Abstract
No part of the human body is immune to tuberculosis, the most common site being the lungs. We report a rare case of primary nasopharyngeal tuberculosis without cervical lymphadenopathy nor pulmonary involvement. The only presenting symptom was an intermittent discomfort in the neck and throat. Several biopsies were performed to exclude nasopharyngeal carcinoma and to reach the final diagnosis of tuberculosis. The patient made full recovery following 6 months of treatment with antibiotics. A multidisciplinary approach by ear, nose and throat, radiology, pathology, and infectious disease colleagues was crucial in reaching the diagnosis and managing the patient.
Keywords: Ear, nose and throat/otolaryngology; TB and other respiratory infections; Pathology; Radiology; Tuberculosis
Background
Tuberculosis (TB) has a global presence.1 It is resurging in developed countries due to AIDS, wide administration of immunosuppressive agents and immigration.2 Patients may be asymptomatic and healthy, without underlying disease and no history of a positive contact.1 2
No part of the human body is immune to it, the most frequent site being the lungs.1 3 TB involving organs other than the lungs is termed ‘extrapulmonary tuberculosis’ (EPTB).4 Up to 10% of TB cases have some manifestations in the head and neck region.1 3 Its most common manifestation in the head and neck region is cervical lymphadenopathy, frequently involving the posterior triangle and the supraclavicular region.3–5 Other ear, nose and throat (ENT) areas that TB may affect are the pharynx, larynx, middle ear, oral and nasal cavity, and submandibular glands.3 4
Nasopharyngeal TB comprises less than 1% of TB cases found in the upper respiratory tract.1 3 6 Nasopharyngeal TB is a rare entity, even in endemic TB areas.4 5 7 TB can involve the nasopharynx primarily without affecting any other system or secondary to pulmonary or extrapulmonary involvement.1 Primary nasopharyngeal TB is very unusual and only a few cases have been reported in the literature.3 Nasopharyngeal TB has many presentations; subtle signs and symptoms may be missed.8 TB should be a differential diagnosis of nasopharyngeal lesions. A biopsy and histological study should be performed in every patient to avoid misdiagnosis.1 7 Repeat biopsies are sometimes necessary, as much for eliminating the diagnosis of a malignant tumour as for confirming TB.9
When treated properly, nasopharyngeal TB carries an excellent prognosis, and complete resolution of the disease is the rule.1
Case presentation
A man in his early 40s presented to the ENT outpatient department with a 2-month history of intermittent pain on the right side of his throat and neck. At the time of presenting to the ENT, he did not complain of hearing loss, odynophagia, dysphonia, cough, fever, night sweats nor weight loss.
He has been a smoker for many years and has not been on foreign travels for the last 4 years. His only medication is topical clobetasol propionate ointment for alopecia areata of the scalp.
His father died of TB 30 years ago. The patient himself has never been treated for TB.
On examination with a flexible nasendoscope, a postnasal mass was identified, more prominent on the right side, with no overlying discharge. Examination of both ears was normal, and no cervical lymph nodes were palpable. At the time of these findings, the most concerning differential diagnosis was nasopharyngeal carcinoma.
Investigations
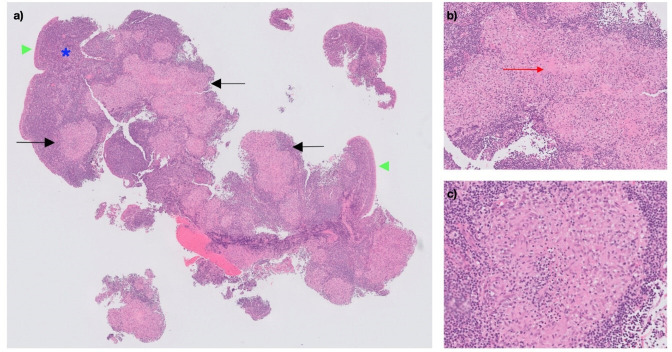
A biopsy under local anaesthetic was taken and sent for histopathology, which revealed no evidence of malignancy but did show granulomas without overt caseation (figure 1). Ziehl-Neelsen (ZN), periodic acid-Schiff (PAS) and Grocott (or Gomori) methenamine silver (GMS) stains were performed, but no mycobacteria or fungal organisms were identified.
Figure 1.
Microscopic images of postnasal biopsy specimen, H&E-stained sections. (A) Respiratory-type epithelium (green arrowheads) overlies a lymphoid stroma (blue asterisk) containing multiple well-formed granulomas (black arrows) (10× magnification). (B) A focus of central degeneration can be seen within a large granuloma (red arrow), with associated acute inflammation (20× magnification). (C) The granulomas, predominantly composed of epithelioid histiocytes, lack the amorphous pink centre of caseous necrosis that is typical of tuberculous granulomas (100× magnification).
Serum ACE and blood calcium were within normal range. Anti-proteinase 3 (anti-PR-3) and anti-myeloperoxidase (anti-MPO) blood tests were also normal. Other routine blood tests showed no abnormal findings of red blood cells, white cell count, C reactive protein, plasma viscosity, electrolytes, and liver and kidney function.
Following initial biopsy and blood tests, another set of biopsies from the postnasal mass was taken for microbiology and histopathology. Microscopy revealed similar well-formed granulomas but no genuine caseous necrosis on a background of normal lymphoid tissue. Again, no acid-fast bacilli or fungal organisms were identified on ZN, PAS and GMS stains.
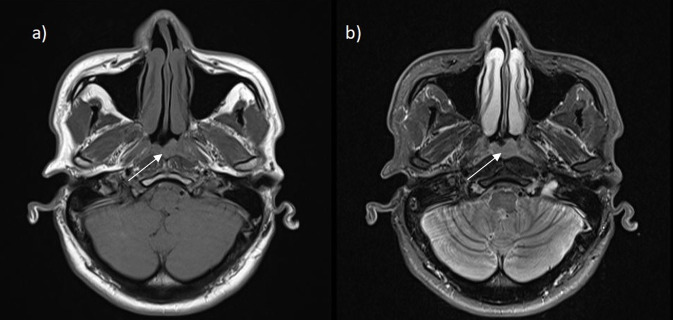
MRI of the sinuses and neck revealed a 12 mm mildly asymmetrical thickening of the posterior nasopharyngeal wall/adenoid area with no cervical lymphadenopathy (figure 2). CT of the chest was normal, with no pulmonary lesions nor mediastinal lymphadenopathy.
Figure 2.
Axial views of MRI head scan: (A) T1-weighted and (B) T2-weighted images. The white arrows point to the asymmetrical thickening of the posterior nasopharyngeal wall/adenoid area measuring up to 12 mm in anterior to posterior dimension.
After 7 weeks, microbiology cultures revealed Mycobacterium tuberculosis sensitive to isoniazid, ethambutol, rifampicin and pyrazinamide.
Differential diagnosis
The most concerning differential diagnosis was nasopharyngeal malignancy. This was excluded on histopathological examination of the biopsies.
As granulomas were found on histopathological examination, sarcoidosis, granulomatosis with polyangiitis and TB were among the differential diagnoses. Sarcoidosis was excluded with normal serum ACE, calcium blood tests and plasma viscosity, as well as normal CT chest imaging. Granulomatosis with polyangiitis was excluded with normal anti-PR-3 and anti-MPO, as well as normal plasma viscosity. TB was only confirmed after 7 weeks of microbiology culture growth, as initial stains had not demonstrated any acid-fast bacilli. As his father died of TB, this was the most likely diagnosis once malignancy was excluded.
Treatment
The patient was referred to infectious disease colleagues for TB treatment. During their workup, HIV, hepatitis B and hepatitis C tests were all negative. He was started on daily rifampicin 720 mg, isoniazid 300 mg, and pyrazinamide 1800 mg and pyridoxine hydrochloride 10 mg (prophylaxis to protect from isoniazid-induced neuropathy) for 2 months. Following this, he was started on daily rifampicin 600 mg, isoniazid 300 mg and pyridoxine hydrochloride 10 mg for 4 months.
Outcome and follow-up
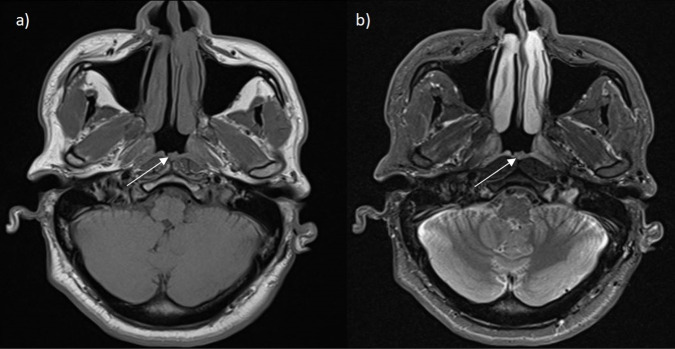
The total course of TB treatment lasted 6 months. During this period, routine liver tests were checked twice, which were normal. Two months into the treatment course, the patient was complaining of intermittent pain on the left side of his throat and neck region, as well as left-sided facial paraesthesia and tenderness over the left eye. As the symptoms were progressing, 1 month before the end of the treatment, an MRI of the brain and neck was performed and revealed no abnormal findings and a marked reduction of the previously noted soft tissue thickening in the nasopharynx (figure 3). As the M. tuberculosis culture was sensitive to the antibiotics used in the treatment, no further tests or treatments were necessary.
Figure 3.
Axial views of MRI head scan: (A) T1-weighted and (B) T2-weighted images. The white arrows highlight the reduced size of previously thickened posterior nasopharyngeal wall/adenoid area.
Discussion
Nasopharyngeal TB seems to be more frequent in women than in men. It occurs in adults, with two peaks of frequency: between 15 and 30 years of age and between 50 and 60 years of age.2 7 9
Several known risk factors for TB have been reported. The most common are immunosuppression, history of TB exposure and ethnic origin.3 5 In some case series, tobacco use and low socioeconomic status have also been reported as risk factors.2 3 9
Some parts of the world have been accepted as an epidemiological risk factor for TB, such as originating from Northwest Africa (Maghrebin) area.9 Therefore, a thorough history must include information on travel, contact and immune status to identify at-risk individuals and plan investigations and management.2 10
Two modes of nasopharyngeal contamination of TB have been described: (1) airway: either directly through nasal ventilation, or secondarily through canalised bacillary expectoration; and (2) haematogenous or lymphatic, from a primary site, most often pulmonary. Lymphatic nasopharyngeal contamination is explained by rich lymphatic network of the Waldeyer ring.6 7 9 This double mode of contamination explains how nasopharyngeal lesions may be primary or secondary to lesions most often of pulmonary origin. Tuberculous lymphadenopathy is always secondary to a pulmonary or nasopharyngeal localisation, but the inoculation site is sometimes too small to identify or already healed.9
TB of the upper respiratory tract and nasopharynx has been observed mainly in patients with active pulmonary TB.2
Nasopharyngeal TB is generally associated with cervical lymphadenopathy or with a pulmonary localisation.1 2 9 The clinical presentation can be the same as a nasopharyngeal tumour.9 The most common presentation of TB in the head and neck is cervical lymphadenopathy, which is present in ∼90% of cases of head and neck TB.4 10 During a workup for cervical tuberculous lymphadenopathy, nasopharyngeal TB may be discovered.2
Some elements of a patient’s medical history may offer clues to the diagnosis: contamination from a contact, absence of vaccination, declining general health, night sweats and associated pulmonary signs and symptoms. Rhinological symptomatology may include unilateral or bilateral nasal obstruction, rhinorrhoea, postnasal drip, nasal bleeding, and rare otological symptoms such as otalgia, hearing loss or unilateral otorrhoea.1 5 7 9 Nasopharyngeal TB is a difficult diagnosis to confirm on clinical suspicion alone.10
Endoscopic examination may reveal a polypoidal mass, ulceration, plaque or diffuse mucosal thickening of the nasopharynx.1 2 7 9 At times, the appearance can be suggestive of ordinary adenoids.9
ZN staining is commonly used to detect acid-fast bacilli on histopathology specimens. However, modified staining methods such as the Fite-Faraco method or alternative techniques such as immunohistochemistry, while perhaps not as widely used, have been shown to provide greater sensitivity in identifying mycobacteria.11 After 4–6 weeks of appropriate microbiological culture, the drug sensitivities of the infecting strain may become apparent.7
A rigid nasendoscopy with multiple biopsies is absolutely necessary for the diagnosis. It allows a pathological study in order to eliminate a malignant tumour, as well as a bacteriological examination.1 5 9 10 Nevertheless, a pathological study is sometimes difficult.9 The diagnosis of mycobacterial infections can be hampered by low organism load, making the organisms difficult to find using histochemical techniques. Typically, giant cell epithelioid granulomas with caseous necrosis are seen on histological examination.7 9 When granulomatous inflammation is confirmed by tissue biopsy, TB should also be one of the differential diagnoses.4 Repeat biopsies are sometimes necessary, both for eliminating a malignant tumour and for confirming TB.7 9
Although the definitive diagnosis is based on microbiological culture, treatment is often commenced based on histological examination. This is because smear and culture tests take up to 2 months to provide a result and are difficult to perform in EPTB due to the low number of bacilli in the specimen.3 5 In our patient’s case, treatment was started after microbiology culture revealing M. tuberculosis with sensitivities to antibiotics as the patient was stable and did not have severe symptoms and signs of TB. Modern laboratory methods such as PCR are proving ever more useful in aiding the diagnosis.3 Tuberculin skin tests, on the other hand, are not specific and often misleading.3 12
CT images usually show either a large or lobulated mass, or irregular soft tissue thickening of the nasopharynx. On MRI, nasopharyngeal TB is seen as either a polypoidal mass of the adenoids or diffuse thickening of the mucosal wall of the nasopharynx.7
It is important to investigate the thorax for evidence of old or new TB.7 10 A chest X-ray is generally obtained at the time of diagnosis to exclude pulmonary TB, although chest CT scan is twice as sensitive in the detection of pulmonary cavities.12 In our patient, pulmonary TB was excluded by CT chest, although the recommended first-line investigation is X-ray.
Patients with TB should be treated with multidisciplinary input, primarily led by infectious disease colleagues.
Involvement of the nasopharynx by TB may be underdiagnosed because it may not produce obvious symptoms or physical signs in all cases. Although rare, it is important to consider TB in the differential diagnosis of nasopharyngeal lesions and take biopsy specimens for histological and bacteriological studies.2 4
Learning points
Tuberculosis (TB) should be one of the differential diagnoses of nasopharyngeal lesions.
Repeat biopsies are sometimes necessary, as much for eliminating the diagnosis of a malignant tumour as for confirming TB.
A multidisciplinary team approach including ear, nose and throat, radiology, pathology, and infectious disease doctors should be followed during the diagnostic and treatment periods.
Footnotes
Contributors: RM wrote the draft, created figures 2 and 3, and revised the manuscript. LJ checked the draft, created figure 1 and revised the manuscript. RG participated in checking and amending the draft, and revised the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Patil C, Kharat Patil R, Deshmukh P, et al. Primary tuberculosis of nasopharynx (adenoid)- a rare presentation. Asian Pac J Trop Med 2013;6:246–8. 10.1016/S1995-7645(13)60033-4 [DOI] [PubMed] [Google Scholar]
- 2.Aktan B, Selimoglu E, Uçüncü H, et al. Primary nasopharyngeal tuberculosis in a patient with the complaint of snoring. J Laryngol Otol 2002;116:301–3. 10.1258/0022215021910609 [DOI] [PubMed] [Google Scholar]
- 3.Mocanu A-I, Mocanu H, Moldovan C, et al. Some manifestations of tuberculosis in otorhinolaryngology - case series and a short review of related data from South-Eastern Europe. Infect Drug Resist 2022;15:2753–62. 10.2147/IDR.S367885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma S, Rana AK. Ent manifestations of tuberculosis: an important aspect of ENT practice. Pan Afr Med J 2020;36:295. 10.11604/pamj.2020.36.295.24823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAllister KA, MacGregor FB. Diagnosis of tuberculosis in the head and neck. J Laryngol Otol 2011;125:603–7. 10.1017/S0022215110002732 [DOI] [PubMed] [Google Scholar]
- 6.Zheng S, Lim KH. An unusual case of a young woman with a postnasal space mass. Am J Trop Med Hyg 2018;99:3–4. 10.4269/ajtmh.17-1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawada N, Inokuchi G, Komatsu H, et al. Nasopharyngeal tuberculosis. J Infect Chemother 2013;19:1158–60. 10.1007/s10156-013-0574-0 [DOI] [PubMed] [Google Scholar]
- 8.Nakao Y, Shibata R, Murohara T, et al. Primary nasopharyngeal tuberculosis: a case report. BMC Infect Dis 2016;16:121. 10.1186/s12879-016-1449-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Percodani J, Braun F, Arrue P, et al. Nasopharyngeal tuberculosis. J Laryngol Otol 1999;113:928–31. 10.1017/S0022215100145621 [DOI] [PubMed] [Google Scholar]
- 10.Pankhania M, Elloy M, Conboy PJ. Nasopharyngeal tuberculosis presenting with auditory symptoms. BMJ Case Rep 2012;2012. 10.1136/bcr-01-2012-5475. [Epub ahead of print: 09 Oct 2012]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crothers JW, Laga AC, Solomon IH. Clinical performance of mycobacterial immunohistochemistry in anatomic pathology specimens. Am J Clin Pathol 2021;155:97–105. 10.1093/ajcp/aqaa119 [DOI] [PubMed] [Google Scholar]
- 12.Nachiappan AC, Rahbar K, Shi X, et al. Pulmonary tuberculosis: role of radiology in diagnosis and management. Radiographics 2017;37:52–72. 10.1148/rg.2017160032 [DOI] [PubMed] [Google Scholar]