Letter to the Editor
Visceral leishmaniasis (VL), or Kala-azar, is one of the most important neglected tropical diseases (NTD). The protozoa of the Leishmania donovani complex cause this disease, the major species being L. donovani. More than 95% of the global VL cases are reported from 10 countries in South Asia, Africa, and South America. In Africa, VL is mainly reported in Sudan, South Sudan, Ethiopia, Kenya, and Somalia. East Africa is second only to India in the number of human cases of VL [1] and has been challenging regarding leishmaniasis control and elimination. The ongoing outbreak of VL in Kenya is a significant setback to the World Health Organization's (WHO) 2021–30 NTD roadmap for the global elimination of visceral leishmaniasis. In addition, it has stretched the fragile healthcare infrastructure in Kenya.
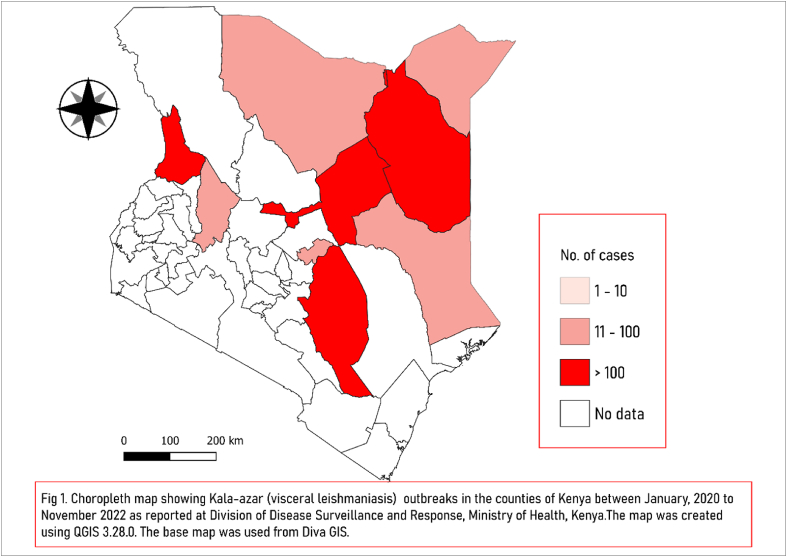
Amongst the 47 counties of Kenya, nine counties, including Marsabit, Garissa, Kitui, Baringo, West Pokot, Mandera, Wajir, Tharaka Nithi and Isiolo, have reported a total of 2037 cases of confirmed and suspected cases of VL since 2020 (Fig 1.), of which ten patients (case fatality rate, CFR, of 0.5%) succumbed to the illness [2]. The outbreak is currently active in four counties of Kitui, West Pokot, Wajir and Isiolo. In the past several years, there have been several outbreaks of VL in Kenya in the counties of Isiolo, Marsabit, Wajir and Baringo Counties in the years 2008, 2011, 2013, 2014, 2017 and 2019 [3]. In addition, the Marsabit county of Kenya has consistently shown an increase in cases for the last few years. For example, in 2014, 136 cases of VL were confirmed with CFR 9.6% [4]. Later, in 2017, 437 were reported, which was followed by a sharp increase in the number of cases to 2338 in 2019. Apart from the rise in the number of cases and geographical shift, there was also an increase in the cases amongst the younger population (mean age 15.3 in 2019 vs 17.6 in 2017) and an increase in the proportion of cases in females and children less than ten years of age. It was suggested that this rise could be because of the more significant number of people being tested due to the availability of new testing facilities [4]. Traditionally, VL was considered more prevalent in arid counties of eastern and northern regions, while CL was more prevalent in the semi-arid counties of western and central areas of Kenya; however, the current outbreak has extended to the western and southern counties as well. The ever-expanding and migrating human population for survival or trade, mainly the pastoral community and refugees, the interaction of humans with the animal population, environmental changes, and social unrest may be some of the factors playing a role in the shift of the disease foci to the earlier non-endemic areas.
Fig. 1.
Choropleth map showing Kala-azar (visceral leishmaniasis) outbreaks in the districts of Kenya between January, 2020 to November, 2022 as reported by the Division of Disease Surveillance and Response, Ministry of Health, Kenya. The map was created using QGIS 3.28.0. The base map was used from Diva GIS.
There are several challenges in the control of VL in Kenya which have led to a persistent increase in the number of cases as well as the geographical shift of the disease. The interventional strategies of diagnosis, management and control of the disease have not proven effective; however, they have remained unchanged even in the face of the changing epidemiology of VL. The diagnostic methods used for VL in east Africa are rK39 antigen-based rapid tests, microscopy of the patient's lymph node punctures, bone marrow and splenic aspirates [5]. The methods requiring invasive sampling are prohibitive and tedious, thus neither efficient nor scalable. On the other hand, the rK-39 rapid diagnostic test (RDT) has been very efficiently used in the Indian subcontinent; however, it has shown suboptimal sensitivity and specificity in Africa [5]. Molecular methods such as polymerase chain reaction (PCR) are highly efficient but expensive for resource-poor countries. The treatment of VL also remains a challenge in Kenya. Earlier, sodium stibogluconate (SSG) was the preferred treatment for VL despite its toxic effects and reports of relapse and unresponsiveness in some patients. Currently, the Ministry of Health of the Republic of Kenya recommends a combination of SSG with paromomycin (PM) as the first-line therapy for VL, as it has the advantage of a shorter duration of treatment and better compliance [3,5]. Other drugs like amphotericin B and miltefosine, although efficacious, are limited in their usage owing to the high cost and teratogenicity in pregnant females, respectively. Also, there are logistic issues in the continuous supply chain of amphotericin B and its preparations. There are several other gaps in understanding the disease, such as the pastoral epidemiology, the effect of natural calamities, animal reservoirs and zoonotic transmission, and refugee movement, which need to be filled to implement adequate control and prevention measures for VL in Kenya. In addition to the already unsurmountable challenges, the COVID-19 pandemic has suffocated countries like Africa concerning healthcare resources and has left the neglected diseases more neglected. During the pandemic, there has been severe disruption in activities such as mass drug administration, case detection, treatment, and vector control [5]. The access of patients to the health care systems has been severely compromised amidst the COVID-19 pandemic regarding timely attention and availability of beds to those who needed them, leading to the unabated community spread of the disease [5].
Despite the many measures taken by the WHO and the government in the prevention of VL by formulating evidence-based policies, facilitating public and private collaborations to aid the management of the disease, provision of medical supplies from the WHO's global emergency stockpile, and other measures, VL remains a problem of public health in Kenya. There are numerous critical areas which need to be urgently addressed. Targeting the improvement of the quality of patient care, provision of more diagnostic facilities and staff training is required, and using shorter and more effective drug combinations will improve clinical outcomes. A prompt response to outbreaks by early case detection, enhanced vector control and surveillance, and robust monitoring and evaluation systems will have to be developed. The logistical issues, such as the supply chain of drugs and diagnostics, will have to be strengthened for resource mobilization—this will require stronger partnerships of ministries and county governments with semi-autonomous government agencies, pharmaceutical manufacturers and global donors. Community participation will have to be strengthened by educating the affected populations on avoiding exposure and improving their health-seeking behaviour. Finally, the research activities will need funding, support from donors, and an initiative from academia to develop more practical diagnostic methods and efficient therapeutics [3].
Along with the WHO roadmap of NTDs, the Ministry of Health, Republic of Kenya, has laid critical strategies for preventing and control to control leishmaniasis by 2025 [3], which encompass the control measures mentioned above. However, an urgent and concentrated effort will be needed to implement the control efforts efficiently, curb the ongoing outbreak, and sustain those gains to prevent the future emergence and reemergence of this dreadful disease.
Funding
None.
Ethical approval
Not applicable.
Declaration of competing interest
None.
Acknowledgements
None.
References
- 1.Alvar J., Vélez I.D., Bern C., Herrero M., Desjeux P., Cano J., et al. vol. 7. 2012 May 31. p. e35671.https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0035671 (Leishmaniasis worldwide and global estimates of its incidence. PLoS one [internet]). [cited 2022 Nov 3] 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kenya: disease outbreak situation report as of 28th Oct 2022 - EPI Week 42 2022 - Kenya | ReliefWeb [Internet] https://reliefweb.int/report/kenya/kenya-disease-outbreak-situation-report-28th-oct-2022-epi-week-42-2022 [cited 2022 Nov 5]
- 3.KSPC-OF-LEISHMANIASIS-STRATEGY-2021-2025.
- 4.de Souza D.K., Picado A., Bessell P.R., Liban A., Wachira D., Mwiti D., et al. Strengthening visceral leishmaniasis diagnosis capacity to improve access to care in Kenya: the example of Marsabit county. Frontiers in Tropical Diseases. 2022 Jan 10:65. 0. [Google Scholar]
- 5.Alvar J., Dagne D.A. Towards the elimination of visceral leishmaniasis as a public health problem in east Africa: reflections on an enhanced control strategy and a call for action. Health Policy. 2022 doi: 10.1016/S2214-109X(21)00392-2. www.thelancet.com/lancetgh www.thelancet.com/lancetgh [Internet]. 2021 [cited 2022 Nov 5];9. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]