Abstract
Objective
Although value-based healthcare (VBHC) views accurate cost information to be crucial in the pursuit of value, little is known about how the costs of care should be measured. The aim of this review is to identify how costs are currently measured in VBHC, and which cost measurement methods can facilitate VBHC or value-based decision making.
Design
Two reviewers systematically search the PubMed/MEDLINE, Embase, EBSCOhost and Web of Science databases for publications up to 1 January 2022 and follow Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines to identify relevant studies for further analysis.
Eligibility criteria
Studies should measure the costs of an intervention, treatment or care path and label the study as ‘value based’. An inductive qualitative approach was used to identify studies that adopted management accounting techniques to identify if or how cost information facilitated VBHC by aiding decision-making.
Results
We identified 1930 studies, of which 215 measured costs in a VBHC setting. Half of these studies measured hospital costs (110, 51.2%) and the rest relied on reimbursement amounts. Sophisticated costing methods that allocate both direct and indirect costs to care paths were seen as able to provide valuable managerial information by facilitating care path adjustments (39), benchmarking (38), the identification of cost drivers (47) and the measurement of total costs or cost savings (26). We found three best practices that were key to success in cost measurement: process mapping (33), expert input (17) and observations (24).
Conclusions
Cost information can facilitate VBHC. Time-driven activity-based costing (TDABC) is viewed as the best method although its ability to inform decision-making depends on how it is implemented. While costing short, or partial, care paths and surgical episodes produces accurate cost information, it provides only limited decision-making information. Practitioners are advised to focus on costing full care cycles and to consider both direct and indirect costs through TDABC.
Keywords: organisation of health services, health policy, health economics, health services administration & management
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Using two independent reviewers, this systematic review analyses all value-based healthcare studies that have to date measured costs to provide a comprehensive comparison of cost measurement methods.
This research operationalises the benefits of cost measurement to practitioners by identifying four mechanisms through which cost information facilitates value-based healthcare.
By comparing the methods used in literature to collect cost information, this study identifies three best practices for practitioners and researchers.
By limiting the search to studies labelled as ‘value based’ in their title or abstract, this review overlooks studies that measure healthcare costs but do not explicitly relate this to value-based healthcare.
The included studies may have achieved value-based healthcare without reporting this explicitly, and therefore, may be overlooked when answering Research Question 2.
Introduction
To make sound value-based decisions in healthcare, hospital practitioners and healthcare providers require patient-level information on the costs incurred and outcomes achieved in hospitals and other healthcare organisations.1 This will enable care providers to steer towards better patient-reported outcome measures, better patient-reported experience measures and clinical outcomes at equal or lower cost.2 With detailed cost and outcome information, care paths can be continuously optimised.3 Consequently, value-based healthcare (VBHC) is considered one solution to the financial pressures our global healthcare system places on managers and administrators1 4 5 based on its promise to streamline care by focusing on desirable outcomes. In addition, hospitals can benefit from cost information by gaining insight into the sources of costs that can then guide cost-containment strategies. Therefore, cost information may facilitate process and quality improvement initiatives pursued by management.6–10 Furthermore, insight into patient-level or treatment-level costs enables hospitals to negotiate appropriate prices with insurance firms, especially given the trend towards new payment models and away from fee-for-service payments.11 12 Finally, it is suggested that such treatment-level cost information enables market-based competition among hospitals based on outcomes and prices.13
Considerable research has addressed the outcome side of Porter’s value equation.14 This value equations suggests that healthcare should pursue ‘value’, where value is defined as desirable and relevant patient level outcomes divided by the costs of delivering care.1 2 Many studies have measured patient-level outcomes from both the patient perspective (eg, patient-reported outcome measures, patient-reported experience measures) and clinical outcome perspective.15 16 Less is known about the cost side of this equation. Often, the term ‘cost’ is conflated with the price paid by insurance firms or patients to the hospital.17 18 However, prices do not reflect the costs incurred by hospitals.6 19–21 Prices paid by insurance firms are negotiated sums that include profit margins for both the insurer and the hospital.22 They are also impacted by political factors, such as the hospital-payor mix23 that refers to the range of private and public insurance schemes that make up the hospital’s income stream. Finally, fee-for-service payments fail to account for patient-level differences in required care. Therefore, reimbursements are considered a poor indicator of costs.
Some authors argue for time-driven activity-based costing (TDABC) as the ‘gold standard’ of cost measurement in healthcare organisations.3 5 15 TDABC, in a fine-grained way, matches direct and indirect costs to activities based on the time an activity takes. A care path is made up of many activities, each generating costs. The costs of a care path can thus be calculated by first identifying all costs relevant to each activity, and then summing these costs across the activities.22
Although the research is growing and results are promising, there is relatively little empirical evidence to support TDABC being the best costing method to enable VBHC since studies rarely compare methods, and often simply use whichever system the investigated hospital or care provider uses. Costing methods differ by how they allocate indirect costs to products or services.24 Moreover, indirect costs cannot causally be attributed to patients, and therefore, need to be appropriately allocated. An example of such indirect costs are the salaries of administrative personnel such as the front office staff who welcome patients, coordinate schedules and manage equipment. While some costing methods ignore this (eg, direct costing), other methods average indirect costs across days or months, or systematically allocate them to patients. These methods range from imprecise to fine-grained, with TDABC towards the fine-grained end of the scale. This insight is particularly relevant to healthcare since indirect costs are high. The most fine-grained method is known as activity-based costing (ABC) and allocates indirect costs based on actual units of resources used per activity. In comparison, TDABC allocates indirect costs based on a per-minute cost, making it considerably easier to implement. Costing methods that ignore the indirect costs of a care path underestimate the true costs of the care delivered.
Previous systematic reviews have found that TDABC was able to facilitate VBHC, often highlighting cost savings as a result but without comparing it to alternative methods.3 4 15 Therefore, we do not know how TDABC compares to other cost measurement methods currently in use. While TDABC may be able to facilitate VBHC,5 15 it is unclear how its benefits compare to other costing methods. For these reasons, the cost side of the value equation remains unclear. To address this challenge, we pose two research questions:
RQ1: Which costing methods are currently being used by practitioners to facilitate VBHC?
RQ2: What are the consequences of applying a specific costing method in VBHC? These consequences may include whether the method enables a cost reduction with equal or better health outcomes or provides sufficient information to further improve a care path.
This comprehensive review draws on management accounting literature to categorise costing methods reported in empirical VBHC literature published over the last two decades (1 January 2003 to 1 January, 2022) into cost measurement methods defined in the literature,24 such as direct costing and absorption costing. Compiling studies in this way revealed four ways through which cost information facilitates VBHC and three best practices.
Materials and methods
Literature search strategy
To identify relevant studies, we systematically searched four major databases: Embase, Medline, Web of Science and CINAHL EBSCOhost. Our search string (online supplemental appendix) was developed by assessing previously identified relevant papers and was designed to catch all studies that address VBHC and measure the costs of an intervention, care path or treatment by including the following specific terms:
bmjopen-2022-066568supp001.pdf (330.7KB, pdf)
*cost*, microcost*, macrocost* AND [meaning in combination with] value-based, value based, OR valuebased
Initial search string testing showed that restricting the search to the phrase “value-based healthcare” excluded too many relevant studies because authors use phases such as “value-based perspective” or “value-based equation” when referring to VBHC. Conversely, the term “value” was too broad and yielded more than 40 000 mostly non-specific results. By using wildcard terms indicated by stars we included many variations on the term ‘cost’.
Eligibility criteria, record selection and data collection
We limited ourselves to peer-reviewed empirical research that measured or estimated costs in a VBHC context. All the inclusion criteria and variables extracted are detailed in online supplemental appendix. The following variables, inspired by Porter2 and the cost measurement methods defined in the accounting literature, were noted:
Cost types included (direct vs indirect).
Cost perspective (provider, payer, patient).
Portion of the care path costed (full, partial).
Cost measurement method used (as labelled by authors, verbatim).
Cost measurement categories based on accounting definitions, for example, direct costing, absorption costing, step-down allocation and other recognised methods.24
Consequences of the costing information generated.
Patient and public involvement
This study did not involve patients or the public in designing, executing or reporting the research.
Results
Record selection
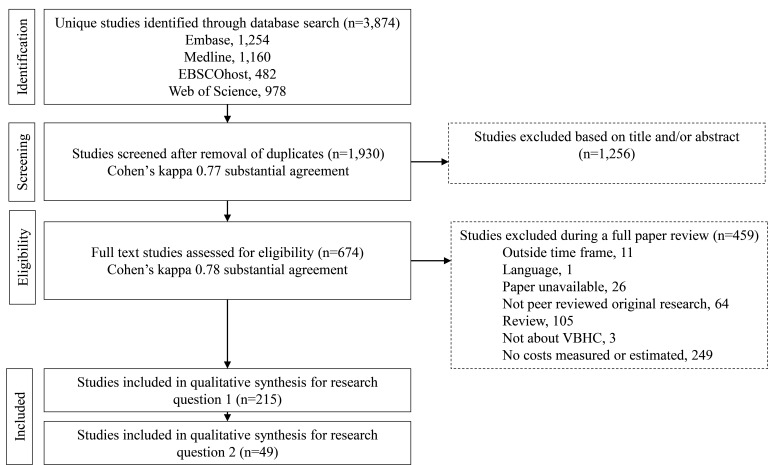
Our four-person (ML, PP, HvE and KA) research group identified 3275 relevant papers, of which 1930 remained after removal of duplicates. We conducted a trial screening of 30 papers to test and further specify screening criteria. The screening process comprised two rounds, as shown in figure 1. In round 1, ML and PP screened the titles and abstracts independently. When there was uncertainty about the eligibility of a paper, it was retained for full-text screening following Bramer.25 We accepted 674 studies based on titles and abstracts, with a Cohen’s kappa inter-rater reliability score of 0.78, indicating substantial agreement.26
Figure 1.
PRISMA flow chart depicting the screening, exclusion and inclusion processes with two reviewers. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; VBHC, value-based healthcare.
In round 2, both ML and PP screened the full text of all 674 studies independently. Of these, 215 studies were seen as relevant for RQ1, with a Cohen’s kappa of 0.76 between ML and PP. HvE was included in any resolution discussions needed. Finally, we assessed whether each paper discussed if or how the costing information facilitated VBHC (RQ2), yielding 49 instances where the costing method facilitated VBHC. This review was not registered.
Descriptive characteristics
An overview of the included studies is provided in table 1. Our earliest study is from 2005, with an upsurge in studies from 2017 onwards. Just under half (n=98, 45.6%) of studies were published in the last 2 years. An overwhelming majority are from the USA (n=178, 82.8%). Europe is the second most common continent with 22 (10.6%) studies of which 9 (4.2%) relate to Dutch healthcare.
Table 1.
Characteristics of value-based healthcare studies that measure costs (n=215)
| Characteristic | n | % | Characteristic | n | % |
| Year published | Topic | ||||
| 2005–2009 | 3 | 1.4 | Cardiology | 5 | 2.3 |
| 2010–2013 | 6 | 2.8 | Dermatology | 1 | 0.5 |
| 2014 | 6 | 2.8 | Emergency and acute care | 11 | 5.1 |
| 2015 | 7 | 3.3 | Endocrinology | 3 | 1.4 |
| 2016 | 9 | 4.2 | Surgical of which | 99 | 46.0 |
| 2017 | 17 | 7.9 | Appendicitis, 2 | ||
| 2018 | 28 | 13.0 | Abdominal, 6 | ||
| 2019 | 41 | 19.1 | Bariatric, 2 | ||
| 2020 | 43 | 20.0 | Cardiac/thoracic, 12 | ||
| 2021 | 51 | 23.7 | Colon/rectal, 2 | ||
| 2022 as per 1/1/2022 | 4 | 1.9 | Endocrine, 2 | ||
| Geography | Ear/nose/throat, 2 | ||||
| Americas | 84.3 | Gallbladder, 2 | |||
| Brazil | 3 | Liver, 2 | |||
| Canada | 1 | Neurosurgical, 5 | |||
| US of which | 178 | Orthopaedic arthroplasty, 25 | |||
| Boston, 8 | Orthopaedic fracture, 12 | ||||
| California, 18 | Orthopaedic rotator cuff repair, 2 | ||||
| New York, 23 | Orthopaedic other, 3 | ||||
| Texas, 12 | Plastic surgery, 2 | ||||
| Pennsylvania, 9 | Spine, 13 | ||||
| Other states, 108 | other surgical, 5 | ||||
| Asia | 2.3 | Geriatrics | 1 | 0.5 | |
| China | 1 | Gynaecology and obstetrics | 8 | 3.7 | |
| Iran | 1 | Infectious disease | 1 | 0.5 | |
| Kuwait | 1 | Internal medicine | 12 | 5.6 | |
| Lebanon | 1 | Multiple | 3 | 1.4 | |
| Singapore | 1 | Nephrology | 1 | 0.5 | |
| Europe | 10.6 | Neurology | 2 | 0.9 | |
| Andalusia | 1 | Oncology | 37 | 17.2 | |
| Germany | 1 | Ophthalmology | 3 | 1.4 | |
| Italy | 3 | Orthopaedic | 1 | 0.5 | |
| Norway | 1 | Pain medicine | 3 | 1.4 | |
| Serbia | 1 | Paediatrics of which | 19 | 8.8 | |
| Spain | 2 | Appendicitis, 3 | |||
| Netherlands | 9 | Emergency and acute care, 2 | |||
| UK | 4 | Neonatal, 3 | |||
| Oceania | 1.9 | Oncology, 1 | |||
| Australia | 4 | Surgical, 5 | |||
| Transcontinental | 0.9 | Surgical, plastic surgery, 2 | |||
| Russia | 1 | Other paediatric, 3 | |||
| Turkey | 1 | Toxicology | 1 | 0.5 | |
| Urology | 4 | 1.9 |
The three largest medical specialty groups represented are surgical (n=99; 46.0%), oncology (n=37; 17.2%) and paediatrics (n=19; 8.8%). A complete list of the 215 studies included is summarised in online supplemental appendix. Extracted data are available in online supplemental file.
bmjopen-2022-066568supp002.xlsx (92.2KB, xlsx)
Which cost measurement methods are currently being used to facilitate VBHC?
To answer RQ1, we look at how costs were measured. A summary of our findings is presented in table 2. The literature contains many overlapping and contradictory terms, as ‘costs’ can refer to insurer costs, reimbursements, hospital costs, or patient costs. About half of the studies (n=110, 51.2%) take a provider perspective, with costs calculated for the hospital or care facility. Many studies use charges or payments because hospital cost data are unavailable, considering charges to be a relevant proxy. Some studies use terms such as ‘costs’, ‘charges’, ‘prices’, ‘payments’ and ‘reimbursements’ interchangeably, making it difficult to differentiate.17 18 27–30 For example, Jain et al17 stated, ‘The terms reimbursement, cost, and payment have been used interchangeably throughout the text to represent actual amounts paid by insurers.’ Similarly, Robles et al30 explained, ‘Total hospital charges were used in this standardised costing analysis. Hospital charge data provides a relative measure of the ‘cost’ of episodes of care, as actual cost data are generally not ascertainable in the healthcare setting.’ When calculating costs using TDABC, Ahluwalia et al31 called these costs ‘prices.’ To try to address this confusion, some recent studies refer to provider costs as the ‘true cost’ of care.6 7 9 19 Some studies that compare several cost types19 20 also differentiate ‘traditional hospital accounting’ costs from ‘true costs’ calculated with TDABC.6 9 20 32 33
Table 2.
Characteristics of costing methods in value-based healthcare
| Characteristic | Studies | Perspectives | ||||
| n | % | n | % | |||
| Cost perspective | ||||||
| Provider | 110 | 51.2 | 111 | 51.6 | ||
| Insurer | 103 | 47.9 | 106 | 49.3 | ||
| Patient | 2 | 0.9 | 5 | 2.3 | ||
| N | 215 | 222 | ||||
| All studies (n=215) | Provider only | Payer only | ||||
| Cost types included | ||||||
| Direct | 28 | 13.0 | 13.0 | 24 | 2 | |
| Direct and indirect | 177 | 81.9 | 81.9 | 84 | 93 | |
| Unspecified | 10 | 4.6 | 4.6 | 2 | 8 | |
| Costs measurement implementation | ||||||
| No, costs measured for purpose of study | 34 | 15.7 | 15.7 | 33 | ||
| Yes, costing method is implemented | 39 | 17.6 | 17.6 | 39 | ||
| Unspecified or not applicable | 142 | 66.2 | 66.2 | 38 | 102 | |
| Costs coverage | ||||||
| Full care path | 47 | 21.8 | 21.8 | 30 | 16 | |
| Full care path (full surgical episode) | 17 | 7.4 | 7.4 | 13 | 4 | |
| Partial care path (full surgical episode) | 22 | 8.3 | 8.3 | 19 | 3 | |
| Partial care path | 86 | 42.1 | 42.1 | 37 | 49 | |
| Unspecified | 43 | 19.9 | 19.9 | 11 | 31 | |
Note: N differs between studies and perspectives because seven studies measured two cost types.
We categorised studies based on the cost types included. Both direct and indirect costs were considered in 177 (81.9%) studies, while 28 (13.0%) papers only included direct costs.
Next, we looked at whether costs were calculated for a complete care path. We found 64 (29.8%) studies that measured costs for a full care path, of which 16 (7.4%) refer to full surgical episodes and label them as such without considering all the presurgical or postsurgical costs. The remaining 86 (42.1%) measure costs of a partial care path.
Table 3 categorises studies based on the costing method used. In those papers measuring costs within a care provider, we identified two clear categories that were in line with the management accounting literature.24 The first is ‘direct costing’ (n=23), where direct costs of care are summed and indirect costs ignored. This implies that, if costs cannot be causally attributed to the treatment of a specific patient, they are not considered and hence overlooked when making managerial decisions.24
Table 3.
Overview of cost measurement methods used in value-based healthcare
| Perspective | Method | n |
| Provider | Direct costs only | |
| Direct costing | 23 | |
| Absorption costing | ||
| ABC | 7 | |
| TDABC | 31 | |
| Other | 47 | |
| Not specified | 3 | |
| Insurer | Charges and reimbursements | |
| Charges, reimbursements, claims | 81 | |
| Charges adjusted with cost-to-charge ratio | 25 | |
| Patient | Out-of-pocket costs to patient | 5 |
Note: The total number of studies here is 222 because 7 studies measure two cost types. Studies are classified based on actual costs included and methods described, not necessarily the labels used by the studies’ authors.
ABC, activity-based costing; TDABC, time-driven activity-based costing.
The second category of studies considers both direct and indirect costs and uses ‘absorption costing’, whereby indirect costs are allocated to patients based on an allocation key (a type of formula used for allocating indirect costs).31 These studies include but are not limited to TDABC (n=31) and ABC (n=7), where costs are allocated to individual care activities (such as a consultation or treatment step). The remaining absorption costing papers (n=47) also consider direct and indirect costs but do not report how indirect costs are allocated to activities. In the absorption costing studies, authors may state that cost information was calculated based on diagnosis-related group costs, microcosting, bottom-up clinical costing or hospital accounting systems not further classified. A full list of all the terms used is presented in online supplemental appendix.
How do these costing methods facilitate VBHC?
To answer RQ2, we extracted all the consequences related to the costing method as described in the papers. Here, like Etges et al,3 we were looking for how the costing information facilitated VBHC. Note that not all the studies included to address RQ1 describe facilitating VBHC or the consequences of the cost information generated. The reported consequences were grouped inductively, revealing four categories:
Identification of cost drivers, in terms of cost items (eg, staff costs, material costs) or activities (eg, surgery, initial consult; n=47).
Comparison of costs across patient groups, care providers or procedures (n=38).
Measured cost difference or cost saving, while achieving equal or better care (n=26).
Suggested or measured care path improvements (n=39).
These studies are presented in table 4. The studies reporting these facilitators used ABC (n=6), TDABC (n=28), other absorption costing methods (n=12) or direct costing (n=3).
Table 4.
Costing method applications, method used and consequences (ordered by year)
| Study characteristics | Best practices | Value-based consequences of costing information | |||||||||||
| Medical specialty | Costing method | period | Centre | Study type | PM | EI | DO | CG | Compare costs across | ICD | MPS | Care path adjustment | |
| 40 | Internal medicine | TDABC | Partial | Single | Retro | Yes | Yes | Items, activities | Yes | Suggested | |||
| 33 | Surgical, orthopaedic, rotator cuff repair | TDABC | Full (FSE) | Single | Retro | Yes | Yes | Items, activities | Surgeons, two alternative treatments | Yes | Yes, ±US$727 about the mean per patient | Suggested | |
| 4 | Cardiology, surgical | TDABC | Full (FSE) | Multi | Retro | Yes | Yes | Yes | Items, activities | Hospitals, procedures | Yes | Yes, estimate 51.0% of procedure cost | Yes |
| 10 | Oncology | TDABC | Full | Single | Retro | Yes | Yes | Yes | Items, activities | Treatment care paths | Yes | US$2302 (25.0%) difference across treatments | Suggested |
| 41 | Surgical, orthopaedic | TDABC | Full (FSE) | Single | Retro | Yes | Items, activities | Patients | Yes | Suggested | |||
| 19 | Surgical, orthopaedic | TDABC | Full (FSE) | Single | Retro | Yes | Yes | Items, activities | Costing methods (TA and TDABC) | Yes | Suggested | ||
| 20 | Surgical, orthopaedic | TDABC | Full (FSE) | Single | Retro | Yes | Yes | Items, activities | Five treatments, cost vs reimbursement | Yes | |||
| 43 | Paediatric, surgical, plastic surgery | TDABC | Full (FSE) | Multi | Pro | Yes | Yes | Items, activities | Treatment care paths | Yes | Up to US8900, but long-term outcomes yet unknown | Suggested | |
| 44 | Emergency and acute care | TDABC | Full (multiple) | Multi | Retro | Yes | Yes | Yes | Items, activities | Eight care paths for acute ureteral stones (patient journeys) | Yes | Yes, US$6614 difference across care paths | Suggested |
| 55 | Surgical, orthopaedic | TDABC | Full (FSE) | Single | Retro | Yes | Yes | Items, activities | Surgeons | Yes | Suggested | ||
| 38 | Oncology | TDABC | Partial | Single | Pro | Yes | Yes | Yes | Items, activities | Treatments and individual care paths | Yes | Yes, cost difference of up to 3.33 times, depending on case mix | Suggested |
| 45 | Oncology (incl. surgery) | TDABC | Full | Single | Retro | Yes | Yes | Items, activities | Yes | Suggested | |||
| 52 | Oncology | TDABC | Partial (FSE) | Single | Retro | Yes | Items | Preimplementation and postimplementation | Yes | Yes, mean cost savings of €309 per patient | Yes | ||
| 65 | Cardiology | AC (other) | Partial | Multi | Retro | Items | Patient journeys | Yes | Suggested | ||||
| 66 | Emergency and acute care | AC (other) | Partial | Single | Retro | Yes | Items | Surgeons | Yes | ||||
| 67 | Surgical, bariatric | AC (other) | Full (FSE) | Single | Retro | Items | Treatment | Yes | |||||
| 68 | Gynaecology and obstetrics | AC (other) | Full | Single | Retro | Yes | Items, activities | Procedures | Yes | Yes, $967 per patient | Suggested | ||
| 58 | Emergency and acute care | AC (other) | Partial | Single | Retro | Items, activities | Yes | ||||||
| 69 | Surgical, colorectal | AC (other) | Partial (FSE) | Single | Retro | Items | Intervention | Yes | Yes, reduced variable cost, similar total cost | Yes | |||
| 21 | Surgical, orthopaedics, fracture | ABC | Partial (FSE) | Single | Retro | Items | Patients, patient groups, demographics | Yes | |||||
| 35 | Surgical, orthopaedic, arthroplasty | ABC | Full (FSE) |
Single | Both | Yes | Yes | Items, activities | Treatment care paths | Yes | Estimate €2 054 000 annually | Yes | |
| 36 | Surgical, spine | ABC | Full | Single | Retro | Yes | Items, activities | Patients, patient groups | Yes | Suggested | |||
| 6 | Paediatric, surgical | TDABC | Full (FSE) |
Single | Both | Yes | Yes | Items, activities | Costing methods (TA and TDABC) | Yes | 20.0% and without care path alteration | Suggested | |
| 46 | Oncology | TDABC | Full (FSE) |
Single | Retro | Yes | Yes | Yes | Items, activities | Treatment care paths | Yes | Yes, estimate for each 10.0% decrease in case duration, total costs could decrease by about 8.0%. | Suggested |
| 31 | Surgical, orthopaedic | TDABC | Full (FSE) |
Single | Pro | Yes | Yes | Yes | Items, activities | Treatment care paths | Yes | £2018 per patient | Suggested |
| 53 | Paediatric, neonatal | TDABC | Partial | Single | Retro | Yes | Yes | Yes | items, activities | Pre and post intervention | Yes | Yes, 36.0% or US$92 000 per tracheostomy care cycle | Yes |
| 70 | Surgical, cardiac/thoracic | AC (other) | Partial | Multi | Retro | Yes | Items | Patients, implant devices | Yes | Suggested | |||
| 71 | Oncology, surgical | AC (other) | Partial | Single | Retro | Items | Yes | Yes, multiple | |||||
| 47 | Multiple | TDABC | Full | Multi, pilot | Retro | Yes | Yes | Yes | Items, activities | Before and after intervention (IPUs) | Yes | Yes, quarterly costs declined | Suggested |
| 48 | Oncology | TDABC | partial (PSE) | Single | Pro | Yes | Yes | Items, activities | Treatment care paths (parallel vs induction design in OR) | Yes | Yes, estimate OR time reduction of 55 min, or US$2818 missed revenue | Suggested | |
| 49 | Surgical, orthopaedics fracture | TDABC | partial (FSE) | Single | Both | Yes | Items | Yes | Suggested | ||||
| 50 | Surgical, foot debridement | TDABC | partial (FSE) | Single | Retro | Yes | Items | Before and after intervention | Yes | Yes | |||
| 39 | Ophthalmology | TDABC | full | Single | Retro | Yes | Yes | Yes | Items, activities | Yes | Suggested | ||
| 56 | Gynaecology and obstetrics, surgical | AC (other) | partial (FSE) | Single | Retro | Items | Yes | Suggested | |||||
| 57 | Gynaecology and obstetrics, surgical | AC (other) | partial (FSE) | Single | Retro | Items | Yes | ||||||
| 60 | Multiple | Direct costing | partial | Single | Retro | items | Yes | ||||||
| 61 | Surgical, orthopaedic | Direct costing | full (FSE) |
Multi | Retro | Items | Intervention | Yes, £255 per patient | Yes | ||||
| 5 | Surgical, carpal tunnel release | TDABC | partial (FSE) | Multi | Retro | Yes | Yes | Items, activities | Multiple treatment care paths | Yes | Yes, 70.9% (US$27,103) and 31.6% (US$178) | Yes | |
| 59 | Surgical, appendicitis | AC (other) | partial (FSE) | Single | Pro | Yes | Yes | Items | Pre and post intervention (dashboard) | Yes | Yes, decreased by $496 per operation | Yes | |
| 62 | Surgical, orthopaedic | Direct costing | partial (FSE) | Single | Retro | Yes | Items | Intervention | Yes | ||||
| 8 | Urology | TDABC | partial | Single | Pro | Yes | Yes | Items, activities | Yes | Yes, estimate 2 hours per cycle | Suggested | ||
| 54 | Paediatrics, appendicitis | TDABC | full (FSE) | Single | Pro | Yes | Yes | Yes | Items, activities | Treatment care paths (pre and post intervention) | Yes | 11.0% cost reduction, and 51.0% hospitalisation time reduction | Yes, several |
| 72 | Urology | AC (other) | partial | Multi | Retro | Yes | Items | ||||||
| 7 | Oncology, surgical, 11 surgeries | TDABC | Partial (FSEs) | Single | Retro | Yes | Items, activities | Potential staffing ratios | Yes | Estimate 13.0%–28.0% per surgery type | Modelled and suggested | ||
| 9 | Oncology | TDABC | full | Single | Retro | Yes | Yes | Yes | Items, activities | Treatments (high-dose vs low-dose brachytherapy) | Yes | US$2668 difference across treatments | Yes |
| 51 | Urology | TDABC | partial (FSE) | Single | Retro | Yes | Yes | Items, activities | Five treatment care paths | Yes | Yes, 400.0% increase from least to most expensive pathways | Suggested | |
| 32 | Surgical, neurosurgery | ABC | partial (FSE) | Single | Retro | Yes | Items, activities | Patients | Yes | Yes, 25.0% | Yes, several | ||
| 34 | Surgical, neurosurgery | ABC | partial (FSE) | Single | Retro | Yes | Items, activities | patients | Yes | Suggested | |||
| 37 | Paediatric plastic surgery | ABC | partial, 1 year | Single | Retro | Items | Patients | Yes | suggested | ||||
| Count | 33 | 17 | 24 | 38 | 47 | 26 | 39 | ||||||
Costing methods are classified based on actual reported costs and methods applied, not necessarily the labels used by authors.
ABC, activity-based costing; AC, absorption costing; CG, cost grouping; DO, direct observation; EI, expert input; FSE, full surgical episode; ICD, identify cost drivers; IPU, integrated practice units; MPS, measured provider cost savings; PM, process mapping; Pro, prospective; PSE, partial surgical episode; Retro, retrospective; TDABC, time-driven activity-based costing.
Activity-based costing
The six studies applying ABC justified this on the basis that it was the care provider’s existing costing method. Three of these studies measured costs for a full surgical episode21 32 34 as part of a longer care path, two measured costs for a full care path,35 36 and one measured costs of a partial care path.37 While these studies all applied ABC, the ability to facilitate VBHC differed. Jacobs et al36 measured costs for a complete care path for patients with adult spinal deformity, a complex care path spanning about 1 year. The authors compared costs across patient groups and patients, identified major cost drivers, and suggested where to concentrate cost containment. Similarly, McLaughlin et al32 34 measured costs, identified cost drivers and evaluated targeted cost containment initiatives. In one paper,32 the cost containment initiatives were informed by the cost information: activities with the highest costs were targeted for savings and a 25% reduction in total costs was achieved. In the other paper,34 they identified comorbidities and demographics that were strongly related to the total costs of patients undergoing neurosurgery, whereas Wise et al21 did not for geriatric hip fracture patients while identifying cost drivers and comparing costs across patient groups. Vanni et al35 successfully predicted about €2 million annual cost savings associated with an enhanced recovery pathway.
Time-driven ABC
The majority of the papers used to answer RQ2 involved TDABC. Significant cost drivers were identified linked to activities in a care path, and some suggested where to target improvement initiatives.4 6–8 10 33 38 39 Many of the TDABC studies were able to suggest6 8 10 19 33 38–51 or measure5 9 31 52–54 care path improvements (see table 4).
The lengths and specificities of the care path costs varied widely. Some studies were narrow in scope, calculating costs for subsections of a single care path or surgical procedure.6 8 44 48 55 Isaacson et al8 calculated costs for cleaning a single reusable piece of equipment, while others costed single surgical days,6 compared alternative surgeons,55 or anaesthesia solutions within a care path.48 Within this group, McClintock et al44 took the broadest perspective by mapping individual patient journeys.
The largest group (n=10) of TDABC studies measured costs across care paths within a single provider and for a single diagnosis.9 10 31 33 38 43 45 46 52 54 Typically, these studies compared costs between a new intervention and the ‘usual’ care,9 10 31 46 53 54 or between alternative care paths33 38 43 52 in order to measure cost savings.
Some studies were broader in scope, costing multiple care paths or treatments within one specialty,5 7 20 51 an entire department,39 40 multiple practice units47 or providers.4 Some compared ‘true costs’ calculated using TDABC across care providers within specialties or care paths,4 43 while others argued that TDABC costs were too subjective to be compared across hospitals.10 44 While most studies compared costs across care paths, some also compared costs across patient groups,19 41 42 or even individual patient journeys.38 44
Technology played a prominent role in studies aiming to reduce costs. One study was able to suggest how to use technology more efficiently,6 and some, by integrating technological investments in the calculated TDABC costs, show how technology can reduce costs.38 43 46
Conversely, studies using unspecified absorption methods56 57 did not include investments in technology, and this is surprising since absorption costing methods require indirect costs to be allocated.
Analyses enabled by activity-based and TDABC
Several of the ABC and TDABC studies compared costs calculated using traditional accounting costs6 19 20 or reimbursement amounts20 21 and found that prices do not equal costs. Some carried out quantitative analyses using cost information generated using ABC or TDABC including regression analyses to identify correlations,7 33 38 41 42 compare patient groups,20 41 42 and compare costs and outcomes across a matched patient sample.38
Two recent studies33 49 have conducted patient-level value analyses, comparing patient-reported outcomes with patient-level TDABC costs. Wise et al33 did so for rotator cuff repair surgery over a period of 1 year, while McCreary et al49 analysed ankle fractures. Both studies found costs to be unrelated to patient-reported outcome measures, highlighting the need for further research. This suggests that patient-reported outcome measures are not strongly associated with the costs of the care delivered, and that patient satisfaction may depend on other factors such as their perceived experience with healthcare professionals.
Other absorption costing methods and direct costing
Other absorption costing methods reported in the studies were labelled as microcosting (n=5), bottom-up clinical costing58 or were described but not labelled (n=6). Most were able to identify cost drivers (n=12, for details, see table 4) and some compared costs within providers. Notably, Robinson et al59 used the cost information to build and evaluate a dashboard that provides real-time feedback to surgeons during operations and monthly summaries and thereby decreases costs significantly. Some studies omitted certain cost categories such as equipment.57 Direct costing enabled cost drivers to be identified,60–62 and in some cases granular cost measurement.
Best practices
Having identified these four facilitators, we compared studies to find common practices. This is particularly useful because costing methods are not labelled consistently. For example, many studies refer to ABC as ‘bottom-up costing.’ To look beyond labels, we compared the actual methodologies used to measure costs. We found that studies that were able to facilitate VBHC used process mapping (n=33), expert input (n=17) and/or direct observations (n=24) when measuring costs. These practices overlap with TDABC best practices, but are not exclusive to TDABC, as shown in table 4.
Studies that made specific care path improvement suggestions used process mapping, and especially those involving multidisciplinary teams reported significant benefits.4 9 10 19 This approach enabled experts (doctors, care professionals, administrators) with the required knowledge and experience to reflect critically on the process,4 9 10 19 resulting in actionable suggestions. In comparison, studies that did not use process mapping tended to suggest minimising high-cost items (eg, total operating time, nursing costs) but were unable to couple these suggestions to specific activities or to chronological points in the care path. Commenting only on cost items, and not identifying chronological points, limits the ability of cost information to steer management towards where to focus process improvement initiatives.
Expert input while creating process maps or measuring costs was often cited by authors as valuable, especially for estimating preparation time or other behind-the-scenes activities that do not involve the patient but are critical to delivering care. Some studies that could not call on expert input cited this as a limitation. A few cases also evaluated the impact of costing information, for example by involving experts to evaluate a dashboard.59
Finally, some studies involved direct observations, particularly those that calculated process times to the minute or measured the costs of individual patient journeys.
Discussion
This review focused on VBHC studies that have measured or estimated costs, and on identifying which costing methods can facilitate VBHC. By assessing the consequences of the costing methods used, we were able to identify characteristics of costing methods that do facilitate VBHC.
Previous research found that TDABC can facilitate VBHC through cost containment and process improvements.3 15 We built on this by comparing value-based consequences across costing methods. While the field is young and alternatives seem limited, we have found considerable evidence that TDABC and ABC can indeed facilitate VBHC. As previously noted, TDABC is considerably easier to implement than ABC, which leads us to recommend it over ABC. We found no well-documented alternatives to TDABC or ABC in our review. However, not all the TDABC studies delivered the facilitating factors we have identified. We, therefore, emphasise the need to follow TDABC guidelines carefully and to explicitly document methods used. Several of the studies in this review simply stated that TDABC was applied, outsourced, used with incomplete costs, or used without listing exact cost rates.
The start and end points of care paths tend to be well documented by authors but are inconsistent. To view costs in relation to outcomes, as suggested by Porter,2 the total costs from start to finish of a trajectory should be included.63 In many studies, the start and end points of cost measurement windows seem somewhat arbitrary but are still labelled as full care paths. Consequently, this results in inconsistencies across studies, hindering comparisons. Encouragingly, some of the more recent studies have measured costs across a genuine full care path and future research should do the same, explicitly defining start and end points. This would enable consistent comparisons across providers. As with the ICHOM standard outcome sets produced by the International Consortium for Health Outcomes Measurement, costs could be catalogued and compared over full care paths. Indeed, in a recent expert consensus study, experts agreed on the need to focus on full care paths.63
Furthermore, we can see a trade-off in the specificity and length of the care path costed. Studies that measure costs for elements of a care path (such as a surgical operation) can provide detailed costs for that portion of the care path, but not total care costs for a patient because the remainder of the care path is not included. Some surgical studies measured costs for partial care paths, and often concluded that operating theatre time should be minimised due to high surgeon and operating theatre costs. However, this conclusion has limited relevance for the value equation13 because it does not provide cost information for an entire care path, or advice on how to circumvent surgery.
Studies that cost complete care paths appear to use less-detailed costing methods (due to the sheer length of the care path) but are able to compute total costs of a patient’s care. This enabled benchmarking across providers, as well as cost comparisons of new versus standard care, or of treatment alternatives. This allowed providers to steer towards lower-cost outcomes to maximise value. Future research should focus on measuring costs for full care paths, and on comparing costs to outcomes as demonstrated in some of the more recently published studies in our review.33 38 49
Our review highlights the need to involve medical professionals in this process, both when implementing costing methods as well as when evaluating the results. Future cost measurement studies, and hospitals looking to implement TDABC, should involve multidisciplinary teams. Studies that have involved medical professionals in the process of measuring costs and then using the findings were able to improve care paths through improvement initiatives and/or dashboards. This suggests that generating and using costing information should be viewed as a process. Future qualitative research should follow this process to better understand the mechanisms through which cost information impacts decision making, and the impact that staff involvement has on cost containment. Previous research suggests that staff involvement is critical as it builds trust in the accuracy of the data.64
Limitations and future research
We must acknowledge several limitations related to the scope, breadth and quality of the included studies. First, our search strategy will have missed studies that measure costs but do not label the study as VBHC-oriented. Not all TDABC studies make value-based claims or contributions and may therefore be overlooked in our review. In addition, not all studies explicitly discuss the impact or consequences of the costing method applied, which may impact our findings. Future qualitative research could usefully investigate TDABC implementations and evaluate whether the facilitating factors found in this review are achieved. Second, sophisticated methods such as TDABC are currently only used with predictable and/or short care paths such as orthopaedic surgery. Further research testing the feasibility and practicality of TDABC in different settings, such as emergency on-call care, or longer care paths such as fertility treatment, is warranted. Further, our findings may have limited generalisability across medical specialties as indicated in table 1. Finally, we have relied on the reporting of authors whose style and quality differs across disciplines and journals. To an extent we circumvented this limitation by looking beyond the cost measurement labels used by authors, extracting the costs included and methods used, and then categorising them using established accounting definitions. However, we cannot exclude the possibility of errors due to a lack of explicit reporting in some of the studies reviewed.
Conclusions
This systematic review reveals that cost information, at the treatment or patient level, for complete care paths does enable value-based decision making through several mechanisms. Such cost information can direct quality and process improvement initiatives alongside informing appropriate reimbursement levels. In the pursuit of VBHC, practitioners and academics are advised to apply ABC or TDABC to estimate costs, using process mapping, expert input and observations, rather than relying on pricing information.
Supplementary Material
Acknowledgments
We thank W.M. Bramer, biomedical information specialist from the Erasmus MC. We also thank our fellow members of the value-based healthcare consortium, involving Amsterdam UMC, Erasmus MC, Radboudumc and Erasmus University Rotterdam, led by J.G.M. Jelsma and M.C. de Bruijne.
Footnotes
Contributors: ML, study design, screening of titles and abstracts for inclusion, data analysis and interpretation, writing. PP: screening of titles and abstracts for inclusion, assisted in writing. KA: study design, assisted in interpretation of results, assisted in writing. HvE: study design, screening of titles and abstracts for inclusion, data analysis and interpretation, assisted in writing. ML is the author acting as guarantor. All authors contributed to and approved of the final manuscript.
Funding: This work was supported by the Dutch Ministry of Health, Welfare and Sport (VWS) through grant number 330843.
Competing interests: The third author, KA, was a speaker at the 2022 TDABC conference and was reimbursed for his stay in Lisbon by the TDABC consortium.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. The data generated in this study is also available in a public, open access repository (Erasmus Data Repository, DOI: 10.25397/eur.20279883).
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Kaplan RS, Porter ME. How to solve the cost crisis in health care. Harv Bus Rev 2011;89:46-52, 54, 56-61 passim. [PubMed] [Google Scholar]
- 2.Porter ME. What is value in health care? N Engl J Med 2010;363:2477–81. 10.1056/NEJMp1011024 [DOI] [PubMed] [Google Scholar]
- 3.Etges APBdaS, Ruschel KB, Polanczyk CA, et al. Advances in value-based healthcare by the application of time-driven activity-based costing for inpatient management: a systematic review. Value Health 2020;23:812–23. 10.1016/j.jval.2020.02.004 [DOI] [PubMed] [Google Scholar]
- 4.da Silva Etges APB, Cruz LN, Schlatter R, et al. Time-Driven activity-based costing as a strategy to increase efficiency: an analyses of interventional coronary procedures. Int J Health Plann Manage 2022;37:189–201. 10.1002/hpm.3320 [DOI] [PubMed] [Google Scholar]
- 5.Martin JA, Mayhew CR, Morris AJ, et al. Using time-driven activity-based costing as a key component of the value platform: a pilot analysis of colonoscopy, aortic valve replacement and carpal tunnel release procedures. J Clin Med Res 2018;10:314–20. 10.14740/jocmr3350w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodar YJL, Srinivasan AK, Shah AS, et al. Time-Driven activity-based costing identifies opportunities for process efficiency and cost optimization for robot-assisted laparoscopic pyeloplasty. J Pediatr Urol 2020;16:460.e1–460.e10. 10.1016/j.jpurol.2020.05.146 [DOI] [PubMed] [Google Scholar]
- 7.French KE, Guzman AB, Rubio AC, et al. Value based care and bundled payments: anesthesia care costs for outpatient oncology surgery using time-driven activity-based costing. Healthc 2016;4:173–80. 10.1016/j.hjdsi.2015.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isaacson D, Ahmad T, Metzler I, et al. Defining the costs of reusable flexible Ureteroscope reprocessing using time-driven activity-based costing. J Endourol 2017;31:1026–31. 10.1089/end.2017.0463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ilg AM, Laviana AA, Kamrava M, et al. Time-Driven activity-based costing of low-dose-rate and high-dose-rate brachytherapy for low-risk prostate cancer. Brachytherapy 2016;15:760–7. 10.1016/j.brachy.2016.08.008 [DOI] [PubMed] [Google Scholar]
- 10.Dziemianowicz M, Burmeister J, Dominello M. Examining the financial impact of altered fractionation in breast cancer: an analysis using time-driven activity-based costing. Pract Radiat Oncol 2021;11:245–51. 10.1016/j.prro.2021.01.003 [DOI] [PubMed] [Google Scholar]
- 11.Cattel D, Eijkenaar F. Value-Based provider payment initiatives combining global payments with explicit quality incentives: a systematic review. Med Care Res Rev 2020;77:511–37. 10.1177/1077558719856775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Counte MA, Howard SW, Chang L, et al. Global advances in value-based payment and their implications for global health management education, development, and practice. Front Public Health 2018;6:379. 10.3389/fpubh.2018.00379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porter ME, Teisberg EO. Redefining competition in health care. Harv Bus Rev 2004;82:136:64–76. [PubMed] [Google Scholar]
- 14.Rathert C, Mittler JN, Lee YSH. Patient-Provider therapeutic connections to improve health care: conceptual development and systematic review of patient measures. Health Care Manage Rev 2022;47:317–29. 10.1097/HMR.0000000000000339 [DOI] [PubMed] [Google Scholar]
- 15.Zanotto BS, Etges APBdaS, Marcolino MAZ, et al. Value-Based healthcare initiatives in practice: a systematic review. J Healthc Manag 2021;66:340–65. 10.1097/JHM-D-20-00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibbons C, Porter I, Gonçalves-Bradley DC, et al. Routine provision of feedback from patient-reported outcome measurements to healthcare providers and patients in clinical practice. Cochrane Database Syst Rev 2021;10:CD011589. 10.1002/14651858.CD011589.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain N, Brock JL, Phillips FM, et al. Chronic preoperative opioid use is a risk factor for increased complications, resource use, and costs after cervical fusion. Spine J 2018;18:1989–98. 10.1016/j.spinee.2018.03.015 [DOI] [PubMed] [Google Scholar]
- 18.Rice-Townsend S, Barnes JN, Hall M, et al. Variation in practice and resource utilization associated with the diagnosis and management of appendicitis at freestanding children's hospitals: implications for value-based comparative analysis. Ann Surg 2014;259:1228–34. 10.1097/SLA.0000000000000246 [DOI] [PubMed] [Google Scholar]
- 19.Fang CJ, Shaker JM, Drew JM, et al. The cost of hip and knee revision arthroplasty by diagnosis-related groups: comparing time-driven activity-based costing and traditional accounting. J Arthroplasty 2021;36:e2673:2674–9. 10.1016/j.arth.2021.03.041 [DOI] [PubMed] [Google Scholar]
- 20.Fang CJ, Shaker JM, Hart P-A, et al. Variation in the profit margin for different types of total joint arthroplasty. J Bone Joint Surg Am 2022;104:459–64. 10.2106/JBJS.21.00223 [DOI] [PubMed] [Google Scholar]
- 21.Wise K, Blaschke BL, Parikh HR, et al. Variation of the inpatient cost of care in the treatment of isolated geriatric Intertrochanteric hip fractures. Geriatr Orthop Surg Rehabil 2020;11:215145932097653. 10.1177/2151459320976533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keel G, Savage C, Rafiq M, et al. Time-Driven activity-based costing in health care: a systematic review of the literature. Health Policy 2017;121:755–63. 10.1016/j.healthpol.2017.04.013 [DOI] [PubMed] [Google Scholar]
- 23.Hoenigl M, Lo M, Coyne CJ, et al. 4th Generation HIV screening in the emergency department: net profit or loss for hospitals? AIDS Care 2021:1–5. 10.1080/09540121.2021.1995838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmerman JL, Yahya-Zadeh M. Accounting for decision making and control. Seventh Edition. McGraw-Hill, 2011: 258–9. http://digilib.umpalopo.ac.id:8080/jspui/handle/123456789/151 10.2308/iace.2011.26.1.258 [DOI] [Google Scholar]
- 25.Bramer WM, Milic J, Mast F. Reviewing retrieved references for inclusion in systematic reviews using endnote. J Med Libr Assoc 2017;105:84–7. 10.5195/jmla.2017.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pérez J, Díaz J, Garcia-Martin J, et al. Systematic literature reviews in software engineering—enhancement of the study selection process using Cohen’s Kappa statistic. J Syst Softw 2020;168:110657. 10.1016/j.jss.2020.110657 [DOI] [Google Scholar]
- 27.Burnett Iii RA, Yang J, Courtney PM, et al. Costs of unicompartmental compared with total knee arthroplasty : a matched cohort study over ten years. Bone Joint J 2021;103-B:23–31. 10.1302/0301-620X.103B6.BJJ-2020-2259.R1 [DOI] [PubMed] [Google Scholar]
- 28.Sun L-lu, Cao D-yan, Yang J-xin, et al. Value-Based medicine analysis on loop electrosurgical excision procedure and CO2 laser vaporization for the treatment of cervical intraepithelial neoplasia 2. J Obstet Gynaecol Res 2012;38:1064–70. 10.1111/j.1447-0756.2011.01832.x [DOI] [PubMed] [Google Scholar]
- 29.Cronin KJ, Mair SD, Hawk GS, et al. Increased health care costs and opioid use in patients with anxiety and depression undergoing rotator cuff repair. Arthroscopy 2020;36:2655–60. 10.1016/j.arthro.2020.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robles AJ, Kornblith LZ, Hendrickson CM, et al. Health care utilization and the cost of posttraumatic acute respiratory distress syndrome care. J Trauma Acute Care Surg 2018;85:148–54. 10.1097/TA.0000000000001926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahluwalia R, Cook J, Raheman F, et al. Improving the efficiency of ankle fracture care through home care and day-surgery units: delivering safe surgery on a value-based healthcare model. Surgeon. 2021;19:e95-e102. 10.1016/j.surge.2020.08.004 [DOI] [PubMed] [Google Scholar]
- 32.McLaughlin N, Upadhyaya P, Buxey F, et al. Value-Based neurosurgery: measuring and reducing the cost of microvascular decompression surgery. J Neurosurg 2014;121:700–8. 10.3171/2014.5.JNS131996 [DOI] [PubMed] [Google Scholar]
- 33.Wise KL, Parikh HR, Okelana B, et al. Measurement of value in rotator cuff repair: patient-level value analysis for the 1-year episode of care. J Shoulder Elbow Surg 2022;31:72–80. 10.1016/j.jse.2021.07.004 [DOI] [PubMed] [Google Scholar]
- 34.McLaughlin N, Martin NA, Upadhyaya P, et al. Assessing the cost of contemporary pituitary care. Neurosurg Focus 2014;37:E7. 10.3171/2014.8.FOCUS14445 [DOI] [PubMed] [Google Scholar]
- 35.Vanni F, Foglia E, Pennestrì F, et al. Introducing enhanced recovery after surgery in a high-volume orthopaedic Hospital: a health technology assessment. BMC Health Serv Res 2020;20:773. 10.1186/s12913-020-05634-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobs K, Dewilde T, Vandoren C, et al. Variability in hospital costs of adult spinal deformity care. Spine 2020;45:1221–8. 10.1097/BRS.0000000000003497 [DOI] [PubMed] [Google Scholar]
- 37.Abbott MM, Meara JG. A microcosting approach for isolated, unilateral cleft lip care in the first year of life. Plast Reconstr Surg 2011;127:333–9. 10.1097/PRS.0b013e3181f95af3 [DOI] [PubMed] [Google Scholar]
- 38.Thaker NG, Boyce-Fappiano D, Ning MS, et al. Activity-Based costing of intensity-modulated proton versus photon therapy for oropharyngeal cancer. Int J Part Ther 2021;8:374–82. 10.14338/IJPT-20-00042.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurt P, Saban M, Cankaya F. Time-Driven activity-based costing in the ophthalmology department of state Hospital: a case study. Fresenius Environ Bull 2019;28:2754–70. [Google Scholar]
- 40.Alibrahim A, Abdulsalam Y, Al Mutawa S, et al. Towards value-based healthcare: establishing baseline pharmacy care costs for diabetes management. Int J Health Plann Manage 2022;37:790–803. 10.1002/hpm.3370 [DOI] [PubMed] [Google Scholar]
- 41.Fang C, Hagar A, Gordon M, et al. Differences in hospital costs among octogenarians and nonagenarians following primary total joint arthroplasty. Geriatrics 2021;6:26. 10.3390/geriatrics6010026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang C, Pagani N, Gordon M, et al. Episode-of-Care costs for revision total joint arthroplasties by Decadal age groups. Geriatrics 2021;6:49. 10.3390/geriatrics6020049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ganske IM, Sanchez K, Le E, et al. Time-Driven, activity-based costing of presurgical infant Orthopedics: a critical component of establishing value of Latham appliance and Nasoalveolar Molding. Plast Reconstr Surg 2021;147:444–54. 10.1097/PRS.0000000000007669 [DOI] [PubMed] [Google Scholar]
- 44.McClintock TR, Friedlander DF, Feng AY, et al. Determining variable costs in the acute urolithiasis cycle of care through time-driven activity-based costing. Urology 2021;157:107–13. 10.1016/j.urology.2021.05.102 [DOI] [PubMed] [Google Scholar]
- 45.Kukreja JB, Seif MA, Mery MW, et al. Utilizing time-driven activity-based costing to determine open radical cystectomy and ileal conduit surgical episode cost drivers. Urol Oncol 2021;39:237.e1–237.e5. 10.1016/j.urolonc.2020.11.030 [DOI] [PubMed] [Google Scholar]
- 46.Ning MS, Venkatesan AM, Stafford RJ, et al. Developing an intraoperative 3T MRI-guided brachytherapy program within a diagnostic imaging suite: methods, process workflow, and value-based analysis. Brachytherapy 2020;19:427–37. 10.1016/j.brachy.2019.09.010 [DOI] [PubMed] [Google Scholar]
- 47.Hernandez A, Kaplan RS, Witkowski ML, et al. Navy medicine introduces value-based health care. Health Aff 2019;38:1393–400. 10.1377/hlthaff.2019.00280 [DOI] [PubMed] [Google Scholar]
- 48.Basto J, Chahal R, Riedel B. Time-Driven activity-based costing to model the utility of parallel induction redesign in high-turnover operating Lists. Healthc 2019;7:100355. 10.1016/j.hjdsi.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 49.McCreary DL, Dugarte AJ, Vang S, et al. Patient-Level value analysis: an innovative approach to optimize care delivery. J Orthop Trauma 2019;33 Suppl 7:S49–52. 10.1097/BOT.0000000000001624 [DOI] [PubMed] [Google Scholar]
- 50.Ahluwalia R, Vainieri E, Tam J, et al. Surgical diabetic foot debridement: improving training and practice utilizing the traffic light principle. Int J Low Extrem Wounds 2019;18:279–86. 10.1177/1534734619853657 [DOI] [PubMed] [Google Scholar]
- 51.Kaplan AL, Agarwal N, Setlur NP, et al. Measuring the cost of care in benign prostatic hyperplasia using time-driven activity-based costing (TDABC). Healthc 2015;3:43–8. 10.1016/j.hjdsi.2014.09.007 [DOI] [PubMed] [Google Scholar]
- 52.Mattar D, Di Filippo A, Invento A, et al. Economic implications of ACOSOG Z0011 trial application into clinical practice at the European Institute of oncology. Eur J Surg Oncol 2021;47:2499–505. 10.1016/j.ejso.2021.06.016 [DOI] [PubMed] [Google Scholar]
- 53.Caloway C, Yamasaki A, Callans KM, et al. Quantifying the benefits from a care coordination program for tracheostomy placement in neonates. Int J Pediatr Otorhinolaryngol 2020;134:110025. 10.1016/j.ijporl.2020.110025 [DOI] [PubMed] [Google Scholar]
- 54.Yu YR, Abbas PI, Smith CM, et al. Time-Driven activity-based costing: a dynamic value assessment model in pediatric appendicitis. J Pediatr Surg 2017;52:1045–9. 10.1016/j.jpedsurg.2017.03.032 [DOI] [PubMed] [Google Scholar]
- 55.Sethi RK, Pumpian RP, Drolet CE, et al. Utilizing lean methodology and time-driven activity-based costing together: an observational pilot study of hip replacement surgery utilizing a new method to study value-based health care. J Bone Joint Surg Am 2021. 10.2106/JBJS.21.00129. [Epub ahead of print: 14 Oct 2021]. [DOI] [PubMed] [Google Scholar]
- 56.Danilyants N, MacKoul P, van der Does L, et al. A value-based evaluation of minimally invasive hysterectomy approaches. Gynecol Surg 2019;16:5. 10.1186/s10397-019-1057-9 [DOI] [Google Scholar]
- 57.Danilyants N, MacKoul P, Baxi R, et al. Value-Based assessment of hysterectomy approaches. J Obstet Gynaecol Res 2019;45:389–98. 10.1111/jog.13853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fernando-Canavan L, Gust A, Hsueh A, et al. Measuring the economic impact of hospital-acquired complications on an acute health service. Aust Health Rev 2021;45:135–42. 10.1071/AH20126 [DOI] [PubMed] [Google Scholar]
- 59.Robinson JR, Carter NH, Gibson C, et al. Improving the value of care for appendectomy through an individual surgeon-specific approach. J Pediatr Surg 2018;53:1181–6. 10.1016/j.jpedsurg.2018.02.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chatfield SC, Volpicelli FM, Adler NM, et al. Bending the cost curve: time series analysis of a value transformation programme at an academic medical centre. BMJ Qual Saf 2019;28:449–58. 10.1136/bmjqs-2018-009068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Featherall J, Brigati DP, Arney AN, et al. Effects of a total knee arthroplasty care pathway on cost, quality, and patient experience: toward measuring the triple aim. J Arthroplasty 2019;34:2561–8. 10.1016/j.arth.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 62.Karns MR, Jones DL, Todd DC, et al. Patient- and Procedure-Specific variables driving total direct costs of outpatient anterior cruciate ligament reconstruction. Orthop J Sports Med 2018;6:232596711878854. 10.1177/2325967118788543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steinmann G, Delnoij D, van de Bovenkamp H, et al. Expert consensus on moving towards a value-based healthcare system in the Netherlands: a Delphi study. BMJ Open 2021;11:e043367. 10.1136/bmjopen-2020-043367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoozée S, Bruggeman W. Identifying operational improvements during the design process of a time-driven ABC system: the role of collective worker participation and leadership style. Manag Acc Res 2010;21:185–98. 10.1016/j.mar.2010.01.003 [DOI] [Google Scholar]
- 65.Bueno H, Bernal JL, Jiménez-Jiménez V, et al. The clinical outcomes, healthcare resource utilization, and related costs (coherent) model. application in heart failure patients. Rev Esp Cardiol 2022;75:585-594. 10.1016/j.rec.2021.08.009 [DOI] [PubMed] [Google Scholar]
- 66.Casey M, Perera D, Enticott J, et al. High utilisers of emergency departments: the profile and journey of patients with mental health issues. Int J Psychiatry Clin Pract 2021;25:316–24. 10.1080/13651501.2021.1904998 [DOI] [PubMed] [Google Scholar]
- 67.Cohen RV, Nishikawa AM, Ribeiro RA, et al. Surgical management of obesity in Brazil: proposal for a value-based healthcare model and preliminary results. Value Health Reg Issues 2021;26:10–14. 10.1016/j.vhri.2020.11.005 [DOI] [PubMed] [Google Scholar]
- 68.Negrini R, da Silva Ferreira RD, Guimarães DZ. Value-Based care in obstetrics: comparison between vaginal birth and caesarean section. BMC Pregnancy Childbirth 2021;21:333. 10.1186/s12884-021-03798-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khanijow AN, Wood LN, Xie R, et al. The impact of an enhanced recovery program (ERP) on the costs of colorectal surgery. Am J Surg 2021;222:186–92. 10.1016/j.amjsurg.2020.11.034 [DOI] [PubMed] [Google Scholar]
- 70.Burnhope E, Waring M, Guilder A, et al. A systematic approach towards implementing value-based health care in heart failure: understandings from retrospective analysis methods in South London. Health Serv Manage Res 2022;35:37-47. 10.1177/0951484820971442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lenfant L, Sawczyn G, Kim S, et al. Single-Institution cost comparison: single-port versus multiport robotic prostatectomy. Eur Urol Focus 2021;7:532-536. 10.1016/j.euf.2020.06.010 [DOI] [PubMed] [Google Scholar]
- 72.Parra E, Arenas MD, Alonso M, et al. Assessing value-based health care delivery for haemodialysis. J Eval Clin Pract 2017;23:477–85. 10.1111/jep.12483 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-066568supp001.pdf (330.7KB, pdf)
bmjopen-2022-066568supp002.xlsx (92.2KB, xlsx)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information. The data generated in this study is also available in a public, open access repository (Erasmus Data Repository, DOI: 10.25397/eur.20279883).