Abstract
Objective: To systematically evaluate the clinical effect and reliability of linezolid in the treatment of pulmonary tuberculosis complicated with severe pneumonia. Methods: A comprehensive search was conducted to screen the published literature on linezolid therapy in pulmonary tuberculosis complicated with severe pneumonia in PubMed, Embase, Web of Science, Cochrane Library, and published databases of China National Knowledge Infrastructure. After screening the internal data of the literature, the quality of the literature was assessed uniformly. RevMan5.3 was used for meta-analysis. Results: The search identified 1202 clinical patients in 24 articles. Meta-analysis results revealed that linezolid treatment was associated with better bacterial clearance rate (OR=3.66 [2.41, 5.58], P<0.001) and superior total clinical effective rate (OR=5.80 [3.92, 8.58], P<0.001) in patients. The linezolid treatment resulted in lower levels of serum inflammatory factors TNF-α (WMD=-10.75 [-13.63, -7.87], P<0.001), IL6 (WMD=-10.16 [-13.50, -6.82], P<0.001), and IL8 (WMD=-8.31 [-10.41, -6.21], P<0.001). There was no obvious distinction in the occurrence of adverse reactions between the linezolid group and the control group (OR=1.34 [0.86, 2.08], P=0.19). Conclusion: Linezolid combined with conventional anti-tuberculosis treatment has a better bacterial clearance rate and clinical total effective rate than conventional anti-tuberculosis programs with reliable safety.
Keywords: Linezolid, pulmonary tuberculosis complicated with severe pneumonia, effectiveness, reliability, meta-analysis
Introduction
Pulmonary tuberculosis (PT) is an infectious disease caused by Mycobacterium tuberculosis. PT damages multiple organs of the patients, with the most common in lung [1]. Patients with pulmonary tuberculosis show typical clinical symptoms including cough, low-grade fever, and expectoration. PT patients commonly suffer declined immunity and are prone to infections by other bacteria, resulting in severe pneumonia. The overall difficulty of the treatment and fatality rate is high [2].
Linezolid is an antibiotic of the oxazolidinone family. The specific mechanism of action is the inhibition of protein synthesis in bacteria. It reduces bacterial growth and controls infection. In recent years, many scholars have studied the clinical effectiveness of linezolid in pulmonary tuberculosis complicated with severe pneumonia. The clinical effectiveness and reliability of linezolid in the therapy of pulmonary tuberculosis complicated with severe pneumonia have not been systematically assessed in evidence-based medicine [3]. This study aimed to verify the effectiveness and reliability of linezolid in the treatment of pulmonary tuberculosis complicated with severe pneumonia by a meta-analysis.
Materials and methods
Literature search
A comprehensive search was conducted to screen the published literature on linezolid therapy in pulmonary tuberculosis complicated with severe pneumonia. The literature searched included PubMed, Embase, Web of Science, Cochrane Library, China Biomedical Documentation, and CNKI, up to March 2022. The search strategy was joint theme keywords and free words: (((pulmonary tuberculosis) OR (pulmonary tuberculosis complicated with severe pneumonia)) OR (pulmonary tuberculosis)) AND (linezolid). The search languages were Chinese and English.
Inclusion and exclusion criteria
Inclusion criteria
RCT studies that investigated the patients with clinically diagnosed pulmonary tuberculosis and severe pneumonia, provided relevant clinical data in terms of overall clinical effectiveness, bacteria clearance rate, inflammatory factors, and adverse reactions. The therapy regimen of the experimental group used linezolid.
Exclusion criteria
Repeated literature; reviews; conference reports, books, case reports, or letters; failure to extract data in a timely manner or insufficient data.
Literature screening and data extraction
For the retrieved literature, Wanfeng Wu and Li Li initially read the titles and abstracts. They preliminarily screened out 366 articles in accordance with the established relevant inclusion and exclusion criteria. After reading the full text, the researchers screened out 24 articles that were included in the meta-analysis. Ambiguities or disagreements were solved by consulting a third person. Li Li and Shaojun Duan were responsible for data extraction and cross-checking. A third researcher reviewed the results. The extracted data from the included studies included publication year, author, country, patient sample size, study site, intervention measures, and outcome variables.
Literature quality assessment
Shaojun Duan and Yunyun Wang analyzed the quality of the included studies based on the Newcastle-Ottawa Scale (NOS). Articles with a score ≥6 were included in this study.
Statistical analysis
RevMan was used for the meta-analysis. For enumeration data, odds ratio (OR) and 95% confidence intervals were used to describe the effect of linezolid on various observing indicators. For the measured data, weighted mean distinction (WMD) and 95% confidence interval were used. I2 test and P value were used to analyze the heterogeneity among multiple studies. If I2>50 or P<0.1, it implied that the included studies had an obvious heterogeneity. A random effect model was selected to analyze the results. If an obvious heterogeneity was not concluded, a fixed effect model was used. The funnel plot was used for the evaluation of publication bias with a test level of α=0.05.
Results
Literature retrieval and screening
A total of 576 studies were identified after a literature search. There were 148 studies removed for duplicate literature. After the check of the title and abstract, 366 articles met the inclusion criteria. There were 217 articles unavailable for clinical data. These included 149 reviews, case reports, conference abstracts, or monographs. We reviewed the full text of the remaining 62 articles. These included 15 articles that were excluded for no study endpoint, 18 articles that were excluded due to insufficient data, and 5 articles that were excluded for NOS scores below 6. After these exclusions, 24 articles involving 1202 clinical patients were eligible for this study. The flow chart of the literature screening process was shown in Figure 1. The basic characteristics of the included literature was shown in Table 1.
Figure 1.

Flow chart of literature retrieval.
Table 1.
General characteristics of included publications
| Author | Study Site | Sample Size | Control Group | Test Group | Observation | Adverse | NOS |
|---|---|---|---|---|---|---|---|
| Zhang Qiuhua [7] | Huizhou, Guangdong | 50/50 | Isoniazid/Rifampicin/Ethambutol | Intravenous linezolid, q12h, 14d | C | 4/1 | 7 |
| Huang Yiming [8] | Wuzhou, Guangxi | 25/25 | Isoniazid/Rifampicin/Ethambutol | Intravenous linezolid, q12h, 14d | C; B | 6/3 | 8 |
| Shi haiyan [9] | Jiamusi, Heilongjiang | 42/42 | Isoniazid/Rifampicin/Pyrazinamide | Intravenous linezolid, q12h, 14d | A; B; D | -- | 6 |
| Wen Yunjie [10] | Cixi, Zhejiang | 30/30 | Isoniazid/Rifampicin/Ethambutol | Intravenous linezolid, q12h, 14d | A; B; C | 5/2 | 6 |
| Pan Lei [5] | Hangzhou, Zhejiang | 49/49 | Isoniazid/Rifapentine/Ethambutol | Intravenous linezolid, q12h, 28d | A; B; C; D | 6/5 | 8 |
| Zhu Guochuan [11] | Taiyuan, Shanxi | 37/37 | Isoniazid | Intravenous linezolid, q12h, 14d | A | 2/3 | 7 |
| Wen Ming [12] | Changsha, Hunan | 34/16 | Isoniazid/Rifapentine/Ethambutol | Intravenous linezolid, q12h, 28d | A | 2/1 | 7 |
| Guan Zhiwei [13] | Lingbao, Henan | 32/32 | Isoniazid/Rifapentine/Ethambutol | Intravenous linezolid, q24h, 28d | A; B | 3/2 | 6 |
| Jiang Yezhou [5] | Changsha, Hunan | 31/31 | Isoniazid/Rifampicin/Pyrazinamide | Intravenous linezolid, q12h, 14d | A; B; D | 4/3 | 7 |
| Li Zhuo [14] | Jinzhou, Liaoning | 54/54 | Isoniazid/Rifampicin/Ethambutol | Intravenous linezolid, q24h, 14d | A; B | 5/3 | 8 |
| Tsering Zhuoga [15] | Tibet | 33/33 | Isoniazid/Rifampicin/Ethambutol | Intravenous linezolid, q8h, 14d | A | 2/3 | 7 |
| Yang Nan [6] | Jinzhou, Liaoning | 80/80 | Isoniazid/Rifampicin/Ethambutol | Intravenous linezolid, q12h, 28d | A; B; C | 2/1 | 7 |
| Gao Yuan [16] | Xinxiang, Henan | 46/46 | Isoniazid/Rifapentine/Ethambutol | Intravenous linezolid, q12h, 28d | A; B | 5/3 | 8 |
| Shi Yunfang [17] | Jincheng, Shanxi | 27/27 | Isoniazid/Rifapentine/Ethambutol | Intravenous linezolid, q12h, 14d | A; B; C | 1/1 | 6 |
| Li Dong [18] | Yili, Xinjiang | 40/40 | Isoniazid/Rifampicin/Ethambutol | Intravenous linezolid, q12h, 28d | A; B; C | -- | 6 |
| Anger [19] | USA | 8/8 | Isoniazid/Rifampicin/Pyrazinamide | Intravenous linezolid, q12h, 28d | 1/1 | 7 | |
| De Lorenzo [20] | Italy | 6/6 | Isoniazid/Rifampicin/Pyrazinamide | Intravenous linezolid, q12h, 28d | -- | 6 | |
| Fortun [21] | Spain | 5/5 | Isoniazid/Rifampicin/Ethambutol | Intravenous linezolid, q12h, 28d | -- | 6 | |
| Migliori [22] | Germany | 5/6 | Isoniazid/Rifampicin/Ethambutol | Intravenous linezolid, q12h, 28d | -- | 7 | |
| Nam [23] | South Korea | 22/22 | Isoniazid/Rifampicin/Ethambutol | Intravenous linezolid, Q24h, 14d | 1/2 | 8 | |
| Schecter [24] | USA | 15/15 | Isoniazid/Rifampicin/Ethambutol | Intravenous linezolid, Q24h, 14d | 1/2 | 7 | |
| Singla [25] | India | 15/14 | Isoniazid/Rifampicin/Pyrazinamide | Intravenous linezolid, q12h, 28d | 1/2 | 8 | |
| Udwadia [26] | India | 9/9 | Isoniazid | Intravenous linezolid, Q24h, 14d | -- | 7 | |
| Villar [27] | Portugal | 8/8 | Isoniazid/Rifampicin/Pyrazinamide | Intravenous linezolid, q12h, 28d | -- | 7 |
Note: Observation index: A, total clinical effective rate; B, serum inflammatory factors; C, bacterial clearance rate; D, immune function protein adverse reactions.
Bacterial clearance rate
Seven studies analyzed the effect of linezolid on bacterial clearance. The meta-analysis found that there was no obvious heterogeneity among the included studies (I2=16%, P=0.31). A fixed-effects model was used for analysis. The results revealed that the bacterial clearance rate in the linezolid experimental group was obviously higher than that in the control group (OR=3.66 [2.41, 5.58], P<0.001) (Figure 2).
Figure 2.
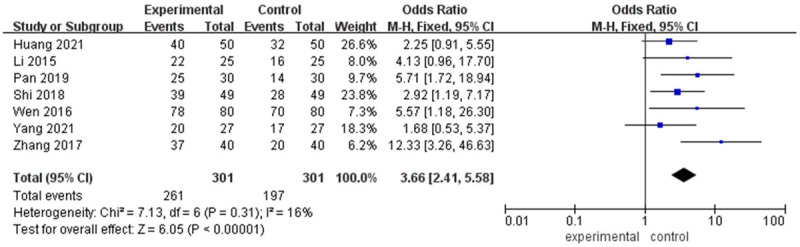
Meta-analysis of the effect of linezolid on bacterial clearance rate in tuberculosis patients with severe pneumonia.
Serum inflammatory factors
Ten studies analyzed the effect of linezolid on the serum levels of TNF-α. There was no obvious heterogeneity among the included studies (I2=0%, P=0.52). A fixed-effect model was selected for data analysis. The results showed that the bacterial clearance rate in the linezolid experimental group was obviously higher than that in the control group (WMD=-7.84 [-10.41, -5.27], P<0.001) (Figure 3).
Figure 3.

Meta-analysis of the effect of linezolid on serum TNF-α level in tuberculosis patients with severe pneumonia.
Six studies investigated the effect of linezolid on the serum level of IL6. There was an obvious heterogeneity among the included studies (I2=98%, P<0.01). A random effect model was carried out for data analysis. The results revealed that serum IL6 in the linezolid experimental group was significantly lower than that in the control group (WMD=-8.10 [-11.57, -4.63], P<0.001) (Figure 4).
Figure 4.

Meta-analysis of the effect of linezolid on serum IL6 level in tuberculosis patients with severe pneumonia.
Six studies examined the effect of linezolid on serum IL8. There was an obvious heterogeneity among the included studies (I2=94%, P<0.01). A random effect model was selected for the analysis. The results revealed that serum IL8 in the linezolid experimental group was obviously lower than that in the control group (WMD=-8.08 [-10.68, -5.49], P<0.001) (Figure 5).
Figure 5.

Meta-analysis of the effect of linezolid on serum IL8 level in tuberculosis patients with severe pneumonia.
Six studies analyzed the effect of linezolid on PCT levels in patients. There was no obvious heterogeneity among the included studies (I2=0%, P=0.97). A fixed effect model was used for analysis. The results revealed that the PCT level of the linezolid experimental group was obviously lower than that in the control group (WMD=-108.17 [-113.07, -103.27], P<0.001) (Figure 6).
Figure 6.

Meta-analysis of the effect of linezolid on the level of PCT in tuberculosis patients with severe pneumonia.
Six studies analyzed the effect of linezolid on white blood cell counts in patients. There was no obvious heterogeneity (I2=0%, P=0.53). A fixed effect model was used for analysis. The results revealed that the number of white blood cells (108/L) in the linezolid experimental group was obviously higher than that in the control group (WMD=62.09 [60.42, 65.38], P<0.001) (Figure 7).
Figure 7.

Meta-analysis of the effect of linezolid on white blood cell count in tuberculosis patients with severe pneumonia.
Total clinical effectiveness
Thirteen studies assessed the therapy effect of linezolid on patients with pulmonary tuberculosis and severe pneumonia by calculating the total clinical response rate. There was no obvious heterogeneity among the included studies (I2=0%, P=0.88). A fixed effect model was applied. The meta analysis results revealed that the total effective rate of patients in the linezolid group was obviously higher than that in the control group (OR=5.80 [3.92, 8.58], P<0.001) (Figure 8).
Figure 8.

Meta-analysis of the total effective rate of patients in the linezolid trial group and the control group.
Adverse reactions
A total of 13 studies recorded the adverse reaction rates during the treatment of pulmonary tuberculosis complicated with severe pneumonia. The meta-analysis found that there was no obvious heterogeneity among the included studies (I2=0%, P=0.99). A fixed effect model was applied. The results revealed that there was no obvious distinction in the incidence of adverse reactions between the linezolid experimental and the control group (OR=1.53 [0.95, 2.47], P=0.08) (Figure 9).
Figure 9.

Meta-analysis of adverse reaction rates in linezolid trial group and control group.
Sensitivity and heterogeneity analysis
A perceptual analysis was implemented by Stata. The data of each study included in the analysis were eliminated sequentially. Their influence on the combined total effect results was observed. The analysis revealed that after excluding each of the studies, the combined effect size did not change significantly. This suggested that the results of this study were stable. The distinctions among the included literature were small, and the results were more reliable (Figure 10).
Figure 10.

Sensitivity and heterogeneity analysis of included studies.
Publication bias
A funnel plot analysis was performed to analyze the publication bias of the included studies. It was found that the scattered dots in the figure were symmetrically distributed, indicating that the included research had no obvious publication bias (Figure 11).
Figure 11.

Publication bias was analyzed by funnel plot.
Discussion
Pulmonary tuberculosis is a slow-onset disease caused by infection of Mycobacterium tuberculosis. Patients experience many complications. For patients with severe pulmonary infection in clinical practice, external ventilation and circulation equipment are needed to support the overall clinical diagnosis and therapy. Antibiotics are the mainstay in clinical treatment of pulmonary tuberculosis and severe pneumonia. Based on the 50S ribosomal subunit of bacterial, linezolid hinders the synthesis of related proteins in bacteria, achieving a broad-spectrum antibacterial effect [4]. Pan et al. [5] conducted a relevant study on 98 patients with severe pulmonary tuberculosis in clinical practice. The study showed that combining essential tuberculosis therapy with linezolid improved the primary treatment efficacy and serum inflammatory factors. and It enhanced the patient’s immunity with reliable safety. Yang et al. [6] analyzed the clinical data of 160 patients with severe pneumonia and suggested that concurrent linezolid treatment decreased the level of related serum inflammatory factors and improved the overall effective rate without increasing the adverse reactions. In this study, a comprehensive meta-analysis was taken to quantitatively and objectively evaluate the effectiveness and reliability of linezolid in patients with pulmonary tuberculosis complicated with severe pneumonia. This study provided reliable evidence-based medical support for clinical practice.
This study found that the overall effective rate of linezolid combined with conventional therapy was better than that of the conventional therapy. In terms of inflammation, the serum levels of TNF-α, IL6, and IL8 in patients taking linezolid combined with conventional therapy were lower than those in the conventional therapy group. The incidence of adverse reactions showed no apparent differences between the linezolid group and others, suggesting that linezolid was safe for patients with tuberculosis complicated with severe pneumonia.
This study had certain limitations: the detection methods of serum inflammatory factors used in various studies were different. This resulted in specific heterogeneity in the outcomes. Some of the data were derived from the calculation of the obtained data. This was insufficient. The overall research in this paper deviated from the original data. There was not much available data.
The preliminary results of this study showed that linezolid combined with the conventional anti-tuberculosis therapy mode is better than the conventional therapy in terms of bacterial clearance rate and total clinical effectiveness.
Disclosure of conflict of interest
None.
References
- 1.Kang W, Yu J, Du J, Yang S, Chen H, Liu J, Ma J, Li M, Qin J, Shu W, Zong P, Zhang Y, Dong Y, Yang Z, Mei Z, Deng Q, Wang P, Han W, Wu M, Chen L, Zhao X, Tan L, Li F, Zheng C, Liu H, Li X, A E, Du Y, Liu F, Cui W, Wang Q, Chen X, Han J, Xie Q, Feng Y, Liu W, Tang P, Zhang J, Zheng J, Chen D, Yao X, Ren T, Li Y, Li Y, Wu L, Song Q, Yang M, Zhang J, Liu Y, Guo S, Yan K, Shen X, Lei D, Zhang Y, Yan X, Li L, Tang S. The epidemiology of extrapulmonary tuberculosis in China: a large-scale multi-center observational study. PLoS One. 2020;15:e0237753. doi: 10.1371/journal.pone.0237753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oh S, Trifonov L, Yadav VD, Barry CE 3rd, Boshoff HI. Tuberculosis drug discovery: a decade of hit assessment for defined targets. Front Cell Infect Microbiol. 2021;11:611304. doi: 10.3389/fcimb.2021.611304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown AN, Drusano GL, Adams JR, Rodriquez JL, Jambunathan K, Baluya DL, Brown DL, Kwara A, Mirsalis JC, Hafner R, Louie A. Preclinical evaluations to identify optimal linezolid regimens for tuberculosis therapy. mBio. 2015;6:e01741-15. doi: 10.1128/mBio.01741-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashemian SMR, Farhadi T, Ganjparvar M. Linezolid: a review of its properties, function, and use in critical care. Drug Des Devel Ther. 2018;12:1759–1767. doi: 10.2147/DDDT.S164515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conradie F, Diacon AH, Ngubane N, Howell P, Everitt D, Crook AM, Mendel CM, Egizi E, Moreira J, Timm J, McHugh TD, Wills GH, Bateson A, Hunt R, Van Niekerk C, Li M, Olugbosi M, Spigelman M. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med. 2020;382:893–902. doi: 10.1056/NEJMoa1901814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeager SD, Oliver JE, Shorman MA, Wright LR, Veve MP. Comparison of linezolid step-down therapy to standard parenteral therapy in methicillin-resistant staphylococcus aureus bloodstream infections. Int J Antimicrob Agents. 2021;57:106329. doi: 10.1016/j.ijantimicag.2021.106329. [DOI] [PubMed] [Google Scholar]
- 7.Zhang QH. Effect of linezolid on patients with pulmonary tuberculosis complicated with severe pneumonia. Chin J Urban Rural Enterprise Hyg. 2017;32:103–104. [Google Scholar]
- 8.Huang YM. Effect and safety analysis of linezolid adjuvant therapy on inflammatory status in patients with pulmonary tuberculosis complicated with severe pneumonia. Mod Med Health Res. 2021;5:54–56. [Google Scholar]
- 9.Guan J, Fu R, Ruan EB, Liang Y, Qu W, Wang GJ, Wang XM, Liu H, Wu YH, Song J, Wang HQ, Xing LM, Shao ZH. Clinical analysis of 102 blood disease patients with gram positive cocci infection treated with linezolid. Zhonghua Xue Ye Xue Za Zhi. 2010;31:527–530. [PubMed] [Google Scholar]
- 10.Dheda K, Limberis JD, Pietersen E, Phelan J, Esmail A, Lesosky M, Fennelly KP, Te Riele J, Mastrapa B, Streicher EM, Dolby T, Abdallah AM, Ben-Rached F, Simpson J, Smith L, Gumbo T, van Helden P, Sirgel FA, McNerney R, Theron G, Pain A, Clark TG, Warren RM. Outcomes, infectiousness, and transmission dynamics of patients with extensively drug-resistant tuberculosis and home-discharged patients with programmatically incurable tuberculosis: a prospective cohort study. Lancet Respir Med. 2017;5:269–281. doi: 10.1016/S2213-2600(16)30433-7. [DOI] [PubMed] [Google Scholar]
- 11.Grudinina SA, Zubkov MM, Krotova LA, Kurdiukova IuP, Kutsenko MA, Marinin VF, Sidorenko SV, Sinpal’nikov AI, Solomatin AS, Sterkhova GV, Fesenko OV, Fomina IG, Chuchalin AG. Comparison of linezolid and vancomycin in nosocomial pneumonia: results of the multicenter double-blind study. Antibiot Khimioter. 2002;47:12–17. [PubMed] [Google Scholar]
- 12.Wang Y, Zhang HJ, Song AJ. Evaluation on efficacy and safety of vancomycin and linezolid for treatment of pulmonaryinfections. Chin J Nosocomiol. 2015;25:2734–2736. [Google Scholar]
- 13.Lee M, Lee J, Carroll MW, Choi H, Min S, Song T, Via LE, Goldfeder LC, Kang E, Jin B, Park H, Kwak H, Kim H, Jeon HS, Jeong I, Joh JS, Chen RY, Olivier KN, Shaw PA, Follmann D, Song SD, Lee JK, Lee D, Kim CT, Dartois V, Park SK, Cho SN, Barry CE 3rd. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med. 2012;367:1508–1518. doi: 10.1056/NEJMoa1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Didilescu C, Craiova UM. Present and future in the use of anti-tubercular drugs. Pneumologia. 2011;60:198–201. [PubMed] [Google Scholar]
- 15.Agyeman AA, Ofori-Asenso R. Efficacy and safety profile of linezolid in the treatment of multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob. 2016;15:41. doi: 10.1186/s12941-016-0156-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang S, Yao L, Hao X, Zhang X, Liu G, Liu X, Wu M, Zen L, Sun H, Liu Y, Gu J, Lin F, Wang X, Zhang Z. Efficacy, safety and tolerability of linezolid for the treatment of XDR-TB: a study in China. Eur Respir J. 2015;45:161–170. doi: 10.1183/09031936.00035114. [DOI] [PubMed] [Google Scholar]
- 17.Luchetti G, Roncaioli JL, Chavez RA, Schubert AF, Kofoed EM, Reja R, Cheung TK, Liang Y, Webster JD, Lehoux I, Skippington E, Reeder J, Haley B, Tan MW, Rose CM, Newton K, Kayagaki N, Vance RE, Dixit VM. Shigella ubiquitin ligase IpaH7.8 targets gasdermin D for degradation to prevent pyroptosis and enable infection. Cell Host Microbe. 2021;29:1521–1530. e1510. doi: 10.1016/j.chom.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pang Y, Zong Z, Huo F, Jing W, Ma Y, Dong L, Li Y, Zhao L, Fu Y, Huang H. In vitro drug susceptibility of bedaquiline, delamanid, linezolid, clofazimine, moxifloxacin, and gatifloxacin against extensively drug-resistant tuberculosis in Beijing, China. Antimicrob Agents Chemother. 2017;61:e0900–17. doi: 10.1128/AAC.00900-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anger HA, Dworkin F, Sharma S, Munsiff SS, Nilsen DM, Ahuja SD. Linezolid use for treatment of multidrug-resistant and extensively drug-resistant tuberculosis, New York City, 2000-06. J Antimicrob Chemother. 2010;65:775–783. doi: 10.1093/jac/dkq017. [DOI] [PubMed] [Google Scholar]
- 20.De Lorenzo S, Centis R, D’Ambrosio L, Sotgiu G, Migliori GB. On linezolid efficacy and tolerability. Eur Respir J. 2012;39:770–772. doi: 10.1183/09031936.00116011. [DOI] [PubMed] [Google Scholar]
- 21.Fortún J, Martín-Dávila P, Navas E, Pérez-Elías MJ, Cobo J, Tato M, De la Pedrosa EG, Gómez-Mampaso E, Moreno S. Linezolid for the treatment of multidrug-resistant tuberculosis. J Antimicrob Chemother. 2005;56:180–185. doi: 10.1093/jac/dki148. [DOI] [PubMed] [Google Scholar]
- 22.Migliori GB, Eker B, Richardson MD, Sotgiu G, Zellweger JP, Skrahina A, Ortmann J, Girardi E, Hoffmann H, Besozzi G, Bevilacqua N, Kirsten D, Centis R, Lange C. A retrospective TBNET assessment of linezolid safety, tolerability and efficacy in multidrug-resistant tuberculosis. Eur Respir J. 2009;34:387–393. doi: 10.1183/09031936.00009509. [DOI] [PubMed] [Google Scholar]
- 23.Nam HS, Koh WJ, Kwon OJ, Cho SN, Shim TS. Daily half-dose linezolid for the treatment of intractable multidrug-resistant tuberculosis. Int J Antimicrob Agents. 2009;33:92–93. doi: 10.1016/j.ijantimicag.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Schecter GF, Scott C, True L, Raftery A, Flood J, Mase S. Linezolid in the treatment of multidrug-resistant tuberculosis. Clin Infect Dis. 2010;50:49–55. doi: 10.1086/648675. [DOI] [PubMed] [Google Scholar]
- 25.Singla R, Caminero JA, Jaiswal A, Singla N, Gupta S, Bali RK, Behera D. Linezolid: an effective, safe and cheap drug for patients failing multidrug-resistant tuberculosis treatment in India. Eur Respir J. 2012;39:956–962. doi: 10.1183/09031936.00076811. [DOI] [PubMed] [Google Scholar]
- 26.Udwadia ZF, Sen T, Moharil G. Assessment of linezolid efficacy and safety in MDR- and XDR-TB: an Indian perspective. Eur Respir J. 2010;35:936–938. doi: 10.1183/09031936.00132009. [DOI] [PubMed] [Google Scholar]
- 27.Villar M, Sotgiu G, D’Ambrosio L, Raymundo E, Fernandes L, Barbedo J, Diogo N, Lange C, Centis R, Migliori GB. Linezolid safety, tolerability and efficacy to treat multidrug- and extensively drug-resistant tuberculosis. Eur Respir J. 2011;38:730–733. doi: 10.1183/09031936.00195210. [DOI] [PubMed] [Google Scholar]


