Abstract
This study has demonstrated the extreme sensitivity of Chlamydia trachomatis growing in McCoy cells to small changes in external amino acid supply. In the absence of cycloheximide, a decrease in the amino acid concentration of medium to 75% of control values was sufficient to induce the growth of enlarged chlamydial forms of reduced infectivity. Morphology became more distorted and the yield of infectious particles from inclusions declined as medium amino acid levels were further reduced. These events correlated with a general decline in intracellular amino acids, as measured by high-performance liquid chromatography, suggesting that chlamydiae require a minimum concentration of each amino acid for normal development. Cycloheximide enhanced the production of normal organisms and increased infectivity yield in media, suggesting that the drug increased the available pool of amino acids. This was supported by intracellular amino acid analyses. Aberrant forms with reduced infectivity were also induced during supply of infected cell cultures with medium containing blood plasma amino acid concentrations, supporting the proposal that nutrient levels in vivo could promote abnormal chlamydial development. Markedly abnormal forms were also observed during glucose deprivation, providing further evidence that aberrant development is a general stress-related response.
The accepted intracellular life cycle of Chlamydia involves alternation between two morphologically distinct forms, the infectious elementary body (EB) and the metabolically active dividing reticulate body (RB). During the developmental cycle, marked ultrastructural and metabolic differences between the two forms are apparent. Size is altered and changes in membrane permeability and chromatin organization occur, probably brought about by expression of developmentally regulated genes, including those for the cysteine-rich proteins in the EB membrane (21, 36, 40) and the histone-like DNA-binding proteins (10, 18, 37).
An important feature of chlamydial infection in vivo is a state in which chronic tissue inflammation is apparent but organisms are undetectable by culture. The increasing use of antigen and nucleic acid probes has, however, facilitated detection of the presence of chlamydiae and/or chlamydial antigens (9, 23, 41). Such noncultivable organisms could provide a constant source of antigen and thus mediate immunopathological damage (34).
The explanation of these phenomena is likely to be based on the parasitic nature of chlamydiae. The organisms obtain high-energy and biosynthetic metabolites, such as ATP and amino acids (AAs), from the host cell. Earlier studies in vitro have demonstrated that the chlamydial developmental cycle is grossly disrupted by removal of all or single AAs from growth medium (1, 2, 11, 12). In particular, abnormally large chlamydial organisms were observed inside small inclusions; these had negligible infectivity (12). Similar aberrant forms have been seen during treatment of cultured cells with gamma interferon (IFN-γ) (5, 7, 8, 43) or tumor necrosis factor alpha (44). Such forms have been suggested to account for the presence of noncultivable chlamydiae associated with chronic disease, supposedly induced via IFN-γ-dependent activation of host cell indoleamine 2,3-dioxygenase and subsequent degradation of intracellular tryptophan (5, 12).
Morphological evidence suggests that aberrant chlamydiae can exist in vivo, with reports of unusual chlamydiae in the synovia of patients with reactive arthritis (35, 42), and in animal models of infection (24, 38, 45). While such forms may be cytokine induced, it has also been proposed that a restriction of available metabolites in vivo could be sufficient to induce production of noninfectious chlamydiae (12, 20, 33). Chlamydiae are routinely cultured in defined media containing cycloheximide (CH) to inhibit host protein synthesis. We have previously reported the adverse effect on chlamydial development of reduction in medium AA levels, in the presence of CH, where inclusions growing in 10% medium AAs and below were aberrant and of low infectivity (12). Here, we have investigated the sensitivity of the chlamydial cycle to reduction in AA supply, using a culture system without CH to allow host cell competition for AAs. We also present data on the host cell AA pools associated with normal and abnormal growth. One way CH is thought to aid chlamydial growth is by increasing the available pool of AAs, normally sequestered by the host cell (20). We have examined this proposal by measuring AA pools in infected cells in the presence or absence of CH.
Concentrations of AAs measured in blood plasma have been reported to be often much lower than the levels in medium used to grow chlamydiae (4, 15, 47). We have thus examined a perhaps more relevant system, by assessing the development of chlamydiae grown in medium with AAs kept at the concentrations found in blood plasma. Finally, much work has focused in recent years on the fact that a variety of bacteria exhibit common morphological, physiological, and biochemical responses to nutrient starvation (carbohydrates, AAs, or phosphate) and other environmental stresses (3, 28–30). Since abnormal chlamydiae have also been observed during in vitro treatment with certain drugs (31, 46) or following heat shock (6, 26), we have further tested the possibility that aberrant chlamydial growth is a general stress response by examining the effect of glucose deprivation on development.
MATERIALS AND METHODS
Growth and purification of chlamydiae.
Chlamydia trachomatis strains L2/434/Bu (strain 434) and E/DK-20/ON (strain DK20) were routinely propagated in CH (Sigma Chemical Co., Dorset, United Kingdom)-treated McCoy cell monolayers (1). Here, monolayers were unirradiated, established in 80-cm2 tissue culture flasks (Nunc, Paisley, United Kingdom), and infected by centrifugation (1,580 × g, 30 min, 37°C).
Cell culture, infection procedure, and media used postinoculation (p.i.).
McCoy cells (ATCC CRL-1696) were obtained from ICN Biomedicals Ltd. (Thame, Oxfordshire, United Kingdom) and were regularly checked for mycoplasmal contamination (detection kit from Boehringer Mannheim Biochemica, Lewes, Sussex, United Kingdom). Cells were routinely cultured at 37°C in Eagle minimal essential medium (MEM) with Earle's salts, to which was added 2 mM l-glutamine (Gibco, Paisley, United Kingdom), 5% (vol/vol) fetal bovine serum (ICN), and streptomycin sulfate (Evans Medical Ltd., Horsham, United Kingdom) at 100 μg ml−1. This medium was considered complete medium (CMEM). For infection studies, monolayers were established on glass coverslips in 24-well trays (Nunc) (2 × 105 cells per well). After overnight incubation, cells were inoculated with organism suspensions (0.3 ml) by centrifugation as described above. The inoculation medium consisted of Earle's salts minus bicarbonate (Gibco), with 25 mM HEPES (Sigma). Supernatants were removed and 0.5 ml of the appropriate medium was added (see below) before monolayers were incubated at 37°C in 5% CO2–air to allow inclusion development.
In AA deprivation studies, media were composed of Earle's salts supplemented with vitamins to concentrations in MEM and the 13 MEM AAs (Sigma tissue-culture grade [11]) at concentrations of 100, 75, 40, 25, and 0%. Glucose-deficient medium was CMEM without glucose but including MEM nonessential AAs (a 100 μM concentration of each; Gibco). Blood plasma medium contained all 20 AAs at the concentrations found in blood plasma, calculated as mean values from data taken from several sources (4, 15, 47). Hence, AA concentrations (micromolar) were as follows: Arg, 200; Cys, 60; Gln, 400; His, 50; Ile, 100; Leu, 150; Lys, 400; Met, 33; Phe, 67; Thr, 250; Trp, 20; Tyr, 75; Val, 175; Ala, 400; Asn, 30; Asp, 50; Glu, 400; Gly, 400; Pro, 300; and Ser, 400. Data for rat plasma were used, since McCoy cells are mouse fibroblasts; however, data for human plasma AA concentrations were noted to be comparable. Monolayers were incubated in plasma medium for 4 h prior to inoculation with chlamydiae. After infection, the medium was changed every 6 h up to 40 h p.i. in order to maintain a constant AA supply. A preliminary experiment demonstrated the relatively stable concentration of most intracellular AAs under these conditions (data not shown), except that Asp, Cys, Gln, and Trp concentrations declined by at least 50%. These AAs may have been utilized more rapidly than the others, or they may have taken longer to equilibrate with extracellular medium at the start of incubations.
Media in nutrient studies contained streptomycin (100 μg ml−1) and 5% (vol/vol) dialyzed fetal bovine serum and were supplemented with CH (1 μg ml−1) in some experiments.
Morphology and infectivity yield of chlamydial inclusions.
Control cell monolayers (those subsequently incubated in CMEM) were always inoculated with organisms such that less than 30% of cells were infected, to avoid multiple infection of cells. Inclusion morphology and infectivity yield were assessed 40 h p.i. Morphology was examined by fluorescence microscopy using acridine orange stain, as previously described (12), and in most experiments by electron microscopy.
Infectivity yields of organisms (per inclusion) were determined by sonication of infected monolayers (70 W, 10 s, 0°C) and determination of the infectivity of the resulting suspension, as inclusion forming units (IFU), by titration on separate monolayers.
Intracellular AA analysis.
Monolayers were established in 80-cm2 flasks and inoculated with organisms by centrifugation, as described above, so that about 80% of cells were infected. Intracellular AA pools were measured 20 h p.i., just before the main burst of chlamydial metabolic activity. On removal of medium from each flask, cells were rapidly chilled on ice and rinsed twice with 10 ml of ice-cold phosphate-buffered saline, so as to avoid depleting intracellular AA pools (19). Monolayers were extracted in ice-cold 5% trichloroacetic acid (2 ml) for 2 h at 4°C. Protein and cell debris was pelleted using a bench top microcentrifuge (20,000 × g, 10 min, 4°C), and soluble samples were taken for AA analysis. This was performed by ion-exchange high-performance liquid chromatography by J. E. Fox and M. Singh of Alta Bioscience in the School of Biochemistry at the University of Birmingham (22). AA detection was done colorimetrically using ninhydrin.
Tryptophan was assayed separately by fluorimetry (13), as it was not always detected by high-performance liquid chromatography. Soluble AA samples were made up to a total volume of 480 μl with 5% trichloroacetic acid in 2-ml screw-cap cryogenic vials (Corning, Sunderland, United Kingdom). Paraformaldehyde (50 μl of 1.8% solution; BDH Laboratory Supplies, Poole, United Kingdom) and 6 mM FeCl3 (25 μl; Fisons, Loughborough, United Kingdom) were added, with rapid vortex mixing. The vials were heated for 60 min at 100°C, resulting in production of the fluorophore norharman. Fluorescence was measured in a Perkin-Elmer 203 fluorimeter with excitation and emission wavelengths of 362 and 452 nm, respectively.
The intracellular concentration of AAs was expressed as nanomoles per microliter of cell water, calculated using a mean value for intracellular water volume of McCoy cells of 4.83 μl/mg of protein. This value was estimated from the steady-state distribution of the nonmetabolizable hexose 3-O-[methyl-14C]-d-glucose, with subsequent inhibition of its efflux with phloretin (Sigma), as described by Kletzien et al. (27) (data not shown). Extracted cell monolayers were dissolved in 0.1 M NaOH for determination of protein content, using bicinchoninic acid (Sigma).
Statistical analyses.
Infectivity data are expressed as means ± standard deviations (SD) for at least 10 fields of view in triplicate samples. Preliminary AA analyses were performed in triplicate, and SD were determined to be generally less than 20% of the means. Data were subsequently expressed as means ± SD for triplicate or duplicate samples. Statistical significance was analyzed by one-way analysis of variance, and the t test was used to make comparisons between individual treatments or comparisons of only two means.
RESULTS
Sensitivity of chlamydial growth to AA supply.
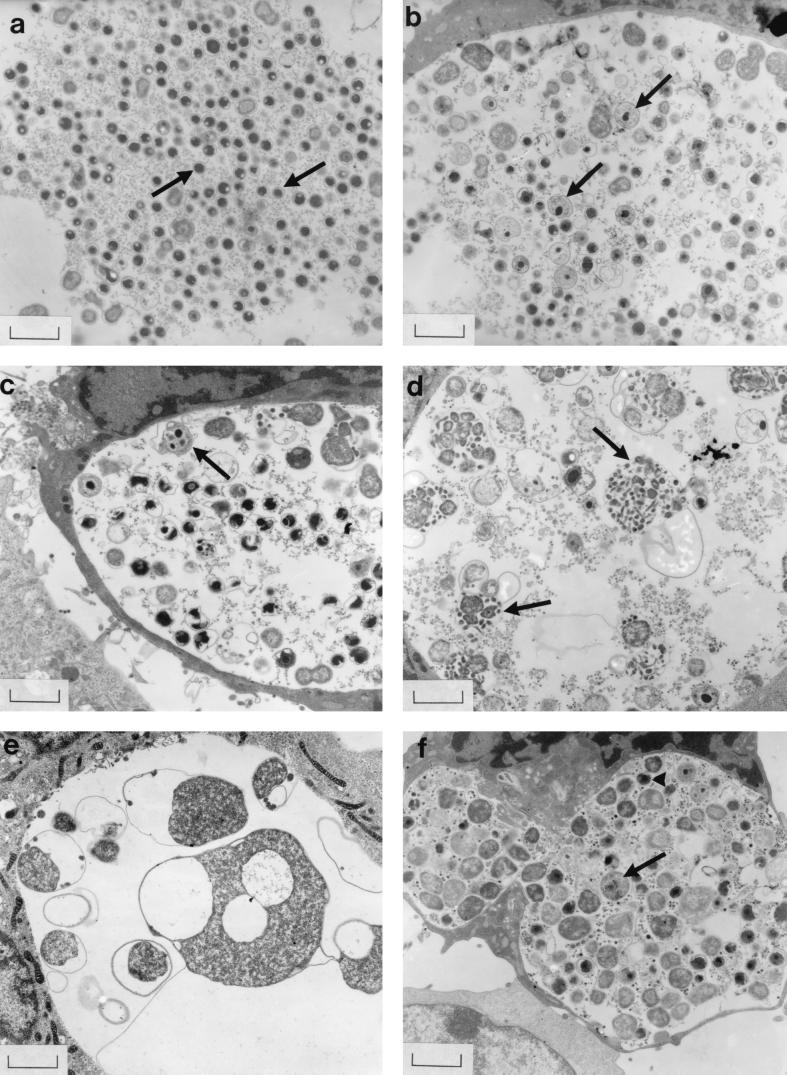
In the absence of CH, preliminary experiments demonstrated that even small changes in the concentration of AAs supplied in medium could result in major changes in chlamydial morphology and infectivity. Media having AA concentrations of 100, 75, 40, 25, or 0% of the AA concentrations in CMEM were selected for detailed study. In McCoy cell monolayers inoculated with C. trachomatis strain 434, the inclusions developing in CMEM (100% AAs) contained mainly normal EBs, as confirmed by DNA staining and by electron microscopy (Fig. 1a). However, a decrease in the concentration of AAs to 75% was associated with the appearance of large RB-like forms, staining for DNA rather than RNA, with some forms containing small particles (Fig. 1b) and with a significant reduction (P < 0.05) in yield of infectious progeny (Table 1). Organisms within inclusions became progressively larger and more distorted (Fig. 1c to e), and the yield of infectious organisms from inclusions steadily declined (Table 1) as medium AAs decreased. Sometimes RB-like forms with multiple dense nucleoid centers were observed (Fig. 1c), and individual large forms often contained small particles (Fig. 1d) or were of bizarre shapes when AAs were absent from the medium (Fig. 1e). Surprisingly, the number of cells infected rose progressively as AA concentrations were decreased (Table 1).
FIG. 1.
Strain 434 inclusions in McCoy cells supplied with medium containing reduced AA concentrations (a to e) or AAs at the concentrations found in blood plasma (f), as viewed by electron microscopy at 40 h p.i. No CH was present. (a) Inclusions in CMEM (100% AAs) contained mainly normal EBs (arrows). (b) AA reduction to 75% of the concentration in CMEM was associated with swollen intermediate forms (arrows). (c to e) Organisms became larger and more distorted as the medium AA supply decreased to 40, 25, and 0%, respectively; individual large forms often contained small particles (particularly noticeable in panel d) (arrows). (f) Many abnormally large chlamydiae (arrow), as well as some normal EBs (arrowhead), were also present in inclusions supplied with blood plasma AA levels Bars = 1.33 μm.
TABLE 1.
Morphology and infectivity yield of chlamydiae (strain 434) during decrease in supplied medium AA concentrationa
| % Medium AAs | No. of cells infected (IFU/field)b
|
Yield (IFU/inclusion)
|
||
|---|---|---|---|---|
| −CH | +CH | −CH | +CH | |
| 100 | 9 ± 0.18c | 23 ± 0.95c | 1,278 ± 57 | 1,938 ± 95 |
| 75 | 14 ± 0.9d* | 23 ± 0.47c | 372 ± 34* | 1,919 ± 37 |
| 40 | 29 ± 2.4e* | 23 ± 0.87c | 34 ± 4.5* | 753 ± 86* |
| 25 | 48 ± 1.7e* | 23 ± 0.87d | 19 ± 2.4* | 316 ± 42* |
| 0 | 62 ± 4.2e* | 20 ± 0.74e | 0.10 ± 0.01* | 0.15 ± 0.01* |
Inoculated monolayers were incubated with medium having AA concentrations of 100, 75, 40, 25, or 0% compared with that in CMEM, in presence or absence of CH. Inclusion morphology (electron microscopy) and infectivity yield were assessed at 40 h p.i. Values are means ± SD (at least 10 fields of view in three replicate wells). A separate experiment gave similar results. *, significantly different from control (P < 0.05).
Mean number of cells per field = 124.
Inclusions with normal morphology and packed with EBs.
Inclusions relatively full but particles larger than normal.
Inclusions with fewer and markedly abnormal particles.
In monolayers treated with CH, there was no such increase in the number of cells infected and the appearance of abnormal chlamydiae was delayed until medium AA concentrations were reduced to 25% of those in CMEM (Table 1). Correspondingly, the inclusion infectivity yield decreased gradually, but at a given medium AA concentration the infectivity yield was always greater than observed in the absence of CH.
Intracellular AA pools during reduction in AA supply.
Infected monolayers were maintained in medium with the AA concentrations indicated above and in the presence or absence of CH until 20 h p.i. and then analyzed for intracellular AAs. This time was judged to precede the major period of chlamydial division and differentiation to EBs, when chlamydial protein synthesis and consumption of AAs would rise to a maximum (35).
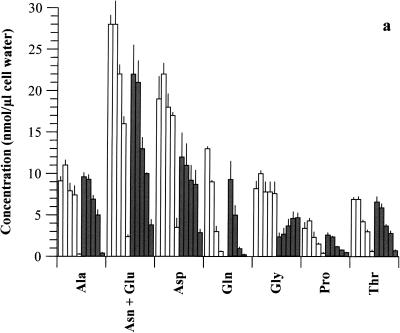
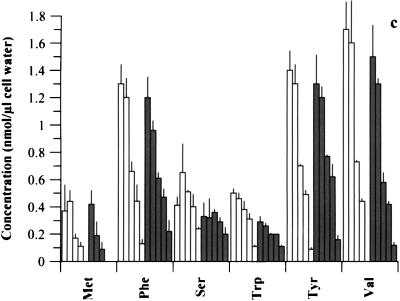
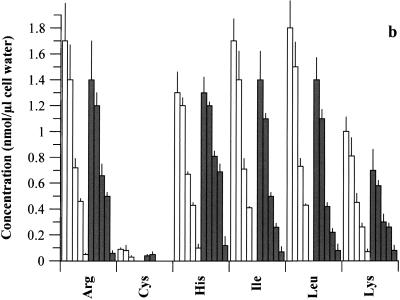
There was a steady decline in the concentration of all intracellular AAs as the medium concentration was reduced, whether CH was present or not (Fig. 2). Possible exceptions were the small but insignificant increases (P > 0.05) in certain AAs as medium AAs declined from 100 to 75% (plus CH) and the steady increase in Gly as medium AAs decreased (no CH). Notably, the intracellular concentration of any single AA in a given medium was generally greater when CH was present; indeed, the total intracellular AA pool for each medium (see Fig. 2 legend) was significantly higher (P < 0.05) in the presence of CH than in untreated cultures, except for medium with 0% AAs, in which it was only slightly higher. Certain intracellular AAs, such as Gln, Met, and Cys, became unmeasurable as their concentration in medium declined, in both the presence and absence of CH.
FIG. 2.
Intracellular AAs available to chlamydiae (strain 434) following graded reduction in medium AA concentrations (100, 75, 40, 25, and 0%), in the presence (open bars) and absence (shaded bars) of CH. Cells were 80% infected with organisms and incubated in medium containing 100 to 0% AAs. Samples were analyzed for AA pools at 20 h p.i. Data for each AA are presented at each medium AA concentration in the order of 100, 75, 40, 25, and 0%. Values are means + SD for triplicate samples, and a separate experiment gave similar results. The total AA pools for 100, 75, 40, 25, and 0% medium AAs in the presence of CH were as follows, in nanomoles per microliter of cell water: 101 ± 5.3, 103 ± 2.5, 72 ± 4.7, 57 ± 6.1, and 16 ± 3.8, respectively; those in the absence of CH were 76 ± 9.9, 67 ± 4.7, 44 ± 1.7, 36 ± 3.5, and 14 ± 1.1, respectively.
Chlamydial development during exposure to medium AAs maintained at the concentrations found in plasma.
Since blood plasma AA concentrations appear to be much lower than those in culture media (4, 15, 47), we examined the effect on chlamydial growth of a more physiologically appropriate system. Monolayers were incubated in a plasma medium containing all 20 AAs at blood plasma AA concentrations for 4 h prior to inoculation with strain 434. To simulate physiological conditions the medium (without CH) was changed every 6 h up to 40 h p.i., with the aim of maintaining a constant supply of AAs (see Materials and Methods). Developing inclusions contained many abnormally large chlamydiae, staining for DNA with acridine orange, as well as some normal EBs (Fig. 1f). The enlarged bodies were bigger than normal RBs, and some swollen forms with smaller particles inside were also present. Moreover, there was a significant (80%) decrease (P < 0.05) in the yield of infectious particles from inclusions cultured in plasma medium (yield reduced from 2,677 ± 84 IFU for inclusions growing in CMEM to 463 ± 37 IFU for those in plasma medium).
Intracellular AA pool data for infected cells in plasma medium (Table 2) are shown in comparison with values previously obtained for cell cultures incubated in CH-free medium with 100% AAs (CMEM; normal chlamydial growth at 40 h) or with 75% AAs (abnormal growth). Of the 13 AAs normally present in CMEM, the intracellular subtotal for infected cells in plasma medium was the same as for cells in 75% AAs, at 20 nmol/μl of cell water (Table 2). This is consistent with the finding, compared with CMEM data, that inclusions in each medium contained abnormally large chlamydiae with a reduced infectivity yield at 40 h p.i. The total pool of all 20 intracellular AAs was actually highest for plasma medium. However, this is inflated by the supplementation of seven AAs (Table 2) that are also synthesized by McCoy cells and are thus in relative excess in plasma medium.
TABLE 2.
Comparison of intracellular AA concentrations available to strain 434 supplied with blood plasma AA levels, CMEM (100% AAs), or 75% medium AAs
| AAs | Intracellular AA concn (nmol/μl of cell water)a
|
||
|---|---|---|---|
| 100% AAs | Plasma AAs | 75% AAs | |
| AAs supplied in CMEM | |||
| Arg | 1.4 ± 0.3 | 0.70 ± 0.10 | 1.2 ± 0.1 |
| Cys | 0.04 ± 0.01 | 0.03 ± 0.009 | 0.05 ± 0.02 |
| Gln | 9.3 ± 2.2 | 6.7 ± 1.77 | 5.0 ± 1.15 |
| His | 1.3 ± 0.12 | 0.48 | 1.2 ± 0.03 |
| Ile | 1.4 ± 0.22 | 0.76 ± 0.01 | 1.1 ± 0.04 |
| Leu | 1.4 ± 0.17 | 0.99 ± 0.02 | 1.1 ± 0.07 |
| Lys | 0.70 ± 0.16 | 1.1 ± 0.16 | 0.58 ± 0.04 |
| Met | 0.42 ± 0.10 | 0.96 ± 0.01 | 0.19 ± 0.10 |
| Phe | 1.2 ± 0.15 | 0.62 ± 0.06 | 0.96 ± 0.07 |
| Thr | 6.6 ± 0.63 | 5.9 ± 0.42 | 5.9 ± 0.47 |
| Trp | 0.29 ± 0.04 | 0.01 ± 0.003 | 0.26 ± 0.02 |
| Tyr | 1.3 ± 0.21 | 0.55 ± 0.01 | 1.2 ± 0.08 |
| Val | 1.5 ± 0.23 | 1.1 ± 0.20 | 1.3 ± 0.04 |
| Subtotal (13 AAs) | 27 | 20 | 20 |
| AAs added to plasma mediumb | |||
| Ala | 9.6 ± 0.51 | 10 ± 0.78 | 9.3 ± 0.55 |
| Asn + Glu | 22 ± 3.5 | 32 ± 2.1 | 21 ± 2.6 |
| Asp | 12 ± 2.9 | 18 ± 4.2 | 11 ± 2.6 |
| Gly | 2.4 ± 0.46 | 17 ± 2.1 | 2.7 ± 0.74 |
| Pro | 2.6 ± 0.3 | 10 ± 0.92 | 2.4 ± 0.06 |
| Ser | 0.33 ± 0.10 | 8.3 ± 1.1 | 0.32 ± 0.14 |
| Total (all 20 AAs) | 76 ± 9.9 | 115 ± 8.5 | 67 ± 4.7 |
Monolayers were incubated in medium containing AAs at the concentration found in blood plasma for 4 h and then 80% infected with strain 434. Medium was changed every 6 h. AA analyses were done at 20 h p.i. Data for infected cells in CMEM or medium with 75% AAs are shown for comparison. Values are means ± SD for triplicate (in the case of CMEM and 75% AAs) or duplicate (in the case of plasma AA concentration) samples (see Materials and Methods).
CMEM was not supplemented with these AAs; however, they were included in plasma medium because they are normally present in blood plasma.
Interestingly, during incubation of infected or uninfected McCoy cells in plasma medium, several AAs had intracellular concentrations many times above external values. Ala, Asn and Glu, Asp, Gln, Gly, Met, Pro, Ser, and Thr were notably highly concentrated in uninfected cells (Table 3), and their values were generally much greater than the concentration ratios reported for rat heart tissue and plasma (4); values for infected cells (not shown) were similar. These AAs could have utilized a variety of transport systems, so the concentrative effect may not have been restricted to one particular system. Only Cys and Trp were not concentrated by McCoy cells.
TABLE 3.
Ratio of intracellular to extracellular AA concentrations in uninfected McCoy cells incubated in plasma medium
| AA(s) | Intracellular/extracellular AA concn ratio in mock-infected cellsa | Rat heart/plasma AA concn ratiob |
|---|---|---|
| Arg | 3.7 | 0.82 |
| Cys | 0.85 | 0.9 |
| Gln | 35 | 9.3 |
| His | 11 | 4.4 |
| Ile | 10 | 1.3 |
| Leu | 8.2 | 1.2 |
| Lys | 2.7 | 1.4 |
| Met | 45 | 2.5 |
| Phe | 10 | 17 |
| Thr | 21 | |
| Trp | 0.75 | |
| Tyr | 10 | 1.7 |
| Val | 6.6 | 0.88 |
| Ala | 28 | 6.1 |
| Asn + Glu | 67 | 48c |
| Asp | 378 | 210 |
| Gly | 32 | 4.5 |
| Pro | 26 | 17 |
| Ser | 25 | 6.9 |
Monolayers were incubated in plasma medium for 4 h before mock infection. Medium was replenished every 6 h, and duplicate AA analyses of both medium and cells were performed at 20 h p.i., enabling calculation of the ratio of the intracellular to the extracellular concentration.
The rat heart tissue/plasma ratios are shown for comparison (4).
Glu only.
Chlamydial growth during glucose deprivation.
To see if abnormal chlamydial organisms could be generated by nutritional means other than AA deficiency, we investigated the effect of glucose deprivation. Monolayers infected with strain 434, or strain DK20 for comparison, were maintained in CMEM without glucose but containing nonessential AAs in the presence or absence of CH. Fluorescence microscopy of both strains revealed the presence of highly abnormal, often bizarre chlamydial forms with negligible infectivity (Table 4), similar to those observed in inclusions deprived of all AAs. As with AA deprivation, in the absence of CH there was a significant increase (P < 0.05) in the number of cells infected during incubation in glucose-free versus normal medium, despite the use of the same inoculum concentration.
TABLE 4.
Morphology and infectivity yield of chlamydiae (strains 434 and DK20) during glucose deprivation and reversibility of abnormal developmenta
| Incubation medium | No. of cells infected (IFU/field)b
|
Yield (IFU/inclusion)
|
||
|---|---|---|---|---|
| Strain 434 | Strain DK20 | Strain 434 | Strain DK20 | |
| With CH | ||||
| CMEM | 21 ± 1.1c | 13 ± 0.7c | 5,102 ± 517 | 640 ± 65 |
| Glucose-free medium | 20 ± 1.5d | 13 ± 0.8d | 17 ± 0.6* | 0.15 ± 0.02* |
| Glucose-free medium (24 h), then CMEM | 22 ± 0.8c | ND | 3,850 ± 163 | ND |
| Without CH | ||||
| CMEM | 6.4 ± 0.3c | 10 ± 0.6c | 4,568 ± 417 | 367 ± 44 |
| Glucose-free medium | 34 ± 0.3d* | 29 ± 2.1d* | 0.63 ± 0.09* | 0.01 ± 0.001* |
| Glucose-free medium (24 h), then CMEM | 35 ± 2.0c* | ND | 2,424 ± 88* | ND |
Monolayers inoculated with strain 434 or DK20 were incubated in medium lacking glucose, with or without CH. After 24 h glucose was introduced to devoid monolayers by addition of CMEM (434 only). Morphology (fluorescence microscopy) and infectivity yield were assessed at 40 h p.i. Values are means ± SD (at least 10 fields of view in three replicate wells). *, significantly different from control (P < 0.05). ND, not determined.
Mean number of cells per field = 121.
Inclusions with normal morphology and packed with EBs.
Inclusions with fewer and markedly abnormal particles.
The reversibility of abnormal development due to glucose deprivation was demonstrated by reintroduction of glucose to strain 434-infected cells at 24 h p.i. Inclusions viewed by fluorescence microscopy at 40 h p.i. appeared to contain normal particles, with at least 50% recovery of infectivity yield from inclusions (Table 4).
Intracellular AA pool sizes were measured in order to assess the potential effects of glucose deficiency on AA metabolism. In the presence or absence of CH, the concentration of most individual AAs was higher during glucose deprivation than when cell cultures were incubated in CMEM (data not shown). Exceptions were Ala and Gln, whose concentrations were closer to those observed with 25% AAs, at which concentration a significant amount of chlamydial infectivity was still observed.
DISCUSSION
Our findings demonstrate the extreme sensitivity of chlamydiae to small changes in external AA concentrations in the absence of CH, with a decrease to 75% AAs sufficient to induce the growth of enlarged forms with reduced infectivity. As medium AA supply was decreased, the increasingly distorted morphology and fall in infectious particle yield were correlated with reduction in intracellular AAs—not to our knowledge previously examined in chlamydial infection. All AAs (except glycine) showed a steady decline as the concentration in medium was reduced. Similar effects were observed when CH was present in media, with both the inclusion infectivity yield and the intracellular concentration of AAs being greater for a given medium than in untreated cultures. Correspondingly, in the presence of CH, enlarged aberrant chlamydiae were absent until AA concentrations were decreased to 25% of those in CMEM. Clearly, an important effect of CH is to spare intracellular AA pools for chlamydiae that would normally be used by the host cell, with resulting increases in inclusion infectivity yield and in production of normal organisms.
The susceptibility of chlamydiae to small changes in host AA pools is further demonstrated by the fact that there was little difference between intracellular pools for medium with 40% AAs plus CH (normal growth; total pool, 72 nmol/μl) and medium with 75% AAs and no CH (abnormal growth; total pool, 67 nmol/μl). There may be a critical concentration for each AA below which aberrant chlamydial development is favored; members of our group have already shown (12) that omission of any AA from medium can lead to abnormal organism growth. Also, the effective disappearance of certain AAs (Gln, Met, and Cys) could have provided the main stimulus for abnormal organism development at low external AA levels.
Of particular importance is the finding that blood plasma AA concentrations induce the development of enlarged, morphologically abnormal chlamydiae and inclusions with significantly reduced infectivity yields. The intracellular concentrations of most of the AAs normally included in media were similar for medium having 75% AAs and plasma medium, and the total concentration was identical for the two. This was as expected, since the chlamydial morphology and infectivity yield in these media were comparable. However, for McCoy cells grown in plasma medium, the total concentration of all 20 intracellular AAs was greater than for McCoy cells supplied with CMEM, with which normal chlamydial growth was observed. Although the AAs added only to plasma medium clearly contributed to the greater pool size, these results support the proposal that the nature of chlamydial growth is determined not by the total AA concentration within cells but by a balance and minimum requirement for each individual AA.
The finding of abnormal chlamydial development during supply with blood plasma AA concentrations leads us to conclude that the accepted developmental cycle of Chlamydia operates exclusively when tissue culture conditions are optimal, with nutrients in excess and maintained at higher levels by the presence of CH. More importantly, our findings provide evidence to support the view that abnormal chlamydial development could occur during natural infections as a result of nutritional deficiency alone (12, 20, 33). A tissue culture model such as this only attempts to mimic the constantly renewed supply of AAs via the bloodstream to inclusions developing in tissues close by. The potential effects of an acute inflammatory response during infection on extracellular AA availability and AA transport can only be guessed at. However, it is not inconceivable that terminally differentiating epithelial cells maintain intracellular AA pools at a basal level, and there is no evidence to suggest that these cells have a particularly high metabolic requirement for AAs. In contrast, the marked ability of McCoy cells to concentrate all AAs (except Cys and Trp) above that normally expected has been demonstrated, and members of our group have also shown that these cells concentrate radiolabeled AAs representative of several AA transport systems (A. Harper, C. I. Pogson, and J. H. Pearce, unpublished data). Therefore, AA concentrations inside infected cells in vivo may be even lower than those detected here in McCoy cells supplied with plasma medium, providing even greater potential for aberrant chlamydial development. The apparent concentrative ability of McCoy cells could be explained by their transformed nature, as transformation has been reported to lead to increases in the transport activity of several cell lines (16, 39).
In the absence of CH, there was a steady increase in the number of cells infected with chlamydiae which correlated with the degree of AA deprivation; similar enhancement was observed following glucose deprivation. CH is routinely used during chlamydial propagation to increase the number of infected cells. We suggest that this now appears unlikely to be due to the increased supply of AAs available to the organisms, which appears rather to promote normal chlamydial growth. A possible explanation is that CH also adversely affects the host cell's response to infection. During infection under normal growth conditions, induction of cytokines may restrict the numbers of infected cells, with progressive enhancement of inclusion formation as AA deprivation weakens the antagonism. Previous studies have demonstrated IFN-α/β and nitric oxide responses following initiation of chlamydial infection in McCoy cells in normal medium; the response is suppressed by CH (14). It was not possible to show conclusively that induction of either component played a part in restricting inclusion formation, although nitric oxide is chlamydiacidal in the presence of IFN-γ (32). Some still-undefined host response to chlamydial challenge may also limit infection in this culture model.
Aberrant chlamydial development appears to involve similar effects on morphology and infectious particle yield, whether the stimulus is the lack of AAs or glucose. These common adverse effects, also seen during drug treatment (31, 46) or after heat shock (6, 26), provide evidence that abnormal development is a general stress-related response. The markedly aberrant growth observed during glucose deprivation was not a result of increased AA metabolism, since the concentration of most intracellular AAs was greater than during incubation with CMEM and was probably due to AA transport and/or proteolysis. Common responses to a variety of stresses, including changes in gene expression and physiological activity, have been reported for a number of bacteria. Many identical starvation proteins are synthesized by Escherichia coli irrespective of whether the response is to deprivation of carbon, phosphate, or nitrogen (17). Similarly, some heat shock proteins are also produced by both E. coli and Salmonella enterica serovar Typhimurium during nutrient deprivation (25). The production of starvation proteins by chlamydiae has not been demonstrated to date, although aberrant forms induced by treatment with IFN-γ appear to contain altered levels of important antigens, with increased synthesis of the 57-kDa chlamydial stress protein relative to the major outer membrane protein, the 60-kDa outer membrane protein, and lipopolysaccharide (5).
It has been reported that the stringent response of bacteria to environmental stresses concomitantly affords them an enhanced resistance to factors such as oxidation, low pH, and hydrogen peroxide (28, 30). Hence, abnormal chlamydial forms could also be more resistant to certain host defense mechanisms, providing them with the potential for survival in host tissues. Conclusive evidence, however, for long-term persistence of chlamydiae in vivo remains to be obtained. Since abnormal development appears to be reversible when more favorable conditions are restored, it may be that during chronic infection there is continual fluctuation between normal (accepted) and aberrant development, dependent on both cytokine production and nutrient availability. While this could provide the inducement for adverse inflammatory responses and tissue damage, it is possible that depending on their location, abnormal forms induced in the absence of an immune response might persist without chronic disease.
ACKNOWLEDGMENTS
This work was supported by The Science and Engineering Research Council (United Kingdom), with sponsorship (A.H.) from Wellcome Research Laboratories.
REFERENCES
- 1.Allan I, Pearce J H. Differential amino acid utilization by Chlamydia psittaci (strain guinea pig inclusion conjunctivitis) and its regulatory effect on chlamydial growth. J Gen Microbiol. 1983;129:1991–2000. doi: 10.1099/00221287-129-7-1991. [DOI] [PubMed] [Google Scholar]
- 2.Allan I, Hatch T P, Pearce J H. Influence of cysteine deprivation on chlamydial differentiation from reproductive to infective life-cycle forms. J Gen Microbiol. 1985;131:3171–3177. doi: 10.1099/00221287-131-12-3171. [DOI] [PubMed] [Google Scholar]
- 3.Almirón M, Link A J, Furlong D, Kolter R. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev. 1992;6:2646–2654. doi: 10.1101/gad.6.12b.2646. [DOI] [PubMed] [Google Scholar]
- 4.Baños G, Daniel P M, Moorhouse S R, Pratt O E. The influx of amino acids into the brain of the rat in vivo: the essential compared with some non-essential amino acids. Proc R Soc Lond. 1973;183:59–70. doi: 10.1098/rspb.1973.0004. [DOI] [PubMed] [Google Scholar]
- 5.Beatty W L, Byrne G I, Morrison R P. Morphologic and antigenic characterization of interferon γ-mediated persistent Chlamydia trachomatis infection in vitro. Proc Natl Acad Sci USA. 1993;90:3998–4002. doi: 10.1073/pnas.90.9.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beatty W L, Belinger T A, Le K D, Desai A A, Morrison R P, Byrne G I. Chlamydial persistence: mechanism of induction and parallels to a stress-related response. In: Orfila J, et al., editors. Chlamydial infections. Proceedings of the Eighth International Symposium on Human Chlamydial Infections. Bologna, Italy: Società Editrice Esculapio; 1994. pp. 415–418. [Google Scholar]
- 7.Beatty W L, Byrne G I, Morrison R P. Repeated and persistent infection with Chlamydia and the development of chronic inflammation and disease. Trends Microbiol. 1994;2:94–98. doi: 10.1016/0966-842x(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 8.Beatty W L, Morrison R P, Byrne G I. Reactivation of persistent Chlamydia trachomatis infection in cell culture. Infect Immun. 1995;63:199–205. doi: 10.1128/iai.63.1.199-205.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell L A, Patten D L, Moore D E, Cappuccio A L, Mueller B A, Wang S. Detection of Chlamydia trachomatis deoxyribonucleic acid in women with tubal infertility. Fertil Steril. 1993;59:45–50. [PubMed] [Google Scholar]
- 10.Christiansen G, Pedersen L B, Koehler J E, Lundemose A G, Birkelund S. Interaction between the Chlamydia trachomatis histone H1-like protein (Hc1) and DNA. J Bacteriol. 1993;175:1785–1795. doi: 10.1128/jb.175.6.1785-1795.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coles A M, Pearce J H. Regulation of Chlamydia psittaci (strain guinea pig inclusion conjunctivitis) growth in McCoy cells by amino acid antagonism. J Gen Microbiol. 1987;133:701–708. doi: 10.1099/00221287-133-3-701. [DOI] [PubMed] [Google Scholar]
- 12.Coles A M, Reynolds D J, Harper A, Devitt A, Pearce J H. Low-nutrient induction of abnormal chlamydial development: a novel component of chlamydial pathogenesis? FEMS Microbiol Lett. 1993;106:193–200. doi: 10.1111/j.1574-6968.1993.tb05958.x. [DOI] [PubMed] [Google Scholar]
- 13.Denckla W D, Dewey H K. The determination of tryptophan in plasma, liver and urine. J Lab Clin Med. 1967;69:160–169. [PubMed] [Google Scholar]
- 14.Devitt A, Lund P A, Morris A G, Pearce J H. Induction of alpha/beta interferon and dependent nitric oxide synthesis during Chlamydia trachomatis infection of McCoy cells in the absence of exogenous cytokine. Infect Immun. 1996;64:3951–3956. doi: 10.1128/iai.64.10.3951-3956.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.East A G, Louis L N, Hoffenberg R. Albumin synthesis by isolated rat liver cells. Exp Cell Res. 1973;76:41–46. doi: 10.1016/0014-4827(73)90416-3. [DOI] [PubMed] [Google Scholar]
- 16.Goenner S, Boutron A, Soni T, Lemonnier A, Moatti N. Amino acid transport systems in the human hepatoma cell line Hep G2. Biochem Biophys Res Commun. 1992;189:472–479. doi: 10.1016/0006-291x(92)91582-b. [DOI] [PubMed] [Google Scholar]
- 17.Groat R G, Schultz J E, Zychlinsky E, Bockman A, Matin A. Starvation proteins in Escherichia coli: kinetics of synthesis and role in starvation survival. J Bacteriol. 1986;168:486–493. doi: 10.1128/jb.168.2.486-493.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hackstadt T, Baehr W, Yuan Y. Chlamydia trachomatis developmentally regulated protein is homologous to eucaryotic histone H1. Proc Natl Acad Sci USA. 1991;88:3937–3941. doi: 10.1073/pnas.88.9.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harvey C M, Ellory J C. Identification of amino acid transporters in the red blood cell. Methods Enzymol. 1989;173:122–160. doi: 10.1016/s0076-6879(89)73010-x. [DOI] [PubMed] [Google Scholar]
- 20.Hatch T P. Competition between Chlamydia psittaci and L cells for host isoleucine pools: a limiting factor in chlamydial multiplication. Infect Immun. 1975;12:211–220. doi: 10.1128/iai.12.1.211-220.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatch T P, Allan I, Pearce J H. Structural and polypeptide differences between envelopes of infective and reproductive life cycle forms of Chlamydia spp. J Bacteriol. 1984;157:13–20. doi: 10.1128/jb.157.1.13-20.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirs C H W, Timasheff S N, editors. Enzyme structure. Methods Enzymol. 1977;47:3–40. [Google Scholar]
- 23.Holland S M, Hudson A P, Bobo L, Whittum-Hudson J A, Viscidi R P, Quinn T C, Taylor H R. Demonstration of chlamydial RNA and DNA during a culture-negative state. Infect Immun. 1992;60:2040–2047. doi: 10.1128/iai.60.5.2040-2047.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hough A J, Rank R G. Pathogenesis of acute arthritis due to viable Chlamydia trachomatis (mouse pneumonitis agent) in C57B1/6 mice. Am J Pathol. 1989;134:903–912. [PMC free article] [PubMed] [Google Scholar]
- 25.Jenkins D E, Schultz J E, Matin A. Starvation-induced cross protection against heat or H2O2 challenge in Escherichia coli. J Bacteriol. 1988;170:3910–3914. doi: 10.1128/jb.170.9.3910-3914.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahane S, Friedman M G. Reversibility of heat shock in Chlamydia trachomatis. FEMS Microbiol Lett. 1992;97:25–30. doi: 10.1016/0378-1097(92)90358-u. [DOI] [PubMed] [Google Scholar]
- 27.Kletzien R F, Pariza M W, Becker J E, Potter V R. A method using 3-O-methyl-d-glucose and phloretin for the determination of intracellular water space of cells in monolayer culture. Anal Biochem. 1975;68:537–544. doi: 10.1016/0003-2697(75)90649-1. [DOI] [PubMed] [Google Scholar]
- 28.Koch A L. Genetic response of microbes to extreme challenges. J Theor Biol. 1993;160:1–21. doi: 10.1006/jtbi.1993.1001. [DOI] [PubMed] [Google Scholar]
- 29.Kunji E R S, Ubbink T, Matin A, Poolman B, Konings W N. Physiological responses of Lactococcus lactis ML3 to alternating conditions of growth and starvation. Arch Microbiol. 1993;159:372–379. [Google Scholar]
- 30.Matin A, Auger E A, Blum P H, Schultz J E. Genetic basis of starvation survival in nondifferentiating bacteria. Annu Rev Microbiol. 1989;43:293–316. doi: 10.1146/annurev.mi.43.100189.001453. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto A, Manire G P. Electron microscopic observations on the effect of penicillin on the morphology of Chlamydia psittaci. J Bacteriol. 1970;101:278–285. doi: 10.1128/jb.101.1.278-285.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayer J, Woods M L, Vavrin Z, Hibbs J., Jr Gamma interferon-induced nitric oxide production reduces Chlamydia trachomatis infectivity in McCoy cells. Infect Immun. 1993;61:491–497. doi: 10.1128/iai.61.2.491-497.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morgan H R. Latent viral infection of cells in tissue culture. I. Studies on latent infection of chick embryo tissues with psittacosis virus. J Exp Med. 1956;103:34–47. doi: 10.1084/jem.103.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrison R P. Chlamydial hsp60 and the immunopathogenesis of chlamydial disease. Semin Immunol. 1991;3:25–33. [PubMed] [Google Scholar]
- 35.Nanagara R, Li F, Beutler A, Hudson A, Schumacher H R. Alteration of Chlamydia trachomatis biologic behaviour in synovial membranes—suppression of surface antigen production in reactive arthritis and Reiter's syndrome. Arthritis Rheum. 1995;38:1410–1417. doi: 10.1002/art.1780381008. [DOI] [PubMed] [Google Scholar]
- 36.Newhall V, W J. Biosynthesis and disulfide cross-linking of outer membrane components during the growth cycle of Chlamydia trachomatis. Infect Immun. 1987;55:162–168. doi: 10.1128/iai.55.1.162-168.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perara E, Ganem D, Engel J N. A developmentally regulated chlamydial gene with apparent homology to eukaryotic histone H1. Proc Natl Acad Sci USA. 1992;89:2125–2129. doi: 10.1073/pnas.89.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phillips D M, Swenson C E, Schachter J. Ultra-structure of Chlamydia trachomatis infection of the mouse oviduct. J Ultrastruct Res. 1984;88:244–256. doi: 10.1016/s0022-5320(84)90122-9. [DOI] [PubMed] [Google Scholar]
- 39.Saier M H, Jr, Daniels G A, Boerner P, Lin J. Neutral amino acid transport systems in animal cells: potential targets of oncogene action and regulators of cellular growth. J Membr Biol. 1988;104:1–20. doi: 10.1007/BF01871898. [DOI] [PubMed] [Google Scholar]
- 40.Sardinia L M, Segal E, Ganem D. Developmental regulation of the cysteine-rich outer-membrane proteins of murine Chlamydia trachomatis. J Gen Microbiol. 1988;134:997–1004. doi: 10.1099/00221287-134-4-997. [DOI] [PubMed] [Google Scholar]
- 41.Schachter J, Moncada J, Dawson C R, Sheppard J, Courtwright P, Said M E, Zaki S, Hafez S F, Lorincz A. Nonculture methods for diagnosing chlamydial infection in patients with trachoma: a clue to the pathogenesis of the disease? J Infect Dis. 1988;158:1347–1352. doi: 10.1093/infdis/158.6.1347. [DOI] [PubMed] [Google Scholar]
- 42.Schumacher H R, Magge S, Cherian P V, Sleckman J, Rothfuss S, Clayburne G, Sieck M. Light and electron microscopic studies on the synovial membrane in Reiter's syndrome. Arthritis Rheum. 1988;31:937–946. doi: 10.1002/art.1780310801. [DOI] [PubMed] [Google Scholar]
- 43.Shemer Y, Sarov I. Inhibition of growth of Chlamydia trachomatis by human gamma interferon. Infect Immun. 1985;48:592–596. doi: 10.1128/iai.48.2.592-596.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shemer-Avni Y, Wallach D, Sarov I. Inhibition of Chlamydia trachomatis growth by recombinant tumor necrosis factor. Infect Immun. 1988;56:2503–2506. doi: 10.1128/iai.56.9.2503-2506.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soloff B L, Rank R G, Barron A L. Ultrastructure studies of chlamydial infection in guinea-pig urogenital tract. J Comp Pathol. 1982;92:547–558. doi: 10.1016/0021-9975(82)90007-x. [DOI] [PubMed] [Google Scholar]
- 46.Tribby I I E, Friis R R, Moulder J W. Effect of chloramphenicol, rifampin, and nalidixic acid on Chlamydia psittaci growing in L cells. J Infect Dis. 1973;127:155–163. doi: 10.1093/infdis/127.2.155. [DOI] [PubMed] [Google Scholar]
- 47.Zunic G, Rolovic Z, Basara N, Simovic M, Vasiljevski M. Decreased plasma proteins, increased total plasma-free amino acids, and disturbed amino acid metabolism in the hereditary severe anemia of the Belgrade laboratory (b/b) rat. Proc Soc Exp Biol Med. 1993;203:366–371. doi: 10.3181/00379727-203-43613. [DOI] [PubMed] [Google Scholar]