2 pathway entertains in the expression of the endogenous antioxidant system, and also in mitochondrial biogenesis and in the improvement of energy metabolism. The silencing of Nrf2 (Nrf2-/-) in male mice subjected to a training protocol of voluntary wheel running for 6-8 weeks is associated with a lower capacity to counteract ROS; the mice were more prone to fatigue and therefore low physical performance. In contrast, wild-type mice (Nrf2+/+) exhibited better aerobic resistance, and although there were no significant differences in mitochondrial content, a greater activity of Cytochrome C Oxidase (COX) and the COXIV subunit was observed [52]. Merry and Ristow [53] reported in male C57/BL6/SJ mice that the gene silencing of NFE2L2 resulted in poor tolerance to exercise, less mitochondrial density, and low SOD activity. The mRNA of the mitochondrial biogenesis markers (nuclear respiratory factor 1 (NRF-1) and mitochondrial transcription factor A (mtTFA)), SOD1 and SOD2, as well as catalase in mouse gastrocnemius muscle, were also blunted. In the same report, acute exposure to H2O2 and NO in C2C12 myoblasts exhibited the increase of mtTFA. These results suggest that NFE2L2 is upregulated by the presence of ROS and NO, promoting the expression of the antioxidant program and mitochondrial biogenesis in skeletal muscle.
In humans, the impact was also evaluated for acute and chronic exercise on the expression of Nrf2 and mitochondrial biogenesis markers. Acute training increased the expression of NRF-1, Nrf2, and TFAM mRNA. Meanwhile, 4 weeks of submaximal interval (Tabata) enhanced CS activity and Nrf2, NRF-1, as well as TFAM protein content, but reduced HO-1 protein content; whatsoever the mode of exercise, the potential role of Nrf2 in mitochondrial biogenesis in human skeletal muscle is highlighted [54]. Another study showed that age can be a critical point in the antioxidant response when young (23±1 y, n = 10) and old (63±1 y, n = 10) men performed 30 min cycling at 70%, and VO2max blood samples were taken before and at 10 min, 30 min, 1 h, 4 h, and 24 h post-exercise. The results indicate that a single bout of submaximal aerobic training elicit the whole cell Nrf2 level; nevertheless, the nuclear accumulation of Nrf2 was significantly higher in the younger men, as was the expression of HMOX1 and NQO1, concluding that Nrf2 target antioxidant genes are diminished with age [55]. It is noteworthy that older individuals have higher baseline levels of Nrf2; contrariwise, lower baseline levels are associated with a more dynamic training response. After an 8-week aerobic-exercise training (3 d/wk, 45 min/d) young (18-28 y, n = 21) and old (≥ 60 y, n = 19) men and women participated in an acute-exercise trial (30 min cycling at 70% VO2max). The results revealed that aerobic exercise improved baseline nuclear levels of Nrf2 in both young and old subjects. Notwithstanding this, better responses for Nrf2 signaling via GCLC protein, NQO1 and GCLC mRNA was found at the lower baseline levels of Nrf2 that were observed in young individuals. These findings support that even old and sedentary individuals can restore Nrf2 signaling and antioxidant response with the practice of exercise training [56].
In young men (25±1 y, n = 16), two different modalities of exercise were evaluated: high-intensity interval and constant workload, and 30-min cycling training. Blood samples were collected before, immediately after, and 30 min post-exercise. The results revealed that both trainings stimulated the activation of Nrf2, the plasma 8-isoprotstane increased significantly in both groups, but GR activity was higher with high-intensity training. Regardless of the modality, exercise elicits Nrf2 activation [57]. Recently, a pilot study using muscle stimulation in mice has suggested that exercise at a high intensity activates Nrf2/ARE in peripheral active tissues and could be a key point for signaling systemic activation [58].
Brief description of phytochemicals
Phytochemicals are metabolites found in plants, fruits, vegetables, and grains. The majority of these are active compounds. Extensive evidence indicates that these bioactive compounds provide health benefits if they are regularly consumed through dietary sources or supplements. These phytochemicals can be sub-divided into five higher-order classes, based on their chemical structure and pharmacological criteria, as follows: carotenoids, phenolic compounds, alkaloids, and nitrogen and organic sulfur compounds (Figure 1) [59-63]. These metabolites offer protection to plants against environmental hazards, such as toxins, pollution, parasites, bacteria, viruses, UV rays, and stressful contaminants [64].
Figure 1.
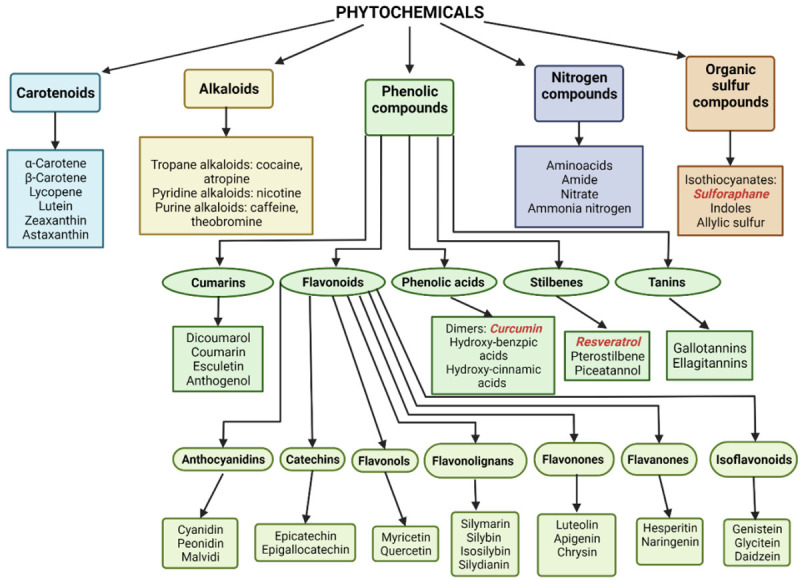
Classification of food and plants phytochemicals. Nrf2 bioactivators used in exercise protocols are marked in red color. Created with BioRender.com.
Over the past years, many of the phytochemical compounds have been characterized, and a large amount of them are not yet identified. To date, more than 5,000 phytochemicals have been identified, and around 400 have been studied in great depth. Diverse studies direct the properties and health benefits of phytochemicals in turn toward antioxidant activity but is very likely that many of their advantages are not yet known to date. The majority of these can react with free radicals and/or reactive species, such as ROS/RNS, which possess a strong relation with the development of several diseases when they are excessively produced. Phytochemicals are identified as multifunctional agents due to their diverse potential to react under different conditions [64,65]. Their most outstanding properties as described in the literature include anti-inflammatory, antioxidant, chemo-preventive, enzyme-modulator, antimicrobial, anticancer, antimutagenic, neuroprotector, glycemic-regulator, cell protector, and immune modulator, among others [64,66].
Phytochemicals and the activation of antioxidant systems
The compounds that can activate molecular mechanisms and intervene in cellular functioning processes could be denominated bioactivators. In particular, the antioxidant signaling pathway is activated by the nuclear translocation of Nrf2, for which the dissociation of Nrf2 from Keap1 is required. Compounds that act by promoting the cleavage of Nrf2 are known as “Nrf2 inducers” or “Nrf2 activators”. They can be the way to hamper Nrf2 ubiquitination and degradation, and they are categorized into electrophilic compounds, protein-protein interaction inhibitors, and multitarget drugs [67]. The first electrophilic compounds oxidizes the cysteine residues present in thiol-rich Keap1 protein or also modifies these by alkylation [68]. Keap1 possesses cysteine thiol groups susceptible to being oxidized (Cys-151, Cys-273, Cys-288, Cys-226, Cys-234, and Cys-613); thus, electrophilic compounds such as ROS or Nrf2 inducers possess the ability to react with sulphydryl groups (-SH), generating low interaction between Nrf2 and Neh2 motifs (DLG/ETGE) and the Keap1 dimer; so, Nrf2/Keap1 bonding is disrupted and Nrf2 translocates into the nucleus [69-71]. Aside from this, upstream kinases phosphorylate threonine Nrf2, breaking Nrf2/Keap1 bonding, and releasing Nrf2 [72].
Some phytochemicals have drawn attention for being electrophilic compounds that can effectively activate Nrf2. Emerging research on Nrf2 activators indicates that these compounds trigger molecular mechanisms to boost cytoprotective defenses in pre-clinical cell-culture studies or in animal models; activation of the Nrf2/Keap1 axis is now the main recognized mechanism by which phytochemicals attenuate oxidative damage and inflammation [73]. The measurement of NQO1 enzymatic activity is used as a reference to estimate the Nrf2 induction and detection of the anticancer activity of phytochemicals utilizing the “CD value”, which is related to the concentration of any compound to double the activity of NQO1 in murine hepatoma cells [74]. NQO1 is an Nrf2 target gene and is considered one of the most important cytoprotective enzymes in cell defenses [74,75]. Bearing in mind that the least amount is required to double NQO1 activity, the most effective of these bioactivators appears to be SFN at a concentration of 0.2 µM, andrographolides (1.43 µM), quercetin (2.5 µM), curcumin (2.7 µM), silymarin (3.6 µM), tamoxifen (5.9 µM), genistein (6.2 µM), beta-carotene (7.2 µM), lutein (17 µM), resveratrol (21 µM), indol-3-carbinol (50 µM), chlorophyll (250 µM), alpha-cryptoxanthin (1.8 mM), zeaxanthin (2.2 mM), epigallocatechin-3-gallate (EGCG) (> 50 µM), and ascorbic acid (> 50 µM) [76]. Remarkably, the CD value of SFN is 13.5-fold greater than that of curcumin, 18-fold superior to that of silymarin, and 105-fold higher than that of resveratrol, phytochemicals that are widely marketed for their frequently stated health-promoting properties [76]. This relevant information might aid in furthering the evaluation of the oral supplements and their active compounds that claim to provide detoxifying or antioxidant properties in favor of preventing cellular aging and contributing to improved general health.
Some of the previously mentioned phytochemicals have been proven to activate Nrf2 signaling, with those studied in greatest depth being SFN [77], curcumin [78], resveratrol [79], and silymarin [80] (Figure 2). Extensive literature supports the numerous benefits to health, such as chemo-preventive, anticancer, antioxidant, anti-inflammatory, and organ protector, as previously mentioned; nonetheless, it should be considered that the majority of these studies were conducted with supra-physiological amounts and achieved little with a normal dietary intake. That is why, to date, that there is no consensus on the effectiveness of phytochemicals in the activation of Nrf2 in humans, and even more so, under conditions of physical exercise.
Figure 2.
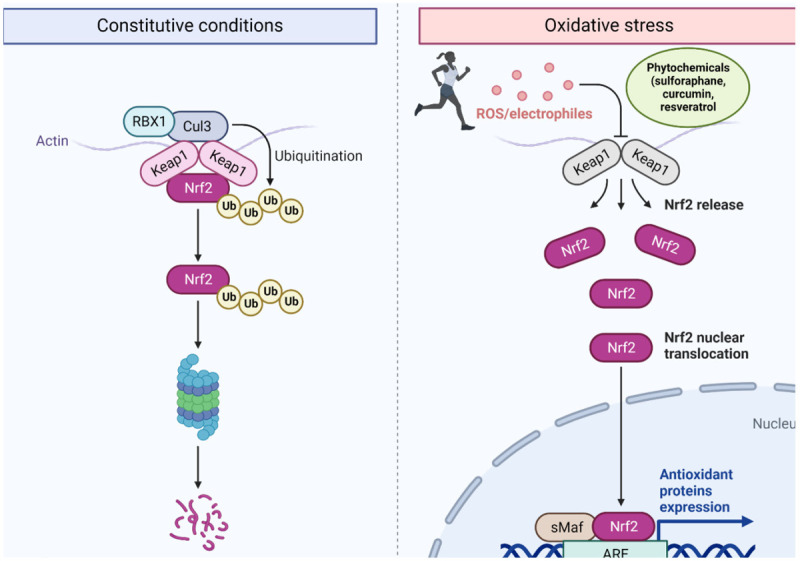
Nrf2/Keap1/ARE signaling activation by ROS exercise-induced and phytochemicals. Under constitutive normal condition Nrf2 is bonding to Keap1 and is ubiquitinated for its proteasomal degradation, in the presence of electrophiles such ROS produced during exercise or some phytochemicals like sulforaphane, curcumin and resveratrol are able to promote Nrf2 dissociation form Keap1 by oxidation of cysteine residues. In consequence Nrf2 is translocated into the nucleus heterodimerized with the musculo-aponeurotic fibrosarcoma proteins (sMaf) in the specific DNA sequence antioxidant response element (ARE) inducing the expression of antioxidant and cytoprotective genes. Created with BioRender.com.
Phytochemicals benefits in sports
Debating the use of antioxidants in exercise
In living systems, physiological levels of harmful events are needed for the activation of cell defenses and for constant-interaction push of cells in order to withstand subsequent insults more efficiently; this latter process is known as hormesis [81]. Thus, exercise-induced ROS/RNS increase oxidative stress and act as messenger molecules, triggering redox-sensitive signaling in the adaptative response. The concept of “exercise preconditioning” is referred as exercise-induced adaptations that are associated with protection against harmful stressors, such as mechanical, oxidative, heat, and metabolic stressors in the organism, and especially in cardiac and muscle fibers [8,82]. Responses also depend on frequency, intensity, and workloads; for example, high-intensity or prologue exercise denotes an increase of stress-response proteins, antioxidant enzymes, heat shock proteins (HSP), transcription factors, adaptor proteins, and mitochondrial biogenesis, which confer protection on the cells in order to counteract upcoming damage [83]. Mitochondria comprise a central element in the exercise-induced stress response; as the core energy provider, the more the demand for ATP in contractile muscle fibers, the more the ROS/RNS production and high oxidative stress. Consequently, sirtuin-1/PGC-1α signaling, antioxidant enzymes and HSP, and the molecular mechanisms of protection such as mitophagy are encouraged, with mitohormesis as the term for this process [49,83]. To a great extent, Nrf2 is in command of mitohormesis by regulating mitochondrial biogenesis, energy metabolism, fatty-acid oxidation, and ATP synthesis [84].
The use of antioxidants in sports has been a discussed topic for decades because, although the use of recognized antioxidants such as vitamin C and vitamin E in the form of dietary supplements has shown to have certain health benefits on the immune system or to be able to prevent the oxidation of cellular structures, in athletes, beyond the scope that they could be of benefit under certain circumstances, they appear to reduce the body’s endogenous capacity to defend itself against oxidative damage, and could even hamper physiological adaptions to exercise [85,86]. Current available data indicate that providing high doses of antioxidants, frequently in the form of supplements, may blunt ROS-mediated physiological events, such as insulin signaling and vasodilatation [87]. A growing body of evidence agrees that antioxidant requirements should be met by dietary sources through a balanced diet according to the recommended dietary allowance (RDA); even so, in repeated heavy workloads, endogenous antioxidant defenses may be surpassed and the risk of damage or fatigue is very high [88].
Apparently in several working laboratories, research carried out on the use of the supplementation of vitamins or minerals with regard to antioxidant activity has produced very similar results under conditions of physical exercise. In general, some of the effects on endogenous antioxidant systems include the attenuation of antioxidant gene expression, the decrease of GPx, SOD, and mRNA levels, and the disruption of PGC-1α and all of the signaling pathways implicated in training-induced cellular adaptations [89,90]. The cell possesses complex cellular systems that maintain functioning and homeostasis. An important part of these are controlled by sensitive redox mechanisms activated during ROS/RNS-producing reactions and at the same time, there is a wide network of elements responsible for neutralization and elimination; therefore, in the presence of high loads of antioxidants such as vitamin C and E, which are not specific to ROS/RNS but are more generalized scavengers, not only will intracellular oxidative stress levels be modified, but also the total number of signaling pathways that must be activated in the presence of ROS/RNS to enhance adaptations as well [3]. This would mean that if exercise positively regulates the expression of the endogenous antioxidant system, to a certain extent the possible negative effects of ROS/RNS would be annulled, with exclusive emphasis on when it comes to physiological adaptations [91] (Figure 3). In this respect, on understanding that many signaling pathways are activated by exercise-induced ROS generation (adenosine monophosphate-activated protein kinase (AMPK), mitogen-activated protein kinase (MAPK), nuclear respiratory factor 2 (NRF2), and PGC-1α) and that they are key for adaptive responses such as mitochondrial biogenesis, energy metabolism, muscle growth, or angiogenesis in skeletal muscle, it must be considered that either the lack or excess of ROS will result in the disruption of cell signaling and adaptation to exercise [92]. Therefore, current studies aim to find novel approaches for preserving the beneficial effects of ROS in cell physiology and, at the same time, avoiding harmful effects [92,93].
Figure 3.
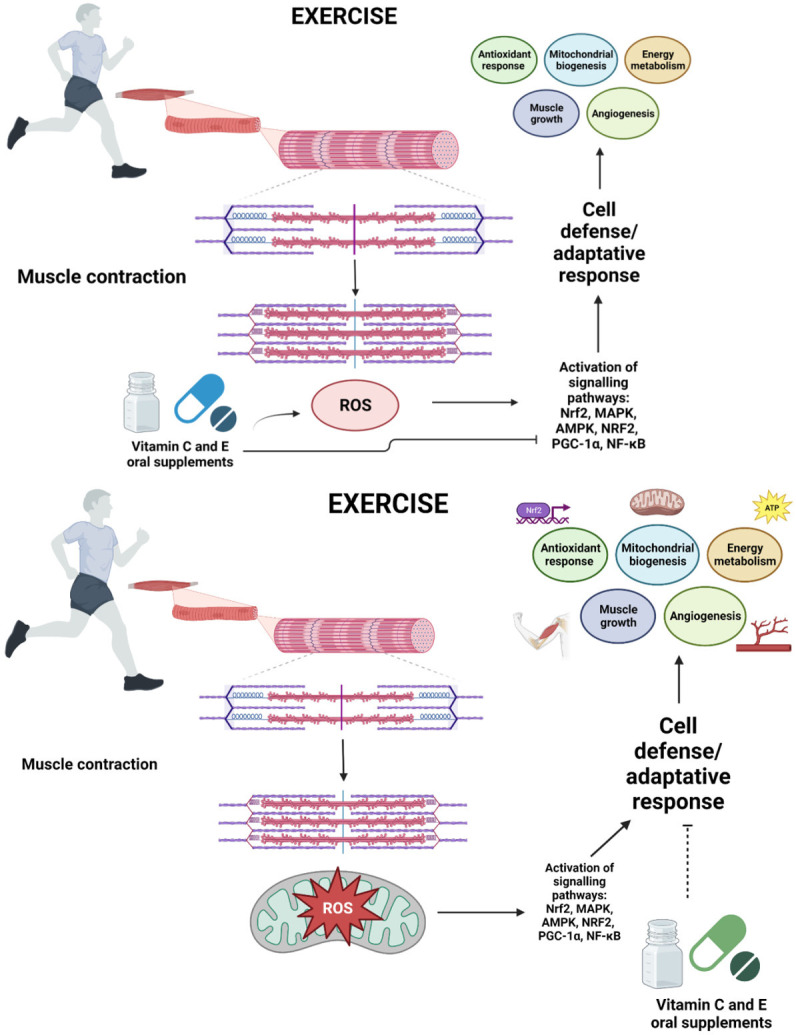
Cell defense and adaptative response in physical exercise. ROS produced along exercise specially in active contractile muscles are necessary for redox-sensitive signaling involved in cell protection and adaptative response, however, vitamin C and E antioxidant supplements seem to blunt physiological adaptations including antioxidant endogenous system. Created with BioRender.com.
Phytochemicals and antioxidant signaling in exercise
Based on the previous statement, it would be interesting to question whether it is appropriate to use antioxidants under physical-training conditions, taking into account that the consumption of antioxidant supplements is often addressed in order to improve the physical condition, prevent damage, or help optimize sports performance. In some way, if it is indeed convenient to ingest antioxidant supplements, what would be the best kind of supplement to take, at what doses, and, above all, under which circumstances should the supplement or antioxidant compound be consumed? Currently, there is no definitive consensus regarding the use of phytochemicals in physical-training situations. In fact, this is an innovative area with a great opportunity to be explored due to the characteristics that have been described in food or plant-derived compounds with potential to activate specific signaling pathways involved in cytoprotective antioxidant response or directly by regulating the genes intricately involved in cellular-adaptation processes that may exert a short/long term impact on athletic performance, regardless of being categorized as antioxidants total physical activity due to their scavenger power against ROS/RNS, as widely described in the literature.
Few phytochemical compounds have been studied in depth in training protocols to assess the antioxidant response, the effects on adaptation, or the enhancement of athletic performance (Table 2). SFN is the most effective bioactivator of the Nrf2 signaling pathway; it has been previously analyzed by Malaguti et al. [94], and these authors found that the 3-day treatment with SFN (25 mg/kg body wt i.p.) in male Wistar rats subjected to a single bout of an exhaustive treadmill-running protocol (+7% slope and 24) significantly increased NQO1, GST, and GR expression and activity, together with a significant expression of Nrf2, as well as total antioxidant capacity and a reduction of creatine phosphokinase (CPK) and lactate dehydrogenase (LDH) activities, suggesting that SFN pre-treatment may exert an impact on the modulation of the antioxidant pathway, preventing exhaustive exercise-induced muscle damage. Later, the study was carried out in 12 to 13-week-old male wild-type mice (Nrf2+/+) and Nrf2-null mice (Nrf2-/-), intraperitoneally (i.p.) injected with SFN (25 mg/kg) or vehicle, which performed an exhaustive running-treadmill test (progressive-continuous all-out). The run distance of the SFN-injected Nrf2+/+ mice was significantly higher than that of uninjected mice. The downstream Nrf2 target genes OH-1, NQO1, CAT, and γ-GCS were upregulated in gastrocnemius and soleus muscle of Nrf2+/+ mice. The muscle-damage markers CPK and LDH were significantly lower in Nrf2+/+ mice; at the same time, the increase in lactate was not significantly high in the same group. Reduction of TBARS was also reported; in contrast, there were no significant differences of SIRT1 and PGC-1α in any group, but AMPKα was significant increased, as well as the number of copies of mitochondrial DNA (mtDNA) in the Nrf2+/+ group. These results suggest that SFN helped attenuate fatigue and exercise-induced oxidative stress and improved aerobic capacity by upregulation of the antioxidant response [95].
Table 2.
Exercise model studies evaluating the effect on antioxidant response and physical performance under phytochemical supplementation
| Model | Methodology | Effects of phytochemical supplementation | Reference | |
|---|---|---|---|---|
|
| ||||
| Antioxidant response | Performance/recovery | |||
| 4-month male Wistar rats | Acute exhaustive exercise treadmill +7% slope and 24 m/min | Increase NQO1, GST and GR expression and activity in vastus laterlis muscle | Reduction of LDH and CPK activities | Malaguti et al. [94] |
| SFN pre-Ex | Increase of Nrf2 expression | |||
| 25 mg/kg bw ip | Elevation of the TAC | |||
| 12-13-week-old male wild type mice (Nrf2+/+) and Nrf2-null mice (Nrf2-/-) on C57BL/6J | Exhaustive (progressive-continuous all-out) treadmill test | Enhance Nrf2 signaling gene expression HO-1, NQO1, CAT and γ-GCS in gastrocnemius and soleus muscle in Nrf2+/+ mice | Improve of running distance | Oh et al. [95] |
| SFN pre-Ex treatment four times for 3 days (72, 48, 24, and 3 h) | Reduction of TBARS | Diminution of LDH and CPK activities | ||
| 25 mg/kg bw i.p. | No differences in SIRT1 and PGC-1α | |||
| Increase of AMPKα and mtDNA copies | ||||
| 4-week-old mdx mice | Grip strength assay | Increase of Phase II enzymes, NQO1 and HO-1 proportional to Nrf2 | Increased of skeletal muscle mass and muscle force 30% | Sun et al. [96] |
| Duchenne muscular dystrophy model | Exercise capacity assay: acute exhaustive exercise treadmill protocol | Attenuation of MDA levels 60% | Improvement of Running distance 20% | |
| 5° slope, increasing speed till 25 m/min | Improvement of GSH-to-GSSG ratio 3.2-fold | Reduction of Plasma CPK 45% and LDH 40% activities | ||
| SFN administration by gavage | Development of Gastrocnemius hypertrophy 25% and myocardial hypertrophy 20% | |||
| 2 mg/kg of body weight/day/4 weeks | Promotion of sarcolemmal integrity | |||
| Attenuation of central nucleation 40%, fiber size variability, and inflammation | ||||
| 16 young men | 6 set of 5 eccentric exercise with the nondominant arm in elbow flexion | Increment on NQO1 mRNA expression in PBMCs | Reduction of DOMS and ROM 2 days after exercise in SFN group | Komine et al. [110] |
| 70% maximum voluntary contraction | Diminution in serum MDA levels 2 days after exercise | |||
| SFN group received 30 mg/day for 2 weeks | ||||
| 8-week-old male Wistar rats | Running exercise treadmill protocol | Enhancement of antioxidant enzyme activity | Reduction of lactate and MDA levels in blood samples | Sahin et al. [111] |
| 25 m/min for 45 min/d for 5 d/wk for 6 weeks | Increase of IκB, PGC-1α, Trx1, SIRT1, GLUT4 and Nrf2 protein levels in muscle of treated group | Improvement in run to exhaustion (minutes) | ||
| Curcuminoids at 20 mg/day for 6 weeks | Diminution in muscle NF-κB levels | |||
| Reduction of HSP70 | ||||
| HF with HFrEF mice model with coronary artery ligation-induced | Adaptation period | Elevation of Nrf2, SOD2 and HO-1, myogenin and MyoD protein expression | Improvement in maximal speed, running distance to exhaustion, and limb grip force | Wafi et al. [112] |
| 10-week male C57BL/6 mice | Exercise capacity treadmill test | Improved the reduced force and rapid fatigue on soleus and extensor digitorum longus muscles | ||
| Whole-body tension test | ||||
| Subcutaneous osmotic minipump curcumin treatment 50 mg·kg-1·day-1-8-weeks | ||||
| 8-week-old C57Bl/6N mice with induced HF | Exercise treadmill exhaustion test | - | Increase of TPA levels and exercise capacity | Sung et al. [113] |
| At a 10 incline, 1 m/min every min the first 5 min, then remained at 15 m/min for 60 min or until exhaustion | Increase of Insulin sensitivity, whole body glucose utilization | |||
| Resveratrol ~450 mg·kg-1·day-1 for 2 weeks | Improve energy metabolism | |||
| Modification of gut microbiota composition | ||||
| Normalized basal and ADP-stimulated O2 consumption in isolated skeletal muscle fibers | ||||
| 8-week-old male Wistar rats | Treadmill exercise | Decrease of NFκB in muscle, liver and heart tissues | - | Pala et al. [119] |
| 1 week of adaptation CE: 25 m/min, 45 min/day, 5 days per week/6 weeks | Rise of Inhibitory protein IκB | |||
| Coenzyme Q10 treatment 300 mg/kg bw i.g. | Increase of Nrf2 and HO-1 in muscle, liver, and heart | |||
| Diminution of plasma triglycerides | ||||
| No changes in metabolites related with CHO and proteins | ||||
SFN: Sulforaphane; pre-Ex: pre-Exercise; NQO1: NADPH Quinone Oxide Reductase 1; GST: Glutathione S Transferase; GR: Glutathione Reductase; TAC: Total Antioxidant Capacity; LDH: Lactate Dehydrogenase; CPK: Creatin Phospho-Kinase; HO-1: Heme Oxygenase-1; CAT: Catalase; γ-GCS: γ-Glutamine Cysteine Synthetase; CHO: Carbohydrates; TBARS: Thiobarbituric Acid Reactive Substances; SIRT1: Sirtuin 1; PGC-1α: Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-Alpha; NF-κB: Nuclear Factor-κB; IκB: Inhibitor of Kappa B; AMPKα: AMP-Activated Protein Kinase Alpha; mtDNA: Mitochondrial DNA; MDA: Malonaldehyde; GSH: Reduced Glutathione; GSSG: Oxidized Glutathione; PBMCs: Peripheral Blood Mononuclear Cells; DOMS: Delayed Onset Muscle Soreness; ROM: Range Of Motion; Trx1: Thioredoxin 1; GLUT4: Glucose Transporter 4; HSP70: Heat Shock Protein 70; HF: Heart Failure; HFrEF: Heart Failure With Low Ejection Fraction; SOD2: Superoxide Dismutase 2; TPA: Total Physical Activity.
Indeed, SFN have proved to be effective in restoring muscle function and protecting from oxidative damage in Duchenne muscular dystrophy in 4-week-old mdx mice. The animals were treated with SFN by gavage (2 mg·kg body wt(-1)·day(-1) for 8 wk); at the end of the treatment, SFN revealed to have improved muscle mass and force in the treated mice. Running-distance capacity and the GSH/GSSG ratio augmented significantly. On the other hand, LDH, CPK, and MDA levels decreased. Sarcolemmal integrity improved, and some cellular features were muddied, such as decreased central nucleation, fiber variability, and inflammation. In sum, SFN treatment may possibly have clinical benefits for the therapy of individuals with muscular dystrophy [96]. In a very similar model, SFN increased the expression of phase-II enzyme HO-1 via activation of Nrf2 and reduced the infiltration of immune cells, proinflammatory cytokines TNF-α, interleukin-1β (IL-1β), interleukin-6 (IL-6), and inflammatory cytokine CD45 in mdx mouse skeletal muscle. The command of the inflammatory signaling pathway by NF-κB was inhibited by the reduced expression of NF-κB (p65) and phosphorylated IκB kinase-α, and augmented inhibitor κB-α expression. Thus, SFN may act as a potent inhibitor of inflammation [97]. SFN is a compound that can also exert protection against oxidative stress by stimulating macroautophagy/autophagy and detoxifying pathways; it accomplishes this through the activation of Nrf2, which is a target gene of the transcription factor EB (TFEB). EB is translocated into the nucleus with a moderate increase of ROS through a Ca2+-dependent, but mTOR (mechanistic target of rapamycin kinase)-independent, mechanism. Then, the essential genes involved in lysosome biogenesis and autophagosome are activated, which are crucial for the removal of damaged mitochondria [98]. During strenuous or high-intensity exercise, the increased level of ROS can generate destabilization and mitochondrial damage; thus, it is important to possess efficient cell-damage repair systems, and SFN could be a potential activator of those systems.
Antioxidant phytochemicals in performance and recovery
Regarding the use of antioxidants in terms of physical activity, the majority of the time this is performed with the intention of helping to reduce muscle-tissue damage and inflammatory processes, to aid in recovery, improve the immune-system response, promote optimal health in the athlete, and, in general to maximize sports performance [88]. Nonetheless, how much of this can really be achieved and, above all, how can this be measured in order to verify that there is a true beneficial effect from the use of antioxidants [99]? It is necessary to take into consideration that in addressing physical exercise, it is easy to recognize the multiplicity of health benefits provided by the practice of regular physical activity, such as the prevention of diseases or perhaps forming part of the treatment of several disorders [100-102]. However, it is important to bear in mind that heavy workloads may cause the eventual disruption of active muscles and probably leads to overtraining syndrome (OTS), which is conceived as prolonged periods of fatigue consequent to intense training or competitions, along with inadequate post-competition-exercise recovery episodes [103]. Therefore, lack of the ability to control exercise-induced oxidative stress and excessive ROS/RNS is closely related to OTS and detrimental athletic performance. Skeletal-muscle fatigue is the result of a diminution in maximal-force production in response to contractile activity. The presence of hydrogen (H+) ions, lactate, inorganic phosphate (Pi), ROS, and heat shock protein (HSP) are metabolic factors that contribute to muscle fatigue [104]. Also, the redox state determines the degree of fatigue in muscle fibers; it has been shown that an oxidized environment and continued exposure to H2O2 favor fatigue and the loss of muscle-force production [105]. Chronic inflammation is related to the sustained oxidized state, which has a negative impact on force production and muscle contraction. Contrariwise, unfatigued and optimal muscle performance is associated with a more reduced state of muscle fibers [105]. While the rise of submaximal force is detected with acute/mild exposure to ROS/RNS in rested myofibers, chronic exposure has the opposite effect [106]. Fatigue fibers often remain in reduced submaximal force, which is induced by a ROS/RNS-dependent decline in sarcoplasmic reticulum Ca2+ release and/or myofibrillar Ca2+ sensitivity [107]. Hence, appropriate functioning of the contractile muscle is determined by, among other issues, factors related to production, type of ROS/RNS, magnitude of time-of-exposure, and the effectiveness with which the endogenous/exogenous antioxidant systems act synergistically [106,107].
Overtraining comprises a high energy demand and the mitochondria might undergo damage, reducing the respiratory capacity and the ability to produce ATP and, in turn, athletic performance. Exhaustive chronic exercise leads to OTS, body-weight loss, muscle catabolism, and a decrease of physical performance in rats after 6 weeks of exhaustive treadmill exercise. Myocardial structure was affected, cellular swelling was detected, and there was the appearance of peroxisomes. Mitochondrial function and a decrease of oxidative phosphorylation (OxPho) were reported by means of a reduced tissue content of cytochrome c [108]. In turn, OTS is not exclusive in skeletal muscle, but can also affect other organs that are highly active throughout exercise, such as the myocardium. There is a clear correlation between high indicators of fatigue and/or OTS and the inability to sustain prolonged or highly intense training episodes [109].
In view of the fact that maintaining a redox imbalance with a greater tendency toward the oxidized state creates adverse effects on muscle-contraction force and enhances fatigue and a low sports performance, it is important to identify the existence of tools that reinforce and improve conditions of the cell environment, support redox balance, and promote athletic performance. In this sense, it is worth mentioning that the supply of antioxidants from the diet or derived from plants or foods such as phytonutrients or phytochemicals that not only function as direct ROS/RNS scavengers, but also activate the signaling pathways responsible for the antioxidant cytoprotective response, would be a suitable option for improving athletic performance and recovery.
In-vivo studies demonstrate that SFN can enhance muscle function and strength, and prevent muscle oxidative damage and inflammation [96]; moreover, SFN interferes with NF-κB expression; consequently, the expression of proinflammatory and inflammatory cytokines is impaired [97]. However, evidence in humans regarding supplementation with SFN in terms of physical exercise is scarce. Recently, a group of 16 young men were randomly divided into an SFN and a control group. SFN was supplemented with 30 mg/1 day for 2 weeks. Then, each group performed six sets of five eccentric exercises with the nondominant arm in elbow flexion at 70% maximal voluntary contraction. The evaluation of delayed-onset muscle soreness (DMSO) and the range of motion (ROM) in the biceps of the SFN group were significantly lower than those in the controls 2 days after exercise. MDA serum levels also decreased significantly compared with those of the control group. Likewise, NQO1 mRNA expression in peripheral blood mononuclear cells (PBMC) was higher. These findings suggest that SFN supplementation for 2 weeks enhances the Nrf2 target gene NQO1, and the effects can be observed as the reduction of DMSO after 2 days of eccentric exercise [110]. At present, it would be too early to state that SFN supplementation had a clear effect on reducing inflammation and the oxidative-stress level, as well as promoting antioxidant response based only on previous evidence. Therefore, additional biomarkers need to be taken into consideration to support these theories.
It is interesting to observe that throughout the trials in which phytochemicals are evaluated under a condition of exercise, it can be reaffirmed that, to a great extent, the underlying effects on physical performance, such as improving aerobic capacity, shortening recovery times, or increasing muscle strength, appear to be linked with the reduction of oxidative stress and damage in skeletal muscle and other organs by the activation signaling pathway relative to the endogenous antioxidant system. Sahin et al. [111] evaluated the effect of curcumin 100 mg/kg CurcuWin(®), by administering 20 mg of curcuminoids daily for 6 weeks to male Wistar rats subjected to a 6-week treadmill-running exercise protocol. During a 5-day period, animals in chronic exercise groups were put through the following different regimens: day 1, 10 m/min for 10 min; day 2, 20 m/min for 10 min; day 3, 25 m/min for 10 min; day 4, 25 m/min for 20 min, and day 5, 25 m/min for 30 min. The animals were exercised at 25 m/min for 45 min/d for 5 d/wk for 6 weeks. The animals treated with curcumin improved significantly in run-to-exhaustion time, as well as in MDA levels. NF-κB and HSP70 were lower than in non-treated animals, whereas PGC-1, SIRT1, Nrf2, and GLUT4 protein levels were significantly higher. The antioxidant-enzyme activities of GPx and SOD were superior, as well as the GSH level, as compared to those of the control group. In conclusion, curcumin treatment is capable of protecting skeletal muscle from damage-induced exhausting exercise by regulating NF-κB Nrf2 signaling, contributing to the enhancement of exercise performance. Wafi et al. [112] evaluated the subcutaneous osmotic-minipump curcumin treatment (50 mg·kg-1·day-1 for 8-weeks) in heart failure (HF) with a low ejection fraction (HFrEF) mouse model with ligation-induced coronary artery, in which intolerance to exercise is a distinctive feature, reporting that the curcumin treatment in HF mice improved maximal speed, running-distance-to-exhaustion, and limb-grip-force significantly; curcumin had a positive impact on the reduced force and rapid fatigue in soleus and extensor digitorum longus muscles. In addition, the protein expressions of Nrf2, SOD2, HO-1, myogenin, and MyoD were enhanced with curcumin. The key findings showed that impairment of the Nrf2 pathway might have strongly influenced exercise intolerance in HFrEF. Furthermore, curcumin could contribute to reducing oxidative stress in the skeletal muscle of HFrEF mice, participating in the prevention of exercise-performance decline by upregulating the antioxidant defense system mediated by triggering Nrf2 signaling. This represents an opportunity to improve exercise tolerance in patients with HFrEF, in that it allows them to have a better clinical prognosis.
Sung et al. [113] administered resveratrol (~450 mg·kg-1·day-1 for 2-weeks) or vehicle in C57Bl/6N mice subjected to either sham or transverse aortic constriction surgery to induce HF. Resveratrol was administered at 3 weeks post-surgery to a cohort of mice with established HF (% ejection fraction < 45). The researchers found that resveratrol supplementation improved exercise intolerance and fatigue. The underlying effects of resveratrol were attributed to the improvement of whole-body glucose utilization and better muscle function and metabolism, as well as changes in gut-microbiota composition. Although molecular mechanisms related to antioxidant signaling were not performed in the study, it is possible to establish a connection with Nrf2, due to the fact that resveratrol has been shown to activate Nrf2 signaling in previous essays, in combination with grape polyphenols, at doses of 100 and 75 mg, respectively [114]. In diabetic patients, 800 mg/day for 2 months significantly increased Nrf2 gene expression [115]; however, the 4-week resveratrol at 500 mg/day had no antioxidant and anti-inflammatory effect on patients with chronic kidney disease [116]. Hence, due to the discrepancies in the emerging literature, it is essential to build a proper design study considering the variables of the exercise protocol, the doses, and the length of the supplementation period, among others.
Currently, there is evidence that supports the use of polyphenols for aiding in fatigue and athletic performance. A precise dose has not yet been established, but in general, an acute intake of ~300 mg from fruit-derived polyphenols 1-2 h prior to exercise is suggested to improve capacity and/or performance in endurance or high-intensity activities such as sprinting, through the activation of antioxidant defenses and vascular functioning. On the other hand, the chronic consumption of > 1,000 mg/day of polyphenols for at least 3 or more days prior to and post-exercise to protect skeletal muscle from damage can promote antioxidant and anti-inflammatory mechanisms, preventing the development of fatigue and enhancing recovery following exercise [117]. It is known that polyphenols derived from fruits possess a potent ability to reduce observed oxidative stress, which can be witnessed by lower oxidative damage markers, such as MDA, TBARS, F2-isoprostanes, lipid hydroperoxides, protein carbonyls, and nitrotyrosine, among others. Due to the low concentration that they achieve in serum and tissues, it is more likely that the underlying effects of polyphenols on the antioxidant response are attributed to supplementation during more than 3 continuous days, thus activating the Nrf2/ARE pathway and the genes involved in the antioxidant response, beyond having a direct antioxidant effect as scavengers of reactive species [118].
Future perspectives and conclusions
For some time now, the study of bioactive compounds derived from food and plants has provided valuable information on health by proving that phytochemicals represent an alternative in the prevention or treatment of a large number of diseases, all of which have been understood from various models of pre-clinical and clinical research. Current studies point to new approaches that seek to preserve the beneficial effects of ROS on cell physiology while avoiding deleterious effects. In this sense, it is worth mentioning that the supply of antioxidants from the diet or derived from plants or foods as phytonutrients or phytochemicals may be an appropriate option to improve sports performance and recovery. The antioxidant approach, such as phytochemicals or phytonutrients in sports, should be carried out from a more complete perspective that considers antioxidants, not only as free radical scavengers, but also as compounds capable of activating the mechanisms involved in both cell-protection systems such as the antioxidant response signaling pathways, or in the inhibition of the inflammatory cascade and the activation of autophagy pathways. In agreement with the available data, Nrf2 is critical for exercise performance and skeletal-muscle functioning, to a greater extent than the role of master regulator of antioxidant and cytoprotective response. In fact, it is implicated in the adaptative response to exercise, and moreover Nrf2 also upregulates enzymes involved in energy metabolism possibly influencing exercise capacity, but more studies are needed to support these theories.
Currently, there is no definitive consensus on the use of phytochemicals in physical training situations. It is not yet clear whether the use of antioxidants in physical training conditions is appropriate, nor has it been established which is the most effective phytopharmaceutical and at what doses are optimal to obtain the desired results. Likewise, efficacy in humans must be supported. Therefore, it is essential to carry out a study with an adequate design that considers the variables of exercise protocol, doses and duration of the supplementation period, as well as other factors that may influence the outcome. It is important to establish protocols for its use and determine under what conditions and with what indications the best results would be obtained. In future, research should be taken into account more indicators related to the activation of signaling pathways and molecular mechanisms, such as the nuclear concentration of transcription factors, target-gene expression, protein expression, and enzymatic activity, together with indicators with respect to physical performance, such as aerobic capacity, endurance, maximal and submaximal muscle force, among others.
In conclusion, there is growing evidence suggesting that supplementation with phytochemicals derived from foods or plants may promote exercise performance by the activation of antioxidant and anti-inflammatory signaling far more than by only exerting antioxidant activity as direct scavengers of ROS/RNS. More investigation is required to determine dosing strategies, the modality, and the intensity and duration of exercise training, for which ergogenic effects may be achieved. According to the CD value, Nrf2 activators are promising phytochemicals that may be potential ergogenic aids; thus, it is suggested that these should be considered for future research. Despite the scarce number of trials performed to evaluate phytochemicals in exercise, current available data show that bioactive compounds are able to contribute to reducing fatigue, improving muscle force and muscle contraction, and attenuating OTS. In this regard, it is important to recognize the type of phtytochemical to use, their blending, the concentration of the compounds, and bioavilability, in order for the specific bioactive compounds and mechanisms of action to be recognized.
Acknowledgements
Nancy Vargas-Mendoza is a scholarship holder for her postgraduate studies by the Consejo Nacional de Ciencia y Tecnologia (CONACyT) and the BEIFI program, Instituto Politécnico Nacional (IPN), Mexico.
Disclosure of conflict of interest
None.
References
- 1.Hughes DC, Ellefsen S, Baar K. Adaptations to endurance and strength training. Cold Spring Harb Perspect Med. 2018;8:a029769. doi: 10.1101/cshperspect.a029769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013;17:162–184. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powers SK, Duarte J, Kavazis AN, Talbert EE. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp Physiol. 2010;95:1–9. doi: 10.1113/expphysiol.2009.050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang S, Lian G. ROS and diseases: role in metabolism and energy supply. Mol Cell Biochem. 2020;467:1–12. doi: 10.1007/s11010-019-03667-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Z, Ren Z, Zhang J, Chuang CC, Kandaswamy E, Zhou T, Zuo L. Role of ROS and nutritional antioxidants in human diseases. Front Physiol. 2018;9:477. doi: 10.3389/fphys.2018.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Meo S, Napolitano G, Venditti P. Mediators of physical activity protection against ROS-linked skeletal muscle damage. Int J Mol Sci. 2019;20:3024. doi: 10.3390/ijms20123024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powers SK, Bomkamp M, Ozdemir M, Hyatt H. Mechanisms of exercise-induced preconditioning in skeletal muscles. Redox Biol. 2020;35:101462. doi: 10.1016/j.redox.2020.101462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powers SK, Deminice R, Ozdemir M, Yoshihara T, Bomkamp MP, Hyatt H. Exercise-induced oxidative stress: friend or foe? J Sport Health Sci. 2020;9:415–425. doi: 10.1016/j.jshs.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powers SK, Ji LL, Kavazis AN, Jackson MJ. Reactive oxygen species: impact on skeletal muscle. Compr Physiol. 2011;1:941–969. doi: 10.1002/cphy.c100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 12.Baar K. Nutrition and the adaptation to endurance training. Sports Med. 2014;44(Suppl 1):S5–12. doi: 10.1007/s40279-014-0146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Orr AL, Brand MD. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol. 2013;1:304–312. doi: 10.1016/j.redox.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brand MD. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic Biol Med. 2016;100:14–31. doi: 10.1016/j.freeradbiomed.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Sakellariou GK, Jackson MJ, Vasilaki A. Redefining the major contributors to superoxide production in contracting skeletal muscle. The role of NAD(P)H oxidases. Free Radic Res. 2014;48:12–29. doi: 10.3109/10715762.2013.830718. [DOI] [PubMed] [Google Scholar]
- 17.Gong MC, Arbogast S, Guo Z, Mathenia J, Su W, Reid MB. Calcium-independent phospholipase A2 modulates cytosolic oxidant activity and contractile function in murine skeletal muscle cells. J Appl Physiol (1985) 2006;100:399–405. doi: 10.1152/japplphysiol.00873.2005. [DOI] [PubMed] [Google Scholar]
- 18.Nethery D, Stofan D, Callahan L, DiMarco A, Supinski G. Formation of reactive oxygen species by the contracting diaphragm is PLA(2) dependent. J Appl Physiol (1985) 1999;87:792–800. doi: 10.1152/jappl.1999.87.2.792. [DOI] [PubMed] [Google Scholar]
- 19.Sakellariou GK, Vasilaki A, Palomero J, Kayani A, Zibrik L, McArdle A, Jackson MJ. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid Redox Signal. 2013;18:603–621. doi: 10.1089/ars.2012.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferreira LF, Laitano O. Regulation of NADPH oxidases in skeletal muscle. Free Radic Biol Med. 2016;98:18–28. doi: 10.1016/j.freeradbiomed.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Culotta VC, Yang M, O’Halloran TV. Activation of superoxide dismutases: putting the metal to the pedal. Biochim Biophys Acta. 2006;1763:747–758. doi: 10.1016/j.bbamcr.2006.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powers SK, Criswell D, Lawler J, Ji LL, Martin D, Herb RA, Dudley G. Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. Am J Physiol. 1994;266:R375–380. doi: 10.1152/ajpregu.1994.266.2.R375. [DOI] [PubMed] [Google Scholar]
- 23.Criswell D, Powers S, Dodd S, Lawler J, Edwards W, Renshler K, Grinton S. High intensity training-induced changes in skeletal muscle antioxidant enzyme activity. Med Sci Sports Exerc. 1993;25:1135–1140. [PubMed] [Google Scholar]
- 24.Deponte M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim Biophys Acta. 2013;1830:3217–3266. doi: 10.1016/j.bbagen.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 25.Jastrząb A, Skrzydlewska E. Thioredoxin-dependent system. Application of inhibitors. J Enzyme Inhib Med Chem. 2021;36:362–371. doi: 10.1080/14756366.2020.1867121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhee SG, Kil IS. Multiple functions and regulation of mammalian peroxiredoxins. Annu Rev Biochem. 2017;86:749–775. doi: 10.1146/annurev-biochem-060815-014431. [DOI] [PubMed] [Google Scholar]
- 27.Fernandes AP, Holmgren A. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid Redox Signal. 2004;6:63–74. doi: 10.1089/152308604771978354. [DOI] [PubMed] [Google Scholar]
- 28.Marinho HS, Real C, Cyrne L, Soares H, Antunes F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014;2:535–562. doi: 10.1016/j.redox.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 2017;11:613–619. doi: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vargas-Mendoza N, Morales-González Á, Madrigal-Santillán EO, Madrigal-Bujaidar E, Álvarez-González I, García-Melo LF, Anguiano-Robledo L, Fregoso-Aguilar T, Morales-Gonzalez JA. Antioxidant and adaptative response mediated by Nrf2 during physical exercise. Antioxidants (Basel) 2019;8:196. doi: 10.3390/antiox8060196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spiegelman BM. Transcriptional control of mitochondrial energy metabolism through the PGC1 coactivators. Novartis Found Symp. 2007;287:60–63. discussion 63-69. [PubMed] [Google Scholar]
- 32.Chinsomboon J, Ruas J, Gupta RK, Thom R, Shoag J, Rowe GC, Sawada N, Raghuram S, Arany Z. The transcriptional coactivator PGC-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proc Natl Acad Sci U S A. 2009;106:21401–21406. doi: 10.1073/pnas.0909131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang KW, Lee SJ, Kim SG. Molecular mechanism of nrf2 activation by oxidative stress. Antioxid Redox Signal. 2005;7:1664–1673. doi: 10.1089/ars.2005.7.1664. [DOI] [PubMed] [Google Scholar]
- 34.Egerman MA, Glass DJ. Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol. 2014;49:59–68. doi: 10.3109/10409238.2013.857291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22:5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 37.Le Moal E, Pialoux V, Juban G, Groussard C, Zouhal H, Chazaud B, Mounier R. Redox control of skeletal muscle regeneration. Antioxid Redox Signal. 2017;27:276–310. doi: 10.1089/ars.2016.6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niture SK, Khatri R, Jaiswal AK. Regulation of Nrf2-an update. Free Radic Biol Med. 2014;66:36–44. doi: 10.1016/j.freeradbiomed.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katoh Y, Iida K, Kang MI, Kobayashi A, Mizukami M, Tong KI, McMahon M, Hayes JD, Itoh K, Yamamoto M. Evolutionary conserved N-terminal domain of Nrf2 is essential for the Keap1-mediated degradation of the protein by proteasome. Arch Biochem Biophys. 2005;433:342–350. doi: 10.1016/j.abb.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 41.Tebay LE, Robertson H, Durant ST, Vitale SR, Penning TM, Dinkova-Kostova AT, Hayes JD. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic Biol Med. 2015;88:108–146. doi: 10.1016/j.freeradbiomed.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eggler AL, Small E, Hannink M, Mesecar AD. Cul3-mediated Nrf2 ubiquitination and antioxidant response element (ARE) activation are dependent on the partial molar volume at position 151 of Keap1. Biochem J. 2009;422:171–180. doi: 10.1042/BJ20090471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raghunath A, Sundarraj K, Nagarajan R, Arfuso F, Bian J, Kumar AP, Sethi G, Perumal E. Antioxidant response elements: discovery, classes, regulation and potential applications. Redox Biol. 2018;17:297–314. doi: 10.1016/j.redox.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47:1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niture SK, Kaspar JW, Shen J, Jaiswal AK. Nrf2 signaling and cell survival. Toxicol Appl Pharmacol. 2010;244:37–42. doi: 10.1016/j.taap.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baird L, Swift S, Llères D, Dinkova-Kostova AT. Monitoring Keap1-Nrf2 interactions in single live cells. Biotechnol Adv. 2014;32:1133–1144. doi: 10.1016/j.biotechadv.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Done AJ, Traustadóttir T. Nrf2 mediates redox adaptations to exercise. Redox Biol. 2016;10:191–199. doi: 10.1016/j.redox.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang P, Li CG, Qi Z, Cui D, Ding S. Acute exercise stress promotes Ref1/Nrf2 signalling and increases mitochondrial antioxidant activity in skeletal muscle. Exp Physiol. 2016;101:410–420. doi: 10.1113/EP085493. [DOI] [PubMed] [Google Scholar]
- 49.Musci RV, Hamilton KL, Linden MA. Exercise-induced mitohormesis for the maintenance of skeletal muscle and healthspan extension. Sports (Basel) 2019;7:170. doi: 10.3390/sports7070170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ji LL, Gomez-Cabrera MC, Vina J. Exercise and hormesis: activation of cellular antioxidant signaling pathway. Ann N Y Acad Sci. 2006;1067:425–435. doi: 10.1196/annals.1354.061. [DOI] [PubMed] [Google Scholar]
- 51.Wang P, Li CG, Qi Z, Cui D, Ding S. Acute exercise induced mitochondrial H2O2 production in mouse skeletal muscle: association with p(66Shc) and FOXO3a signaling and antioxidant enzymes. Oxid Med Cell Longev. 2015;2015:536456. doi: 10.1155/2015/536456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crilly MJ, Tryon LD, Erlich AT, Hood DA. The role of Nrf2 in skeletal muscle contractile and mitochondrial function. J Appl Physiol (1985) 2016;121:730–740. doi: 10.1152/japplphysiol.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merry TL, Ristow M. Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates exercise-induced mitochondrial biogenesis and the anti-oxidant response in mice. J Physiol. 2016;594:5195–5207. doi: 10.1113/JP271957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Islam H, Bonafiglia JT, Turnbull PC, Simpson CA, Perry CGR, Gurd BJ. The impact of acute and chronic exercise on Nrf2 expression in relation to markers of mitochondrial biogenesis in human skeletal muscle. Eur J Appl Physiol. 2020;120:149–160. doi: 10.1007/s00421-019-04259-7. [DOI] [PubMed] [Google Scholar]
- 55.Done AJ, Gage MJ, Nieto NC, Traustadóttir T. Exercise-induced Nrf2-signaling is impaired in aging. Free Radic Biol Med. 2016;96:130–138. doi: 10.1016/j.freeradbiomed.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 56.Ostrom EL, Traustadóttir T. Aerobic exercise training partially reverses the impairment of Nrf2 activation in older humans. Free Radic Biol Med. 2020;160:418–432. doi: 10.1016/j.freeradbiomed.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Done AJ, Newell MJ, Traustadóttir T. Effect of exercise intensity on Nrf2 signalling in young men. Free Radic Res. 2017;51:646–655. doi: 10.1080/10715762.2017.1353689. [DOI] [PubMed] [Google Scholar]
- 58.Ostrom EL, Valencia AP, Marcinek DJ, Traustadóttir T. High intensity muscle stimulation activates a systemic Nrf2-mediated redox stress response. Free Radic Biol Med. 2021;172:82–89. doi: 10.1016/j.freeradbiomed.2021.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.López-Romero D, Izquierdo-Vega JA, Morales-González JA, Madrigal-Bujaidar E, Chamorro-Cevallos G, Sánchez-Gutiérrez M, Betanzos-Cabrera G, Alvarez-Gonzalez I, Morales-Gon-zález Á, Madrigal-Santillán E. Evidence of some natural products with antigenotoxic effects. Part 2: plants, vegetables, and natural resin. Nutrients. 2018;10:1954. doi: 10.3390/nu10121954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vargas-Mendoza N, Sandoval-Gallegos EM, Madrigal-Santillán EO, Morales-Martínez M, Soriano-Ursúa MA, Angeles-Valencia M, Morales-González Á, Portillo-Reyes J, Morales-González JA. The cytoprotective activity of Nrf2 is regulated by phytochemicals (sulforaphane, curcumin, and silymarin) In: Ekiert HM, Ramawat KG, Arora J, editors. Plant Antioxidants and Health. Cham: Springer International Publishing; 2020. pp. 1–52. [Google Scholar]
- 61.Turner ER. The movement of organic nitrogen compounds in plants: with two figures in the text. Ann Bot. 1960;24:387–396. [Google Scholar]
- 62.Maoka T. Carotenoids as natural functional pigments. J Nat Med. 2020;74:1–16. doi: 10.1007/s11418-019-01364-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Truzzi F, Tibaldi C, Zhang Y, Dinelli G, D Amen E. An overview on dietary polyphenols and their biopharmaceutical classification system (BCS) Int J Mol Sci. 2021;22:5514. doi: 10.3390/ijms22115514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang YJ, Gan RY, Li S, Zhou Y, Li AN, Xu DP, Li HB. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 2015;20:21138–21156. doi: 10.3390/molecules201219753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr. 2004;134(Suppl):3479S–3485S. doi: 10.1093/jn/134.12.3479S. [DOI] [PubMed] [Google Scholar]
- 66.Chun KS, Raut PK, Kim DH, Surh YJ. Role of chemopreventive phytochemicals in NRF2-mediated redox homeostasis in humans. Free Radic Biol Med. 2021;172:699–715. doi: 10.1016/j.freeradbiomed.2021.06.031. [DOI] [PubMed] [Google Scholar]
- 67.Robledinos-Antón N, Fernández-Ginés R, Manda G, Cuadrado A. Activators and inhibitors of NRF2: a review of their potential for clinical development. Oxid Med Cell Longev. 2019;2019:9372182. doi: 10.1155/2019/9372182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Satoh T, McKercher SR, Lipton SA. Reprint of: Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radic Biol Med. 2014;66:45–57. doi: 10.1016/j.freeradbiomed.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 69.Saito R, Suzuki T, Hiramoto K, Asami S, Naganuma E, Suda H, Iso T, Yamamoto H, Morita M, Baird L, Furusawa Y, Negishi T, Ichinose M, Yamamoto M. Characterizations of three major cysteine sensors of Keap1 in stress response. Mol Cell Biol. 2015;36:271–284. doi: 10.1128/MCB.00868-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamamoto T, Suzuki T, Kobayashi A, Wakabayashi J, Maher J, Motohashi H, Yamamoto M. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol Cell Biol. 2008;28:2758–2770. doi: 10.1128/MCB.01704-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baird L, Llères D, Swift S, Dinkova-Kostova AT. Regulatory flexibility in the Nrf2-mediated stress response is conferred by conformational cycling of the Keap1-Nrf2 protein complex. Proc Natl Acad Sci U S A. 2013;110:15259–15264. doi: 10.1073/pnas.1305687110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baird L, Dinkova-Kostova AT. Diffusion dynamics of the Keap1-Cullin3 interaction in single live cells. Biochem Biophys Res Commun. 2013;433:58–65. doi: 10.1016/j.bbrc.2013.02.065. [DOI] [PubMed] [Google Scholar]
- 73.Clifford T, Acton JP, Cocksedge SP, Davies KAB, Bailey SJ. The effect of dietary phytochemicals on nuclear factor erythroid 2-related factor 2 (Nrf2) activation: a systematic review of human intervention trials. Mol Biol Rep. 2021;48:1745–1761. doi: 10.1007/s11033-020-06041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Houghton CA, Fassett RG, Coombes JS. Sulforaphane: translational research from laboratory bench to clinic. Nutr Rev. 2013;71:709–726. doi: 10.1111/nure.12060. [DOI] [PubMed] [Google Scholar]
- 75.Ross D, Siegel D. The diverse functionality of NQO1 and its roles in redox control. Redox Biol. 2021;41:101950. doi: 10.1016/j.redox.2021.101950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Houghton CA, Fassett RG, Coombes JS. Sulforaphane and other nutrigenomic Nrf2 activators: can the clinician’s expectation be matched by the reality? Oxid Med Cell Longev. 2016;2016:7857186. doi: 10.1155/2016/7857186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vanduchova A, Anzenbacher P, Anzenbacherova E. Isothiocyanate from broccoli, sulforaphane, and its properties. J Med Food. 2019;22:121–126. doi: 10.1089/jmf.2018.0024. [DOI] [PubMed] [Google Scholar]
- 78.Shahcheraghi SH, Salemi F, Peirovi N, Ayatollahi J, Alam W, Khan H, Saso L. Nrf2 regulation by curcumin: molecular aspects for therapeutic prospects. Molecules. 2021;27:167. doi: 10.3390/molecules27010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Farkhondeh T, Folgado SL, Pourbagher-Shahri AM, Ashrafizadeh M, Samarghandian S. The therapeutic effect of resveratrol: focusing on the Nrf2 signaling pathway. Biomed Pharmacother. 2020;127:110234. doi: 10.1016/j.biopha.2020.110234. [DOI] [PubMed] [Google Scholar]
- 80.Vargas-Mendoza N, Morales-González Á, Morales-Martínez M, Soriano-Ursúa MA, Delgado-Olivares L, Sandoval-Gallegos EM, Madrigal-Bujaidar E, Álvarez-González I, Madrigal-Santillán E, Morales-Gonzalez JA. Flavolignans from silymarin as Nrf2 bioactivators and their therapeutic applications. Biomedicines. 2020;8:122. doi: 10.3390/biomedicines8050122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Calabrese E, Iavicoli I, Calabrese V. Hormesis: its impact on medicine and health. Hum Exp Toxicol. 2013;32:120–152. doi: 10.1177/0960327112455069. [DOI] [PubMed] [Google Scholar]
- 82.Radák Z, Sasvári M, Nyakas C, Pucsok J, Nakamoto H, Goto S. Exercise preconditioning against hydrogen peroxide-induced oxidative damage in proteins of rat myocardium. Arch Biochem Biophys. 2000;376:248–251. doi: 10.1006/abbi.2000.1719. [DOI] [PubMed] [Google Scholar]
- 83.Lawler JM, Rodriguez DA, Hord JM. Mitochondria in the middle: exercise preconditioning protection of striated muscle. J Physiol. 2016;594:5161–5183. doi: 10.1113/JP270656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Holmström KM, Kostov RV, Dinkova-Kostova AT. The multifaceted role of Nrf2 in mitochondrial function. Curr Opin Toxicol. 2016;1:80–91. doi: 10.1016/j.cotox.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morrison D, Hughes J, Della Gatta PA, Mason S, Lamon S, Russell AP, Wadley GD. Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic Biol Med. 2015;89:852–862. doi: 10.1016/j.freeradbiomed.2015.10.412. [DOI] [PubMed] [Google Scholar]
- 86.de Oliveira DCX, Rosa FT, Simões-Ambrósio L, Jordao AA, Deminice R. Antioxidant vitamin supplementation prevents oxidative stress but does not enhance performance in young football athletes. Nutrition. 2019;63-64:29–35. doi: 10.1016/j.nut.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 87.Peternelj TT, Coombes JS. Antioxidant supplementation during exercise training. Sports Med. 2011;41:1043–1069. doi: 10.2165/11594400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 88.Antonioni A, Fantini C, Dimauro I, Caporossi D. Redox homeostasis in sport: do athletes really need antioxidant support? Res Sports Med. 2019;27:147–165. doi: 10.1080/15438627.2018.1563899. [DOI] [PubMed] [Google Scholar]
- 89.Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Viña J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;87:142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 90.Gomez-Cabrera MC, Domenech E, Viña J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med. 2008;44:126–131. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 91.Merry TL, Ristow M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J Physiol. 2016;594:5135–5147. doi: 10.1113/JP270654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bouviere J, Fortunato RS, Dupuy C, Werneck-de-Castro JP, Carvalho DP, Louzada RA. Exercise-stimulated ROS sensitive signaling pathways in skeletal muscle. Antioxidants (Basel) 2021;10:537. doi: 10.3390/antiox10040537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gomez-Cabrera MC, Salvador-Pascual A, Cabo H, Ferrando B, Viña J. Redox modulation of mitochondriogenesis in exercise. Does antioxidant supplementation blunt the benefits of exercise training? Free Radic Biol Med. 2015;86:37–46. doi: 10.1016/j.freeradbiomed.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 94.Malaguti M, Angeloni C, Garatachea N, Baldini M, Leoncini E, Collado PS, Teti G, Falconi M, Gonzalez-Gallego J, Hrelia S. Sulforaphane treatment protects skeletal muscle against damage induced by exhaustive exercise in rats. J Appl Physiol (1985) 2009;107:1028–1036. doi: 10.1152/japplphysiol.00293.2009. [DOI] [PubMed] [Google Scholar]
- 95.Oh S, Komine S, Warabi E, Akiyama K, Ishii A, Ishige K, Mizokami Y, Kuga K, Horie M, Miwa Y, Iwawaki T, Yamamoto M, Shoda J. Nuclear factor (erythroid derived 2)-like 2 activation increases exercise endurance capacity via redox modulation in skeletal muscles. Sci Rep. 2017;7:12902. doi: 10.1038/s41598-017-12926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun C, Yang C, Xue R, Li S, Zhang T, Pan L, Ma X, Wang L, Li D. Sulforaphane alleviates muscular dystrophy in mdx mice by activation of Nrf2. J Appl Physiol (1985) 2015;118:224–237. doi: 10.1152/japplphysiol.00744.2014. [DOI] [PubMed] [Google Scholar]
- 97.Sun CC, Li SJ, Yang CL, Xue RL, Xi YY, Wang L, Zhao QL, Li DJ. Sulforaphane attenuates muscle inflammation in dystrophin-deficient mdx mice via NF-E2-related factor 2 (Nrf2)-mediated inhibition of NF-κB signaling pathway. J Biol Chem. 2015;290:17784–17795. doi: 10.1074/jbc.M115.655019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98.Li D, Shao R, Wang N, Zhou N, Du K, Shi J, Wang Y, Zhao Z, Ye X, Zhang X, Xu H. Sulforaphane activates a lysosome-dependent transcriptional program to mitigate oxidative stress. Autophagy. 2021;17:872–887. doi: 10.1080/15548627.2020.1739442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Margaritis I, Rousseau AS. Does physical exercise modify antioxidant requirements? Nutr Res Rev. 2008;21:3–12. doi: 10.1017/S0954422408018076. [DOI] [PubMed] [Google Scholar]
- 100.Miko HC, Zillmann N, Ring-Dimitriou S, Dorner TE, Titze S, Bauer R. Effects of physical activity on health. Gesundheitswesen. 2020;82:S184–S195. doi: 10.1055/a-1217-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Poirel E. Psychological benefits of physical activity for optimal mental health. Sante Ment Que. 2017;42:147–164. [PubMed] [Google Scholar]
- 102.Grazioli E, Dimauro I, Mercatelli N, Wang G, Pitsiladis Y, Di Luigi L, Caporossi D. Physical activity in the prevention of human diseases: role of epigenetic modifications. BMC Genomics. 2017;18(Suppl 8):802. doi: 10.1186/s12864-017-4193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheng AJ, Jude B, Lanner JT. Intramuscular mechanisms of overtraining. Redox Biol. 2020;35:101480. doi: 10.1016/j.redox.2020.101480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wan JJ, Qin Z, Wang PY, Sun Y, Liu X. Muscle fatigue: general understanding and treatment. Exp Mol Med. 2017;49:e384. doi: 10.1038/emm.2017.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol. 1998;509:565–575. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Debold EP. Potential molecular mechanisms underlying muscle fatigue mediated by reactive oxygen and nitrogen species. Front Physiol. 2015;6:239. doi: 10.3389/fphys.2015.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cheng AJ, Yamada T, Rassier DE, Andersson DC, Westerblad H, Lanner JT. Reactive oxygen/nitrogen species and contractile function in skeletal muscle during fatigue and recovery. J Physiol. 2016;594:5149–5160. doi: 10.1113/JP270650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kadaja L, Eimre M, Paju K, Roosimaa M, Põdramägi T, Kaasik P, Pehme A, Orlova E, Mudist M, Peet N, Piirsoo A, Seene T, Gellerich FN, Seppet EK. Impaired oxidative phosphorylation in overtrained rat myocardium. Exp Clin Cardiol. 2010;15:e116–127. [PMC free article] [PubMed] [Google Scholar]
- 109.Urhausen A, Gabriel HH, Weiler B, Kindermann W. Ergometric and psychological findings during overtraining: a long-term follow-up study in endurance athletes. Int J Sports Med. 1998;19:114–120. doi: 10.1055/s-2007-971892. [DOI] [PubMed] [Google Scholar]
- 110.Komine S, Miura I, Miyashita N, Oh S, Tokinoya K, Shoda J, Ohmori H. Effect of a sulforaphane supplement on muscle soreness and damage induced by eccentric exercise in young adults: a pilot study. Physiol Rep. 2021;9:e15130. doi: 10.14814/phy2.15130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sahin K, Pala R, Tuzcu M, Ozdemir O, Orhan C, Sahin N, Juturu V. Curcumin prevents muscle damage by regulating NF-κB and Nrf2 pathways and improves performance: an in vivo model. J Inflamm Res. 2016;9:147–154. doi: 10.2147/JIR.S110873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wafi AM, Hong J, Rudebush TL, Yu L, Hackfort B, Wang H, Schultz HD, Zucker IH, Gao L. Curcumin improves exercise performance of mice with coronary artery ligation-induced HFrEF: Nrf2 and antioxidant mechanisms in skeletal muscle. J Appl Physiol (1985) 2019;126:477–486. doi: 10.1152/japplphysiol.00654.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sung MM, Byrne NJ, Robertson IM, Kim TT, Samokhvalov V, Levasseur J, Soltys CL, Fung D, Tyreman N, Denou E, Jones KE, Seubert JM, Schertzer JD, Dyck JR. Resveratrol improves exercise performance and skeletal muscle oxidative capacity in heart failure. Am J Physiol Heart Circ Physiol. 2017;312:H842–H853. doi: 10.1152/ajpheart.00455.2016. [DOI] [PubMed] [Google Scholar]
- 114.Ghanim H, Sia CL, Korzeniewski K, Lohano T, Abuaysheh S, Marumganti A, Chaudhuri A, Dandona P. A resveratrol and polyphenol preparation suppresses oxidative and inflammatory stress response to a high-fat, high-carbohydrate meal. J Clin Endocrinol Metab. 2011;96:1409–1414. doi: 10.1210/jc.2010-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Seyyedebrahimi S, Khodabandehloo H, Nasli Esfahani E, Meshkani R. The effects of resveratrol on markers of oxidative stress in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. Acta Diabetol. 2018;55:341–353. doi: 10.1007/s00592-017-1098-3. [DOI] [PubMed] [Google Scholar]
- 116.Saldanha JF, Leal VO, Rizzetto F, Grimmer GH, Ribeiro-Alves M, Daleprane JB, Carraro-Eduardo JC, Mafra D. Effects of resveratrol supplementation in Nrf2 and NF-κB expressions in nondialyzed chronic kidney disease patients: a randomized, double-blind, placebo-controlled, crossover clinical trial. J Ren Nutr. 2016;26:401–406. doi: 10.1053/j.jrn.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 117.Bowtell J, Kelly V. Fruit-derived polyphenol supplementation for athlete recovery and performance. Sports Med. 2019;49(Suppl 1):3–23. doi: 10.1007/s40279-018-0998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huang Y, Li W, Su ZY, Kong AN. The complexity of the Nrf2 pathway: beyond the antioxidant response. J Nutr Biochem. 2015;26:1401–1413. doi: 10.1016/j.jnutbio.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pala R, Orhan C, Tuzcu M, Sahin N, Ali S, Cinar V, Atalay M, Sahin K. Coenzyme Q10 supplementation modulates NFκB and Nrf2 pathways in exercise training. J Sports Sci Med. 2016;15:196–203. [PMC free article] [PubMed] [Google Scholar]


