Abstract
Patients with critical illness may present with electrocardiogram (ECG) findings with bizarre QRS morphology or abnormal amplitude. This article provides ECG examples from such clinical scenarios and discusses their clinical characteristics and significance. (Level of Difficulty: Beginner.)
Key Words: cardiac tamponade, electrocardiogram, flecainide toxicity, hyperkalemia
Abbreviations and Acronyms: AKI, acute kidney injury; ECG, electrocardiogram
Central Illustration


Patients with critical illness may present with electrocardiogram (ECG) findings with bizarre QRS morphology or abnormal amplitude.
Learning Objectives
-
•
To recognize and generate differential diagnosis for ECGs with bizarre morphology or abnormal amplitude changes.
-
•
To understand the clinical significance of ECGs with bizarre morphology or abnormal amplitude for management.
Case 1
A 34-year-old woman presented with diabetic ketoacidosis, metabolic acidosis, and acute renal failure. ECG showed STE-segment elevation (STE) in V1, V2 mimicking ST-segment elevation myocardial infarction or a Brugada pattern with narrow peaked and tall T waves suggestive of hyperkalemia (Figure 1A). The patient’s serum potassium level was 9 mmol/L.
Figure 1.
Electrocardiogram Features of Hyperkalemia
(A) ST-segment elevation in V1, V2 mimicking ST-segment elevation myocardial infarction or Brugada pattern and QRST complexes in V5, V6 mimicking de Winter pattern in this electrocardiogram (ECG) from a patient with serum K level of 9 mmol/L. Brugada-type ST-segment elevation in right precordial leads may also be caused by acute occlusion of the conus branch of right coronary artery. (B) Another ECG from a patient with end-stage renal disease and a potassium level of 9.5 mmol/L. (C) Rapid reversal of hyperkalemic ECG patterns shown in (B) 20 minutes after intravenous calcium, insulin, and dextrose. (D) Increased latency period between ventricular pacing spike and onset of QRS complex and widening of QRS complex when potassium level was 7.7 mmol/L (upper); both latency period and width of QRS complex reduced (lower) after partial reversal of potassium level to 5.7 mmol/L.
Case 2
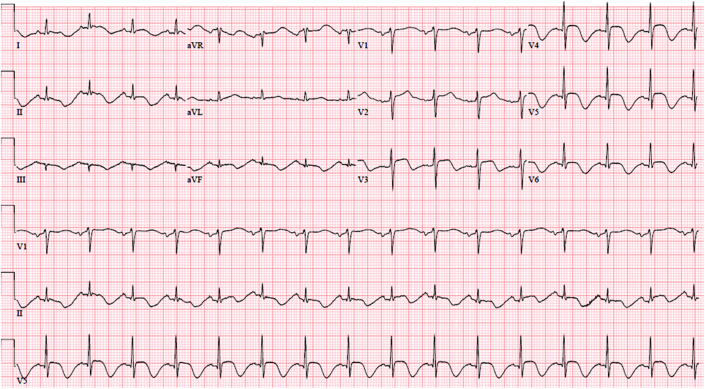
A 72-year-old woman presented to the emergency department describing “not feeling and eating well” and “difficulty in breathing,” Her ECG is shown in Figure 2. She had previously undergone surgical mitral valve repair for mitral regurgitation caused by mitral valve prolapse, with normal left ventricular function and no coronary artery disease. She took flecainide to control paroxysmal atrial fibrillation. She had a new acute kidney injury (AKI), with creatinine of 3.7 mg/dL (baseline 0.9 mg/dL) and potassium of 5.5 mmol/l. Given her normal left ventricular function and high-normal serum potassium level, a diagnosis of flecainide toxicity was made and treated as such. Her subsequent ECG showed atrial fibrillation.
Figure 2.
Electrocardiogram of Flecainide Toxicity
Electrocardiogram from a patient taking flecainide as rhythm control strategy for paroxysmal atrial fibrillation with a newly developed acute kidney injury leading to flecainide toxicity.
Case 3
A 46-year-old woman with a history of metastatic breast cancer described increasing shortness of breath and need for supplemental oxygen for 4 days. Her ECG (Figure 3) showed low QRS voltage and electric alternans, with cardiac tamponade on echocardiogram.
Figure 3.
Electrocardiogram of Cardiac Tamponade
Electrocardiogram from a patient with cardiac tamponade showing 1:1 alternation of QRS complexes (red arrows) and cyclic QRS voltage changes from respiration (blue bars).
Discussion
When the EKG suggests hyperkalemia
As the circulating potassium level exceeds 5.5 mmol/L, terminal repolarization starts to accelerate, resulting in ”peaking” of T waves. When the potassium level exceeds 6.5 mmol/L, QRS duration prolongs with bizarre morphology. When potassium level is higher than 7 mmol/L, the P-wave amplitude decreases, and the duration of P waves prolongs because of slower conduction in the atria. The PR interval also increases because of slower atrioventricular conduction. Conduction block (intraventriclar, fascicular, bundle branch) may also occur. As potassium level progresses higher, sinoventricular rhythm (sine-wave pattern), ventricular fibrillation, or asystole occur, similar to the ECG recorded in a dying heart.
Common illnesses associated with hyperkalemia are renal failure, massive muscle injury, and metabolic acidosis due to sepsis, shock, or multiorgan failure. These patients often are critically ill with altered mentation, unable to provide a clear medical history, and a laboratory value of potassium level may not be available to treating physicians before a review of the ECG. Hence, prompt recognition of hyperkalemic ECG patterns is crucial in the initial phase of management. Hyperkalemic ECG changes can be rapidly reversed within minutes with intravenous calcium and insulin/dextrose (Figures 1B and 1C). Inasmuch as the reversing effect of these treatments on ECG is not long lasting, definitive treatment for hyperkalemia such as emergent dialysis or treatment of precipitating illness should be initiated immediately.
Hyperkalemia also affects the performance of implanted cardiac devices. In patients with pacemakers, hyperkalemia can cause widening of paced QRS complex, failure to capture or sense or increased latency period between pacemaker spike and onset of QRS complex during ventricular pacing (Figure 1D). Hyperkalemia could affect implantable cardioverter-defibrillator performance as a result of oversensing of paced or spontaneous T-wave causing inappropriate shock. The impedance between right ventricular coil to SVC coil may increase as well.1
What to look for when ECG shows a bizarre and wide QRS complex
Class I antiarrhythmic agents such as flecainide are frequently used to maintain sinus rhythm in patients with a history of atrial fibrillation without structural heart disease. Occasionally these patients may experience a concomitant illness, leading to poor oral intake, dehydration, electrolyte imbalance, and AKI. They may also be using polypharmacy, including diuretics, which worsens their volume status in such situations. Given that flecainide is renally excreted, this may lead to flecainide toxicity.
Flecainide is a sodium channel blocker with a high affinity to activated sodium channels and a relatively long half-life. It has a narrow therapeutic index, and the incidence of adverse effects increases with increased flecainide plasma levels, not consistently related to the dose of flecainide.2,3 It prolongs PR interval and QRS duration on ECG, slows conduction in AV node and the His-Purkinje system, and also prolongs action potential duration in both ventricular and atrial muscle fibers by inhibiting delayed potassium rectifier current. It has a negative inotropic effect on cardiac muscles and hence is contraindicated in patients with heart failure.
The cardiac toxic effects of flecainide include prolongation of PR interval, widening of QRS duration, bradycardia, sinoatrial block, and asystol.4 The morphology of widened QRS complex is related to QRS duration. If QRS duration is <200 ms, it tends to be of right bundle branch block morphology, whereas if QRS duration is >200 ms, it is likely to be of left bundle branch block morphology.5 Given its ability to widen QRS duration, supraventricular tachycardia can manifest as a tachycardia with bizarre QRS morphology (Figure 2). It can be mistakenly identified as ventricular tachycardia, leading to inappropriate treatment. Flecainide-induced QRS widening is more likely to occur when the heart rate is rapid as a result of use dependence phenomenon.
The differential diagnosis of ECG with bizarre wide QRS complexes, in addition to toxicity from class I antiarrhythmic agents, includes hyperkalemia, tricyclic antidepressant overdose, or advanced cardiomyopathy, and rarely plant intoxication with yew.6 These patients commonly have an altered sensorium, and a detailed history is not immediately available during presentation. ER physicians and cardiologists being consulted need to have a high index of suspicion of the aforementioned differential diagnosis because many conditions are reversible. A recently described ECG pattern called spiked helmet pattern, named after the Bavarian military helmet, or Piklehaub (Figure 4), also has bizarre QRS morphology often associated with noncardiac critical illness.
Figure 4.
Electrocardiogram From a Patient With Confirmed Diagnosis of Takotsubo Cardiomyopathy
QRS morphology in multiple leads (II, V3-V6) mimics “spiked helmet” sign previously reported in patients with noncardiac critical illness.
When the EKG suggests pericardial effusion with acute cardiac tamponade
The classic description of ECG manifestations of pericardial effusion with cardiac tamponade includes sinus tachycardia, low voltage, and electrical alternans. Among these ECG signs, electrical alternans has been described most frequently in published reports. Electrical alternans depicts the alteration of any components of the ECG. It can be changing individual wave forms, intervals, or a combination of the 2, with a constant interval between alternating complexes originating from the same pacemaker and independent of respiratory activity. If alternation is seen in P-QRS-T complexes, it is called total electrical alternans. The alternation usually is 1:1, but 2:1, 3:1, or varying degrees of alternation in ECG complexes have been described.7 The amplitude of alternation can be subtle or obvious, and alternant beats may be seen in only some leads on 12-lead ECG; hence, electrical alternans can be missed if only rhythm strips are reviewed for detection. In addition to electrical alternation, sometimes cyclic changes of ECG amplitude caused by respiratory distress can be seen on 12-lead ECG (Figure 3).
Although electrical alternans is often reported in patients with cardiac tamponade, its prevalence in cardiac tamponade is not high, ranging between 17% and 23%.8,9 Electrical alternans is not specific to pericardial effusion or cardiac tamponade. It has been described in supraventricular tachycardia, ventricular tachycardia, Wolff-Parkinson-White syndrome, chronic obstructive pulmonary disease, pleural effusion, hypothermia, digitalis intoxication, or coronary ischemia.
Inasmuch as the mechanism underlying electrical alternans in cardiac tamponade has been attributed to swinging of the heart in the pericardial fluid, causing the recorded ECG voltage to change, electrical alternans commonly disappears immediately after pericardiocentesis. Interestingly, low voltage can remain unchanged immediately after pericardiocentesis and may take several days to recover.10 Low QRS voltage may also be seen with cardiac transplant rejection, hypothyroidism, and amyloid cardiomyopathy.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Barold S.S., Herweg B. The effect of hyperkalaemia on cardiac rhythm devices. Europace. 2014;16:467–476. doi: 10.1093/europace/eut383. [DOI] [PubMed] [Google Scholar]
- 2.Salerno D.M., Granrud G., Sharkey P., et al. Pharmacodynamics and side effects of flecainide acetate. Clin Pharmacol Ther. 1986;40:101–107. doi: 10.1038/clpt.1986.145. [DOI] [PubMed] [Google Scholar]
- 3.Tamargo J., Le Heuzey J.Y., Mabo P. Narrow therapeutic index drugs: A clinical pharmacological consideration to flecainide. Eur J Clin Pharmacol. 2015;71:549–567. doi: 10.1007/s00228-015-1832-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newson J.M., Santos C.D., Walters B.L., Todd B.R. The case of flecainide toxicity: What to look for and how to treat. J Emerg Med. 2020;59:e43–e47. doi: 10.1016/j.jemermed.2020.04.052. [DOI] [PubMed] [Google Scholar]
- 5.Valentino M.A., Panakos A., Ragupathi L., Williams J., Pavri B.B. Flecainide toxicity: A case report and systematic review of its electrocardiographic patterns and management. Cardiovasc Toxicol. 2017;17:260–266. doi: 10.1007/s12012-016-9380-0. [DOI] [PubMed] [Google Scholar]
- 6.Cerrato N., Calzolari G., Tizzani P., Actis P.E., Dellavalle A., Aluffi E. Bizarre and scary ECG in yew leaves poisoning: Report of successful treatment. Ann Noninvasive Electrocardiol. 2018;23 doi: 10.1111/anec.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niarchos A.P. Electrical alternans in cardiac tamponade. Thorax. 1975;30:228–233. doi: 10.1136/thx.30.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M.L., Liao W.B., Bullard M.J., et al. Cardiac tamponade in Taiwan. Jpn Circ J. 1997;61:767–771. doi: 10.1253/jcj.61.767. [DOI] [PubMed] [Google Scholar]
- 9.Chandra A., Marhefka G.D., DeCaro M.V. Clinical significance of 12 lead ECG changes in patients undergoing pericardiocentesis for cardiac tamponade. Acta Cardiol. 2021;76:76–79. doi: 10.1080/00015385.2019.1700336. [DOI] [PubMed] [Google Scholar]
- 10.Bruch C., Schmermund A., Dagres N., et al. Changes in QRS voltage in cardiac tamponade and pericardial effusion: Reversibility after pericardiocentesis and after anti-inflammatory drug treatment. J Am Coll Cardiol. 2001;38:219–226. doi: 10.1016/s0735-1097(01)01313-4. [DOI] [PubMed] [Google Scholar]