Abstract
Objective: To determine the effect of Danhong injection combined with tirofiban on cardiac function, myocardial enzyme spectrum and lipoprotein-associated phospholipase A2 (Lp-PLA2) level in patients with acute myocardial infarction (AMI). Methods: The clinical data of 124 AMI patients who were treated in the Second Affiliated Hospital of Wenzhou Medical University from August 2019 to April 2021 were collected and analyzed retrospectively. Among them, 58 patients treated with routine thrombolysis combined with tirofiban were assigned to the control group, and the other 66 patients treated with Danhong injection on the basis of treatment to the control group were assigned to the observation group. Treatment efficacy, cardiac function, myocardial enzyme spectrum, and Lp-PLA2 level before and after treatment, and adverse cardiovascular events during treatment were compared between the two groups. The patients were further grouped into an occurrence group and a non-occurrence group in the light of the occurrence of adverse cardiovascular events after treatment, and then the risk factors of adverse cardiovascular events were analyzed by logistic regression. Results: The control group showed a notably lower total effective rate than the observation group (P=0.015). After treatment, the observation group showed a higher left ventricular ejection fraction (LVEF) level and a lower left ventricular end-diastolic dimension (LVEDD) than the control group (both P < 0.05). In addition, the observation group showed lower levels of CK, CK-MB and Lp-PLA2 than the control group (all P < 0.05). A significantly higher incidence of adverse cardiovascular events was found in the control group than that in the observation group (P=0.039), and Logistic regression analysis showed that NYHA grade, LVEF, LVEDD, CK-MB and Lp-PLA2 were independent risk factors (P < 0.05). The prediction model =-86.255 + (4.645*NYHA grade) + (-0.581*LVEF) + (1.058*LVEDD) + (0.263*CK-MB) + (0.121*Lp-PLA2). According to the ROC curve analysis, the area under the curve of the model in predicting adverse cardiovascular events among patients was 0.970. Conclusion: Danhong injection combined with tirofiban can improve the cardiac function, myocardial enzyme spectrum and Lp-PLA2 level in AMI patients.
Keywords: Danhong injection, tirofiban, patients with acute myocardial infarction, cardiac function, myocardial enzyme spectrum, Lp-PLA2
Introduction
Acute myocardial infarction (AMI) is a common cardiovascular disease with high morbidity and mortality [1]. The major cause of AMI is coronary artery occlusion, which is usually triggered by the rupture, thrombosis or erosion of coronary atherosclerotic plaque and results in a serious shortage of myocardial blood flow and oxygen supply [2]. Currently, percutaneous coronary intervention (PCI) is the first choice for AMI [3]. Reportedly, PCI can restore occluded blood vessels and reduce the mortality of AMI [4], but patients still face a certain risk of in-hospital death [5]. According to studies [6,7], during PCI, some ruptured thrombi may fall off and flow to the distal micro vessels along with the blood flow, which easily hinders perfusion in coronary artery or causes slow blood flow, triggering coronary artery non-perfusion and local myocardial ischemia. Prior research has revealed that serious adverse cardiovascular events may occur after PCI, such as angina pectoris, heart failure and even sudden cardiac death [8]. Moreover, some patients have PCI contraindications, so they can only receive thrombolytic therapy, but the therapeutic effects of different methods are different [9].
Tirofiban hydrochloride is a reversible antagonist of non-peptide platelet glycoprotein IIb/IIIa receptor, which can suppress the common pathway of platelet aggregation and thrombosis. It is suitable for the treatment of patients with unstable angina pectoris or non-Q wave myocardial infarction [10]. According to one study [11], tirofiban hydrochloride can substantially lower the incidence of acute coronary syndrome and myocardial ischemia after PCI and contribute to favourable prognosis. Moreover, tirofiban can also inhibit the release of a large number of inflammatory factors and vasoconstrictive substances during platele activation [12]. Danhong injection, as the extract of Salvia miltiorrhiza root (Salvia miltiorrhiza) and safflower, is a compound injection that is extensively used to treat many diseases including AMI [13]. According to prior research, salvia miltiorrhiza in Danhong injection can dilate blood vessels, reduce vascular resistance and blood viscosity, while safflower has been verified to have many pharmacological properties, including vasodilation, antioxidation, calcium antagonism and oxygen free radical scavenging [14]. Recent studies have found positive effects of Danhong injection in inhibiting platelet aggregation and improving hemodynamics and endothelial function of AMI patients [15]. Therefore, Danhong injection is a potential effective drug for the treatment of AMI.
However, the efficacy and safety of Danhong injection combined with tirofiban on AMI have not been systematically evaluated. Accordingly, this study was conducted to evaluate the efficacy and safety of their combination in AMI patients.
Methods and data
Clinical data
The clinical data of 124 AMI patients in The Second Affiliated Hospital of Wenzhou Medical University from August 2019 to April 2021 were collected and analyzed retrospectively. Among them, 58 patients treated with routine thrombolysis combined with tirofiban were seen as control group, and the other 66 patients treated with additional Danhong injection on the basis of control group were seen as the observation group. This study was performed with permission from the Medical Ethics Committee of the Second Affiliated Hospital of Wenzhou Medical University, with ethnical approval number of LLSH20193354.
Inclusion and exclusion criteria
Inclusion criteria
Patients who met the relevant diagnostic criteria in the Guidelines for diagnosis and treatment of AMI [16]; patients who experienced a time window from onset to admission less than 4.5 hours; patients with contraindications to PCI; patients who were treatment-naive; and patients with complete clinical data.
Exclusion criteria
End-stage patients with malignant tumor or other diseases; patients with diabetes or heart, liver or kidney failure; patients with a previous history of cardiac insufficiency or heart disease; patients with mental disorders; patients with active visceral bleeding in the last 2 weeks; patients allergic to the drugs used in this study; patients complicated with infectious or immune diseases; or pregnant women.
Therapeutic regimen
For the control group
Tirofiban (Lunambert Pharmaceutical Co., LTD., State Food and Drug Administration (SFDA) approval number: H20090328) was given at an infusion rate of 0.4 μg/kg·min within 30 min, followed by maintenance at 0.1 μg/kg·min for 14 days.
For the observation group
On the basis of treatment in the control group, the patient was additionally treated with 30 mL Danhong injection (Shandong Danhong Pharmaceutical Co., Ltd., SFDA approval number: Z20026866) fully diluted with 250 mL 0.9% sodium chloride injection, through intravenous drip, once a day, for 14 days.
Cardiac function test
The cardiac function-associated indexes, including left ventricular ejection fraction (LVEF) and left ventricular end-diastolic dimension (LVEDD), were determined using a Philips IU22 color ultrasonic diagnostic instrument before treatment and after 14 days of treatment. LVEF and LVEDD are commonly used in clinical evaluation of cardiac function. The normal range of LVEF is 50%-70%, and the normal range of LVEDD is 35-50 mm.
Biological index test
Before and after the treatment, 5 mL peripheral venous blood was collected and centrifuged (2000 r/min) for 5 min to obtain the serum, which was stored at low temperature for subsequent analysis. Creatine phosphokinase (CK) and creatine kinase isoenzyme (CK-MB) in the serum were quantified through an automatic biochemistry analyzer (Beckman Coulter) under instructions, and Lp-PLA2 in the serum was quantified using enzyme-linked immuno-sorbent assay (ELISA; USA; Sigma, RAB1564) under instructions of the kits from corresponding instrument supporting company.
Outcome measures
Primary outcome measures: The efficacy of the two groups was compared after treatment. The total effective rate = (the number of remarkably effective cases + effective cases) × 100%/total number of cases. The evaluation criteria for efficacy are shown in Table 1. The changes in the cardiac function before and after therapy were compared between the two groups. In addition, the myocardial enzyme spectrum and Lp-PLA2 were also compared between the two groups.
Table 1.
Clinical efficacy evaluation criteria
| Efficacy grading | Evaluation criteria |
|---|---|
| Markedly effective | The patient’s disease signs and clinical symptoms disappeared, without attack of angina pectoris or with decrease of the number of attacks by more than 80%, and the ECG returned to normal. |
| Effective | The patient’s disease signs and clinical symptoms were greatly alleviated, with decrease in the number of angina pectoris attacks by less than 80% but more than 50% or 50%, and the T wave and ST segment of ECG were improved. |
| Ineffective | The patient’s disease signs and clinical symptoms were not alleviated or even worsen, with decrease in the number of angina pectoris attacks by < 50%, and the ECG showed no improvement. |
Secondary outcome measures: The baseline data and adverse cardiovascular events during treatment were compared between the two groups. The patients were further grouped into an occurrence group and a non-occurrence group in the light of the occurrence of adverse cardiovascular events after treatment, and then the risk factors of adverse cardiovascular events were analyzed through logistic regression.
Statistical analysis
This study adopted SPSS 20.0 (SPSS Inc., Chicago, IL, USA) for analysis of collected data, and GraphPad Prism 8 for visualization of data. The Kolmogorov-Smirnov test was adopted for evaluation of the normal distribution. Data in normal distribution were described by mean ± SD and analyzed using the t test. Inter-group comparison and intra-group comparison were conducted using the independent sample t test and paired t test, respectively. Classified variables were compared by chi-square test. Logistic regression test was used to analyze the risk factors of adverse cardiovascular events, and the value of risk factors in predicting the occurrence of adverse cardiovascular events was analyzed by receiver operating characteristic (ROC) curves. P < 0.05 indicated a significant difference.
Results
Comparison of clinical data
No notable differences were found in age, sex, body mass index (BMI), course of disease, New York Heart Association classification (NYHA), previous medical history and smoking history between the two groups (all P > 0.05, Table 2), indicating the comparability between the two groups.
Table 2.
Comparison of baseline data
| Factor | Control group (n=58) | Observation group (n=66) | χ2 value | P-value |
|---|---|---|---|---|
| Age | 0.698 | 0.403 | ||
| ≥ 60 years old | 41 | 51 | ||
| < 60 years old | 17 | 15 | ||
| Gender | 0.606 | 0.265 | ||
| Male | 37 | 45 | ||
| Female | 21 | 21 | ||
| BMI | 0.777 | 0.377 | ||
| ≥ 23 kg/m2 | 21 | 19 | ||
| < 23 kg/m2 | 37 | 47 | ||
| Course of disease | 0.305 | 0.580 | ||
| ≥ 3 h | 23 | 23 | ||
| < 3 h | 35 | 43 | ||
| NYHA classification | 0.260 | 0.878 | ||
| Class II | 17 | 20 | ||
| Class III | 21 | 26 | ||
| Class IV | 20 | 20 | ||
| Past medical history | ||||
| Hypertension | 30 | 33 | 0.036 | 0.848 |
| Diabetes | 22 | 27 | 0.114 | 0.735 |
| History of smoking | 0.715 | 0.397 | ||
| Yes | 40 | 50 | ||
| No | 18 | 16 |
Note: BMI: Body Mass Index; NYHA: New York Heart Association Classification.
Comparison of clinical efficacy
The clinical efficacy on the two groups after treatment was evaluated, and the control group showed a lower total clinical effective rate than the observation group after treatment (P=0.015, Table 3).
Table 3.
Efficacy evaluation
| Group | Markedly effective | Effective | Ineffective | Total effective rate |
|---|---|---|---|---|
| Control group (n=58) | 27 (46.55%) | 19 (32.76%) | 12 (20.69) | 46 (79.31%) |
| Observation group (n=66) | 36 (54.55%) | 26 (39.39%) | 4 (6.06) | 62 (93.94%) |
| χ2 value | 5.879 | |||
| P value | 0.015 | |||
Changes of cardiac function before and after treatment
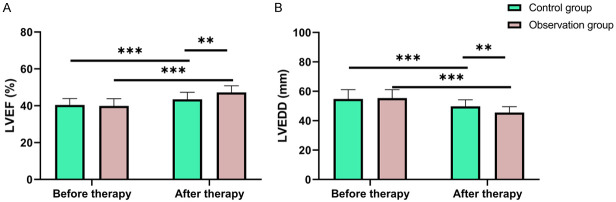
The two groups were not significantly different in LVEF and LVEDD levels before therapy (both P > 0.05), but after therapy, both groups showed notably increased LVEF level and decreased LVEDD level (both P < 0.05), with a higher LVEF level and a lower LVEDD level in the observation group than those in the control group (all P < 0.05, Figure 1).
Figure 1.
Changes in cardiac function of the patients before and after therapy. A. Changes of LVEF in the patients before and after treatment. B. Changes of LVEDD in the patients before and after treatment. Notes: LVEF: Left Ventricular Ejection Fraction; LVEDD: Left Ventricular End-Diastolic Dimension. ** suggests P < 0.01, *** suggests P < 0.001.
Comparison of myocardial enzyme spectrum and Lp-PLA2 in patients before and after treatment
Before therapy, the levels of CK, CK-MB and Lp-PLA2 were not greatly different between the two groups (all P > 0.05). However, after the therapy, those levels in both groups decreased significantly (all P < 0.05), with notably lower levels in the observation group than those in the control group (all P < 0.05, Figure 2).
Figure 2.
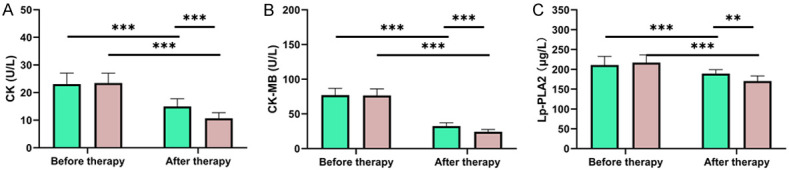
Changes of myocardial enzyme spectrum and Lp-PLA2 in patients before and after treatment. A. Changes of CK in patients before and after treatment. B. Changes of CK-MB in patients before and after treatment. C. Changes of Lp-PLA2 in patients before and after treatment. Note: Lp-PLA2: Lipoprotein-Associated Phospholipase A2; CK: Creatine Phosphokinase; CK-MB: Creatine Kinase Isoenzyme. **P < 0.01, ***P < 0.001.
Comparison of adverse cardiovascular events in patients
The control group showed a notably higher incidence of adverse cardiovascular events than the control group (P=0.039, Table 4).
Table 4.
Adverse cardiovascular events
| Group | Cardiac failure | Angina pectoris | Arrhythmia | Total incidence rate |
|---|---|---|---|---|
| Control group (n=58) | 2 (3.44%) | 3 (5.19%) | 4 (6.88%) | 9 (15.51%) |
| Observation group (n=66) | 1 (1.52%) | 1 (1.52%) | 1 (1.52%) | 3 (4.56) |
| X2 value | 4.252 | |||
| P value | 0.039 | |||
Analysis of risk factors for adverse cardiovascular events
According to the occurrence of adverse cardiovascular events, the patients were grouped into the occurrence group (n=12) and the non-occurrence group (n=112), and NYHA grade, LVEF, LVEDD, CK, CK-MB, Lp-PLA2 showed great differences between the two group (all P < 0.05, Table 5). Further logistic regression analysis showed that NYHA grade, LVEF, LVEDD, CK-MB and Lp-PLA2 were independent risk factors for adverse cardiovascular events (all P < 0.05, Table 6). Then, through the regression equation, we constructed a prediction model: =-86.255 + (4.645*NYHA grade) + (-0.581*LVEF) + (1.058*LVEDD) + (0.263*CK-MB) + (0.121*Lp-PLA2). According to ROC curve-based analysis, the area under the curve (AUC) of the model in predicting adverse cardiovascular events was 0.970, indicating that the model was an ideal prediction model (Figure 3).
Table 5.
Multivariate analysis
| Factor | Occurrence group (n=12) | Non-occurrence group (n=112) | χ2/t value | P value |
|---|---|---|---|---|
| Age | 2.119 | 0.145 | ||
| ≥ 60 years old (n=92) | 11 | 81 | ||
| < 60 years old (n=32) | 1 | 31 | ||
| Gender | 1.543 | 0.214 | ||
| Male (n=82) | 6 | 76 | ||
| Female (n=42) | 6 | 36 | ||
| BMI | 0.007 | 0.933 | ||
| ≥ 23 kg/m2 (n=40) | 4 | 36 | ||
| < 23 kg/m2 (n=84) | 8 | 76 | ||
| Course of disease | 0.080 | 0.776 | ||
| ≥ 3 h (n=46) | 4 | 42 | ||
| < 3 h (n=78) | 8 | 70 | ||
| NYHA classification | 7.519 | 0.023 | ||
| Grade II (n=37) | 1 | 36 | ||
| Grade III (n=47) | 3 | 44 | ||
| Grade IV (n=40) | 8 | 32 | ||
| Past medical history | ||||
| Hypertension (n=63) | 7 | 56 | 0.301 | 0.583 |
| Diabetes mellitus (n=49) | 4 | 45 | 0.212 | 0.644 |
| History of smoking | 0.039 | 0.843 | ||
| Yes (n=90) | 9 | 81 | ||
| No (n=34) | 3 | 31 | ||
| LVEF (%) | 49.48±4.47 | 55.74±5.86 | 3.580 | 0.001 |
| LVEDD (mm) | 43.23±3.30 | 39.81±3.63 | 3.123 | 0.002 |
| CK (U/L) | 26.00±2.68 | 23.00±3.76 | 2.688 | 0.008 |
| CK-MB (U/L) | 84.79±8.34 | 76.00±9.34 | 3.126 | 0.002 |
| Lp-PLA2 (μg/L) | 229.91±19.16 | 212.46±19.96 | 2.887 | 0.004 |
Notes: BMI: Body Mass Index; NYHA: New York Heart Association; LVEF: Left Ventricular Ejection Fraction; LVEDD: Left Ventricular End-Diastolic Dimension; Lp-PLA2: Lipoprotein-Associated Phospholipase A2; CK: Creatine Phosphokinase; CK-MB: Creatine Phosphokinase Isoenzyme.
Table 6.
Multivariate analysis
| Factor | B | SE | Wals | Sig. | Exp (B) | 95% CI of EXP (B) | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Lower limit | Upper limit | ||||||
| NYHA classification | 4.645 | 1.908 | 5.927 | 0.015 | 104.076 | 2.473 | 4380.376 |
| LVEF | -0.581 | 0.240 | 5.842 | 0.016 | 0.559 | 0.349 | 0.896 |
| LVEDD | 1.058 | 0.389 | 7.403 | 0.007 | 2.880 | 1.344 | 6.169 |
| CK | 0.647 | 0.354 | 3.341 | 0.068 | 1.909 | 0.954 | 3.819 |
| CK-MB | 0.263 | 0.118 | 4.989 | 0.026 | 1.301 | 1.033 | 1.639 |
| Lp-PLA2 | 0.121 | 0.050 | 5.839 | 0.016 | 1.129 | 1.023 | 1.246 |
Note: LVEF: Left Ventricular Ejection Fraction; LVEDD: Left Ventricular End-Diastolic Dimension; Lp-PLA2: Lipoprotein-Associated Phospholipase A2; CK: Creatine Phosphokinase; CK-MB: Creatine Phosphokinase Isoenzyme; NYHA: New York Heart Association.
Figure 3.
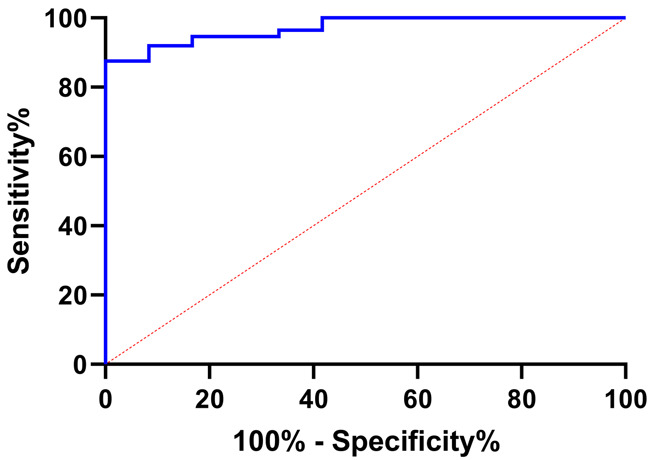
ROC of risk model in predicting patients’ adverse cardiovascular events. Note: ROC: Receiver Operating Characteristic.
Discussion
In the past decades, great progress has been achieved in the treatment of AMI by revascularization, which greatly lowers the risk of death for AMI patients [17]. Over the past ten years, effective progress has been made in the reperfusion therapy of AMI. Some scholars have pointed out that about 90% of AMI cases are caused by coronary artery thrombosis, so thrombolytic therapy is an effective means to restore myocardial reperfusion and limit myocardial necrosis in AMI patients [18]. Rt-PA thrombolytic therapy can activate fibrin-binding plasminogen in thrombus, degrade fibrin and fibrinogen, promote thrombolysis and relieve coronary artery occlusion [19]. However, according to prior research [20], rt-PA thrombolysis has high selectivity, with little effect on the fibrinolytic activity of the whole body during the treatment process, but it brings the risks of vascular re-occlusion and ischemia-reperfusion injury.
With a history of thousands of years in China, Chinese herbal medicine is extensively used in the treatment of AMI-related diseases, and it is considered as a natural product with good curative effect and few side effects [21]. Danhong injection is one of the most extensively adopted traditional Chinese medicines for treating AMI in China [22]. Reportedly, Danhong injection can inhibit platelet activation and aggregation, both of which take a crucial part in the pathogenesis of AMI [23]. Moreover, Danhong injection has been found to be able to protect ischemic myocardium from myocardial ischemia/reperfusion injury [24]. In this study, Danhong injection effectively improved the clinical efficacy of tirofiban combined with conventional treatment, and effectively inhibited the level of LVEDD and myocardial enzyme spectrum of patients, and improved LVEF. Qi et al. [25] have revealed that Danhong injection can reduce the myocardial infarction area of patients with acute ST-segment elevation myocardial infarction who are at high risk of no-reflow during direct PCI. Lv et al. [26] have found that Danhong injection combined with Naoxintong Tablet can reduce the inflammatory reaction in patients with acute coronary syndrome and improve their cardiac function 3 months after discharge. All these studies suggest that Danhong injection can alleviate the condition of AMI, promote thrombolysis and improve the curative effect. The reasons may be as follows: Danhong injection is composed of two kinds of traditional Chinese medicines, Salvia miltiorrhiza and safflower, both of which can dilate blood vessels, lower vascular resistance, improve microcirculation and reduce blood viscosity, etc. The main component of safflower is chalcone compounds, which can dilate blood vessels, increase blood flow and inhibit platelet aggregation, thus improving the therapeutic effect [27].
Lp-PLA2, also known as platelet activating factor acetylhydrolase, is secreted by mature lymphocytes and macrophages, which can eventually produce oxidized free fatty acids and lysophospholipids and can also promote the expression of various inflammatory factors and aggravate the injury of arterial intima, finally inducing the formation of unstable plaques [28,29]. In this study, after treatment, the serum Lp-PLA2 level in patients after treatment declined greatly, and declined more greatly in the observation group treated with additional Danhong injection, indicating that the combined treatment could effectively regulate Lp-PLA2 and protect the cardiac function.
Finally, we analyzed the risk factors of adverse cardiovascular events after treatment, and found that NYHA grade, LVEF, LVEDD, CK-MB and Lp-PLA2 were independent risk factors for adverse cardiovascular events. In the study by Wu et al. [30], LVEF was found to be independently bound up with adverse cardiovascular events. In the community-based cohort of patients with acute coronary syndrome, Li et al. [31] found strong association of Lp-PLA2 with major cardiac adverse events and gradually increased risk identification. These studies are basically similar to the results of our analysis. Therefore, the indexes before treatment can be used as reference indexes for evaluating the occurrence of adverse cardiovascular events in patients. In order to further predict the occurrence of adverse events in patients, this study constructed a prediction model by logistic regression. The AUC of the model in forecasting the patients’ adverse events was 0.970, indicating that the model is an ideal model and worthy of clinical popularization.
This study determined through analysis that Danhong injection combined with tirofiban can improve the cardiac function, myocardial enzyme spectrum and Lp-PLA2 level of AMI patients and constructed an ideal prediction model. However, this study still has some limitations. First, the study is a retrospective study with a limited sample size, so the samples can’t be as uniform as that in a randomized controlled experiment. Secondly, although this study constructed a model, external samples have not been collected for verification. Finally, in this study, the patients cannot be followed up, so the long-term prognosis of the patients remains unclear. Therefore, it is hoped to carry out more experiments and follow-up in the future to improve the research conclusions.
To sum up, Danhong injection combined with tirofiban can improve the cardiac function, myocardial enzyme spectrum and Lp-PLA2 level in AMI patients.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81770292) and Natural Foundation of ZheJiang Provincial China (LGF18H300005) and Natural Foundation of JiangXi Provincial China (20202BABL216078/20195656).
Disclosure of conflict of interest
None.
References
- 1.Henry TD, Tomey MI, Tamis-Holland JE, Thiele H, Rao SV, Menon V, Klein DG, Naka Y, Pina IL, Kapur NK, Dangas GD American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; and Council on Cardiovascular and Stroke Nursing. Invasive management of acute myocardial infarction complicated by cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2021;143:e815–e829. doi: 10.1161/CIR.0000000000000959. [DOI] [PubMed] [Google Scholar]
- 2.Zhao J, Zeng Y, Shen X. Efficacy and safety of early initiation of Sacubitril/Valsartan in patients after acute myocardial infarction: a meta-analysis. Clin Cardiol. 2021;44:1354–1359. doi: 10.1002/clc.23717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiele H, Desch S, de Waha S. Acute myocardial infarction in patients with ST-segment elevation myocardial infarction: ESC guidelines 2017. Herz. 2017;42:728–738. doi: 10.1007/s00059-017-4641-7. [DOI] [PubMed] [Google Scholar]
- 4.Zhang F, Su S, Hou Y, Zhao L, Wang Z, Liu F, Wu F, Zhang L. Effects (MACE and bleeding events) of ticagrelor combined with omeprazole on patients with acute myocardial infarction undergoing primary PCI. Hellenic J Cardiol. 2020;61:306–310. doi: 10.1016/j.hjc.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Matsuo H, Uemura S, Kadota K, Hikichi Y, Tsujita K, Ako J, Nakagawa Y, Morino Y, Hamanaka I, Shiode N, Shite J, Honye J, Matsubara T, Kawai K, Igarashi Y, Okamura A, Ogawa T, Shibata Y, Tsuji T, Yajima J, Iwabuchi K, Komatsu N, Sugano T, Yamaki M, Yamada S, Hirase H, Miyashita Y, Yoshimachi F, Kobayashi M, Aoki J, Oda H, Katahira Y, Ueda K, Nishino M, Nakao K, Michishita I, Ueno T, Inohara T, Kohsaka S, Ismail TF, Serruys PW, Nakamura M, Yokoi H, Ikari Y Task Force on Primary Percutaneous Coronary Intervention (PCI) of the Japanese Cardiovascular Interventional Therapeutics (CVIT) CVIT expert consensus document on primary percutaneous coronary intervention (PCI) for acute myocardial infarction (AMI) in 2018. Cardiovasc Interv Ther. 2018;33:178–203. doi: 10.1007/s12928-018-0516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeitouni M, Silvain J, Guedeney P, Kerneis M, Yan Y, Overtchouk P, Barthelemy O, Hauguel-Moreau M, Choussat R, Helft G, Le Feuvre C, Collet JP, Montalescot G ACTION Study Group. Periprocedural myocardial infarction and injury in elective coronary stenting. Eur Heart J. 2018;39:1100–1109. doi: 10.1093/eurheartj/ehx799. [DOI] [PubMed] [Google Scholar]
- 7.Cho MS, Ahn JM, Lee CH, Kang DY, Lee JB, Lee PH, Kang SJ, Lee SW, Kim YH, Lee CW, Park SW, Park DW, Park SJ. Differential rates and clinical significance of periprocedural myocardial infarction after stenting or bypass surgery for multivessel coronary disease according to various definitions. JACC Cardiovasc Interv. 2017;10:1498–1507. doi: 10.1016/j.jcin.2017.05.051. [DOI] [PubMed] [Google Scholar]
- 8.Brener SJ, Dambrink JH, Maehara A, Chowdhary S, Gershlick AH, Genereux P, Koolen J, Mehran R, Fahy M, Gibson CM, Stone GW. Benefits of optimising coronary flow before stenting in primary percutaneous coronary intervention for ST-elevation myocardial infarction: insights from INFUSE-AMI. EuroIntervention. 2014;9:1195–1201. doi: 10.4244/EIJV9I10A201. [DOI] [PubMed] [Google Scholar]
- 9.Sim DS, Jeong MH, Kang JC. Current management of acute myocardial infarction: experience from the Korea Acute Myocardial Infarction Registry. J Cardiol. 2010;56:1–7. doi: 10.1016/j.jjcc.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Jang SH, Sohn SI, Park H, Lee SJ, Kim YW, Hong JM, Kim CH, Choi JW, Kang DH, Kim YS, Hwang YH, Lee JS, Hong JH. The safety of intra-arterial tirofiban during endovascular therapy after intravenous thrombolysis. AJNR Am J Neuroradiol. 2021;42:1633–1637. doi: 10.3174/ajnr.A7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang L, Tang X, Yang Q. The application of tirofiban in the endovascular treatment of acute ischemic stroke: a meta-analysis. Cerebrovasc Dis. 2021;50:121–131. doi: 10.1159/000512601. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Zhang L, Yang Y. Tirofiban hydrochloride sodium chloride injection combined with cardiovascular intervention in the treatment of Acute Myocardial Infarction. Pak J Med Sci. 2020;36:54–58. doi: 10.12669/pjms.36.2.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng X, Li Y, Wang Y, Li L, Little PJ, Xu SW, Liu S. Danhong injection in cardiovascular and cerebrovascular diseases: pharmacological actions, molecular mechanisms, and therapeutic potential. Pharmacol Res. 2019;139:62–75. doi: 10.1016/j.phrs.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Feng C, Wan H, Zhang Y, Yu L, Shao C, He Y, Wan H, Jin W. Neuroprotective effect of Danhong injection on cerebral ischemia-reperfusion injury in rats by activation of the PI3K-AKT pathway. Front Pharmacol. 2020;11:298. doi: 10.3389/fphar.2020.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui X, Han S, Jin XL, Wang ZF, Zhang Q, Xie YM. Clinical comprehensive evaluation of Danhong injection in treatment of stroke with blood stasis syndrome. Zhongguo Zhong Yao Za Zhi. 2021;46:6096–6104. doi: 10.19540/j.cnki.cjcmm.20210930.506. [DOI] [PubMed] [Google Scholar]
- 16.Damluji AA, van Diepen S, Katz JN, Menon V, Tamis-Holland JE, Bakitas M, Cohen MG, Balsam LB, Chikwe J American Heart Association Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Surgery and Anesthesia; and Council on Cardiovascular and Stroke Nursing. Mechanical complications of acute myocardial infarction: a scientific statement from the American Heart Association. Circulation. 2021;144:e16–e35. doi: 10.1161/CIR.0000000000000985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu Y, Yan Y, Liu X. Effects of alprostadil combined with tanshinone IIa injection on microcirculation disorder, outcomes, and cardiac function in AMI patients after PCI. Ann Palliat Med. 2021;10:97–103. doi: 10.21037/apm-20-2147. [DOI] [PubMed] [Google Scholar]
- 18.Phrommintikul A, Abdel-Aty H, Schulz-Menger J, Friedrich MG, Taylor AJ. Acute oedema in the evaluation of microvascular reperfusion and myocardial salvage in reperfused myocardial infarction with cardiac magnetic resonance imaging. Eur J Radiol. 2010;74:e12–17. doi: 10.1016/j.ejrad.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Che R, Zhao W, Ma Q, Jiang F, Wu L, Yu Z, Zhang Q, Dong K, Song H, Huang X, Ji X. Rt-PA with remote ischemic postconditioning for acute ischemic stroke. Ann Clin Transl Neurol. 2019;6:364–372. doi: 10.1002/acn3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marder VJ. Comparison of thrombolytic agents: selected hematologic, vascular and clinical events. Am J Cardiol. 1989;64:2A–7A. doi: 10.1016/0002-9149(89)90921-1. discussion 24A-26A. [DOI] [PubMed] [Google Scholar]
- 21.Lu CY, Lu PC, Chen PC. Utilization trends in traditional Chinese medicine for acute myocardial infarction. J Ethnopharmacol. 2019;241:112010. doi: 10.1016/j.jep.2019.112010. [DOI] [PubMed] [Google Scholar]
- 22.Liang J, He X, Zhou H, Liang P. Effects of Danhong injection on cardiac function and blood lipid in patients with angina pectoris of coronary heart disease: a protocol for randomized, double-blind, placebo-controlled clinical trial. Medicine (Baltimore) 2021;100:e27479. doi: 10.1097/MD.0000000000027479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Shi X, Gao J, Zhou R, Guo F, Zhang Y, Fan F, Zhai Q, Sun M, Yang H. Danhong injection and trimetazidine protect cardiomyocytes and enhance calcium handling after myocardial infarction. Evid Based Complement Alternat Med. 2021;2021:2480465. doi: 10.1155/2021/2480465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo H, Li MJ, Liu QQ, Guo LL, Ma MM, Wang SX, Yu B, Hu LM. Danhong injection attenuates ischemia/reperfusion-induced brain damage which is associating with Nrf2 levels in vivo and in vitro. Neurochem Res. 2014;39:1817–1824. doi: 10.1007/s11064-014-1384-1. [DOI] [PubMed] [Google Scholar]
- 25.You Q, Wang J, Dong W, Tian F, Liu HX, Jing J, Chen YD. Protective effect of Danhong injection in patients with acute myocardial infarction at a high risk of no-reflow during primary percutaneous coronary intervention. J Geriatr Cardiol. 2019;16:406–413. doi: 10.11909/j.issn.1671-5411.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lv Y, Pan Y, Gao Y, Lu J, Li Y, Bai J, Zhai J. Effect of Danhong injection combined with Naoxintong tablets on prognosis and inflammatory factor expression in acute coronary syndrome patients undergoing percutaneous coronary intervention. Acta Cardiol Sin. 2015;31:301–307. doi: 10.6515/ACS20150502A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li-Jun H, Ai-Hua L, Cong-Zhao F, Jing-Yuan S. Production and quality control of original herbal materials of Danhong injection. Zhongguo Zhong Yao Za Zhi. 2020;45:5443–5451. doi: 10.19540/j.cnki.cjcmm.20200915.302. [DOI] [PubMed] [Google Scholar]
- 28.Bonnefont-Rousselot D. Lp-PLA2, a biomarker of vascular inflammation and vulnerability of atherosclerosis plaques. Ann Pharm Fr. 2016;74:190–197. doi: 10.1016/j.pharma.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Ait-Oufella H, Mallat Z, Tedgui A. Lp-PLA2 and sPLA2: cardiovascular biomarkers. Med Sci (Paris) 2014;30:526–531. doi: 10.1051/medsci/20143005015. [DOI] [PubMed] [Google Scholar]
- 30.Wu C, Huo X, Liu J, Zhang L, Bai X, Hu S, Li X, Lu J, Zheng X, Li J, Zhang H. Development and validation of a risk prediction model for in-hospital major cardiovascular events in patients hospitalised for acute myocardial infarction. BMJ Open. 2021;11:e042506. doi: 10.1136/bmjopen-2020-042506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li N, Li S, Yu C, Gu S. Plasma Lp-PLA2 in acute coronary syndrome: association with major adverse cardiac events in a community-based cohort. Postgrad Med. 2010;122:200–205. doi: 10.3810/pgm.2010.07.2187. [DOI] [PubMed] [Google Scholar]