Abstract
Objective
The purpose of the systematic review was to assess the effectiveness of remote patient monitoring (RPM) follow-up compared with standard care, for patients with chronic kidney disease (CKD) who perform dialysis at home.
Methods
We conducted a systematic review in accordance with international guidelines. We performed systematic searches for publications from 2015 to 2021 in five databases (eg, Medline, Cinahl, Embase) and a search for grey literature in reference lists. Included effect measures were quality of life, hospitalisation, technical failure as the cause for transfer to a different dialysis modality, infections and time patients use for travel. Screening of literature, data extraction, risk-of-bias assessment and certainty of evidence assessment (using the Grading of Recommendations Assessment, Development and Evaluation approach) were done by two researchers. We conducted meta-analyses when possible.
Results
Seven studies met the inclusion criteria, of which two were randomised controlled trials and five were retrospective cohort studies with control groups. The studies included 9975 participants from 5 countries, who were a good representation of dialysis patients in high-income and upper-middle-income countries. The patients were on peritoneal dialysis (six studies) or home haemodialysis (one study). There was very low certainty of evidence for the outcomes, except for hospitalisations: there was low certainty evidence from three cohort studies for fewer hospitalisation days in the RPM group. No studies included data for time patients used for travel.
Conclusion
We found low to very low certainty evidence that indicate there may be positive effects of RPM follow-up, in comparison to standard care only, for adult patients with CKD who perform dialysis at home. Offering RPM follow-up for home dialysis patients as an alternative or supplement to standard care appears to be safe and provide health benefits such as fewer hospitalisation days. Future implementation should be coupled with robust, high-quality evaluations.
PROSPERO registration number
CRD42021281779.
Keywords: Telemedicine, Dialysis, End stage renal failure, Adult nephrology, Chronic renal failure, Health informatics
STRENGTH AND LIMITATIONS OF THIS STUDY.
To our knowledge, this is the first systematic review to assess the effectiveness and safety of remote patient monitoring follow-up for adult patients with dialysis-dependent chronic kidney disease on home dialysis (haemodialysis and peritoneal dialysis).
Our systematic review was conducted in line with guidelines from the Cochrane and Grading of Recommendations Assessment, Development and Evaluation working group. The researchers specialise in systematic review research, one researcher is a registered nurse with long and diverse nephology experience, and the searches were conducted by a search specialist.
Due to study heterogeneity, inconsistent measurement and reporting, our ability to conduct meta-analyses was limited.
Introduction
Chronic kidney disease (CKD) is a significant public health concern, with 8%–16% of the world’s population affected.1 It is characterised by a need for close monitoring, poor health outcomes and a high economic burden for society as well as for the individual.2 The world’s population is growing older, and with CKD prevalence rising parallel with age,2 an increasing number of people will continue to need monitoring and treatment with dialysis. There are two main types of dialysis: peritoneal dialysis (PD) and haemodialysis (HD). Both are suitable treatment options when the kidneys are unable to filter the blood sufficiently.3
With the use of technology, there are encouraging possibilities for thorough patient follow-up, and at the same time, human resource savings.4–6 Both PD and HD can be performed at home. With home dialysis, the patients receive comprehensive training arranged by staff at a dialysis centre to ensure that they have the skills and knowledge required to perform the treatment at home.3 7 While dialysis is time-consuming regardless of location, patients on home dialysis are not dependent on hospital service hours and may experience more freedom than patients receiving in-centre dialysis.8 9 Additionally, for patients on in-centre dialysis, the burden of time spent commuting between home and hospital can be extensive. They often also spend a substantial amount of time waiting for transport and waiting to be assisted by hospital staff for connection and disconnection from HD. Research shows that travel time to dialysis exceeding 60 min is associated with significantly decreased health-related quality of life (QoL) and significantly increased mortality risk compared with patients who travel 15 min or less.10 With dialysis at home, it is reasonable to expect considerable time-savings for the patients as well as improved health-related QoL.
In healthcare, there is increasing interest in using technology‐based interventions. Telemedicine and e-health are broad terms used when medical treatment, examination or patient follow-up is done from a distance.11 Homecare telehealth is another related term, and remote patient monitoring (RPM) is a subcategory thereof. RPM uses computer systems or software application technology that transfers patient-generated data to healthcare professionals.12 Given the intervention considered in this systematic review is internet dependent, we will use the term RPM. RPM can give the patient quick access to medical expertise, independent of the distance to a treatment centre and provides healthcare teams with valuable information about the patient’s condition. Thus, RPM can be a tool to empower patients in self-care and for healthcare providers to offer support from a distance.11 Qualitative studies from the UK and Norway suggest that patients on home dialysis have a positive attitude towards the use of RPM and believe that this could decrease anxiety and make it easier for more patients to choose home dialysis.13 14 In a recent pilot study from Italy, patients overcame physical, cognitive and psychological barriers to PD by RPM follow-up.15 Strategies to switch more patients to home dialysis may have positive impacts on the patients’ daily life,14 16 decrease mortality17 and offer economic savings for the patients as well as for society.16 18 RPM holds much promise for enhancing follow-up of patients with CKD on dialysis and it is critical to determine whether and which strategies are effective at improving outcomes. RPM patient follow-up is seemingly already expanding its reach. Our Google Scholar search in December 2021 showed that there has been a 200% increase in records about e-health home dialysis from 2018 to 2021. Although interest in nephrology and e-health, including RPM, is increasing, to date, there are no systematic reviews about the effectiveness and safety of RPM follow-up including adult patients with dialysis-dependent CKD on home dialysis (HD and PD). We aimed to conduct a systematic review on the effectiveness of RPM follow-up compared with standard care, for adult patients with CKD who perform dialysis at home.
Methods
We conducted this systematic review in accordance with guidelines set forth in the Cochrane Handbook for Systematic Reviews of Interventions V.6.2.19 The prespecified protocol was registered in PROSPERO (CRD42021281779) and we report in line with the Preferred Reporting for Systematic Reviews and Meta-analyses statement.20
Search strategy and selection
The reviewers (HTN and RCB) prepared the search strategy in collaboration with a research librarian (LN), and a second research librarian peer-reviewed the search strategy. The librarian (LN) conducted searches in August 2021 in CINAHL (EBSCO), EMBASE (OVID), Medline (OVID), Cochrane Database of Systematic Reviews and CENTRAL. The search included both subject headings (eg, MeSH in Medline) and text words (available in online supplemental appendix 1). In addition, the two reviewers conducted hand searches in the reference lists of the included studies.
bmjopen-2022-061772supp001.pdf (64KB, pdf)
The basis for the search was the inclusion criteria. We applied the (S)PICO model, which directs attention to the study design, population, intervention, comparison and outcomes.21 Eligible study designs were primary intervention studies with a control group. That is, randomised controlled trials (RCTs), non-randomised controlled studies, controlled before–after studies and cohort studies with a control group. Study participants needed to be 18 years or older, with dialysis-dependent CKD who performed dialysis at home (HD or PD). The patients could perform dialysis independently or with assistance of family or other carers. CKD did not have to be the only disease of the study participant. This is because patients with CKD are known to have a higher burden of comorbidities than the average population.22 The eligible intervention was RPM, understood as internet-dependent technology used to transfer treatment data from the patient’s home to a healthcare institution.12 This included video consultations, applications installed on the patient’s phone, computer or a tablet as well as technology that transferred treatment data directly from the dialysis machine to healthcare providers.12 RPM that was not directly treatment related was excluded. This included, but was not limited to, apps for lifestyle changes, interventions for blood pressure control and interventions for diabetes management. The comparator was standard care, understood as patients performing dialysis in-centre or at home and having regular in-person consultations at a HD or PD centre. Included effect measures were QoL (measured with any type of QoL assessment tool), hospitalisation (all-cause, disease-specific and number of hospitalisation days), technical failure as the cause for transfer to a different dialysis modality, hospital registered infections not requiring hospitalisation and time patients use for travel. Lastly, studies had to be published in a Scandinavian or English language, in 2015–2021 because we wanted to identify all studies relevant to the question and today’s clinical situation, being cognisant that technology is rapidly improving.
We imported all records from the searches into an EndNote library and removed all duplicate entries. Two researchers (HTN and RCB) independently screened all titles and abstracts from the literature searches in accordance with the predetermined inclusion and exclusion criteria. All abstracts that appeared to fit the inclusion criteria or did not provide enough information were promoted to full-text screening. At each level, we evaluated the identified records independently of one another using a predeveloped inclusion form. The final determination to include or exclude was made together and any disagreements were solved by discussion. Excluded studies with justifications are available in online supplemental appendix 2.
bmjopen-2022-061772supp002.pdf (23.5KB, pdf)
Risk-of-bias (RoB) assessment and data extraction
To assess the included studies for RoB, we used two different instruments: The Newcastle–Ottawa Scale for cohort studies23 and Cochrane Risk of Bias Tool for RCTs.19 Two researchers (HTN and RCB) conducted independent RoB assessments and then agreed on a final RoB evaluation, with disagreements solved by discussion.
One researcher (HTN) created a standard extraction form and extracted data from all included studies. The information extracted from the studies was: title, authors, publication details, study design, aim of the study, study setting (location and time the study was conducted), characteristics of included participants (age, gender etc), characteristics of the intervention, study setting, outcomes and results. Whenever information was available, dichotomous and continuous data for all eligible outcomes were extracted. HTN contacted several authors for additional data, but did not receive a reply. RCB assessed the extracted data for completeness and accuracy and any disagreements were solved by further inspection of the publication and discussion.
Analysis and assessment of the certainty of the evidence (GRADE)
We extracted crude outcome data for all eligible outcomes when postscores for both intervention and control groups were available and, when such data were available, adjusted outcome data (adjusted comparison (effect) estimates and their standard errors or 95% CI). We provide dichotomous outcomes as the number of events and number of people in groups as proportions, RR, incident RR (IRR) or OR as appropriate. Continuous outcomes are shown as mean difference and SD or the most appropriate presentation based on the available data in the included studies.
We evaluated the characteristics of the studies’ (S)PICO and when they were considered sufficiently similar, and data were available, we conducted meta-analyses. The judgments about whether meta-analyses were appropriate were based on recommendations in the Cochrane Handbook.19 We used Mantel-Haenszel random effects meta-analysis for dichotomous outcomes and we presented the relative risks and their corresponding 95% CI (it was not possible to meta-analyse any continuous outcomes). We also examined between-study heterogeneity using visual inspection of CIs, the χ2 test and I2 statistic, quantifying the degree of heterogeneity as described in the Cochrane Handbook.19 We used RevMan V.5.4, the latest version of the Cochrane meta-analysis software.24 When the studies’ (S)PICOs or results were too heterogeneous to pool statistically, or data were unavailable, we reported the results narratively, in text and tables. We planned to perform a subgroup analysis for the outcome technical failure, but this was not possible due to lack of data.
We assessed the certainty of the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework.25 With regard to results that could not be meta-analysed, we followed the Synthesis Without Meta-analysis guideline.26
Patient and public involvement
Due to the nature of the study (systematic review), no patients were involved.
Results
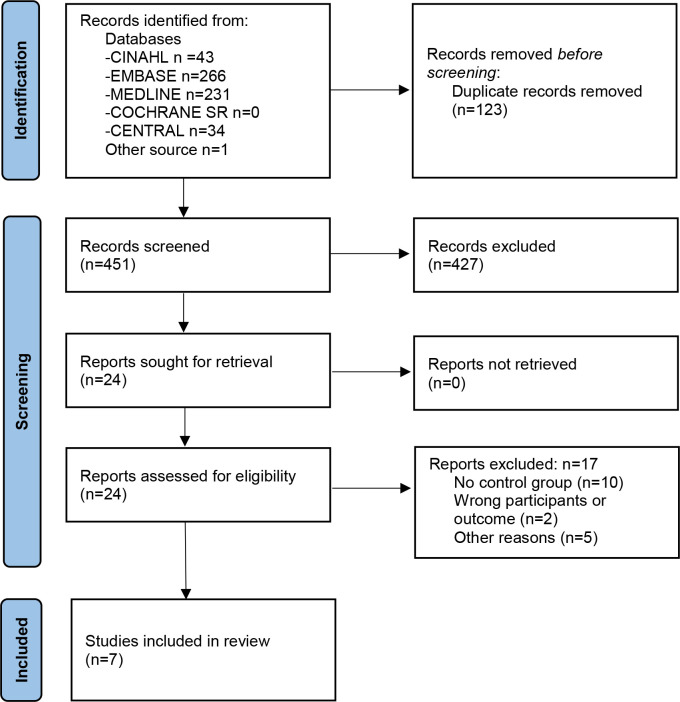
The searches returned 451 references after removal of duplicates (figure 1). We read 24 reports in full text, including 1 study identified from the hand search in reference lists. The most common primary reasons for exclusion were that there was no control group or it was the wrong participants or outcomes. Seven studies published between 2018 and 2021 were eligible for inclusion.27–33
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram for selection of studies.
Description of the studies
The seven included studies consisted of two RCTs and five retrospective cohort studies (table 1). They were conducted in five different countries. There were two studies each from Columbia and USA, and one study each from China, Italy and South Korea. Overall, 3 were set in a single PD centre, 4 took place in 2 or more renal care centres and the 2 largest studies took place in the USA with 1 including 55 home HD centres and another 931 Fresenius PD clinics.
Table 1.
Description of the included studies (n=7)
| Author, (country, setting) Study design |
Population | Intervention and comparator (follow-up time) | Outcomes | Risk of bias |
| Cao (China: 1 PD centre) RCT27 |
N=160, on CAPD Men 58% Mean age 52 |
RPM versus SC Instant messaging application for support and education (Mean 11.4 mo FU) |
Hospitalisations Infections Technical failure |
Moderate |
| Chaudhuri (USA: 931 renal centres) Cohort28 |
N=6343, on PD Men 73% Mean age 57 |
RPM versus SC ‘Patient hub’ application—patients add and access treatment data (12 mo FU) |
Hospitalisations Technical failure |
Low |
| Corzo (Columbia: 5 renal centres) Cohort29 |
N=558, on APD Men 60% Mean age 54 |
RPM versus SC Cloud-based software—prescriptions can be changed remotely (Mean 8.3 mo FU) |
Technical failure | Low |
| Jung (South Korea: 6 renal centres) RCT30 |
N=57, on APD Men 60% Mean age 47 |
RPM versus SC Cloud-based software—prescriptions can be changed remotely (6 mo FU) |
QoL | Moderate |
| Milan Manani (Italy: 1 PD centre) Cohort31 |
N=73, on APD Men 75% Median age 60 |
RPM versus SC Cloud-based software—prescriptions can be changed remotely (6 mo FU) |
Hospitalisations Technical failure QoL |
Low/moderate |
| Sanabria (Columbia: 28 Baxter renal care centres) Cohort32 |
N=360, on APD Men 66% Mean age 57 |
RPM versus SC Cloud-based software—prescriptions can be changed remotely (Mean 9 mo FU) |
Hospitalisations Technical failure |
Low |
| Weinhandl (USA: 55 HHD centres) Cohort33 |
N=2424, on HHD Men 63% Mean age 53 |
RPM versus SC Nx2me telehealth platform—staff can do remote ‘troubleshooting’ during HHD (Mean 11 mo FU) |
Technical failure | Low |
APD, automated peritoneal dialysis; CAPD, continuous peritoneal dialysis; FU, follow-up; HHD, home haemodialysis; mo, months; PD, peritoneal dialysis; QoL, quality of life; RCT, randomised controlled trial; RPM, remote patient monitoring; SC, standard care.
With respect to the population, all in all, there were 9975 patients with dialysis-dependent CKD in the studies. The range was 57–6343 patients, thus there was imbalance in sample sizes across the studies. The two largest studies, cohorts from the USA, made up 88% of the total number of study participants. In all the studies, most patients were men (range 53%–75%) and the mean age of the study participants was about 55. In all studies except one, the patients were on PD, they lived at home, and performed dialysis independently or with the assistance of a carer.
As per our inclusion criteria, the intervention was RPM with different types of software that collected treatment data and transferred it to a treatment centre (added by the patients or automatically collected). The specific type of RPM varied across the studies. Four studies, Corzo et al,29 Jung et al,30 Milan Manani et al31 and Sanabria et al,32 used the automated PD system from Baxter: Homechoice Claria, connected to the Sharesource platform. Milan Manani et al31 additionally used the sleep-safe harmony home bridge system from Fresenius for half of the patients. Weinhandl & Collins33 used the Nx2me telehealth platform for home HD patients. The software collects treatment data and transmits it to the healthcare providers, and the prescription can be changed ‘from afar’. Chaudhuri et al28 used the ‘Patient hub’ application. The PD patients can see their prescription, laboratory results and enter treatment data, and the app transmits the patient-entered data to the healthcare providers. Cao et al27 used the ‘kidney cleaning group’ instant messaging software. Technical support, nurse support, physician support and support from fellow patients were available through chat and video. The patients were divided into smaller groups and one experienced PD patient with few complications was the group leader. Educational resources were also available in the platform. In addition, in all studies, all patients had or were likely to receive some level of standard care. This was generally described as in-person follow-up at the hospital. However, the frequency of standard care ranged from weekly (n=1) to every 3 months (n=1). Most studies had or were likely to have an in-person review monthly (n=5). The follow-up time ranged from 6 to 12 months.
RoB of included studies
The RCTs had moderate RoB, while the retrospective cohort studies were rated fair-to-good methodological quality, that is, having low-to-moderate RoB (table 1 and online supplemental appendix 3). With respect to the studies’ sources of funding, three of the observational studies received financial support from the provider of the intervention (online supplemental appendix 3).
bmjopen-2022-061772supp003.pdf (100.6KB, pdf)
Effect of RPM versus standard care
Across the studies, there were data on four of our five predetermined outcomes: Hospitalisation,27 28 31 32 infections,27 technical failure as the cause for transfer to a different dialysis modality,27–29 31–33 and QoL.30 31 Due to the inconsistent measurement of outcomes, and inconsistent and incomplete reporting of outcome results in the studies, our ability to synthesise data was limited. The results are described in the text below, table 2 and figure 2. The GRADE assessments in table 3 show that there was low to very low certainty of evidence for all of the outcomes. This means that the effects are largely uncertain. No publications included data for the outcome ‘time patients used for travel’.
Table 2.
Study outcomes and effect estimates
| Study | Outcome | Result/effect estimate (95% CI) |
| Hospitalisations | ||
| Chaudhuri et al28 2020 Milan Manani et al31 2020 Sanabria et al32 2019 |
Hospitalisation days (12 mo) Hospitalisation days (6 mo) Hospitalisation days (9 mo) |
Adj. IRR 0.68 (0.55 to 0.83) Median 5 days difference p 0.55 Adj. IRR 0.46 (0.23 to 0.92) |
| Cao et al27 2018 Chaudhuri et al28 2020 Milan Manani et al31 2020 Sanabria et al32 2019 |
Hospitalisation all-cause (11 mo) Hospitalisation all-cause (12 mo) Hospitalisation all-cause (11 mo) Hospitalisation all-cause (9 mo) |
RR 0.57 (0.17 to 1.88) Adj. IRR 0.74 (0.66 to 0.83) RR 1.33 (0.63 to 2.81) Adj. IRR 0.61 (0.39 to 0.95) |
| Infections | ||
| Cao et al27 2018 | Infections (11 mo) | More peritonitis (60 in RPM group vs 40 in control group per patient month) but less exit site infections with RPM (RR=0.45, 0.12 to 1.68) |
| Technical failure as cause for transfer to a different dialysis modality | ||
| Cao et al27 2018 Chaudhuri et al28 2020 Corzo et al29 2020 |
Technical failure (11 mo) Technical failure (12 mo) Technical failure (8 mo) |
RR 1.00 (0.26 to 3.86) Adj. HR 0.79 (0.63 to 1.00) IRR 0.88 (0.41 to 1.74) |
| Sanabria et al32 2019 Weinhandl et al 33 2018 |
Technical failure (subgroup) (9 mo) Technical failure (subgroup) (11 mo) |
RR 0.97 (0.42 to 2.25) Adj. HR 0.66 (0.50 to 0.86) |
| Quality of life | ||
| Jung et al30 2021 Milan Manani et al31 2020 |
KDQOL—Patient satisfaction questions (6 mo) KDQOL—Patient satisfaction questions (6 mo) |
Mean 75.5 in RPM group versus 73.7 in SC group, p 0.64 Median 83.3 in both groups, p 0.99 |
| Jung et al30 2021 Milan Manani et al31 2020 |
KDQOL—Dialysis staff encouragement (6 mo) KDQOL—Dialysis staff encouragement (6 mo) |
Mean 93.1 in RPM group versus 97.1 in SC group, p 0.05 Median 100 in both groups, p 0.16 |
Adj, adjusted (listed in online supplemental appendix 3); IRR, incident rate ratio (compares the incidence rates between two different groups and shows if exposure to something increases or decreases the rate of some incidence—if IRR is 1 then there is no difference); KDQOL, kidney disease quality of life; mo, months; RPM, remote patient monitoring; RR, relative risk; SC, standard care.
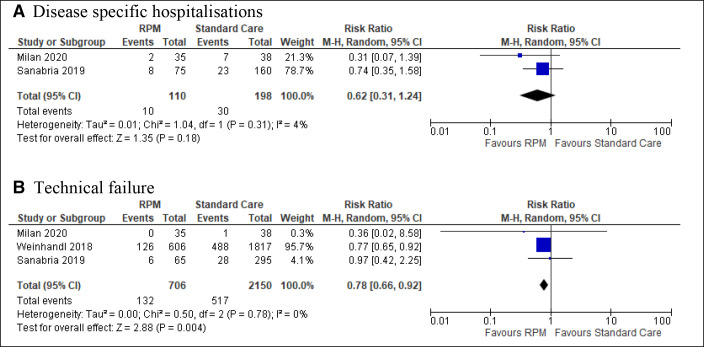
Figure 2.
Meta-analyses of outcomes disease-specific hospitalisations and technical failure. RPM, remote patient monitoring.
Table 3.
Summary of findings (GRADE)
| Population: patients with CKD Countries: China, Columbia, Italy, South Korea, USA Intervention: RPM Comparison: Standard care | |||||
| Outcome, follow-up time | Anticipated absolute effects (95% CI) | Relative effect (95% CI) |
Participants, n (Studies) |
Quality of evidence (GRADE) |
|
| Assumed risk with control | Assumed risk with RPM | ||||
| Hospitalisations (6–12 months) | |||||
| Days | All 3 cohort studies showed that there were fewer hospitalisation days in the RPM group (table 2) | 6736 (3) | ⊕⊕ΟΟ low | ||
| All-cause | 3 of 4 studies (1 RCT, 3 cohort) showed that there were fewer hospitalisations in the RPM group (table 2) | 6936 (4) | ⊕ΟΟΟ very low† | ||
| Disease-specific | 30/198 (15.2%) | 10/110 (9.1%) | RR 0.62 (0.31 to 1.24) |
308 (2 cohort) | ⊕ΟΟΟ very low‡ |
| Infections (11 months) | |||||
| 1 RCT reported more peritonitis but fewer exit site infections with RPM (table 2) | 160 (1) | ⊕ΟΟΟ very low§ | |||
| Technical failure (6–12 months) | |||||
| 521/2230 (23.4%) | 136/786 (17.3%) | RR 0.78 (0.66 to 0.93) |
2856 (3 cohort) | ⊕ΟΟΟ very low¶ | |
| 2 of 3 studies (1 RCT, 2 cohort) reported fewer failures with RPM (table 2) | 7161 (3) | ||||
| Quality of life (6 months) | |||||
| Patient satisfaction | 1 RCT found higher QoL in the RPM group, 1 cohort found QoL was similar in the two groups (table 2) | 130 (2) | ⊕ΟΟΟ very low** | ||
| Dialysis staff encouragement | 1 RCT found higher QoL in the RPM group, 1 cohort found QoL was similar in the two groups (table 2) | 130 (2) | ⊕ΟΟΟ very low** | ||
| Travel time | 0 studies assess this outcome | No evidence | |||
*The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
†Downgraded by 1 level because of moderate risk of bias in 1 study and inconsistency.
‡Downgraded by 1 level because of imprecision.
§Downgraded by 3 levels because of moderate risk of bias, inconsistency, imprecision.
¶Downgraded by 1 level because of moderate risk of bias in 1 study and imprecision.
**Downgraded by 1 level because of inconsistency and imprecision.
CKD, chronic kidney disease; GRADE, Grading of Recommendations Assessment, Development and Evaluation; RCT, randomised controlled trail; RPM, remote patient monitoring.
Hospitalisations
One RCTs and three observational studies from Italy, Colombia, China and the USA examined the effect of RPM on hospitalisations.27 28 31 32 However, the outcome was reported differently across the studies, as hospitalisation days/days admitted, all-cause hospitalisations and disease-specific hospitalisations (caused by overhydration, access dysfunction and infections).
Hospitalisation days
The three observational studies, Chaudhuri et al,28 Milan Manani et al31 and Sanabria et al,32 all found fewer hospitalisation days in the RPM group than the control group (table 2). The results in Sanabria et al32 were from a matched sample, as data for the whole sample was not available. This study showed the largest effect with a difference of six hospitalisation days (IRR 0.46, 0.23–0.92).
All-cause hospitalisations
One RCT27 and three observational studies28 31 32 had data on general, all-cause hospitalisations. While three of the four studies showed that RPM users had less all-cause hospitalisations than patients with standard care only, the fourth study favoured standard care (table 2).
Disease-specific hospitalisations
The results on disease-specific hospitalisations from two observational studies, Milan Manani et al31 and Sanabria et al,32 could be pooled in a meta-analysis (figure 2). The non-significant result suggested there were fewer disease-specific hospitalisations in the RPM group than in the control group (RR 0.62, 95% CI 0.31 to 1.24). Milan Manani et al31 defined disease-specific hospitalisations as infections (peritonitis and exit site), overhydration and access dysfunction. Sanabria et al32 provided numbers for hospitalisations due to peritonitis and overhydration.
Infections
Only one RCT, from China, examined the effectiveness of RPM follow-up for PD patients on infections.27 The result for this outcome was inconclusive, as Cao et al found more peritonitis but fewer exit site infections with RPM. It was not specified whether the infections were treated at home or in the hospital.
Technical failure as the cause for transfer to a different dialysis modality
One RCT from China27 found no difference between the groups while five observational studies from the USA,28 33 Colombia29 32 and Italy31 consistently reported less technical failure as cause for transfer to a different dialysis modality in the RPM group compared with the control group (table 2). Three of the cohort studies could be pooled in a meta-analysis; the result implies benefit of RPM (0.78, 95% CI 0.66 to 0.92) (figure 2). Two of the studies32 33 gave data on novice patients with less than 3-month treatment duration at baseline, indicating a positive, but non-significant effect of RPM in new patients (table 2).
Self-reported QoL
Both studies, one RCT30 and one observational study,31 reporting on QoL used the tool ‘The short form of kidney disease QoL’, which is an adaptation of the 36-Item Short Form Survey Instrument (SF-36).34 All answers were transformed into precoded numeric values with a range from 0 to 100, where 100 was the highest QoL.35 Neither of the studies offered an overall total score across the questions/areas, and we selected the two questions/areas that we considered most relevant (patient satisfaction and dialysis staff encouragement). For both patient satisfaction and dialysis staff encouragement, Milan Manani et al31 found the same score in both groups, while Jung et al30 found a higher score in the RPM group than the control group concerning patient satisfaction, but opposite for dialysis staff encouragement (table 2).
Discussion
Principal findings
This systematic review advances the evidence on the effects of RPM for patients with dialysis-dependent CKD on home dialysis, including home HD and PD. Our findings are in line with previous research36 37 and document that there is no conclusive evidence, but that positive effects of RPM are suggested for clinical outcomes, technical failure and QoL.
The results consistently suggest that RPM reduces hospitalisations and the number of days the patient is admitted. It was especially convincing that Milan Manani et al31 observed a median difference of five fewer hospitalisation days in the RPM group over 6 months, because the patients on RPM had a worse comorbidity score. Furthermore, except for one study that found the same number of technical failures in both groups, the other five studies found less technical failure in the RPM group. In four of the studies measuring this outcome, prescriptions could be changed from the hospital without in-person consultations. In effect, RPM allows resolving technical issues early, thus preventing progression of technical failure to the stage where the patient would need to transfer to a different dialysis modality. Research has found great advantages with the technology displaying possible causes and solutions to problems, alarm indicators showing who to contact for guidance (nurse or technician) and reminders of activities that need to be performed.13–15 Concerning QoL, only two studies assessed this and the results showed the scores were comparable for the patients on RPM and usual care. Encouragingly, scores for QoL improved slightly and patient satisfaction was higher than neutral. This is in line with a study from the USA that found that RPM increased patients’ confidence and satisfaction with treatment because they felt more closely supported.38 Lastly, no studies assessed time patients use for travel. However, research suggests that health-related QoL and time patients use for travel are intertwined10 and that dialysis free time and reduction of fatigue are highly valued outcomes by patients.9 39 40 This could reflect positively on QoL.
Our results mirror two earlier systematic reviews on e-health interventions in PD patients36 and in people with CKD.37 Both reviews, with literature searches in 2018–2019, included a wide range of patients and e-health modalities, including mobile or tablet application, text or email messages, electronic monitors, internet/websites and video or DVD. Consequently, there was minimal overlap in included studies: only one review36 included two of our included studies. Both reviews concluded that the quality of evidence for the effectiveness of e-health was low with uncertain effects, but that no adverse effects were indicated. Of note, a recent modelling analysis projected that in a cohort of 100 patients on automated PD over 1 year, RPM would lead to 27 fewer hospitalisations, 518 fewer hospitalisation days, 31 additional months free of complications and 6 fewer peritonitis episodes.41
Implications
Overall, the low to very low certainty of evidence on the effects of RPM for patients with dialysis-dependent CKD on home dialysis prevents strong recommendations. Given RPM seems comparable to usual care, the absence of adverse effects and promising clinical effects, it seems advisable cautiously to implement RPM while concomitantly evaluating outcomes important for patients. Prior to recommending RPM for patients with CKD on home dialysis, more trials are needed to be certain of its benefits over standard care, and to establish equity and cost-effectiveness. A modelling analysis from the payer perspective has found that RPM is cost-effective,41 but economic evaluations of e-health interventions are scarce and highlights an important area for further research.5 42 Additionally, patient groups should be involved in RPM implementation and evaluation to maximise the potential for modification and ultimately effect.
Our review highlights the need for robust, high-quality research on both PD and home HD, but especially for patients on home HD and patients whose home is in a nursing home. To our knowledge, home HD in nursing homes is rare, while PD is common. It is likely that nursing home staff aided by RPM support from specialist nurses at dialysis centres could provide invaluable assistance to frail patients with CKD with great need for follow-up. For such patients and others with dialysis-dependent CKD on home dialysis, time used for travel and dialysis free time is a patient-important outcome that warrants further research. It is reasonable to suspect substantial time-savings when follow-up is performed from afar and evidence from video consultations in patient follow-up is positive.15 43 We encourage research on the combined use of video consultations and cloud-based technology on outcomes such as travel time, technical failure and hospitalisations. Standardised outcomes in nephrology (SONG) have identified and prioritised outcomes for both HD and PD patients and can be a useful tool when planning outcomes in future research.44
Strengths and limitations
Our systematic review was conducted in line with guidelines from the Cochrane and GRADE working group. The outcome selection was in alignment with core outcomes recommended by the SONG initiative.44 The researchers specialise in systematic review research, one researcher is a registered nurse with long and diverse nephrology experience, and the searches were conducted by a search specialist. Yet, it is possible that relevant studies have been missed and relevant studies have been published after our last search. Due to study heterogeneity, variability in intervention characteristics, inconsistent measurement and reporting, our ability to conduct meta-analyses was limited. Therefore, it was neither possible to improve precision to any great extent nor statistically assess potential differences across groups, such as type of platform or HD and PD. We contacted several authors asking for more data, but did not receive a reply. The low number of studies meant that we were unable to statistically check for publication bias. Given the modestly positive but varied results, we believe the potential for publication bias is low, but we recommend future reviews of a higher number of included studies to assess this potential bias. The imbalance in sample sizes across the studies, with two studies having a considerably larger sample size than the other five, influenced the results related to hospitalisations and technical failure. Four, including these two studies had low RoB, one had low-moderate and two had moderate RoB.
Conclusion
This systematic review summarises and presents low to very low evidence that indicate there may be positive effects of RPM follow-up, in comparison to standard care only, for adult patients with CKD who perform dialysis at home. Offering RPM follow-up for home dialysis patients as an alternative or supplement to standard care appears to be safe and provide health benefits, but future implementation should be coupled with robust, high-quality evaluations. Despite the high interest in RPM and increasing demands for nephrology services, good quality evidence is still needed to determine their effectiveness.
Supplementary Material
Acknowledgments
We are grateful to Elisabet Hafstad, Norwegian Institute of Public Health, for peer review of the systematic search strategies
Footnotes
Twitter: @Globelotte
Contributors: HTN wrote the first draft. RCB and HTN contributed equally to the rest of the work. LN prepared and conducted the systematic searches and contributed with inputs on the final draft. HTN is responsible for the overall content as the guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA 2019;322:1294–304. 10.1001/jama.2019.14745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tonelli M, Riella M. Chronic kidney disease and the aging population. Indian J Nephrol 2014;24:71–4. 10.4103/0971-4065.127881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinnakirouchenan R, Holley JL. Peritoneal dialysis versus hemodialysis: risks, benefits, and access issues. Adv Chronic Kidney Dis 2011;18:428–32. 10.1053/j.ackd.2011.09.001 [DOI] [PubMed] [Google Scholar]
- 4.Meld. St. 7 (2019–2020) . Nasjonal helse- OG sykehusplan 2020–2023: Helse- OG omsorgsdepartementet. Available: https://www.regjeringen.no/no/dokumenter/meld.-st.-7-20192020/id2678667/ [Accessed 09 Dec 2021].
- 5.Kitsiou S, Paré G, Jaana M, et al. Effectiveness of mHealth interventions for patients with diabetes: an overview of systematic reviews. PLoS One 2017;12:e0173160. 10.1371/journal.pone.0173160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Widmer RJ, Collins NM, Collins CS, et al. Digital health interventions for the prevention of cardiovascular disease: a systematic review and meta-analysis. Mayo Clin Proc 2015;90:469–80. 10.1016/j.mayocp.2014.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helsedirektoratet . Nyresvikt - dialysepasienter som får hjemmedialyse: Helsedirektoratet, 2018. Available: https://www.helsedirektoratet.no/statistikk/kvalitetsindikatorer/behandling-av-sykdom-og-overlevelse/andel-dialysepasienter-som-har-hjemmedialyse [Accessed 12 Dec 2021].
- 8.Helsedirektoratet . Handlingsplan for forebygging OG behandling AV kronisk nyresykdom (2011-2015), 2011. Available: http://www.nephro.no/foreningsnytt/Handlingsplan_forebygging_behandling_kronisk_nyresykdom.pdf [Accessed 09 Nov 2021].
- 9.Urquhart-Secord R, Craig JC, Hemmelgarn B, et al. Patient and caregiver priorities for outcomes in hemodialysis: an international nominal group technique study. Am J Kidney Dis 2016;68:444–54. 10.1053/j.ajkd.2016.02.037 [DOI] [PubMed] [Google Scholar]
- 10.Moist LM, Bragg-Gresham JL, Pisoni RL, et al. Travel time to dialysis as a predictor of health-related quality of life, adherence, and mortality: the dialysis outcomes and practice patterns study (DOPPS). Am J Kidney Dis 2008;51:641–50. 10.1053/j.ajkd.2007.12.021 [DOI] [PubMed] [Google Scholar]
- 11.Braut GS . Telemedisin store medisinske leksikon. Available: https://sml.snl.no/telemedisin [Accessed 10 Oct 2021].
- 12.DelVecchio A . Definition, remote patient monitoring (RPM): Tech target, search health it. Available: https://searchhealthit.techtarget.com/definition/remote-patient-monitoring-RPM [Accessed 10 Oct 2021].
- 13.Rajkomar A, Farrington K, Mayer A, et al. Patients' and carers' experiences of interacting with home haemodialysis technology: implications for quality and safety. BMC Nephrol 2014;15:195–95. 10.1186/1471-2369-15-195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rygh E, Arild E, Johnsen E, et al. Choosing to live with home dialysis-patients' experiences and potential for telemedicine support: a qualitative study. BMC Nephrol 2012;13:13. 10.1186/1471-2369-13-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viglino G, Neri L, Barbieri S, et al. Videodialysis: a pilot experience of telecare for assisted peritoneal dialysis. J Nephrol 2020;33:177–82. 10.1007/s40620-019-00647-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.François K, Bargman JM. Evaluating the benefits of home-based peritoneal dialysis. Int J Nephrol Renovasc Dis 2014;7:447–55. 10.2147/IJNRD.S50527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marshall MR, Polkinghorne KR, Kerr PG, et al. Temporal changes in mortality risk by dialysis modality in the Australian and New Zealand dialysis population. Am J Kidney Dis 2015;66:489–98. 10.1053/j.ajkd.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 18.Walker RC, Howard K, Morton RL. Home hemodialysis: a comprehensive review of patient-centered and economic considerations. Clinicoecon Outcomes Res 2017;9:149–61. 10.2147/CEOR.S69340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JPT TJ, Chandler J, Cumpston M. Cochrane Handbook for systematic reviews of interventions version 6.2 (updated February 2021): cochrane, 2021. Available: www.training.cochrane.org/handbook [Accessed 09 Dec 2021].
- 20.PRISMA transperant reporting of systematic reviews and meta-analyses. Available: http://www.prisma-statement.org/ [Accessed 11 Aug 2021].
- 21.Straus SE, Glasziou P, Richardson WS. Evidence-Based medicine E-book: how to practice and teach EBM: Elsevier health sciences, 2018. [Google Scholar]
- 22.Tonelli M, Wiebe N, Guthrie B, et al. Comorbidity as a driver of adverse outcomes in people with chronic kidney disease. Kidney Int 2015;88:859–66. 10.1038/ki.2015.228 [DOI] [PubMed] [Google Scholar]
- 23.Wells GA, Shea B, O'Connell D. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses: the Ottawa Hospital research Institute. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Accessed 21 Oct 2021].
- 24.Cochrane RevMan Cochrane Training [updated Latest verion of RevMan 5.4.1, 2020. Available: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman [Accessed 10 Oct 2021].
- 25.GRADE . The grade Working group; 2004-2021. Available: https://www.gradeworkinggroup.org/ [Accessed 21 Oct 2021].
- 26.Campbell M, McKenzie JE, Sowden A, et al. Synthesis without meta-analysis (swim) in systematic reviews: reporting guideline. BMJ 2020;368:l6890. 10.1136/bmj.l6890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao F, Li L, Lin M, et al. Application of instant messaging software in the follow-up of patients using peritoneal dialysis, a randomised controlled trial. J Clin Nurs 2018;27:3001–7. 10.1111/jocn.14487 [DOI] [PubMed] [Google Scholar]
- 28.Chaudhuri S, Han H, Muchiutti C, et al. Remote treatment monitoring on hospitalization and technique failure rates in peritoneal dialysis patients. Kidney360 2020;1:191–202. 10.34067/KID.0000302019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corzo L, Wilkie M, Vesga JI, et al. Technique failure in remote patient monitoring program in patients undergoing automated peritoneal dialysis: a retrospective cohort study. Perit Dial Int 2022;42:896860820982223.:288–96. 10.1177/0896860820982223 [DOI] [PubMed] [Google Scholar]
- 30.Jung H-Y, Jeon Y, Kim YS, et al. Outcomes of remote patient monitoring for automated peritoneal dialysis: a randomized controlled trial. Nephron 2021;145:702–10. 10.1159/000518364 [DOI] [PubMed] [Google Scholar]
- 31.Milan Manani S, Baretta M, Giuliani A, et al. Remote monitoring in peritoneal dialysis: benefits on clinical outcomes and on quality of life. J Nephrol 2020;33:1301–8. 10.1007/s40620-020-00812-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanabria M, Buitrago G, Lindholm B, et al. Remote patient monitoring program in automated peritoneal dialysis: impact on hospitalizations. Perit Dial Int 2019;39:472–8. 10.3747/pdi.2018.00287 [DOI] [PubMed] [Google Scholar]
- 33.Weinhandl ED, Collins AJ. Relative risk of home hemodialysis attrition in patients using a telehealth platform. Hemodial Int 2018;22:318–27. 10.1111/hdi.12621 [DOI] [PubMed] [Google Scholar]
- 34.Wong FKY, Chow SKY, Chan TMF. Evaluation of a nurse-led disease management programme for chronic kidney disease: a randomized controlled trial. Int J Nurs Stud 2010;47:268–78. 10.1016/j.ijnurstu.2009.07.001 [DOI] [PubMed] [Google Scholar]
- 35.Kidney Disease Quality of Life Instrument (KDQOL) . The Rand Corporation. Available: https://www.rand.org/health-care/surveys_tools/kdqol.html [Accessed 14 Sep 2021].
- 36.Cartwright EJ, Zs Goh Z, Foo M, et al. eHealth interventions to support patients in delivering and managing peritoneal dialysis at home: a systematic review. Perit Dial Int 2021;41:32–41. 10.1177/0896860820918135 [DOI] [PubMed] [Google Scholar]
- 37.Stevenson JK, Campbell ZC, Webster AC, et al. eHealth interventions for people with chronic kidney disease. Cochrane Database Syst Rev 2019;8:CD012379. 10.1002/14651858.CD012379.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magnus M, Sikka N, Cherian T, et al. Satisfaction and improvements in peritoneal dialysis outcomes associated with telehealth. Appl Clin Inform 2017;8:214–25. 10.4338/ACI-2016-09-RA-0154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manera KE, Johnson DW, Craig JC, et al. Patient and caregiver priorities for outcomes in peritoneal dialysis. CJASN 2019;14:74–83. 10.2215/CJN.05380518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evangelidis N, Tong A, Manns B, et al. Developing a set of core outcomes for trials in hemodialysis: an international Delphi survey. American Journal of Kidney Diseases 2017;70:464–75. 10.1053/j.ajkd.2016.11.029 [DOI] [PubMed] [Google Scholar]
- 41.Ariza JG, Walton SM, Sanabria M, et al. Evaluating a remote patient monitoring program for automated peritoneal dialysis. Perit Dial Int 2020;40:377–83. 10.1177/0896860819896880 [DOI] [PubMed] [Google Scholar]
- 42.Sanyal C, Stolee P, Juzwishin D, et al. Economic evaluations of eHealth technologies: a systematic review. PLoS One 2018;13:e0198112. 10.1371/journal.pone.0198112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gallar P, Vigil A, Rodriguez I, et al. Two-Year experience with telemedicine in the follow-up of patients in home peritoneal dialysis. J Telemed Telecare 2007;13:288–92. 10.1258/135763307781644906 [DOI] [PubMed] [Google Scholar]
- 44.SONG . Standardised outcomes in nephrology. Available: https://songinitiative.org/ [Accessed 23 Apr 2022].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-061772supp001.pdf (64KB, pdf)
bmjopen-2022-061772supp002.pdf (23.5KB, pdf)
bmjopen-2022-061772supp003.pdf (100.6KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.