Abstract
The invasion and metastasis of malignant tumors are major causes of death. The most common metastases of cancer are lymphatic metastasis and hematogenous metastasis. Hematogenous metastasis often leads to rapid tumor dissemination. The mechanism of hematogenous metastasis of malignant tumors is very complex. Some experts have found that platelets play an important role in promoting tumor hematogenous metastasis. Platelets may be involved in many processes, such as promoting tumor cell survival, helping tumor cells escape immune surveillance, helping tumors attach to endothelial cells and penetrating capillaries for distant metastasis. However, recent studies have shown that platelets can also inhibit tumor metastasis. At present, the function of platelets in tumor progression has been widely studied, and they not only promote tumor cell metastasis, but also have an inhibitory effect. Therefore, in-depth and summary research of the molecular mechanism of platelets in tumor cell metastasis is of great significance for the screening and treatment of cancer patients. The following is a brief review of the role of platelets in the process of malignant tumor metastasis.
Keywords: Platelets, Malignant tumors, Metastasis, Invasion
Platelets; Malignant tumors; Metastasis; Invasion.
1. Introduction
Metastasis is the most dangerous stage in the development of malignant tumors, and approximately 60% of the deaths in patients with solid tumors are caused by metastasis [1, 2]. Platelets are the smallest circulating blood cell components, and their main function is to participate in the processes of coagulation, hemostasis, and thrombosis upon vascular or tissue injury. Platelets undergo deformation, paracrine and cytokine release during maturation, which are related to the invasion and metastasis of malignant tumors [3, 4].
With the rapid development of science and technology and numerous researches, the view that platelets promote tumor metastasis has been widely accepted in the medical community and is supported by substantial research data. However, the detailed mechanisms involved are not fully understood, which has made research on the relationship between platelets and tumors a hot topic in oncology in recent years. In 1865, the association between platelets and cancer was named Trousseau's syndrome [5]. Later, Billroth accidently found a potential relationship between tumor cells and platelets during autopsies. He noted that tumor metastasis was often accompanied by the formation of platelet thrombosis, which was similar to the "armor" of tumor cells. He then made the following bold statement: “the process of tumor metastasis is closely related to platelets”. However, this conjecture was not confirmed by researchers such as Gasic and Li until more than half a century later. Gasic et al. [6] established an in vivo model of thrombocytopenia by administering anti-platelet antibody plasma to mice and adding a tumor cell suspension to the in vivo model and then observed the metastasis of tumor cells. The results showed that the metastasis of cancer cells in the mouse model of thrombocytopenia was significantly reduced. Li et al. [7] injected platelets together with tumor cells into mice, and the results showed that the number of tumor metastases in mice infused with platelets increased significantly compared with mice infused with only tumor cells. The above studies preliminarily confirmed that platelets are involved in the process of hematogenous metastasis of malignant tumors, which laid the foundation for further studies on the relationship between tumor cells, platelets and metastasis. Due to the role of platelets in the hematogenous metastasis of malignant tumors, drugs targeting platelets in anti-tumor therapy have become a hotspot in the scientific research field. Pharmaceutical studies showed that aspirin [8, 9, 10], clopidogrel [11, 12] and ticagrelor [13, 14] all inhibit platelets, but while they inhibit platelets, many functions of platelets will also be damaged. To balance the advantages and disadvantages of these drugs, liposomal nanoparticles bearing tumor-homing pentapeptide CREKA (Cys-Arg-Glu-Lys-Ala) have been developed to avoid the negative effects of direct infusion of drugs in patients. Liposomal nanoparticles can deliver platelet inhibiting drugs to tumor tissues, thereby specifically inhibiting tumor-related platelets, and the functions of non-tumor related platelets will not be affected, such as hemostasis and coagulation [15].
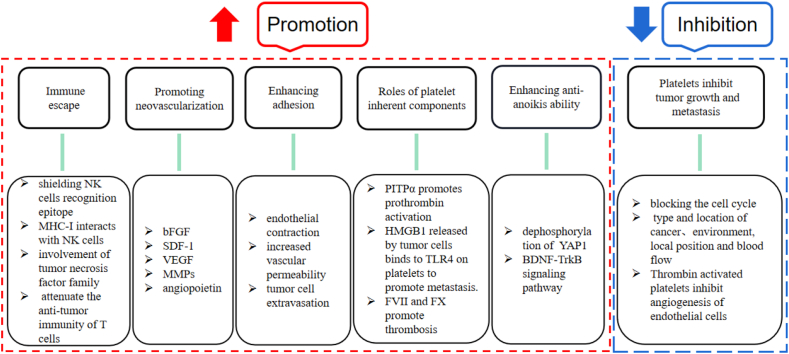
The interaction between tumor cells and platelets is a prerequisite for successful hematogenous metastasis of tumors [16]. There is a complex two-way communication pathway between platelets and tumor cells. The interaction between tumor cells and platelets leads to platelet activation, which releases factors conducive to the survival and proliferation of tumor cells [17, 18]. There are various ways in which tumor cells activate platelets. For example, they can directly secrete thrombin, the most effective agonist for platelet activation, and thrombin regulates platelets through protein kinase receptor feedback, resulting in a platelet aggregation waterfall reaction [7]. Tumor cells can also secrete a series of tissue factors to activate platelets, which starts the process of external coagulation, promotes thrombosis, and then protects tumor cells [19]. In addition, tumor cells can induce platelet activation by releasing the metabolite ADP [20], which is a way of indirectly activating platelets. Platelets activated by tumor cells can promote the survival and invasion of tumor cells through a variety of mechanisms (Figure 1). But recent studies have shown that platelets also play an inhibitory role in tumor growth and metastasis (Figure 1). For example, platelets inhibit tumor cell proliferation by blocking the cell cycle [21]. Platelet factor (PF4) promotes apoptosis and vascular degeneration by binding to its receptor CXCR3B, leading to inhibition of tumor metastasis [22].
Figure 1.
Roles of platelets in tumor invasion and metastasis summarized in this review. The red part is the mechanisms of platelets promoting metastasis, and the blue part is the summaries of platelets inhibiting tumor growth and metastasis.
2. Mechanisms by which platelets promote tumor metastasis
Platelets promote tumor metastasis as a result of platelet-tumor cell interactions. Tumor cells activate platelets, which in turn can stimulate the distant metastasis of tumor cells in a systematic process. Studies have shown that platelets can aid in tumor cell immune escape [23], avoid the influence of blood flow shear force, enhance the ability of tumor cells to resist anoikis and apoptosis [24], promote vascular remodeling, and help tumor cells to enter the blood circulation and metastasize [25]. Therefore, studying the mechanism by which platelets promote tumor metastasis is a crucial step.
2.1. Platelets fight the immune effects of NK cells and T cells
In the blood circulation, cancer cells are monitored and killed by peripheral immune cells such as natural killer (NK) cells [26, 27, 28], T cells [29, 30] and dendritic cells (DCs) [31, 32]. When NK cells encounter tumor cells, NK cells release perforin and granzyme, triggering apoptosis. In addition, CD8+ T cells secrete tumor necrosis factor α (TNF-α) and interferon γ (IFN-γ), hindering tumor metastasis [33]. DCs also secrete various cytokines to enhance cellular immune response [34]. Tumor cells, as antigens in the tumor immune microenvironment, will significantly cause the above reactions, so that immune-related cells can accurately identify, kill, and clear tumor cells. Therefore, platelets assisting tumor cell immune escape is the key to achieve tumor cell metastasis, the current platelet assisted tumor cell immune escape mechanisms are mostly concentrated on NK cells and T cells, which is achieved through the following mechanisms:
2.1.1. Platelets can shield epitopes that NK cells recognize on the tumor cell surface
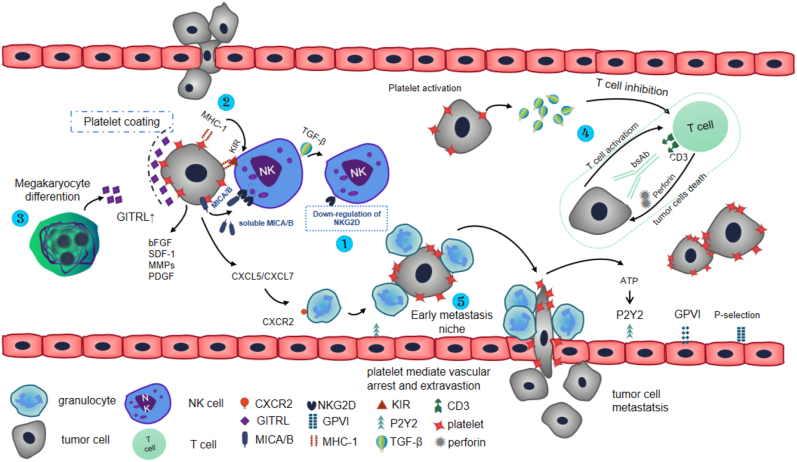
Natural killer cell group 2D (NKG2D) is the surface receptor of NK cells that activates NK cells in the blood, and platelets induce tumor cells to express ligands that can bind to NKG2D receptors, thereby masking the recognition epitopes of NK cells to tumor cells. At the same time, some signal activation events are also significantly inhibited, such as NK cell degranulation and the production of INF-γ, which effectively inhibit the immune clearance of NK cells to tumor cells. Platelet-derived transforming growth factor beta (TGF-β) down-regulates the expression of NK cell activation receptor NKG2D, resulting in the inability of NK cells to be activated [35]. In addition, after tumor-platelet interactions, NKG2D ligands are sheded from the surface of tumor cells [36], resulting in impaired “self-inducing” recognition patterns of NK cells [37]. A study showed that the platelet surface and the post-activation release of ADAM10 promotes NKG2D receptor expression [38]. MICA and MICB, located on the surface of tumor cells, are ligands of NKG2D receptors. Platelets interact with tumor cells, causing the secretion of MICA and MICB into the tumor microenvironment, which can inhibit the expression of NKG2D receptor on NK cells, resulting in blocked activation of NK cells and immune escape of tumor cells [39] (Figure 2①). Platelets covering the surface of circulating tumor cells (CTCs) are equivalent to the “protective umbrella” of tumor cells. Platelets gather and wrap on the surface of tumor cells to avoid direct contact between NK cells and tumor cells, preventing NK cells from recognizing the tumor cells, platelets can also aggregate fibrin around CTCs through activated thrombin, doubly increasing the physical barrier to NK cells and protecting tumor cells from the attack of blood turbulence and damage due to blood flow shear force [40].
Figure 2.
Mechanism by which platelets promote tumor metastasis. Tumor cells separate from the primary tumor and invade the blood circulation. Tumor cells immediately activate and interact with platelets in the first minutes. ① Platelets secrete TGF-β upon activation which induces NK cell dysfunction due to NKG2D down-regulation. In addition, the ligands MICA and MICB of NKG2D are lost from the surface of tumor cells in a platelet dependent manner, resulting in the down-regulation of NKG2D receptor function. ② After platelets interact with tumor cells, platelet-derived MHC-1 molecules translocate to the surface of tumor cells. KIR is a specific inhibitory receptor on the surface of NK cells, and inhibitory KIR binds specifically to MHC-1 to inhibit the immunogenicity of NK cells. ③ GITRL on the platelet surface binds to GITR receptor, which inhibits the activity of NK cells. ④ Tumor-associated platelets down-regulate T cell recruitment bispecific antibody (BsAb)-mediated reactivity of CD4+ T cells and CD8+ T cells, resulting in degranulation of T cells, impaired perforin secretion and destruction of anti-tumor cytotoxicity of T cells. The green dashed box represents the anti-tumor response mediated by BsAb under normal conditions. ⑤ Granulocytes are recruited to the aggregation areas of platelets and tumor cells by chemokines CXCL5 and CXCL7 to form an early metastasis niche. Finally, platelets mediate the stagnation of tumor cells in the vascular wall through P-selectin and GPVI, and promote the extravasation of tumor cells to the subendothelial matrix of distant organs by activating endothelial P2Y2 receptor. Furthermore, platelets secrete bFGF, SDF-1, MMPs and PDGF to promote angiogenesis, creating favorable conditions for tumor cell metastasis.
2.1.2. Major histocompatibility complex-I (MHC-I) interacts with NK cells to inhibit NK cell immunogenicity
NK cells express killer cell immunoglobulin-like receptors (KIRs), which are specific inhibitory receptors for MHC-I molecules. KIRs are divided into two haplotypes, A and B. Haplotype A includes KIR3DL3, KIR2DL3, KIR2DP1, KIR2DL1, KIR3DP1, KIR2DL4, KIR3DL1, KIR2DS4, KIR3DL2 and pseudogene KIR2DP1. In group A haplotypes, with the exception of KIR2DS4, the others are inhibitory KIRs. Group B haplotypes include KIR2DS1, KIR2DS2, KIR2DS3, KIR2DS5, KIR3DS1, KIR2DL2 and KIR2DL5. In group B haplotypes, with the exception KIR2DL2 and KIR2DL5, the others are activating KIRs [41]. After the interaction between platelets and tumor cells, platelet-derived MHC-I molecules specifically bind to the inhibitory receptor KIR, inhibitory receptor KIR is activated and NK cell immune responses are suppressed (Figure 2②). In addition, MHC-I molecules can also prevent NK cells from recognizing tumor cells through the TGF-β and nuclear factor-kB pathways [42]. This can also indicate that in cells with low expression of MHC-I molecules, the immunogenicity of NK cells can be activated. The reactivity of NK cells can be largely restored by blocking MHC-I molecules to prevent tumor progression [37, 43].
2.1.3. Tumor necrosis factor family members are involved in platelet-mediated tumor cell escape from NK cell attack
Zheng et al. [44] showed that tumor necrosis factor related ligand (GITRL), induced by glucocorticoids, is produced during megakaryocyte differentiation, resulting in the expression of GITRL by megakaryocytes and their platelet offspring. When tumor cells interact with platelets, the platelets are activated and GITRL rapidly aggregates on the surface of platelets and binds to GITR expressed by NK cells (Figure 2③). The interaction of GITR-GITRL will lead to a significant reduction in the immunogenicity of NK cells [45, 46, 47], and this interaction can also inhibit NK cells from producing INF-γ [48]. Zheng et al. showed that blocking GITR expressing NK cells can significantly reverse NK cell reactivity. However, in the absence of platelets, blocking GITR-GITRL interaction will not change NK cell reactivity. In addition, the generation of INF-γ will be partially recovered by adding anti-GITR antibody. These phenomena confirmed the inhibitory effect of platelets derived GITRL on NK cell reactivity. In addition, activated GITR is involved in inhibiting IL-15 mediated signal activation events in NK cells, resulting in blocked production of cytokines and cytotoxic particles, and weakened proliferative capacity of tumor cells [49], enabling cancer cells to escape the attack of NK cells and to metastasize distantly [50]. Tumor-associated platelets can up-regulate the expression of RANKL, a member of the TNF family, and RANKL can inhibit the anti-tumor activity of normal NK cells [51], but there have been few studies on this subject.
2.1.4. Platelets attenuate the anti-tumor immunity of T cells
Lutz et al. showed that tumor-associated platelets down-regulate T cell recruitment bispecific antibody (BsAb)-mediated reactivity of CD4+ T cells and CD8+ T cells in a TGF-β-dependent manner, specifically manifested by T cell degranulation, impaired perforin secretion, and destruction of T cell anti-tumor cytotoxic effects, that is, the main mechanism inside the green box in Figure 2④ is broken [52]. Platelet derived soluble factors such as lactate and TGF-β suppress the function of CD4+ T cells and CD8+ T cells, enabling tumor cells to escape the immune surveillance of T cells [53]. In addition, TGF-β converts CD4+ T cells into induced regulatory T cells (ITregs) [54], and ITregs have the ability to kill activated T cells through a granzyme B-dependent mechanism [55, 56]. Polasky et al. confirmed that platelets can reduce the expression of programmed cell death protein-1 (PD1) on CD4+ T cells, while inhibiting the production of INF-γ and TNF-α [57], which is conducive to the successful metastasis of cancer cells, that is, platelets promote cancer cell metastasis by inhibiting the immune response of T cells.
2.2. Platelets secrete a variety of bioactive factors to promote the formation of neovascularization
The growth of tumors and the formation of metastasis depend on the formation of new blood vessels. There are abundant vessels in the primary tumor, and platelets in blood vessels can secrete angiogenic regulatory factors such as stromal cell-derived factor-1 (SDF-1) [58], basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) [59], which affect angiogenesis and distribution in tumor tissue. Tumor-associated platelets can also secrete matrix metalloproteinases (MMPs) [60], which are very helpful for tumor vascular remodeling and angiogenesis. The enhanced expression of MMPs degrades the extracellular matrix, and interstitial tumor cells leave the primary site and invade the peripheral blood circulation with the help of 5-hydroxytryptamine and histamine released by platelets. Studies have shown that activated platelets release angiopoietin [61], VEGF [62], secretogranin III, cyclophilin A, and calumenin [63], which promote angiogenesis, vascular proliferation and vascular remodeling, maintain the integrity of tumor blood vessels, promote the growth and proliferation of tumor cells. In addition, platelet-derived growth factor (PDGF) is a relatively new angiogenesis signal molecule that mediates vascular maturation and stability [64]. Neovascularization is similar to the “nutrition absorption center” of tumor cells, creating favorable conditions for the hematogenous metastasis of tumor cells.
2.3. The interaction between platelets and tumor cells promotes the cross-endothelial migration of tumor cells
The adhesion between vascular endothelial cells and tumors is very low, but platelet activation increases the expression of P-selectin [65] on the plasma membrane, platelet surface integrins such as αIIbβ3 [66] and α6β1 [67], resulting in a sudden increase in adhesion between mucin on the surface of tumor cells and vascular endothelial cells, which greatly helps tumor cells implant at the metastatic site. Tumor cells then penetrate the capillary canal walls and follow the blood circulation to the target organ. The specific mechanisms of action are as follows: (1) CD97 is an adhesion G protein-coupled receptor (GPCR) and over-expresses tumor antigen in a variety of cancers. The CD97-platelet interaction coordinates tumor invasion and endothelial cell contraction, resulting in increased inter-cellular space and promoting the diffusion of tumor cells into the blood circulation [42]. (2) Following induced activation, platelets secrete prostaglandins, resulting in increased vascular permeability. (3) Galectin-3 on the tumor surface binds to platelet glycoprotein 4 (GPVI), which can induce a series of changes, such as platelet activation, shape change, degranulation and ATP secretion. The released ATP then acts on the endothelial purine receptor P2Y2, thereby increasing vascular permeability and further enhancing the cross-endothelial migration of tumor cells [68]. (4) After platelet-tumor cell interactions, platelets secrete CXCL5 and CXCL7 chemotactic factors, and granulocytes are recruited by platelet-derived CXCL5/7 chemokines through the granulocyte receptor CXCR2. Platelets and granulocytes are recruited in an orderly manner to tumor cells to form an “early metastasis niche” [69] (Figure 2⑤), which promotes tumor migration out of endothelial cells. In conclusion, the interactions between platelets and tumor cells create favorable conditions for tumor cell cross-endothelial migration.
2.4. Inherent components on platelets promote tumor metastasis
Many studies have shown that some inherent components of platelets have positive significance for tumor hematogenous metastasis. Phosphatidylinositol transfer protein-α (PITPα) in platelets plays an important role in the process of platelet phosphatidyl inositol signal transduction, PITPα is essential for the synthesis of higher-order phosphatidylinositol-PtdIns (4,5) P2, when stimulated by thrombin, phospholipase C hydrolyzes phosphatidylinositol-PtdIns (4,5) P2, producing inositol triphosphate (IP3). The production of IP3 within platelets stimulates an increase in cytoplasmic calcium, resulting in exposure to phosphatidylserine on the surface of platelets, and then the prothrombin is activated to start the endogenous coagulation process, which facilitates the formation of platelet-fibrin complexes that envelop tumor cells and allows tumor cells to metastasize remotely [70]. High-mobility group Box 1 (HMGB1) released by tumor cells is a key factor that interacts with platelet toll-like receptor 4 (TLR4), mediating platelet-tumor cell interactions and promoting migration and invasion, suggesting that TLR4 and its endogenous ligand HMGB1 may be the target of anti-metastatic therapy [42]. In addition, tissue factors expressed by tumor cells interact with platelet coagulation factor VII (FVII) and coagulation factor X (FX) to produce thrombin. Thrombin not only leads to the formation of fibrin and the activation of platelet receptors but also promotes thrombosis, and then the thrombus wraps tumor cells to achieve distant metastasis [71].
2.5. Platelets enhance the ability of tumor cells to resist anoikis
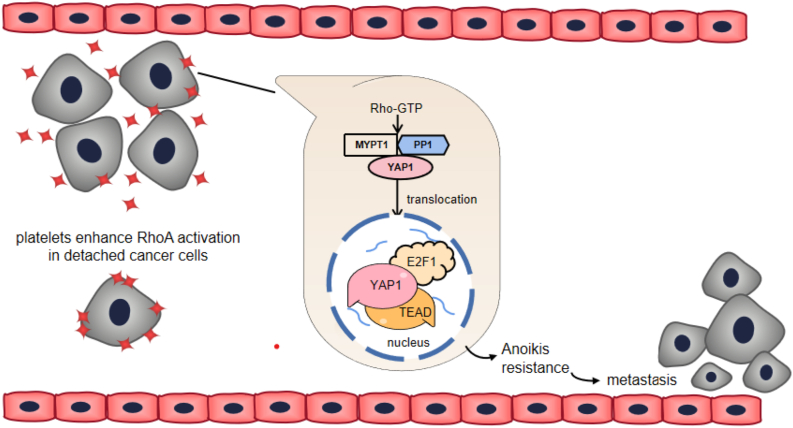
Anoikis induces cell death through the traditional apoptotic pathway, which was named for the first time in 1994. Its core feature in the failure of anchoring between cells or between cells and the matrix, resulted in cell autonomous death. Anoikis plays roles in homeostasis regulation, disease occurrence and tumor metastasis, and reduces the probability of tumor cells implanting in unsuitable places. Therefore, reducing anoikis is a necessary condition for tumor cells to achieve successful invasion and metastasis. Platelets not only induce tumor cells to fail contact with the extracellular matrix, but also destroy tumor cells to adhere to other cells, causing tumor cells to survive in a paracrine or autocrine manner to resist natural apoptosis and restore the ability to attach, spread, metastasize, and invade. Platelets can secrete TGF-β and PDGF to maintain epithelial mesenchymal transformation (EMT) of CTCs. PDGF can participate in the maintenance of EMT by activating signal transducer and activator of transcription (STAT) [72]. In pancreatic cancer, PDGF activates the Notch signaling pathway. Notch is a relatively conserved ligand receptor pathway that induces EMT [73], while TGF-β signaling enhances the expression of PDGF [74], and the two work together to enhance the anti-apoptotic ability of tumor cells [9]. After dephosphorylation of Yes-associated protein 1 (YAP1) mediated by the platelet-activated RhoA-MYPT1-PP1 pathway, YAP1 translocates to the nucleus and binds to transcription factors E2F1 and TEAD2/4, promoting proliferation and inhibiting apoptosis (Figure 3). Interestingly, tumor cells that do not adhere to platelets do not undergo dephosphorylation of YAP1, that is, YAP1 activity is the main reason for the platelet mediated enhanced survival of cancer cells [24]. Tanaka et al. showed that in colorectal cancer, tumor cells can resist anoikis apoptosis through their own BDNF-TrkB (brain-derived neurotrophic factor-tyrosine kinase receptors) signaling pathway [75]. At present, little is known about the mechanism of platelet resistance to anoikis apoptosis.
Figure 3.
Platelets enhance the ability of tumor cells to resist anoikis. Platelet activated YAP1 translocates to the nucleus and binds to transcription factors E2F1 and TEAD2/4 to inhibit apoptosis and promote metastasis.
Thus, platelets play an important role in the proliferation and metastasis of tumor cells. On the primary side of the tumor, platelets in blood vessels secrete pro-angiogenic regulatory factors such as SDF-1, bFGF and VEGF to promote the proliferation of cancer cells and create favorable conditions for cell migration. Platelets infiltrating around tumor tissue secrete TGF-β, while activating TGF-β/Smad and NF-κB pathways in cancer cells, causing cancer cells to acquire a mesenchymal-like phenotype and metastasis [76]. It has been confirmed that GPVI and P-selectin (Figure 2) on the surface of platelets can help metastatic cells colonize at the distance [77, 78]. Studies have shown that the number of platelets in most patients with solid tumors is abnormal. For example, in gastric cancer, platelets directly contact gastric cancer cells and promote the malignant behavior of cancer cells [79]. In a mouse model of breast cancer, it was found that the activation level of platelets and the degree of interaction between platelets and cancer cells increased with tumor progression [80]. Therefore, platelets have become one of the markers for judging the poor prognosis of tumor patients [81, 82, 83, 84].
3. Inhibition of platelet functions and blockage of tumor metastasis
Platelets promote tumor metastasis by inducing EMT in cancer cells and protecting CTCs from immune-mediated clearance. Therefore, inhibition of platelet function provides a potential new method for treatment centered on eliminating tumor metastasis [15]. On the one hand, inhibiting platelet function can accelerate platelet apoptosis to inhibit the metastasis and invasion of cancer cells. Wang et al. [85] showed that tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) plays an important role here, that is, TRAIL receptor-DR5 is expressed on the surface of platelets. The combination of the two promotes platelet apoptosis and hinders tumor cell migration, which provides an innovative way for us to identify potential new targets to inhibit tumor metastasis. However, Greer et al. [86] confirmed that the anti-cancer properties of TRAIL are also limited, as it causes liver toxicity, and many tumor cells will develop drug resistance to TRAIL. Further study have found that recombinant TRAIL protein can function better, inhibit the growth of tumor cells, and even cause tumors to regress without obvious damage to the host, suggesting that it has potential good application prospects in tumor therapy. On the other hand, as previously described, liposomal nanoparticles can be used to import platelet inhibitory drugs into tumor tissues to inhibit the function of tumor-associated platelets [15, 87]. The safety of these nanoparticles has been verified and has wide and good application prospects in the clinic.
4. Mechanisms by which platelets inhibit tumor growth and metastasis
Although the view that platelets promote tumor metastasis is widely accepted and supported by a large number of studies, the role of platelets in tumor growth is still controversial [21]. Moreover, the latest research shows that platelets can inhibit tumor metastasis in some ways [88] (Figure 1). However, the mechanism is unclear at present and requires further investigation.
4.1. Platelets inhibit tumor proliferation and metastasis
4.1.1. Platelets inhibit tumor cell proliferation by blocking the cell cycle
Platelet infusion is a necessary supportive treatment for cancer patients, as platelets inhibit tumor cell proliferation by blocking the G0/G1 phase of the cell cycle, and this inhibitory effect increases with increasing infusion time [21]. By analyzing the cell cycle, it was found that platelets affect tumor proliferation by blocking the cell cycle and inhibiting DNA synthesis. Animal experiments showed that platelets inhibit the growth of tumor cells in vitro in a non-MHC-1-dependent manner. This inhibition is not related to tumor type or the apoptosis pathway, but is closely related to the cell cycle [89].
4.1.2. Platelet factors inhibit tumor metastasis
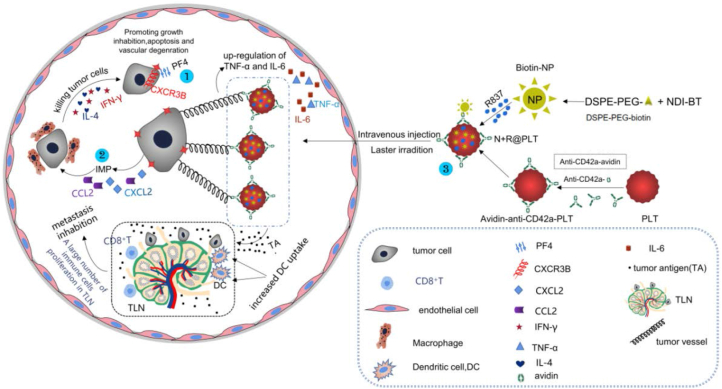
Platelet factor (PF4) plays an inhibitory role in the process of tumor metastasis, mainly by binding with receptor CXCR3B [22]. CXCR3B is a G protein-coupled receptor that is mostly found in the cytoplasm of tumor cells. After binding, they mediate the anti-tumor effect by promoting growth inhibition, cell apoptosis and vascular degeneration [90] (Figure 4①). Glycoprotein Ibalpha (GPIbα), located on the surface of platelets, is a part of the receptor complex GPIb-V-IX, and Erpenbeck et al. [91] showed that inhibition of GPIbα leads to a significant increase in lung metastasis. Therefore, we suspect that targeting certain receptors on the surface of platelets may have an inhibitory effect on tumor metastasis. Platelets and platelet-derived particles have anti-proliferative and cytotoxic effects on many tumor cells [92], thus promoting cell cycle arrest, but the presence or absence of cytotoxic effects is closely related to cancer types. Current studies have shown that the role of platelets in tumor development is more complex than originally expected.
Figure 4.
Mechanism by which platelets inhibit tumor growth and metastasis and the construction of N + R@PLT. ① PF4 binds to CXCR3B in the cytoplasm of tumor cells, promoting tumor growth inhibition, cell apoptosis, vascular degeneration and hindering tumor metastasis. ② Direct interaction between tumor cells and platelets produces IMPs, IMPs produce CCL2 and CXCL2, which are responsible for recruiting macrophages. With the assistance of IL-4 and IFN-γ, macrophages kill tumor cells. ③ Schematic illustration of N + R@PLT construction (Through Suzuki reaction, thiophene and naphthalene diimide were partially integrated into the polymer skeleton to synthesize the photothermal polymer (NDI-BT). Then, hybrid nanoparticles were constructed with the photothermal copolymer and the hydrophilic polyethylene glycol (PEG) segment of distearoyl ethanolamine phosphate (DSPE)-PEG, and then the nanoparticles were modified with biotin. After that, CD42a on PLT membrane was pretreated with avidin labeled anti-CD42a antibody, the highly specific interaction between biotin and avidin will trigger the binding of nanoparticles with anti-CD42a antibody and promote the internalization of nanoparticles into PLT through CD42a molecule. The immunostimulator R837 hydrochloride uniformly dispersing in the medium was also imported during the internalization process, N + R@PLT successfully built). Laser irradiation after intravenous injection, N + R@PLTs interact with tumor vessel, resulting a large amount of exposed tumor antigen was transported to the tumor-draining lymph node (TLN), increasing uptake by DCs. In addition, CD8+T cells and other immune cells also proliferate in TLN, and tumor metastasis is inhibited.
4.2. Platelet inhibition of tumor metastasis is related to many factors
4.2.1. Platelet-induced promotion or inhibition of tumor metastasis is closely related to the type and location of cancer
A study on the effect of the combined removal of NK cells and platelets on liver and lung metastases of melanoma and breast cancer cells showed that the incidence of lung metastasis in mice with combined removal of platelets and NK cells was significantly lower than that in mice with only NK cells removed. In contrast, mice lacking NK cells and platelets had a significantly increased incidence of melanoma liver metastases compared with mice lacking only NK cells [93]. Bulla et al. [94] showed that both thrombolytic products and platelet release products can inhibit the migration of canine osteosarcoma cells, suggesting that platelets may antagonize the migration of canine osteosarcoma. However, there are no studies on specific platelet release products or thrombolytic products, which needs further investigation. Studies have preliminarily confirmed that whether platelets promote or inhibit tumor metastasis is closely related to the location and type of cancer.
4.2.2. The promotion or inhibition of tumor metastasis by platelets is closely related to the environment, local position and blood flow
When platelets infiltrate the tumor microenvironment, the interaction between platelets and tumor cells will lead to the production of three types of microparticles (MPs), including tumor-derived microparticles (TMP), platelet-derived microparticles (PMP) and mixed MPs with both platelet and tumor cell features (T + PMPs). These particles are collectively referred to as IMPs. IMPs produce chemokines CCL2 and CXCL12, which are responsible for recruiting macrophages, and with the assistance of IL-4 and IFN-γ, tumor killing ability of macrophages is activated, thereby inhibiting the growth of primary tumors. Studies have confirmed that there are IMPs in colon cancer [95, 96] (Figure 4②). However, IMPs activate endothelial cells in the circulating blood flow, promote the adhesion of tumor cells to endothelial cells, produce EMT and promote tumor metastasis. Therefore, we speculate that the promotion or inhibition of tumor metastasis by platelets is closely related to the environment, local position and blood flow. In addition, platelets can destroy the angiogenesis of cancer cells [97] and hinder the vascular reconstruction of cancer cells.
4.3. Thrombin-activated platelets inhibit angiogenesis of endothelial cells
It has been clinically confirmed that cancer patients have abundant tissue factors to promote the conversion of prothrombin to thrombin [71], thrombin is one of the most effective platelet agonists, a study using an in vitro cultured vascular endothelial cell (EC) model demonstrated that thrombin-activated platelets (TAPLT) target vascular cell adhesion molecule-1 (VCAM-1), resulting in a decrease in reactive oxygen species production in endothelial cells induced by tumor conditioned medium (TCM), both permeability and angiogenesis were inhibited, and the migration of cancer cells across ECs was blocked [98].
5. Clinical application of platelets
5.1. Liquid biopsy
Tumor-acting platelets play an important role in tumor development, but also change the mRNA profile of platelets, through platelet mRNA sequencing, which can better distinguish between local and distant metastases in patients. Moreover, this is significantly related to the target drug related indicators, such as HER2-positive, MET and EGFR, and has diagnostic potential, providing a potential marker for the diagnosis of various cancers. Clinically, blood tests based on tumor associated platelet biomarkers can not only detect early cancer, but also closely correlate with personalized treatment and prediction of recurrence [83]. Liquid biopsy has the advantage of overcoming the limitations of tissue collection, and has been studied in breast cancer [99], pancreatic ductal adenocarcinoma [100] and liver cancer [101].
5.2. Development of an anti-cancer platelet-based biomimetic formulation
In addition to being an indicator of clinical diagnosis, some studies have shown that platelets can be used as effective anticancer carriers through mechanisms, such as vascular-endothelial cell adhesion, surgical injury-induced aggregation and activated secretory nanovesicles [102, 103, 104]. A block copolymer, naphthalene diimide-bithiophene derivative (NDI-BT), was used as a photothermal material, and then photothermal nanoparticles were synthesized and introduced into platelets together with immunostimulant R837 hydrochloride to construct engineered PLTs (N + R@PLTs) [105] (Figure 4③), which rely on NDI-BT to induce tumor cells to release antigens [106], R837 to activate the immune response and tumor killing cells. The new anticancer biomimetic formulation takes platelets as the carrier and can effectively remove primary and secondary tumors, and has broad clinical application prospects.
5.3. The role of the bi-directional function of platelets in clinical treatment
Tumor-associated platelets have a bi-directional function in the process of cancer progression. In the clinic, we can serve patients individually according to this feature, first of all, treatment can be carried out according to different malignancies, such as the study by Servais et al. [107], which confirmed that platelets promote the occurrence of colon cancer by inhibiting the immune response, while Lucy et al. [108] found that platelets can significantly enhance melanoma in lung metastasis, but do not affect liver metastasis, however, in the absence of platelets, liver metastasis is significantly enhanced, demonstrating that the function of tumor-associated platelets is organ-specific. Therefore, in the clinical treatment process, we can formulate treatment plans for different cancer types according to the function of tumor-associated platelets. In addition, tumor-associated platelet function is also different in different stages of the same tumor. Katrin et al. [109] proposed that the platelet fibrinogen receptor glycoprotein IIb (GPIIb) can support the initiation of lung metastasis by promoting the aggregation of platelets and tumor cells, but weakening the subsequent distant planting and proliferation of tumor cells. Therefore, during the process of clinical diagnosis and treatment, the functions and characteristics of tumor-related platelets still need to be further explored, and then individualized and targeted assessment of the prognosis and treatment of patients is needed to improve the quality and survival time of treatment.
6. Summary
In recent years, an increasing number of researchers have paid attention to the dual role of platelets in the occurrence and development of tumors. Some important platelet-related molecules are involved in the whole process of tumor metastasis, invasion, screening, diagnosis and prognosis evaluation, and their potential biological values have gradually emerged. However, due to the complexity of the interactions between platelets and tumor cells, the specific mechanisms still need further exploration and elucidation, including how to balance and develop tumor cells, immune cells, platelets and inflammatory cells. In different types of metastatic cancer cells, what are the typical mechanisms of action of platelets? In the process of promoting metastasis, which mechanism plays a key role and which main molecules are involved in different stages still require further research data for verification. In addition, the choice of anti-platelet drugs is also a hot topic in the current medical community, and the choice of drug types and doses and their safety will be the future direction of key exploration and breakthroughs in the scientific research field. Platelets not only promote tumor metastasis but also hinder tumor metastasis to a certain extent. The mechanism of this functional duality of platelets is still unclear. The specific mechanism of platelets in tumor metastasis still needs further research.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
Minghui Zhang was supported by the natural science foundation of inner Mongolia [2020MS08084], the inner mongolia science & technology plan project [201802133 & 2020GG0297].
Data availability statement
No data was used for the research described in the article.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Minghui Zhang, Email: cfzhangminghui@163.com.
Lei Zhang, Email: zhangleijiang8848@163.com.
References
- 1.Dillekås H., Rogers M.S., Straume O. Are 90% of deaths from cancer caused by metastases? Cancer Med. 2019;8:5574–5576. doi: 10.1002/cam4.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riggi N., Aguet M., Stamenkovic I. Cancer metastasis: a reappraisal of its underlying mechanisms and their relevance to treatment. Annu. Rev. Pathol. 2018;13:117–140. doi: 10.1146/annurev-pathol-020117-044127. [DOI] [PubMed] [Google Scholar]
- 3.Gaertner F., Massberg S. Patrolling the vascular borders: platelets in immunity to infection and cancer. Nat. Rev. Immunol. 2019;19:747–760. doi: 10.1038/s41577-019-0202-z. [DOI] [PubMed] [Google Scholar]
- 4.Labelle M., Hynes R.O. The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discovery. 2012;2:1091–1099. doi: 10.1158/2159-8290.CD-12-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varki A. Trousseau’s syndrome: multiple definitions and multiple mechanisms. Blood. 2007;110:1723–1729. doi: 10.1182/blood-2006-10-053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gasic G.J., Gasic T.B., Stewart C.C. Antimetastatic effects associated with platelet reduction. Proc. Natl. Acad. Sci. U. S. A. 1968;61:46–52. doi: 10.1073/pnas.61.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Studies on Tumor-Cell-Induced Platelet Aggregation in Human Lung Cancer Cell Lines, (n.d.). https://pubmed.ncbi.nlm.nih.gov/8954171/(accessed August 26, 2022). [DOI] [PubMed]
- 8.Aspirin Inhibits Platelets from Reprogramming Breast Tumor Cells and Promoting Metastasis, (n.d.). https://pubmed.ncbi.nlm.nih.gov/30670536/(accessed August 26, 2022). [DOI] [PMC free article] [PubMed]
- 9.Lou X.-L., Deng J., Deng H., Ting Y., Zhou L., Liu Y.-H., Hu J.-P., Huang X.-F., Qi X.-Q. Aspirin inhibit platelet-induced epithelial-to-mesenchymal transition of circulating tumor cells (Review) Biomed. Rep. 2014;2:331–334. doi: 10.3892/br.2014.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patrignani P., Patrono C. Aspirin, platelet inhibition and cancer prevention. Platelets. 2018;29:779–785. doi: 10.1080/09537104.2018.1492105. [DOI] [PubMed] [Google Scholar]
- 11.Le Quellec S., Bordet J.-C., Negrier C., Dargaud Y. Comparison of current platelet functional tests for the assessment of aspirin and clopidogrel response. A review of the literature. Thromb. Haemostasis. 2016;116:638–650. doi: 10.1160/TH15-11-0870. [DOI] [PubMed] [Google Scholar]
- 12.Ma Q., Chen G.-Z., Zhang Y.-H., Zhang L., Huang L.-A. Clinical outcomes and predictive model of platelet reactivity to clopidogrel after acute ischemic vascular events. Chin. Med. J. 2019;132:1053–1062. doi: 10.1097/CM9.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gareau A.J., Brien C., Gebremeskel S., Liwski R.S., Johnston B., Bezuhly M. Ticagrelor inhibits platelet-tumor cell interactions and metastasis in human and murine breast cancer. Clin. Exp. Metastasis. 2018;35:25–35. doi: 10.1007/s10585-018-9874-1. [DOI] [PubMed] [Google Scholar]
- 14.Meng X., Liu W., Yang H., Cao Z., He H., Zheng K., Chen Y., Su J., Lv J., Sun J., Li P., Ding S., Ahmad N., Qian J., Zhou Y. Ticagrelor prevents tumor metastasis via inhibiting cell proliferation and promoting platelet apoptosis. Anti Cancer Drugs. 2020;31:1012–1017. doi: 10.1097/CAD.0000000000000925. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y., Wei J., Liu S., Wang J., Han X., Qin H., Lang J., Cheng K., Li Y., Qi Y., Anderson G.J., Sukumar S., Li S., Nie G. Inhibition of platelet function using liposomal nanoparticles blocks tumor metastasis. Theranostics. 2017;7:1062–1071. doi: 10.7150/thno.17908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Role of platelets and platelet receptors in cancer metastasis. https://pubmed.ncbi.nlm.nih.gov/30305116/ (n.d.) (accessed August 26, 2022) [DOI] [PMC free article] [PubMed]
- 17.Rickles F.R. Mechanisms of cancer-induced thrombosis in cancer. Pathophysiol. Haemostasis Thrombosis. 2006;35:103–110. doi: 10.1159/000093551. [DOI] [PubMed] [Google Scholar]
- 18.Placke T., Örgel M., Schaller M., Jung G., Rammensee H.-G., Kopp H.-G., Salih H.R. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res. 2012;72:440–448. doi: 10.1158/0008-5472.CAN-11-1872. [DOI] [PubMed] [Google Scholar]
- 19.Tissue factor in cancer progression and angiogenesis, (n.d.). https://pubmed.ncbi.nlm.nih.gov/20434002/(accessed August 26, 2022).
- 20.Kim S., Kunapuli S.P. P2Y12 receptor in platelet activation. Platelets. 2011;22:56–60. doi: 10.3109/09537104.2010.497231. [DOI] [PubMed] [Google Scholar]
- 21.Pu F., Li X., Wang S., Huang Y., Wang D. Platelet supernatant with longer storage inhibits tumor cell growth. Transfus. Apher. Sci. Off. J. World Apher. Assoc. Off. J. Eur. Soc. Haemapheresis. 2021;60 doi: 10.1016/j.transci.2020.103042. [DOI] [PubMed] [Google Scholar]
- 22.CXCR3 in carcinoma progression, (n.d.). https://pubmed.ncbi.nlm.nih.gov/25663474/(accessed August 27, 2022).
- 23.Zeidman I. The fate of circulating tumors cells. I. Passage of cells through capillaries. Cancer Res. 1961;21:38–39. [PubMed] [Google Scholar]
- 24.Platelets Reduce Anoikis and Promote Metastasis by Activating YAP1 Signaling, (n.d.). https://pubmed.ncbi.nlm.nih.gov/28827520/(accessed August 26, 2022). [DOI] [PMC free article] [PubMed]
- 25.Stellos K., Langer H., Daub K., Schoenberger T., Gauss A., Geisler T., Bigalke B., Mueller I., Schumm M., Schaefer I., Seizer P., Kraemer B.F., Siegel-Axel D., May A.E., Lindemann S., Gawaz M. Platelet-derived stromal cell-derived factor-1 regulates adhesion and promotes differentiation of human CD34+ cells to endothelial progenitor cells. Circulation. 2008;117:206–215. doi: 10.1161/CIRCULATIONAHA.107.714691. [DOI] [PubMed] [Google Scholar]
- 26.Chiossone L., Dumas P.-Y., Vienne M., Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018;18:671–688. doi: 10.1038/s41577-018-0061-z. [DOI] [PubMed] [Google Scholar]
- 27.NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. https://pubmed.ncbi.nlm.nih.gov/29429633/ (n.d.) (accessed August 26, 2022) [DOI] [PMC free article] [PubMed]
- 28.Melaiu O., Lucarini V., Cifaldi L., Fruci D. Influence of the tumor microenvironment on NK cell function in solid tumors. Front. Immunol. 2019;10:3038. doi: 10.3389/fimmu.2019.03038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leslie M. T cells target pancreatic tumors. Cancer Discovery. 2016;6:8. doi: 10.1158/2159-8290.CD-NB2015-167. [DOI] [PubMed] [Google Scholar]
- 30.CD4+ T Cells Induce Rejection of Urothelial Tumors after Immune Checkpoint Blockade, (n.d.). https://pubmed.ncbi.nlm.nih.gov/30518683/(accessed August 26, 2022). [DOI] [PMC free article] [PubMed]
- 31.Chiang C.L.-L., Kandalaft L.E. In vivo cancer vaccination: which dendritic cells to target and how? Cancer Treat. Rev. 2018;71:88–101. doi: 10.1016/j.ctrv.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elliman D. Whooping cough immunisation for children with cerebral irritation or damage in the neonatal period. Br. Med. J. Clin. Res. Ed. 1986;293:1569. doi: 10.1136/bmj.293.6561.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang W., Green M., Choi J.E., Gijón M., Kennedy P.D., Johnson J.K., Liao P., Lang X., Kryczek I., Sell A., Xia H., Zhou J., Li G., Li J., Li W., Wei S., Vatan L., Zhang H., Szeliga W., Gu W., Liu R., Lawrence T.S., Lamb C., Tanno Y., Cieslik M., Stone E., Georgiou G., Chan T.A., Chinnaiyan A., Zou W. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma C., Su M., Shen K., Chen J., Ning Y., Qi C. Key genes and pathways in tumor-educated dendritic cells by bioinformatical analysis. Microbiol. Immunol. 2020;64:63–71. doi: 10.1111/1348-0421.12747. [DOI] [PubMed] [Google Scholar]
- 35.Kopp H.-G., Placke T., Salih H.R. Platelet-derived transforming growth factor-beta down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res. 2009;69:7775–7783. doi: 10.1158/0008-5472.CAN-09-2123. [DOI] [PubMed] [Google Scholar]
- 36.Maurer S., Kropp K.N., Klein G., Steinle A., Haen S.P., Walz J.S., Hinterleitner C., Märklin M., Kopp H.-G., Salih H.R. Platelet-mediated shedding of NKG2D ligands impairs NK cell immune-surveillance of tumor cells. Oncoimmunology. 2018;7 doi: 10.1080/2162402X.2017.1364827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gasser S., Raulet D.H. Activation and self-tolerance of natural killer cells. Immunol. Rev. 2006;214:130–142. doi: 10.1111/j.1600-065X.2006.00460.x. [DOI] [PubMed] [Google Scholar]
- 38.Maurer S., Kopp H.-G., Salih H.R., Kropp K.N. Modulation of immune responses by platelet-derived ADAM10. Front. Immunol. 2020;11:44. doi: 10.3389/fimmu.2020.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cluxton C.D., Spillane C., O’Toole S.A., Sheils O., Gardiner C.M., O’Leary J.J. Suppression of Natural Killer cell NKG2D and CD226 anti-tumour cascades by platelet cloaked cancer cells: implications for the metastatic cascade. PLoS One. 2019;14 doi: 10.1371/journal.pone.0211538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Platelets and Fibrinogen Facilitate Each Other in Protecting Tumor Cells from Natural Killer Cytotoxicity, (n.d.). https://pubmed.ncbi.nlm.nih.gov/19302289/(accessed August 26, 2022). [DOI] [PMC free article] [PubMed]
- 41.Meazza R., Falco M., Canevali P., Loiacono F., Colomar-Carando N., Muntasell A., Rea A., Mingari M.C., Locatelli F., Moretta L., Lopez-Botet M., Pende D. Characterization of KIR+ NK cell subsets with a monoclonal antibody selectively recognizing KIR2DL1 and blocking the specific interaction with HLA-C. HLA. 2022;100:119–132. doi: 10.1111/tan.14640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu L.-X., Yan L., Yang W., Wu F.-Q., Ling Y., Chen S.-Z., Tang L., Tan Y.-X., Cao D., Wu M.-C., Yan H.-X., Wang H.-Y. Platelets promote tumour metastasis via interaction between TLR4 and tumour cell-released high-mobility group box1 protein. Nat. Commun. 2014;5:5256. doi: 10.1038/ncomms6256. [DOI] [PubMed] [Google Scholar]
- 43.Vivier E., Raulet D.H., Moretta A., Caligiuri M.A., Zitvogel L., Lanier L.L., Yokoyama W.M., Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Placke T., Salih H.R., Kopp H.-G. GITR ligand provided by thrombopoietic cells inhibits NK cell antitumor activity. J. Immunol. Baltim. Md. 2012;189:154–160. doi: 10.4049/jimmunol.1103194. [DOI] [PubMed] [Google Scholar]
- 45.Baltz K.M., Krusch M., Bringmann A., Brossart P., Mayer F., Kloss M., Baessler T., Kumbier I., Peterfi A., Kupka S., Kroeber S., Menzel D., Radsak M.P., Rammensee H.-G., Salih H.R. Cancer immunoediting by GITR (glucocorticoid-induced TNF-related protein) ligand in humans: NK cell/tumor cell interactions. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2007;21:2442–2454. doi: 10.1096/fj.06-7724com. [DOI] [PubMed] [Google Scholar]
- 46.Baltz K.M., Krusch M., Baessler T., Schmiedel B.J., Bringmann A., Brossart P., Salih H.R. Neutralization of tumor-derived soluble glucocorticoid-induced TNFR-related protein ligand increases NK cell anti-tumor reactivity. Blood. 2008;112:3735–3743. doi: 10.1182/blood-2008-03-143016. [DOI] [PubMed] [Google Scholar]
- 47.Buechele C., Baessler T., Wirths S., Schmohl J.U., Schmiedel B.J., Salih H.R. Glucocorticoid-induced TNFR-related protein (GITR) ligand modulates cytokine release and NK cell reactivity in chronic lymphocytic leukemia (CLL) Leukemia. 2012;26:991–1000. doi: 10.1038/leu.2011.313. [DOI] [PubMed] [Google Scholar]
- 48.Lanier L.L. NK cell recognition. Annu. Rev. Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 49.Sakurai T., Okuyama Y., Kobayashi S., Phung H.T., Asao A., Kawabe T., Ndhlovu L.C., Riccardi C., Kudo H., Wada M., Nio M., So T., Ishii N. GITR controls intestinal inflammation by suppressing IL-15-dependent NK cell activity. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020;34:14820–14831. doi: 10.1096/fj.202001675R. [DOI] [PubMed] [Google Scholar]
- 50.GITR Ligand provided by Thrombopoietic Cells Inhibits NK Cell Antitumor Activity, (n.d.). https://pubmed.ncbi.nlm.nih.gov/22649191/(accessed August 26, 2022). [DOI] [PubMed]
- 51.Nakanishi T., Inaba M., Inagaki-Katashiba N., Tanaka A., Vien P.T.X., Kibata K., Ito T., Nomura S. Platelet-derived RANK ligand enhances CCL17 secretion from dendritic cells mediated by thymic stromal lymphopoietin. Platelets. 2015;26:425–431. doi: 10.3109/09537104.2014.920081. [DOI] [PubMed] [Google Scholar]
- 52.Platelets Subvert Antitumor Efficacy of T Cell-Recruiting Bispecific Antibodies, (n.d.). https://pubmed.ncbi.nlm.nih.gov/35110356/(accessed August 26, 2022). [DOI] [PMC free article] [PubMed]
- 53.Rachidi S., Metelli A., Riesenberg B., Wu B.X., Nelson M.H., Wallace C., Paulos C.M., Rubinstein M.P., Garrett-Mayer E., Hennig M., Bearden D.W., Yang Y., Liu B., Li Z. Platelets subvert T cell immunity against cancer via GARP-TGFβ axis. Sci. Immunol. 2017;2:eaai7911. doi: 10.1126/sciimmunol.aai7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang S., Li Z., Xu R. Human cancer and platelet interaction, a potential therapeutic target. Int. J. Mol. Sci. 2018;19:E1246. doi: 10.3390/ijms19041246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gondek D.C., Lu L.-F., Quezada S.A., Sakaguchi S., Noelle R.J. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J. Immunol. Baltim. Md. 2005;174:1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 56.Human T Regulatory Cells Can Use the Perforin Pathway to Cause Autologous Target Cell Death, (n.d.). https://pubmed.ncbi.nlm.nih.gov/15485635/(accessed August 26, 2022). [DOI] [PubMed]
- 57.Polasky C., Wendt F., Pries R., Wollenberg B. Platelet induced functional alteration of CD4+ and CD8+ T cells in HNSCC. Int. J. Mol. Sci. 2020;21:E7507. doi: 10.3390/ijms21207507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stellos K., Langer H., Daub K., Schoenberger T., Gauss A., Geisler T., Bigalke B., Mueller I., Schumm M., Schaefer I., Seizer P., Kraemer B.F., Siegel-Axel D., May A.E., Lindemann S., Gawaz M. Platelet-derived stromal cell-derived factor-1 regulates adhesion and promotes differentiation of human CD34+ cells to endothelial progenitor cells. Circulation. 2008;117:206–215. doi: 10.1161/CIRCULATIONAHA.107.714691. [DOI] [PubMed] [Google Scholar]
- 59.Yan M., Lesyk G., Radziwon-Balicka A., Jurasz P. Pharmacological regulation of platelet factors that influence tumor angiogenesis. Semin. Oncol. 2014;41:370–377. doi: 10.1053/j.seminoncol.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 60.Mott J.D., Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell Biol. 2004;16:558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Daly C., Qian X., Castanaro C., Pasnikowski E., Jiang X., Thomson B.R., Quaggin S.E., Papadopoulos N., Wei Y., Rudge J.S., Thurston G., Yancopoulos G.D., Davis S. Angiopoietins bind thrombomodulin and inhibit its function as a thrombin cofactor. Sci. Rep. 2018;8:505. doi: 10.1038/s41598-017-18912-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holmes C.E., Huang J.C., Pace T.R., Howard A.B., Muss H.B. Tamoxifen and aromatase inhibitors differentially affect vascular endothelial growth factor and endostatin levels in women with breast cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008;14:3070–3076. doi: 10.1158/1078-0432.CCR-07-4640. [DOI] [PubMed] [Google Scholar]
- 63.Coppinger J.A., Cagney G., Toomey S., Kislinger T., Belton O., McRedmond J.P., Cahill D.J., Emili A., Fitzgerald D.J., Maguire P.B. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood. 2004;103:2096–2104. doi: 10.1182/blood-2003-08-2804. [DOI] [PubMed] [Google Scholar]
- 64.Rubenstein D.A., Yin W. Platelet-activation mechanisms and vascular remodeling. Compr. Physiol. 2018;8:1117–1156. doi: 10.1002/cphy.c170049. [DOI] [PubMed] [Google Scholar]
- 65.Shao B., Wahrenbrock M.G., Yao L., David T., Coughlin S.R., Xia L., Varki A., McEver R.P. Carcinoma mucins trigger reciprocal activation of platelets and neutrophils in a murine model of Trousseau syndrome. Blood. 2011;118:4015–4023. doi: 10.1182/blood-2011-07-368514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lonsdorf A.S., Krämer B.F., Fahrleitner M., Schönberger T., Gnerlich S., Ring S., Gehring S., Schneider S.W., Kruhlak M.J., Meuth S.G., Nieswandt B., Gawaz M., Enk A.H., Langer H.F. Engagement of αIIbβ3 (GPIIb/IIIa) with ανβ3 integrin mediates interaction of melanoma cells with platelets: a connection to hematogenous metastasis. J. Biol. Chem. 2012;287:2168–2178. doi: 10.1074/jbc.M111.269811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Platelet Integrin α 6 β 1 Controls Lung Metastasis through Direct Binding to Cancer Cell-Derived ADAM9, (n.d.). https://pubmed.ncbi.nlm.nih.gov/27699237/(accessed August 26, 2022). [DOI] [PMC free article] [PubMed]
- 68.Mammadova-Bach E., Gil-Pulido J., Sarukhanyan E., Burkard P., Shityakov S., Schonhart C., Stegner D., Remer K., Nurden P., Nurden A.T., Dandekar T., Nehez L., Dank M., Braun A., Mezzano D., Abrams S.I., Nieswandt B. Platelet glycoprotein VI promotes metastasis through interaction with cancer cell-derived galectin-3. Blood. 2020;135:1146–1160. doi: 10.1182/blood.2019002649. [DOI] [PubMed] [Google Scholar]
- 69.Platelets Guide the Formation of Early Metastatic Niches, (n.d.). https://pubmed.ncbi.nlm.nih.gov/25024172/(accessed August 26, 2022). [DOI] [PMC free article] [PubMed]
- 70.Phosphatidylinositol Transfer Protein-α in Platelets Is Inconsequential for Thrombosis yet Is Utilized for Tumor Metastasis, (n.d.). https://pubmed.ncbi.nlm.nih.gov/29084966/(accessed August 27, 2022). [DOI] [PMC free article] [PubMed]
- 71.Ruf W., Mueller B.M. Thrombin generation and the pathogenesis of cancer. Semin. Thromb. Hemost. 2006;32(Suppl 1):61–68. doi: 10.1055/s-2006-939555. [DOI] [PubMed] [Google Scholar]
- 72.Autocrine PDGFR Signaling Promotes Mammary Cancer Metastasis, (n.d.). https://pubmed.ncbi.nlm.nih.gov/16741576/(accessed August 27, 2022). [DOI] [PMC free article] [PubMed]
- 73.Notch-1 Induces Epithelial-Mesenchymal Transition Consistent with Cancer Stem Cell Phenotype in Pancreatic Cancer Cells, (n.d.). https://pubmed.ncbi.nlm.nih.gov/21463919/(accessed August 27, 2022). [DOI] [PMC free article] [PubMed] [Retracted]
- 74.Gotzmann J., Fischer A.N.M., Zojer M., Mikula M., Proell V., Huber H., Jechlinger M., Waerner T., Weith A., Beug H., Mikulits W. A crucial function of PDGF in TGF-beta-mediated cancer progression of hepatocytes. Oncogene. 2006;25:3170–3185. doi: 10.1038/sj.onc.1209083. [DOI] [PubMed] [Google Scholar]
- 75.Brain-derived Neurotrophic Factor (BDNF)-induced Tropomyosin-Related Kinase B (Trk B) Signaling Is a Potential Therapeutic Target for Peritoneal Carcinomatosis Arising from Colorectal Cancer, (n.d.). https://pubmed.ncbi.nlm.nih.gov/24801982/(accessed August 27, 2022). [DOI] [PMC free article] [PubMed]
- 76.Direct Signaling between Platelets and Cancer Cells Induces an Epithelial-mesenchymal-like Transition and Promotes Metastasis, (n.d.). https://pubmed.ncbi.nlm.nih.gov/22094253/(accessed August 27, 2022). [DOI] [PMC free article] [PubMed]
- 77.Contribution of Platelets to Tumour Metastasis, (n.d.). https://pubmed.ncbi.nlm.nih.gov/21258396/(accessed August 27, 2022).
- 78.Microenvironmental Regulation of Tumor Progression and Metastasis, (n.d.). https://pubmed.ncbi.nlm.nih.gov/24202395/(accessed August 27, 2022).
- 79.Saito R., Shoda K., Maruyama S., Yamamoto A., Takiguchi K., Furuya S., Hosomura N., Akaike H., Kawaguchi Y., Amemiya H., Kawaida H., Sudo M., Inoue S., Kono H., Suzuki-Inoue K., Ichikawa D. Platelets enhance malignant behaviours of gastric cancer cells via direct contacts. Br. J. Cancer. 2021;124:570–573. doi: 10.1038/s41416-020-01134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Time-dependent Interactions of Blood Platelets and Cancer Cells, Accompanied by Extramedullary Hematopoiesis, Lead to Increased Platelet Activation and Reactivity in a Mouse Orthotopic Model of Breast Cancer - Implications for Pulmonary and Liver Metastasis, (n.d.). https://pubmed.ncbi.nlm.nih.gov/32191918/(accessed August 27, 2022). [DOI] [PMC free article] [PubMed]
- 81.Miao Y., Xu Z., Feng W., Zheng M., Xu Z., Gao H., Li W., Zhang Y., Zong Y., Lu A., Zhao J. Platelet infiltration predicts survival in postsurgical colorectal cancer patients. Int. J. Cancer. 2022;150:509–520. doi: 10.1002/ijc.33816. [DOI] [PubMed] [Google Scholar]
- 82.Augustine T.N. The aegis: platelets as biomarkers of tumor progression. Biomarkers Med. 2020;14:573–585. doi: 10.2217/bmm-2019-0514. [DOI] [PubMed] [Google Scholar]
- 83.Best M.G., Sol N., Kooi I., Tannous J., Westerman B.A., Rustenburg F., Schellen P., Verschueren H., Post E., Koster J., Ylstra B., Ameziane N., Dorsman J., Smit E.F., Verheul H.M., Noske D.P., Reijneveld J.C., Nilsson R.J.A., Tannous B.A., Wesseling P., Wurdinger T. RNA-seq of tumor-educated platelets enables blood-based pan-cancer, multiclass, and molecular pathway cancer diagnostics. Cancer Cell. 2015;28:666–676. doi: 10.1016/j.ccell.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tomimaru Y., Eguchi H., Gotoh K., Kawamoto K., Wada H., Asaoka T., Noda T., Yamada D., Ogawa H., Umeshita K., Nagano H., Doki Y., Mori M. Platelet count is more useful for predicting posthepatectomy liver failure at surgery for hepatocellular carcinoma than indocyanine green clearance test. J. Surg. Oncol. 2016;113:565–569. doi: 10.1002/jso.24166. [DOI] [PubMed] [Google Scholar]
- 85.TRAIL Inhibits Platelet-Induced Colorectal Cancer Cell Invasion, (n.d.). https://pubmed.ncbi.nlm.nih.gov/30621488/(accessed August 27, 2022). [DOI] [PMC free article] [PubMed]
- 86.Greer Y.E., Gilbert S.F., Gril B., Narwal R., Peacock Brooks D.L., Tice D.A., Steeg P.S., Lipkowitz S. MEDI3039, a novel highly potent tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) receptor 2 agonist, causes regression of orthotopic tumors and inhibits outgrowth of metastatic triple-negative breast cancer. Breast Cancer Res. 2019;21:27. doi: 10.1186/s13058-019-1116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Geranpayehvaghei M., Shi Q., Zhao B., Li S., Xu J., Taleb M., Qin H., Zhang Y., Khajeh K., Nie G. Targeting delivery of platelets inhibitor to prevent tumor metastasis. Bioconjugate Chem. 2019;30:2349–2357. doi: 10.1021/acs.bioconjchem.9b00457. [DOI] [PubMed] [Google Scholar]
- 88.Foss A., Muñoz-Sagredo L., Sleeman J., Thiele W. The contribution of platelets to intravascular arrest, extravasation, and outgrowth of disseminated tumor cells. Clin. Exp. Metastasis. 2020;37:47–67. doi: 10.1007/s10585-019-10009-y. [DOI] [PubMed] [Google Scholar]
- 89.Wang Y., Zhang H. Platelet-induced inhibition of tumor cell growth. Thromb. Res. 2008;123:324–330. doi: 10.1016/j.thromres.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 90.Platelet Factor 4 Is Produced by Subsets of Myeloid Cells in Premetastatic Lung and Inhibits Tumor Metastasis, (n.d.). https://pubmed.ncbi.nlm.nih.gov/27223426/(accessed August 27, 2022). [DOI] [PMC free article] [PubMed]
- 91.Erpenbeck L., Nieswandt B., Schön M., Pozgajova M., Schön M.P. Inhibition of platelet GPIb alpha and promotion of melanoma metastasis. J. Invest. Dermatol. 2010;130:576–586. doi: 10.1038/jid.2009.278. [DOI] [PubMed] [Google Scholar]
- 92.Platelet Microparticles Infiltrating Solid Tumors Transfer miRNAs that Suppress Tumor Growth, (n.d.). https://pubmed.ncbi.nlm.nih.gov/28500171/(accessed August 27, 2022). [DOI] [PMC free article] [PubMed]
- 93.Platelets and P-Selectin Control Tumor Cell Metastasis in an Organ-specific Manner and Independently of NK Cells, (n.d.). https://pubmed.ncbi.nlm.nih.gov/22836751/(accessed August 27, 2022). [DOI] [PubMed]
- 94.Bulla S.C., Badial P.R., Silva R.C., Lunsford K., Bulla C. Platelets inhibit migration of canine osteosarcoma cells. J. Comp. Pathol. 2017;156:3–13. doi: 10.1016/j.jcpa.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 95.L P., D M., L C., E C., S R., S C., N B., W E., F D.-G., C D., L P.-D. The interaction of platelets with colorectal cancer cells inhibits tumor growth but promotes metastasis. Cancer Res. 2020;80 doi: 10.1158/0008-5472.CAN-19-1181. [DOI] [PubMed] [Google Scholar]
- 96.Wang X., Zhao S., Wang Z., Gao T. Platelets involved tumor cell EMT during circulation: communications and interventions. Cell Commun. Signal. CCS. 2022;20:82. doi: 10.1186/s12964-022-00887-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Platelets Disrupt Vasculogenic Mimicry by Cancer Cells, (n.d.). https://pubmed.ncbi.nlm.nih.gov/32246008/(accessed August 27, 2022). [DOI] [PMC free article] [PubMed]
- 98.Thrombin-Activated Platelets Protect Vascular Endothelium against Tumor Cell Extravasation by Targeting Endothelial VCAM-1, (n.d.). https://pubmed.ncbi.nlm.nih.gov/35408794/(accessed August 27, 2022). [DOI] [PMC free article] [PubMed]
- 99.Yoo T.-K. Liquid biopsy in breast cancer: circulating tumor cells and circulating tumor DNA. Adv. Exp. Med. Biol. 2021;1187:337–361. doi: 10.1007/978-981-32-9620-6_17. [DOI] [PubMed] [Google Scholar]
- 100.Lee J.-S., Park S.S., Lee Y.K., Norton J.A., Jeffrey S.S. Liquid biopsy in pancreatic ductal adenocarcinoma: current status of circulating tumor cells and circulating tumor DNA. Mol. Oncol. 2019;13:1623–1650. doi: 10.1002/1878-0261.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Temraz S., Nasr R., Mukherji D., Kreidieh F., Shamseddine A. Liquid biopsy derived circulating tumor cells and circulating tumor DNA as novel biomarkers in hepatocellular carcinoma. Expert Rev. Mol. Diagn. 2022;22:507–518. doi: 10.1080/14737159.2022.2094706. [DOI] [PubMed] [Google Scholar]
- 102.Brown T.P., Forouzan O., Shevkoplyas S.S., Khismatullin D.B. Histamine reduces GPIbα-mediated adhesion of platelets to TNF-α-activated vascular endothelium. Thromb. Res. 2013;131:150–157. doi: 10.1016/j.thromres.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 103.Han X., Chen J., Chu J., Liang C., Ma Q., Fan Q., Liu Z., Wang C. Platelets as platforms for inhibition of tumor recurrence post-physical therapy by delivery of anti-PD-L1 checkpoint antibody. J. Control. Release Off. J. Control. Release Soc. 2019;304:233–241. doi: 10.1016/j.jconrel.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 104.Hu Q., Sun W., Wang J., Ruan H., Zhang X., Ye Y., Shen S., Wang C., Lu W., Cheng K., Dotti G., Zeidner J.F., Wang J., Gu Z. Conjugation of haematopoietic stem cells and platelets decorated with anti-PD-1 antibodies augments anti-leukaemia efficacy. Nat. Biomed. Eng. 2018;2:831–840. doi: 10.1038/s41551-018-0310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lv Y., Li F., Wang S., Lu G., Bao W., Wang Y., Tian Z., Wei W., Ma G. Near-infrared light-triggered platelet arsenal for combined photothermal-immunotherapy against cancer. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abd7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang F., Lu G., Wen X., Li F., Ji X., Li Q., Wu M., Cheng Q., Yu Y., Tang J., Mei L. Magnetic nanoparticles coated with polyphenols for spatio-temporally controlled cancer photothermal/immunotherapy. J. Control. Release Off. J. Control. Release Soc. 2020;326:131–139. doi: 10.1016/j.jconrel.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 107.Servais L., Wéra O., Dibato Epoh J., Delierneux C., Bouznad N., Rahmouni S., Mazzucchelli G., Baiwir D., Delvenne P., Lancellotti P., Oury C. Platelets contribute to the initiation of colitis-associated cancer by promoting immunosuppression. J. Thromb. Haemost. JTH. 2018;16:762–777. doi: 10.1111/jth.13959. [DOI] [PubMed] [Google Scholar]
- 108.Coupland L.A., Chong B.H., Parish C.R. Platelets and P-selectin control tumor cell metastasis in an organ-specific manner and independently of NK cells. Cancer Res. 2012;72:4662–4671. doi: 10.1158/0008-5472.CAN-11-4010. [DOI] [PubMed] [Google Scholar]
- 109.Echtler K., Konrad I., Lorenz M., Schneider S., Hofmaier S., Plenagl F., Stark K., Czermak T., Tirniceriu A., Eichhorn M., Walch A., Enders G., Massberg S., Schulz C. Platelet GPIIb supports initial pulmonary retention but inhibits subsequent proliferation of melanoma cells during hematogenic metastasis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0172788. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.