Abstract
Life experiences and environmental conditions in childhood can change the physiology and behaviour of exposed individuals and, in some cases, of their offspring. In rodent models, stress/trauma, poor diet, and endocrine disruptors in a parent have been shown to cause phenotypes in the direct progeny, suggesting intergenerational inheritance. A few models also examined transmission to further offspring and suggested transgenerational inheritance, but such multigenerational inheritance is not well characterized. Our previous work on a mouse model of early postnatal stress showed that behaviour and metabolism are altered in the offspring of exposed males up to the 4th generation in the patriline and up to the 2nd generation in the matriline. The present study examined if symptoms can be transmitted beyond the 4th generation in the patriline. Analyses of the 5th and 6th generations of mice revealed that altered risk-taking and glucose regulation caused by postnatal stress are still manifested in the 5th generation but are attenuated in the 6th generation. Some of the symptoms are expressed in both males and females, but some are sex-dependent and sometimes opposite. These results indicate that postnatal trauma can affect behaviour and metabolism over many generations, suggesting epigenetic mechanisms of transmission.
Keywords: mouse model, postnatal trauma, risk-taking, weight, glucose metabolism, patriline, offspring, 5th and 6th generations, transgenerational inheritance
Introduction
The environment strongly influences physiology in plants and animals. Many environmental factors can modify phenotypes persistently and affect mental and physical health in mammals. They represent serious health risks, and it is estimated that 12.6 M global deaths per year are due to modifiable environmental factors [1]. If germ cells are affected and their alterations persist until conception, they may be transferred to the embryo at fertilization and result in symptoms of exposure in the progeny. Such inheritance is not due to changes in the genetic sequence but likely involves epigenetic factors and mechanisms. Transmission of environmentally induced traits has been extensively documented in plants, ciliates, chicken, fish, Neurospora crassa, Caenorhabditis elegans, Drosophila, and mammals [2–9]. In humans, large epidemiological studies on historical cohorts such as the Överkalix, Dutch Hunger Winter Famine, and Avon Longitudinal Study of Parents and Children suggested that food supply and famine in prenatal life or childhood of grandparents have an incidence on the risk for cardiovascular diseases, obesity, and mortality in descendants [10–13]. Likewise, endocrine-disrupting chemicals, smoking, and lead can affect health across generations in humans [14–18].
Further to conditions involving food and chemicals, emotional and psychological factors can also strongly affect health. Up to 45% of children in developed countries and over 50% in emerging economies are exposed to life adversity, such as emotional, physical, or sexual abuse, household violence, neglect, or parental loss. Such conditions increase the risk for depression, personality and mood disorders, addiction, and comorbidities, such as cardiometabolic and autoimmune diseases and cancer, in exposed individuals [19–21] and their descendants [17, 18, 22, 23]. Childhood trauma is one of the leading causes of premature death in adulthood [24]. While maternal or caregiver behaviour and nursing are possible ways by which the effects of adversity can be transferred to children [25–27], transmission via gametes is another possible route [28, 29]. The likely involvement of the parental germline is supported by adoption studies in humans [30] and cross-fostering in animals [31, 32], and implies that the offspring may be affected independently of parental care.
Several rodent models have been developed to examine the effects of early life stress across generations. We established a model of postnatal trauma in mice based on unpredictable maternal separation combined with unpredictable maternal stress (MSUS) [33] that mimics childhood trauma resulting from parental neglect, unreliable care, and poor affective attachment in humans. MSUS consists in separating mothers (F0) from their newborn pups (F1) each day for 3 h at an unpredictable time, starting 1 day after birth until postnatal day (PND) 14 (Fig. 1a). During separation, dams are also exposed unpredictably to a stressor (acute swim or restraint chosen randomly) to further increase distress (Fig. 1b). When adult, the exposed animals have profound behavioural, cognitive, and metabolic symptoms that are transmitted to their offspring, via both females and males [32–39]. Phenotypes include risk-taking behaviours, depressive-like symptoms, altered social abilities, memory deficits, insulin/glucose dysregulation and altered bone homeostasis, but also, in some conditions, stress resilience and improved behavioural flexibility. Recently, we showed that some of the symptoms induced by MSUS are transmitted to the 4th generation via males [40].
Figure 1:
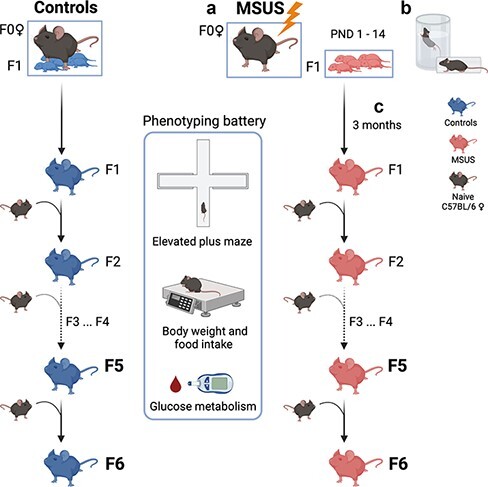
MSUS paradigm. (a) For MSUS, naïve males and primiparous females are mated, and starting 1 day after birth until PND14 (PND1–14), pups (F1) are separated from their mother (F0) for 3 h daily at an unpredictable time. (b) During separation, dams are unpredictably exposed to either an acute (5 min) swim in 18°C water or a 20-min physical restraint in a tube anytime (unpredictably) during the 3 h. Between PND15 and weaning at PND21, pups are left undisturbed with their mother and are then raised normally until adulthood. Control animals are produced and raised normally (left). (c) When adult (>3 months), F1 males are mated with naïve primiparous control females to produce F2 offspring, and breeding is repeated with males of each generation to produce F3, F4, F5, and F6 offspring. Male breeders are removed from the mating cage after 1 week and are never in contact with their offspring. MSUS is applied only to F1 pups but not to any of the following offspring. When adult, animals from each generation undergo behavioural and metabolic testing. The present study used F5 and F6 animals. Created with BioRender.com
To extend our previous findings, we examined if behavioural and metabolic symptoms induced by MSUS can be transmitted to animals beyond the 4th generation. We produced 5th and 6th generation MSUS and control offspring from the patriline by breeding 4th and 5th generation males to naïve females, respectively (Fig. 1c), and assessed their behaviour and metabolic responses. The results show that altered risk-taking behaviours and glucose/insulin responses are still manifested by animals of the 5th generation, in a sex-dependent manner and with opposite effects for glucose/insulin responses. The symptoms are, however, attenuated in animals of the 6th generation.
Material and Methods
Mice
C57BI/6JRj mice (Elevage Janvier, Le Genest Saint Isle, France) are maintained in a temperature- and humidity-controlled facility on a 12-h reversed light–dark cycle (white light from 8 pm to 8 am and darkness from 8 am to 8 pm) in individually ventilated cages (SealSafe PLUS, Tecniplast, Germany) with food (M/R Haltung Extrudat, Provimi Kliba SA, Switzerland, Cat. #3436) and water ad libitum. Cages contain wood chip bedding (LIGNOCEL SELECT, J. Rettenmaier & Söhne), paper tissue as nesting material, and a plastic house. All procedures are carried out during the animals’ active phase (darkness) in accordance with guidelines and regulations of the cantonal veterinary office in Zürich and the Swiss Animal Welfare Act (Tierschutzgesetz). All animal experiments are approved by cantonal veterinary authorities (licence 57/2015 and 83/2018).
Unpredictable Maternal Separation Combined with Unpredictable Maternal Stress (MSUS)
Naïve C57BI/6JRj males and primiparous females (3–4 months old) are bred in a one-to-one pairing. After a week, the male is removed from the cage to avoid any interference with gestation. At delivery, each dam (F0) and pups (F1) are assigned to MSUS or control groups randomly while taking into consideration the number of male/female pups in each litter to have a comparable group size. MSUS pups are separated from their mother daily for 3 h at an unpredictable time during the active phase starting at PND 1 until PND14 (Fig. 1a). During separation, MSUS dams are randomly and unpredictably subjected to either an acute swim in cold water (18°C for 5 min) or restraint for 20 min in a plastic tube (3.18 cm in diameter with a sliding nose restraint and air holes, Midsci) (Fig. 1b). Control animals are left undisturbed. Both control and MSUS mice have weekly cage changes and weight measurements. Pups are weaned at PND21 and reared in social groups (3–5 mice/cage, controls or MSUS) but from different dams to avoid litter effects. To produce offspring, adult control and MSUS males at each generation (n = 15–30) are mated with naïve primiparous C57BI/6JRj females (one-to-one), and F2, F3, F4, F5, and F6 MSUS offspring are successively obtained (Fig. 1c). The MSUS paradigm is applied only to F0 dams and their F1 pups. F2, F3, F4, F5, and F6 MSUS animals are not exposed to any treatment.
Breeding Size and Litter Numbers
The number of males used for breeding at each generation ranges from 10 to 40 depending on the number of offspring needed for the experiments [40]. Litters with 4–10 male and female pups are used for the final cage assignment. Litters with less than 4 or more than 10 pups are excluded. Only litters used for experiments are reported (see Results and Supplementary Table S1). Breeding is conducted after phenotyping, following 2–3 weeks of rest after the last test.
Behavioural Testing
For the elevated plus maze, mice are singly housed 1 h before testing. This avoids the collective stress of cage-mates taken away for testing, reduces experimental variability, and improves data quality [41]. The animals are moved from the colony room to the experimental room right before testing. The elevated plus maze consists of a dark grey polyvinyl chloride platform with two open (length: 30 cm, width: 5 cm) and two closed (with 15 cm walls) arms, elevated 60 cm above the floor. A video camera is placed directly above the centre of the maze, and two overhead white lights are positioned to obtain different levels of illumination for the open (18 ± 1 Lux) and closed (9 ± 1 Lux) arms. Animals are gently removed from their home cage by the tail and carefully placed directly into the centre of the maze (central platform 5 × 5 cm) facing the open arm opposite from the experimenter. Tracking/recording lasts 5 min and is remotely initiated when the mouse is on the platform. The time spent in open and closed arms and the total distance covered are automatically recorded by a video-tracking system (ViewPoint Behaviour Technology). The latency to first enter an open arm is manually scored. Animals that do not enter an open arm are assigned a latency of 300 s (equal to the duration of the entire test). The maze is wiped with a tissue after each animal. The experimenter is blind to the identity and treatment of animals. Testing is conducted on adult animals (3–5 months old) and during the animals’ active phase (darkness). Experiments in males and females are run on different days to avoid confounding effects of olfactory cues between sexes.
Metabolic Assays
Body weight is measured under dim red light, while blood sampling is done in white light. Experimenters are blind to the identity and treatment of animals.
Food Intake and Body Weight
Food pellets are weighed in each cage every day for 72 h and averaged by the number of animals (maximum 4 animals/cage). Every 24 h, pellets are replaced with fresh pellets to limit crumb spillage. Animals are weighed before the experiment and right after the last food measurement. Caloric intake is calculated as the mean amount of food consumed during 72 h over the mean body weight per cage. Caloric intake was measured in 4.5-month-old F5 males and 6-month-old females.
Glucose Level in Response to Restraint
The test is conducted as described in [40]. Each mouse is placed for 30 min in a cylindrical plastic tube (3.18 cm diameter with a sliding nose restraint and air holes, Midsci) for physical restraint, and the tail is fixed on the table with tape. Blood is drawn at 0, 15, 30, and 90 min by tail prick, within 1 cm of tail end using a 22 G needle. After 30 min, the animal is placed in a temporary cage for 1 h. For the 90-min measurement, each mouse is confined under an inverted 1-l glass beaker (14 cm high and 12 cm diameter), with its tail protruding from under the spout to allow access by the experimenter for the last blood sampling. The animal is then immediately placed back into its cage. Glucose is measured from fresh blood droplets with an Accu-Chek Aviva glucometer (Roche).
Glucose Tolerance Test and Insulin Tolerance Test
Mice are temporarily singly housed and fasted for 5 h before the experiment. Glucose is measured in blood samples at 0, 15, 30, 90, and 120 min after an intraperitoneal injection of glucose (Glucose Tolerance Test, GTT) or insulin (Insulin Tolerance Test, ITT). For blood sampling, each animal is confined under an inverted 1-l glass beaker (14 cm high and 12 cm diameter) with the tail exiting from under the spout. For GTT, 2 mg/g body weight glucose in 0.45% (wt/vol) saline is injected. For ITT, 1 mU of insulin (NovoRapid Novo Nordisk A/S) in sterile 0.9% saline per gram body weight (1 mU/g) is injected. If blood glucose falls below 1.7 mM/ml, the animal is rescued with an intraperitoneal injection of 2 mg/gram glucose and removed from the experiment. Since many F5 females had to be rescued after insulin injection at 1 mU/g, a dose of 0.6 mU/g was used for F6 females. For both GTT and ITT, each mouse is placed in a temporary cage after 30 min and confined again for 90 min after the initial injection to conduct the last two blood samplings. The glucose level is determined in fresh tail blood using an Accu-Chek Aviva glucometer (Roche).
Statistics
Statistical analyses are performed using GraphPad Prism software, versions 8 and 9. Sample size is estimated based on previous experiments on the MSUS model [33, 37, 40]. Data are screened for outliers using Prism’s robust regression and outlier removal test (ROUT, Q set at 5%) for weight, distance covered on the elevated plus maze, and food intake. Animals identified as outliers are excluded from the analyses. For all other tests, mice are excluded only in cases of technical problems (e.g. unexpected noise during behavioural testing and interruption of tasks to rescue the animal; Supplementary Table S2). Data distribution is examined using D’Agostino–Pearson and/or Shapiro–Wilk tests. When data follow a Gaussian distribution, parametric tests, e.g. the two-tailed Student’s t-test and repeated-measures Analysis of Variance (ANOVA), are used to compare two groups. Welch’s correction is applied if the variance is not homogeneous between groups. In the case of a non-parametric distribution, the Mann–Whitney U test is used to compare two groups. The Geisser–Greenhouse correction is applied to repeated-measures ANOVA to account for deviations from sphericity. The correction parameter epsilon (ε) and adjusted P-values are reported in Supplementary Tables S3 and S4. Significant effects in ANOVAs are further analysed using Šidák’s multiple comparison test. Significance is set at P < 0.05 for all tests and indicated by asterisks. P < 0.1 is considered a trend and is indicated by a hashtag. Reported n represents data after outliers’ removal. Control mice are in blue, whereas MSUS mice are in red.
Results
Production of 5th and 6th Generation Offspring
15 control and 16 MSUS male mice (8.5 months old) from the 4th generation (F4) [40], obtained by breeding of the grand-offspring (F3) of exposed males (F1), were each paired with one naïve primiparous female to generate F5 offspring (Supplementary Table S1). The breeding produced 14 control and 13 MSUS F5 litters. For the phenotyping of the F5 cohort, a total of 104 males (57 control and 47 MSUS) and 48 females (24 control and 24 MSUS) were tested. To obtain F6 offspring, 15 control and 16 MSUS F5 males (10 months old) were each mated one-to-one with a naïve primiparous female and produced 6 control and 10 MSUS litters. F6 phenotyping was conducted on 60 males (30 control and 30 MSUS) and 40 females (20 control and 20 MSUS; Supplementary Table S1).
Anxious Behaviours in MSUS Males of the 5th Generation
We assessed the response to aversive conditions in F5 and F6 adult animals using an elevated plus maze. F5 MSUS males spent significantly less time in the open arms of the maze than controls (Fig. 2a) but had a similar latency to first enter an open arm (Fig. 2b). Notably, the reduced time in open arms is opposite to that previously observed in F1, F2, F3, and F4 males, who spent more time in open arms [37, 40]. The difference between controls and MSUS remains statistically significant even when the control male who spent more than 140 s in the open arms is removed from the analysis (Controls n = 29, MSUS n = 30, t57 = 2.013, *P = 0.048. For outliers’ exclusion criteria, see Material and Methods). Time spent in open arms and latency to first enter an open arm in F6 MSUS males were not significantly different from controls despite a trend for an increase (P = 0.0842, Fig. 2c and d). F5 and F6 MSUS females did not show any behavioural alteration, suggesting no apparent transmission of risk-taking traits beyond F4 (Fig. 2) [40]. Locomotor activity in male and female mice of both F5 and F6 generations was not affected by MSUS (Supplementary Fig. S1). We also compared the performance of control males across generations to assess the reliability of the elevated plus maze test. Overall, control males from F1 to F6 generations spent a comparable amount of time on the open arms, although F4 males had a slightly decreased time (Supplementary Fig. S2).
Figure 2:
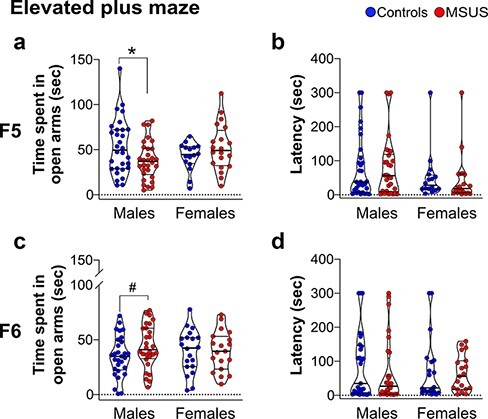
performance of F5 and F6 adult mice on an elevated plus maze. F5 MSUS males (a) spend less time in the open arms of the maze than control males (Controls n = 30, MSUS n = 30, t58 = 2.279, P = 0.0263) but (b) have comparable latency to first enter an open arm (Controls n = 30, MSUS n = 30, U = 441, P = 0.8978). F5 control and MSUS females (a) spend a comparable amount of time in open arms (Controls n = 16, MSUS n = 20, t34 = 1.346, P = 0.1873) and (b) have comparable latency to first enter an open arm to control females (Controls n = 16, MSUS n = 20, U = 125, P = 0.2722). (c and d) There is a trend for F6 MSUS males to spend more time in open arms than control males (Controls n = 29, MSUS n = 28, t55 = 1.759, P = 0.0842) but no difference in females (Controls n = 20, MSUS n = 19, t37 = 0.0815, P = 0.9354). Latency to first enter an open arm is comparable in control and MSUS males (Controls n = 29, MSUS n = 28, U = 360, P = 0.4677) and females (Controls n = 20, MSUS n = 19, U = 166.5, P = 0.5177). Truncated violin plots illustrate individual values and data distribution (kernel probability density). The thick horizontal bar represents the median, and the thin bars indicate the 1st and 3rd quartiles. #P < 0.1, *P < 0.05
Altered Body Weight in MSUS Males and Females of the 5th Generation
F5 MSUS males and females were overweight from PND7 until early adulthood (4.5 months) when compared with control animals (Fig. 3a and b). This is consistent with the increased weight observed in adult F4 MSUS males [40] but opposite to the reduced weight in adult MSUS mice from F2 and F3 generations. The overweight phenotype was no longer observed in MSUS females at 7.5 months (Fig. 3b). We examined if the increased body weight is due to higher food consumption by measuring caloric intake. F5 MSUS males consumed an amount of food comparable to control males but F5 MSUS females consumed slightly more than control females (although not significantly P = 0.081, Fig. 4a). This suggested that the increased weight in adulthood (Fig. 4b) is not due to excessive food intake but most likely due to metabolic dysregulation. F6 MSUS animals had normal weight (Fig. 3c and d) and food intake compared to controls (data not shown).
Figure 3:
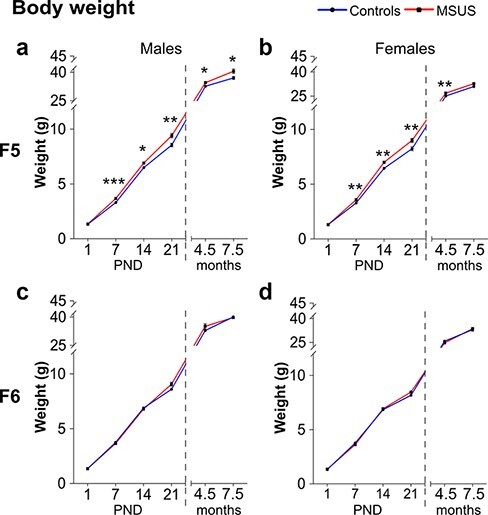
weight across development in F5 and F6 mice. (a) F5 MSUS males weigh more than controls at PND7 (Controls n = 54, MSUS n = 46, t98 = 3.932, P = 0.0002), PND14 (Controls n = 56, MSUS n = 46, t100 = 2.527, P = 0.0131), PND21 (Controls n = 56, MSUS n = 45, t99 = 3.080, P = 0.0027), 4.5 months (Controls n = 28, MSUS n = 22, Welch’s t28.33 = 2.331, P = 0.0271), and 7.5 months (Controls n = 16, MSUS n = 16, t30 = 2.435, P = 0.0210) but not at PND1 (Controls n = 58, MSUS n = 46, t102 = 1.084, P = 0.2808). (b) F5 MSUS females weigh more than controls at PND7 (Controls n = 41, MSUS n = 42, t81 = 3.401, P = 0.0010), PND14 (Controls n = 48, MSUS n = 42, t88 = 3.059, P = 0.0029), PND21 (Controls n = 48, MSUS n = 43, t89 = 2.887, P = 0.0049), and 4.5 months (Controls n = 17, MSUS n = 20, Welch’s t26.44 = 3.068, P = 0.0049) but not at PND1 (Controls n = 49, MSUS n = 46, t93 = 0.0074, P = 0.9940) or at 7.5 months (Controls n = 18, MSUS n = 20, t36 = 1.614, P = 0.1153). No weight differences are observed in F6 MSUS (c) males (PND1 Controls n = 35, MSUS n = 50, t83 = 0.0716, P = 0.9431; PND7 Controls n = 34, MSUS n = 51, U = 806.5, P = 0.5907; PND14 Controls n = 34, MSUS n = 42, t74 = 0.3191, P = 0.7506; PND21 Controls n = 34, MSUS n = 42, t74 = 1.164, P = 0.2481; 4.5 months Controls n = 16, MSUS n = 16, t30 = 1.392, P = 0.1741; 7.5 months Controls n = 16, MSUS n = 16, t30 = 0.0015, P = 0.9988) or (d) females (PND1 Controls n = 25, MSUS n = 69, t92 = 1.042, P = 0.3003; PND7 Controls n = 29, MSUS n = 66, U = 894, P = 0.6140; PND14 Controls n = 24, MSUS n = 54, U = 570, P = 0.4025; PND21 Controls n = 23, MSUS n = 53, Welch’s t56.75 = 1.663, P = 0.1011; 4.5 months Controls n = 15, MSUS n = 15, t28 = 1.692, P = 0.1018; 7.5 months Controls n = 20, MSUS n = 20, t38 = 0.5016, P = 0.6189). Numbers variation among PND1–7–14–21 time points might be due to outliers removal (see also Material and Methods and Statistics), wrong gender identification, or perinatal death. Weight was measured only a subgroup of adult animals (4.5 and 7.5 months). Data represent mean ± SEM. #P < 0.1, *P < 0.05, **P < 0.01, ***P < 0.001
Figure 4:
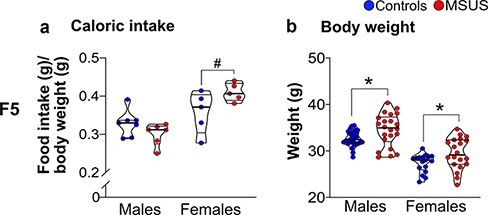
caloric intake in adult F5 mice. The average caloric intake/cage is comparable in F5 control and MSUS males (Controls n = 7 cages, MSUS n = 6 cages, t11 = 1.578, P = 0.1429, 4.5 months) and females (Controls n = 5 cages, MSUS n = 5 cages, t8 = 1.996, P = 0.0810, 6 months) but weight is higher in F5 MSUS than control males (Controls n = 28, MSUS n = 22, Welch’s t28.33 = 2.331, P = 0.0271) and females (Controls n = 17, MSUS n = 20, Welch’s t31.45 = 2.347, P = 0.0254). Truncated violin plots illustrate individual values and data distribution (kernel probability density). The thick horizontal bar represents the median and thin bars indicate the 1st and 3rd quartiles. #P < 0.1, *P < 0.05
Dysregulated Glucose Homeostasis in F5 and F6 MSUS Mice
We examined glucose response in F5 and F6 animals using a GTT [42]. The glucose level in F5 MSUS females but not males was lower than that of controls at the peak of the curve, suggesting that MSUS females have a more efficient glucose clearance (Fig. 5a and b and Supplementary Table S3). We then used an ITT to assess the glucose response after an insulin injection [42]. Insulin provoked a more pronounced decline in blood glucose in control than in MSUS F5 males, even if the overall trend of the response was not changed (Fig. 5c, Supplementary Table S3). Control and MSUS F5 females did not show any difference on the ITT (Fig. 5d, Supplementary Table S3) although only half of the animals completed the test (6 controls and 7 MSUS), while the other half had to be rescued by glucose injection due to signs of distress. Consistent with F5 MSUS males, F6 MSUS males had a lower glucose response to insulin during ITT (Fig. 5e, Supplementary Table S4) but no change during GTT (Supplementary Table S3). No alteration in glucose response after GTT or ITT was observed in F6 MSUS females (Fig. 5f, Supplementary Table S4). We next examined the glucose response after an acute stress using a restraint test. Although blood glucose was transiently increased in F5 and F6 MSUS and control animals, there was no significant overall difference between the groups (Supplementary Tables S3 and S4). This suggests that the blunted increase in blood glucose observed in F2 and F4 MSUS mice [37, 40] is not transferred to subsequent generations or is compensated for.
Figure 5:
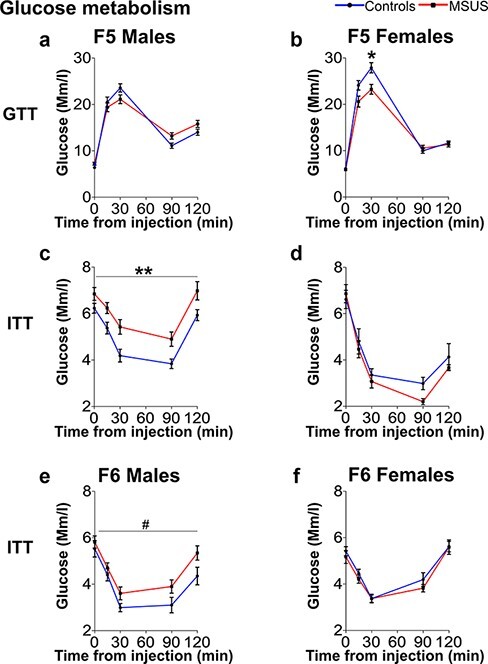
metabolic assessment in F5 and F6 mice. (a, b) On GTT, (a) F5 control and MSUS males have comparable level of blood glucose (Controls n = 15, MSUS n= 14, interaction F(4108) = 4.469, P = 0.0022; post-hoc test: P > 0.05) but (b) F5 MSUS females have blood glucose at the peak of the curve lower than controls (Controls n = 13, MSUS n = 14, interaction F(4100) = 4.423, P = 0.0025; post-hoc test: P30 = 0.0285). (c–f) On ITT, insulin response is (c) significantly blunted in F5 MSUS males compared to controls (Controls n = 16, MSUS n = 15, group effect: F(1, 29) = 10.17, P = 0.0034) but (e) not in F6 MSUS males (Controls n = 12, MSUS n = 14, group effect F(1,24) = 3.549, P = 0.0718) or (d) in F5 (Controls n = 6, MSUS n = 7) and (f) F6 (Controls n = 13, MSUS n = 14) females. Data represent mean ± SEM. #P < 0.1, *P < 0.05, **P < 0.01, ***P < 0.001. Detailed results are presented in Supplementary Tables S3 (F5) and S4 (F6)
Discussion
The current study extends our previous findings that postnatal traumatic stress in mice causes behavioural and metabolic changes that persist across three generations of offspring (till F4). The MSUS model is characterized by increased risk-taking, which is the most penetrant trait observed in directly exposed males and their offspring up to the 4th generation [40]. This trait is, however, not manifested in animals of the 5th generation, but F5 males have increased anxiety. Mice of the 6th generation have nonetheless shown a trend for increased risk-taking. Notably, we observed in the past that some MSUS traits can be manifested in one generation but not in the following one, e.g. depressive-like behaviour is not detected in F2 mice but is detected in F3 mice [33]. Other models of exposure, e.g. to endocrine disruptors [43], maternal immune activation [44], or neonatal over-nutrition [45], were reported to have different phenotypes across generations. Similarly, the different phenotypes in F5 and F6 MSUS animals compared to previous generations might be a form of adaptation or overcompensation. The advanced age of F5 fathers at breeding (10 months) might also contribute to the attenuation of the MSUS phenotype from the 5th to the 6th generation and the reduced number of F6 litters.
Animals of the 5th generation have an altered glucose homeostasis similar to mice from previous generations [37, 40]. The reduced glucose level observed on GTT in MSUS females may involve different mechanisms such as increased efficiency of glucose in stimulating its own uptake and suppressing its production in basal/constant insulin concentration [42, 46, 47]. The increased glucose level on ITT in MSUS males may also result from a reduced sensitivity to insulin or insulin resistance, suggesting a pathological state similar to metabolic syndrome or type 2 diabetes [48]. It may also be due to changes in other factors such as insulin counter-regulatory hormones like glucagon, epinephrine, cortisol, or growth hormone, which sustain plasma glucose during fasting conditions [49]. Additional methods such as the glucose clamp technique or direct quantification of insulin level could be envisaged to fully assess changes in glucose metabolism in MSUS animals. Our results show that glucose response is not altered in animals of the 6th generation, suggesting that the underlying factors of transmission in the germline were corrected or compensated for, perhaps progressively across generations. The fact that MSUS males are bred with non-exposed naïve females at each generation probably favours such a correction. The manifestation of metabolic phenotypes after early life trauma that persist across generations is highly relevant to humans and is reminiscent of the increased risk for physical health problems in people exposed to childhood trauma [50]. Besides the differential manifestation of phenotypes across generations, we also observed differences between males and females in F5 and F6 generations. Sex-specific inheritance of traits is a phenomenon that was previously observed in our model [33, 37, 40] and others [51, 52]. The underlying mechanisms are not known but may involve different regulatory pathways controlling sexual development, sex chromosomes, and sex-specific metabolic or hormonal influences [53].
The mechanisms and evolutionary implications of such transmission are not fully understood and may be different depending on the type of exposure. Challenging environmental conditions can increase fitness by inducing phenotypic adaptation to these conditions. But, if the conditions change, the progeny is then maladapted and may express deficits or negative phenotypes [54]. Furthermore, not all phenotypes have the same depth of penetrance, causing some features to be transmitted from the exposed parent to direct offspring but not further. The scarcity of mammalian models with transgenerational effects of environmental exposure beyond the 2nd or 3rd generation limits our full understanding of these effects. Among models of prenatal stress [55], environmental toxicants [2], obesogens [56], and drugs [57, 58], MSUS has several advantages and unique features. First, it does not involve any invasive manipulation and is based on emotional and physical mistreatment, which is highly similar to aspects of childhood trauma in humans. Second, it uses a short exposure in early postnatal life (PND1–14) unlike many models that have extensive exposure sometimes spanning preconception to postnatal or even adult life [59–62]. MSUS does not interfere with prenatal development or epigenetic reprogramming during embryogenesis [29], and its restricted time window makes it easier to identify the developmental processes or cells likely to be affected. For instance, in early postnatal life in mice, developing gonads mostly have spermatogonial cells but no differentiated spermatocytes yet [29, 63]. Furthermore, the patriline design eliminates confounding factors such as the intrauterine milieu and maternal behaviours that can mask germline-dependent transmission [41, 64–66]. It allowed us to identify sperm as a carrier of molecular signals of exposure from father to offspring [37]. So far, sperm RNA is one of the best documented vectors of epigenetic inheritance [7], which we demonstrated to be causally responsible for the transmission of the effects of MSUS to the offspring [37, 67]. Recently, we also provided evidence that some of the effects of exposure in the offspring can be reproduced by intravenous injection of MSUS serum in adult control males [68], suggesting that blood components can modify the germline [4, 69, 70]. Developing other models of exposure with both patriline and matriline transmission like MSUS would help gain a deeper understanding of germline-dependent mechanisms of epigenetic inheritance.
Supplementary Material
Acknowledgements
We thank Gretchen van Steenwyk and Rodrigo Gacel Arzate-Mejia for critical discussion of the results, Alberto Corcoba and Deepak Tanwar for help with statistical analyses, and Yvonne Zipfel and the LASC team for excellent animal care.
Contributor Information
Chiara Boscardin, Laboratory of Neuroepigenetics, Brain Research Institute, Faculty of Medicine of the University Zürich, Winterthurerstrasse 190, Zürich 8057, Switzerland; Institute for Neuroscience, Department of Health Science and Technology of ETH Zürich, Centre for Neuroscience Zürich, Winterthurerstrasse 190, Zürich 8057, Switzerland.
Francesca Manuella, Laboratory of Neuroepigenetics, Brain Research Institute, Faculty of Medicine of the University Zürich, Winterthurerstrasse 190, Zürich 8057, Switzerland; Institute for Neuroscience, Department of Health Science and Technology of ETH Zürich, Centre for Neuroscience Zürich, Winterthurerstrasse 190, Zürich 8057, Switzerland.
Isabelle M Mansuy, Laboratory of Neuroepigenetics, Brain Research Institute, Faculty of Medicine of the University Zürich, Winterthurerstrasse 190, Zürich 8057, Switzerland; Institute for Neuroscience, Department of Health Science and Technology of ETH Zürich, Centre for Neuroscience Zürich, Winterthurerstrasse 190, Zürich 8057, Switzerland.
Supplementary data
Supplementary data is available at EnvEpig online.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
We thank the University of Zürich, the Swiss Federal Institute of Technology Zürich, the Swiss National Science Foundation (grant number 31003A_175742/1), ETH grant ETH-25 19-2, the National Center of Competence in Researcn (NCCR) funded by SNSF (grant number 182880/Phase 2 and 205601/Phase 3), EU Horizon 2020 Research and Innovation Programme grant number 848158 EarlyCause, and Escher Family Fund for supporting this research.
Data availability
The data that support the findings of this study are available from the corresponding author, I.M.M., upon reasonable request.
Authors’ Contribution
C.B., F.M., and I.M.M. conceived and designed the study. C.B. and F.M. conducted MSUS paradigm, performed behavioural and metabolic experiments, and collected the results. F.M. organized and managed breeding. C.B. compiled and analysed raw data and prepared figures. C.B., F.M., and I.M.M. wrote the manuscript. I.M.M. raised funds to support this project.
References
- 1. Prüss-Üstün A, Wolf J, Corvalan CF. et al. Preventing Disease Through Healthy Environments: A Global Assessment of the Burden of Disease From Environmental Risks . Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 2. Anway MD, Cupp AS, Uzumcu N. et al. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 2005;308:1466–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Castro Barbosa T, Ingerslev LR, Alm PS. et al. High-fat diet reprograms the epigenome of rat spermatozoa and transgenerationally affects metabolism of the offspring. Mol Metab 2016;5:184–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zeybel M, Hardy T, Wong YK. et al. Multigenerational epigenetic adaptation of the hepatic wound-healing response. Nat Med 2012;18:1369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu S, Guo W, Li X. et al. Paternal chronic folate supplementation induced the transgenerational inheritance of acquired developmental and metabolic changes in chickens. Proc R Soc B Biol Sci 2019;286:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rechavi O, Houri-Ze’evi L, Anava S. et al. Starvation-induced transgenerational inheritance of small RNAs in C. elegans. Cell 2014;158:277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rassoulzadegan M, Grandjean V, Gounon P. et al. RNA-mediated non-mendelian inheritance of an epigenetic change in the mouse. Nature 2006;441:469–74. [DOI] [PubMed] [Google Scholar]
- 8. Buescher JL, Musselman LP, Wilson CA. et al. Evidence for transgenerational metabolic programming in Drosophila. Dis Model Mech 2013;6:1123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nowacki M, Landweber LF. Epigenetic inheritance in ciliates. Curr Opin Microbiol 2009;12:638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bygren LO, Tinghög P, Carstensen J. et al. Change in paternal grandmothers’ early food supply influenced cardiovascular mortality of the female grandchildren. BMC Genet 2014;15:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pembrey ME, Bygren LO, Kaati G. et al. Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet 2006;14:159–66. [DOI] [PubMed] [Google Scholar]
- 12. van den Berg GJ, Pinger PR. Transgenerational effects of childhood conditions on third generation health and education outcomes. Econ Hum Biol 2016;23:103–20. [DOI] [PubMed] [Google Scholar]
- 13. Vågerö D, Pinger PR, Aronsson V. et al. Paternal grandfather’s access to food predicts all-cause and cancer mortality in grandsons. Nat Commun 2018;9:5124–30. Author Correction: Nat Comm 2021;12:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Cauwenbergh O, Di Serafino A, Tytgat J. et al. Transgenerational epigenetic effects from male exposure to endocrine-disrupting compounds: a systematic review on research in mammals. Clin Epigenetics 2020;12:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Golding J, Ellis G, Gregory S. et al. Grand-maternal smoking in pregnancy and grandchild’s autistic traits and diagnosed autism. Sci Rep 2017;7:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sen A, Heredia N, Senut M-C. et al. Multigenerational epigenetic inheritance in humans: DNA methylation changes associated with maternal exposure to lead can be transmitted to the grandchildren. Sci Rep 2015;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thumfart KM, Jawaid A, Bright K. et al. Epigenetics of childhood trauma: long term sequelae and potential for treatment. Neurosci Biobehav Rev 2021;132:1049–66. [DOI] [PubMed] [Google Scholar]
- 18. Jawaid A, Jehle KL, Mansuy IM. Impact of parental exposure on offspring health in humans. Trends Genet 2021;37:373–88. [DOI] [PubMed] [Google Scholar]
- 19. Salas J, van den Berk-clark C, Skiöld-Hanlin S. et al. Adverse childhood experiences, depression, and cardiometabolic disease in a nationally representative sample. J Psychosom Res 2019;127:1–8. [DOI] [PubMed] [Google Scholar]
- 20. Merrick MT, Ford DC, Ports KA. et al. Vital signs: estimated proportion of adult health problems attributable to adverse childhood experiences and implications for prevention—25 states, 2015–2017. Morb Mortal Wkly Rep 2019;68:999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Teicher MH, Gordon JB, Nemeroff CB. Recognizing the importance of childhood maltreatment as a critical factor in psychiatric diagnoses, treatment, research, prevention, and education. Mol Psychiatry 2021;4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Wert M, Anreiter I, Fallon BA. et al. Intergenerational transmission of child abuse and neglect: a transdisciplinary analysis. Gend Genome 2019;3:1–21. [Google Scholar]
- 23. Lünnemann MKM, Horst FCP, Van Der, Prinzie P. et al. The intergenerational impact of trauma and family violence on parents and their children. Child Abuse Negl 2019;96:1–12. [DOI] [PubMed] [Google Scholar]
- 24. Felitti VJ, Anda RF, Nordenberg D. et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults in the adverse childhood experiences (ACE) study. Am J Prev Med 1998;14:245–58. [DOI] [PubMed] [Google Scholar]
- 25. Erickson N, Julian M, Muzik M. Perinatal depression, PTSD, and trauma: impact on mother–infant attachment and interventions to mitigate the transmission of risk. Int Rev Psychiatry 2019;31:245–63. [DOI] [PubMed] [Google Scholar]
- 26. Francis D, Diorio J, Liu D. et al. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science 1999;286:1155–8. [DOI] [PubMed] [Google Scholar]
- 27. Creutzberg KC, Kestering-Ferreira É, Viola TW. et al. Corticotropin-releasing factor infusion in the bed nucleus of the stria terminalis of lactating mice alters maternal care and induces behavioural phenotypes in offspring. Sci Rep 2020;10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pembrey M, Saffery R, Bygren LO. Human transgenerational responses to early-life experience: potential impact on development, health and biomedical research. J Med Genet 2014;51:563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bohacek J, Mansuy IM. Molecular insights into transgenerational non-genetic inheritance of acquired behaviours. Nat Rev Genet 2015;16:641–52. [DOI] [PubMed] [Google Scholar]
- 30. Skandrani S, Harf A, El Husseini M. The impact of children’s pre-adoptive traumatic experiences on parents. Front Psychiatry 2019;10: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. López-Rodríguez D, Aylwin CF, Delli V. et al. Multi- and transgenerational outcomes of an exposure to a mixture of endocrine-disrupting chemicals (EDCs) on puberty and maternal behavior in the female rat. Env Health Perspect 2021;129:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weiss IC, Franklin TB, Vizi S. et al. Inheritable effect of unpredictable maternal separation on behavioral responses in mice. Front Behav Neurosci 2011;5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Franklin TB, Russig H, Weiss IC. et al. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry 2010;68:408–15. [DOI] [PubMed] [Google Scholar]
- 34. Bohacek J, Farinelli M, Mirante O. et al. Pathological brain plasticity and cognition in the offspring of males subjected to postnatal traumatic stress. Mol Psychiatry 2015;20:621–31. [DOI] [PubMed] [Google Scholar]
- 35. Franklin TB, Linder N, Russig H. et al. Influence of early stress on social abilities and serotonergic functions across generations in mice. PLoS One 2011;6:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gapp K, Corcoba A, van Steenwyk G. et al. Brain metabolic alterations in mice subjected to postnatal traumatic stress and in their offspring. J Cereb Blood Flow Metab 2017;37:2423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gapp K, Jawaid A, Sarkies P. et al. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci 2014;17:667–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Razoux F, Russig H, Mueggler T. et al. Transgenerational disruption of functional 5-HT 1A R-induced connectivity in the adult mouse brain by traumatic stress in early life. Mol Psychiatry 2017;22:519–26. [DOI] [PubMed] [Google Scholar]
- 39. Wuertz-Kozak K, Roszkowski M, Cambria E. et al. Effects of early life stress on bone homeostasis in mice and humans. Int J Mol Sci 2020;21:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Steenwyk G, Roszkowski M, Manuella F. et al. Transgenerational inheritance of behavioral and metabolic effects of traumatic experiences in early postnatal life in mice: evidence in the 4th generation. Environ Epigenetics 2018;4:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bohacek J, Mansuy IM. A guide to designing germline-dependent epigenetic inheritance experiments in mammals. Nat Methods 2017;14:243–9. [DOI] [PubMed] [Google Scholar]
- 42. Ayala JE, Samuel VT, Morton GJ. et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech 2010;3:525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Beck D, Sadler-Riggleman I, Skinner MK. Generational comparisons (F1 versus F3) of vinclozolin induced epigenetic transgenerational inheritance of sperm differential DNA methylation regions (epimutations) using MeDIP-Seq. Environ Epigenetics 2017;3:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weber-Stadlbauer U, Richetto J, Zwamborn RAJ. et al. Transgenerational modification of dopaminergic dysfunctions induced by maternal immune activation. Neuropsychopharmacology 2021;46:404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pentinat T, Ramon-Krauel M, Cebria J. et al. Transgenerational inheritance of glucose intolerance in a mouse model of neonatal overnutrition. Endocrinology 2010;151:5617–23. [DOI] [PubMed] [Google Scholar]
- 46. Virtue S, Vidal-Puig A. GTTs and ITTs in mice: simple tests, complex answers. Nat Metab 2021;3:883–6. [DOI] [PubMed] [Google Scholar]
- 47. Dube SL, Errazuriz-Cruzat I, Basu A. et al. The forgotten role of glucose effectiveness in the regulation of glucose tolerance. Curr Diab Rep 2015;15:1–6. [DOI] [PubMed] [Google Scholar]
- 48. Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 2000;106:473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Boden G, Reichard GA, Hoeldtke RD. et al. Severe insulin-induced hypoglycemia associated with deficiencies in the release of counterregulatory hormones. NEJM 1981;305:1200–5. [DOI] [PubMed] [Google Scholar]
- 50. Mariani N, Borsini A, Cecil CAM. et al. Identifying causative mechanisms linking early-life stress to psycho-cardio-metabolic multi-morbidity: the EarlyCause project. PLoS One 2021;16:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Faraji J, Karimi M, Soltanpour N. et al. Intergenerational sex-specific transmission of maternal social experience. Sci Rep 2018;8:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sarker G, Berrens R, von Arx J. et al. Transgenerational transmission of hedonic behaviors and metabolic phenotypes induced by maternal overnutrition. Transl Psychiatry 2018;8:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gabory A, Attig L, Junien C. Sexual dimorphism in environmental epigenetic programming. Mol Cell Endocrinol 2009;304:8–18. [DOI] [PubMed] [Google Scholar]
- 54. Bohacek J, Mansuy IM. Epigenetic inheritance of disease and disease risk. Neuropsychopharmacology 2013;38:220–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kiss D, Ambeskovic M, Montina T. et al. Stress transgenerationally programs metabolic pathways linked to altered mental health. Cell Mol Life Sci 2016;73:4547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chamorro-Garcia R, Diaz-Castillo C, Shoucri BM. et al. Ancestral perinatal obesogen exposure results in a transgenerational thrifty phenotype in mice. Nat Commun 2017;8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Choi CS, Gonzales EL, Kim KC. et al. The transgenerational inheritance of autism-like phenotypes in mice exposed to valproic acid during pregnancy. Sci Rep 2016;6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Govorko D, Bekdash RA, Zhang C. et al. Male germline transmits fetal alcohol adverse effect on hypothalamic proopiomelanocortin gene across generations. Bio Psychiatry 2012;72:378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fullston T, Teague EMCO, Palmer NO. et al. Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content. FASEB J 2013;27:4226–43. [DOI] [PubMed] [Google Scholar]
- 60. Eaton SA, Aiken AJ, Young PE. et al. Maternal obesity heritably perturbs offspring metabolism for three generations without serial programming. Int J Obes 2018;42:911–4. [DOI] [PubMed] [Google Scholar]
- 61. Grandjean V et al. RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders. Sci Rep 2015;5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Huypens P, Sass S, Wu M. et al. Epigenetic germline inheritance of diet-induced obesity and insulin resistance. Nat Genet 2016;48:497–9. [DOI] [PubMed] [Google Scholar]
- 63. Picut CA, Remick AK, de Rijk EPCT. et al. Postnatal development of the testis in the rat: morphologic study and correlation of morphology to neuroendocrine parameters. Toxicol Pathol 2015;43:326–42. [DOI] [PubMed] [Google Scholar]
- 64. Drickamer LC, Gowaty PA, Holmes CM. Free female mate choice in house mice affects reproductive success and offspring viability and performance. Anim Behav 2000;59:371–8. [DOI] [PubMed] [Google Scholar]
- 65. Weaver ICG, Cervoni N, Champagne FA. et al. Epigenetic programming by maternal behavior. Nat Neurosci 2004;7:847–54. [DOI] [PubMed] [Google Scholar]
- 66. Champagne FA, Francis DD, Mar A. et al. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav 2003;79:359–71. [DOI] [PubMed] [Google Scholar]
- 67. Gapp K, van Steenwyk G, Germain PL. et al. Alterations in sperm long RNA contribute to the epigenetic inheritance of the effects of postnatal trauma. Mol Psychiatry 2020;25:2162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. van Steenwyk G et al. Involvement of circulating factors in the transmission of paternal experiences through the germline. EMBO J 2020;39:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Villeda SA, Luo J, Mosher KI. et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011;477:90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Castellano JM, Mosher KI, Abbey RJ. et al. Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature 2017;544:488–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, I.M.M., upon reasonable request.


