Abstract

A solvent extraction-based technique has been utilized to study the separation of ruthenium from simulated alkaline solution using Aliquat 336 as the extractant and isodecyl alcohol (IDA) as the phase modifier in n-dodecane. The effects of various experimental parameters such as solution pH, mixing time, concentration of Aliquat 336 and IDA, role of citric acid as the aqueous phase modifier/complexing agent, and stripping agents have been evaluated. It was observed that with the increase in the solution pH, the extraction efficiency increases gradually. However, when citric acid was added into the aqueous solution, an overall increase (from ∼20 to 91%) in ruthenium extraction is observed. 20 min of the mixing time was found to be sufficient to reach the extraction equilibrium. Solution composition was optimized as 50% Aliquat 336 and 10% IDA in n-dodecane (v/v) for maximum extraction. The stripping of ruthenium from the loaded organic phase has been studied using HCl and HNO3. The result indicates that in the presence of 8 M HNO3, ∼73% of ruthenium can be back extracted to the aqueous phase in a single contact. The stripping efficiency of HNO3 was found to be higher than that of HCl. Active studies with 106Ru as the radiotracer were also performed and monitored using a HPGe detector. The same method was implemented for extraction studies with real waste solution in the presence of other radionuclides such as 137Cs, 90Sr, and 125Sb. The presence of the chemical species in aqueous as well as organic phase has been identified using UV–vis spectrophotometry, Fourier transform infrared spectroscopy, and Raman spectroscopy. Density functional theory-based quantum mechanical calculations have been performed in order to unravel the extraction mechanism with the present solvent system.
1. Introduction
Ruthenium (Ru) belongs to the platinum group element in the periodic table. During the nuclear fission of 235U in nuclear reactors, a large amount of fission products are formed among which ruthenium isotopes, 103Ru (t1/2: 39.25 d, fission yield: 3.12%), 105Ru (t1/2: 4.44 h, fission yield: 0.92%), 106Ru (t1/2: 368 d, fission yield: 0.4%), and 107Ru (t1/2: 3.8 m, fission yield: 0.17%), are well known.1−6 Therefore, these ruthenium isotopes altogether contribute a significant yield which is a waste management-related concern at the back end of nuclear technology due to their high specific activity, radiological toxicity, and moderate to high aqueous solubility. Among all, ruthenium-106 is a troublesome nuclide in the back end of the nuclear fuel cycle due to its relatively long half-life and moderate fission yield.7−9 Separation of ruthenium from either acidic high level liquid waste (HLLW) or alkaline intermediate level liquid waste (ILLW) is a long-term challenge to the separation scientists due to the existence of ruthenium in different oxidation states and variation in speciation in aqueous solution.10−12 In a nitric acid solution, Ru can exist with many oxidation states from +3 to +8 with the major formation of various nitroso nitrate complexes with the general formula [Ru(NO)(NO3)x(NO2)y(OH)z(H2O)5–x−y−z]3–x−y−z.1,13 The complexes also get co-extracted in the organic medium along with other metal ions during extraction of U and Pu in the PUREX process when 30% tributyl phosphate (TBP) in n-dodecane is used as the solvent.14,15 The extraction and stripping behaviors of ruthenium are not predictable and therefore complicate the PUREX process.16−18 This sometimes contaminates the final product streams of U and Pu.19,20 On the other hand, the fully oxidized ruthenium tetroxide is highly volatile in nature and escapes to the vapor phase which is subsequently deposited as a black RuO2 residue to the inner pipe, equipment used for the ventilations during HLLW evaporation and/or vitrification.16 Many numbers of reports are available on the extraction and recovery of ruthenium from the acidic medium. Similarly, separation of ruthenium from a chloride medium has been well established by several researchers due to the propensity of formation of the anionic chloro complex in a chloride/HCl medium.21,22 Various anion exchangers containing quaternary ammonium halide and trialkylamines as active functional groups were utilized to separate the anionic chloro complexes from the aqueous chloride medium.23,24 Separation of ruthenium from aqueous nitric acid is more difficult, which makes the ruthenium separation very challenging at the nuclear back end cycle where the overall medium is 2–3 M HNO3.25,26 Ruthenium tetroxide is found to be soluble in certain organic solvents such as CCl4.27 The removal of ruthenium in the form of ruthenium tetroxide from the PUREX process waste stream has been reported with paraffin oil. The final product has been collected as ruthenium dioxide after filtration through an ordinary filter paper.
The separation of ruthenium from an alkaline medium is equally important as it is present in substantial quantity (∼2 × 10–2–2 mCi/L depending on fuel cooling period) in intermediate level liquid waste.25−28 There are many sources of this waste which are as follows: (i) metallic decladding operation of spent nuclear fuel at the reprocessing site, (ii) evaporation and neutralization of process condensate during the evaporation of acidic HLLW to concentrate, and (iii) purification of degraded TBP solvent through carbonate washing. The ruthenium separation from this alkaline waste is more difficult than acidic HLLW because of alkalinity and high salt content.29−31 For this reason, very limited literature is available on the Ru separation from alkaline ILLW. The total dissolved solid (TDS) in this type of waste sometimes reaches up to 20–30 gm/L, especially when any metallic clad other than the zirconium alloy is processed. In an alkaline medium, ruthenium exists mainly in the form of RuO4– and RuO42– in the pH range of 9–13, especially in the oxidizing environment.31−35 Therefore, most of the reports of ruthenium separation from alkaline waste streams are based on the ion exchange process.36,37 Although a few reports are available on the recovery of ruthenium from an alkaline medium, no report is available on the recovery of ruthenium through liquid–liquid extraction using a liquid anion exchanger in the presence of any aqueous phase modifier. In a recent publication, a chromatographic separation/solid phase extraction of ruthenium from an alkaline medium using Aliquat 336 as the extractant loaded on Chromosorb W resin has been reported, where ruthenium is oxidized to RuO42– using sodium hypochlorite as the oxidizing agent.36 However, it is quite unlikely that Ru will be present with this type of oxidizing agent in an alkaline medium. Infact, there is always a chance of presence of various ruthenium nitrosyl nitrate complexes in most of the wastes related to the back end fuel cycle as mentioned already (vide supra). The formal oxidation state of Ru in these types of complexes is generally found to be +3. It is also reported that alkali addition to the ruthenium nitrosyl nitrate complexes systematically replaces the nitrite, nitrate, and water molecules from the Ru primary coordination sphere by hydroxide ions.38−40 However, there is no emphasis on the extraction of this type of species from the alkaline medium, which is more practical in the nuclear industry.
In the present report, a liquid–liquid extraction method has been utilized for the separation of ruthenium from simulated alkaline intermediate level waste using Aliquat 336 as the extractant at pH ∼13. The effect of various experimental parameters such as solution pH, equilibration time, extractant concentration, and complexing agent concentration on extraction of ruthenium has been discussed. The speciation of ruthenium complexes in aqueous as well as in organic phases has been studied using various spectroscopic techniques. UV–visible spectrophotometry has been utilized for the characterization of ruthenium species after systematic addition of citric acid in the alkaline medium. Similarly, Fourier transform infrared (FTIR) spectrometry has been used to characterize the complexation behavior of ruthenium with the organic phase. Furthermore, Raman spectroscopy is used for the determination of the nature of ruthenium complexes in aqueous as well as in organic medium under various chemical conditions. The stripping of ruthenium from the loaded organic phase has been studied using various concentrations of nitric acid and hydrochloric acid. With the optimized condition, the separation of ruthenium has been performed from actual intermediate level waste solution successfully.
2. Experimental Section
2.1. Reagents
Ruthenium(III) nitrosyl nitrate solution [Ru(NO)(NO3)x(OH)y, x + y = 3 in dilute nitric acid] has been purchased from Sigma-Aldrich. Aliquat 336 (91%, Acros organics), isodecyl alcohol (IDA) (98%, Prabhat Chemical, Mumbai), citric acid monohydrate (Loba Chemie Pvt. Ltd, Mumbai), and n-dodecane (99%, Alfa Aesar) have been used without further purification. Electronic grade HNO3 (69–71%, Research-Lab Fine Chem Industries, Mumbai) and NaOH (98%, Chenkem, Mumbai) are used for maintaining the pH of various stock solutions used in the experiments. All the dilutions were done with the help of ultrapure water (LaboStar PRO TWF water purifiers, Evoqua Water Technology, GmbH). 106Ru tracer was used for the active experiments.
The organic phase was prepared by mixing Aliquat 336 and IDA at different proportions (v/v %) in n-dodecane as the diluent. Required amount of citric acid was added into the aqueous feed solution before performing the solvent extraction experiments.
2.2. Ruthenium Stock Solution
The inactive stock solution of ruthenium (∼120 ppm) was prepared by diluting the requisite amount of ruthenium(III) nitrosyl nitrate solution in ultrapure water in a 50 mL glass beaker and pH of the solution was adjusted to ∼13 with the help of NaOH solutions. Concentration of ruthenium in feed and raffinate solution was measured using ICP–OES instruments calibrated with ruthenium standard solutions. For active experiments, 106Ru radiotracer was added and measured by radioactive γ-counting using a HPGe detector connected with a multichannel analyzer.
For experiments with alkaline ILLW, actual waste was received and used as the stock solution with the following composition (Table 1). As it can be seen that the 137Cs content in the waste is so high (mCi/L level) with respect to 106Ru (10–2 mCi/L), 137Cs was removed from the solution initially by passing the solution through a glass column filled with resorcinol–formaldehyde (RF) polycondensate resin preconditioned with 0.1 M NaOH solution. This is because in the presence of high 137Cs, 106Ru measurement was practically impossible due to the interference of large Compton continuum of 137Cs (photo peaks at 661 keV) with 106Ru γ-ray photo peaks (511 and 621 keV). Due to the RF column run, the 137Cs, 106Ru, and 125Sb activity was reduced to 0.12, 1.44 × 10–2, and 1.06 × 10–2 mCi/L, respectively, in which 106Ru estimation was possible with the addition of pure 106Ru tracer externally to the waste solution to maintain the final concentration of 106Ru at ∼0.25 mCi/L.
Table 1. Composition of Actual Alkaline ILLW.
| properties | values |
|---|---|
| pH | 13.1 |
| TDS | 19.3 |
| gross βγ (mCi/L) | 2.66 |
| gross α (mCi/L) | 1.23 × 10–5 |
| Cs-137(mCi/L) | 2.06 |
| Ru-106(mCi/L) | 2.3 × 10–2 |
| Sb-125(mCi/L) | 1.23 × 10–2 |
| Al (g/L) | 8.57 |
| carbonate (M) | 0.31 |
2.3. Instrumentation
All the UV–vis absorption experiments were carried out on a JASCO V-630 model spectrophotometer in duplicate. To a fixed concentration of ruthenium (2.00 mL of 0.984 × 10–4 M), citric acid solution (0.1 M) was added in increments of 0.01–0.05 mL, and the absorption profile was recorded over the wavelength range of 300–600 nm. Both metal and ligand solutions were prepared in 0.1 M NaOH solution. The absorption data were analyzed using HypSpec programming41 to estimate the species formed during the course of reaction and the respective stabilities. The program needs an initial input of probable species that could coexist under the present experimental condition with the approximate thermodynamic stabilities (log K values). Citric acid is a tribasic acid and thus can exist in different protonated (such as Cit-H, Cit-H2, and Cit-H3) forms and as completely deprotonated form (Cit), which could potentially bind with ruthenium to form a variety of Ru–CitHn (n = 0–3) complexes. As the experiments were carried out with citric acid in 0.1 M NaOH solution, where it predominantly exist in the fully deprotonated form (Cit), the possibility of existence of Ru–CitHn (n = 1–3) species are ruled out. Thus, the speciation model consisted of 1:1 and 1:2 Ru/Cit species and was found to obey the convergence.
The FTIR spectra have been recorded using Bruker FTIR model V-70. The spectra were recorded at room temperature from 4000 to 500 cm–1 under vacuum conditions using Diamond ATR. The Raman spectra of complexes formed in aqueous and organic phases have been recorded using WITec Oxford Raman spectrometer, model: alpha300 R equipped with a 532 nm YAG laser. Each measurement was carried out in triplicate for a period of 120 s in the range between 100 and 2500 cm–1.
3. Results and Discussion
3.1. Effect of Solution pH
Determination of optimum solution pH is a very important parameter which will ascertain the working pH range of the solution under study. The experiments were conducted to evaluate optimum pH of the aqueous solution for maximum recovery of ruthenium (135 ppm) from aqueous media containing 0.5 M citric acid as the complexing agent using 50% Aliquat 336 and 10% IDA in dodecane. The results have been compared without the addition of citric acid. Figure 1 represents the variation of % extraction of ruthenium with pH of the aqueous feed solution for 30 min of equilibration time. With the increase in pH of the feed solution from 3 to 13, there is a continuous increase in % extraction of ruthenium from ∼8 to ∼20% in the absence of citric acid. A similar trend can be observed for the solution mixed with citric acid although with a much faster rate of increment and reaches the maximum extraction of ∼91% at pH 13. This clearly signifies that citric acid has a positive role in ruthenium extraction from the alkaline medium. For the subsequent stages of study, the pH of the aqueous feed solution was kept constant at 13. As per the literature report, [Ru(NO)(OH)5]2– is the predominant complex of ruthenium in an alkaline medium in the pH range of 9–13 when acidic Ru(NO)(NO3)x(OH)y, x + y = 3 is made alkaline.38−40 In the presence of citric acid, there is a chance of formation of various ruthenium citrate complexes [Ru–citrate]n− with varying anionic charges [vide infra, UV–vis spectroscopy and density functional theory (DFT) study].
Figure 1.
Effect of solution pH on the extraction of ruthenium from an alkaline medium in the presence and absence of citric acid; feed: [Ru] = 135 ppm, organic phase composition = 50% Aliquat 336 + 10% IDA + 40% n-dodecane, contact time = 30 min, O/A = 1:1 (v/v).
[Ru(NO)(OH)5]2– and ruthenium citrates of [Ru–citrate]n− type are anionic complexes which may get extracted by anion exchanger Aliquat 336 according to the following equation
| 1 |
| 2 |
The organic affinity of the ruthenium citrates are found to be more with respect to [Ru(NO)(OH)5]2– may be due to the higher anionic charges of ruthenium citrates [Ru–citrate]n− than dianionic [Ru(NO)(OH)5]2–, higher lipophilicity of citrates toward organic solvent, and lower charge density (lesser hydration energy) of the citrates. For this reason, an overall higher extraction of ruthenium is observed when citric acid is present in the aqueous solution (as complexing agent) prior to the extraction process.
3.2. Extraction Kinetics
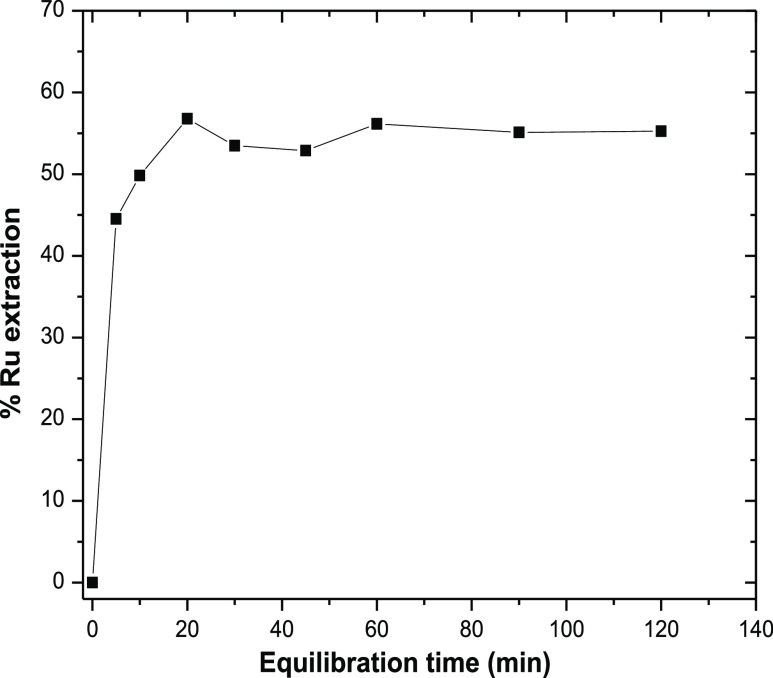
The reaction kinetics of any extraction process takes a vital role and not only determines the optimum reaction time but also provides an idea about practical applicability of the process. The effect of equilibrium time for liquid–liquid extraction of ruthenium from alkaline solution at pH = 13 containing 135 μg/mL of Ru was studied over a period of time in the presence of ∼0.5 M citric acid using 10% Aliquat 336 and 10% IDA in dodecane. The reaction was continued up to 2 h and the samples were collected at different time intervals after a settling period of 5 min for Ru estimation. Figure 2 shows the experimental results of % extraction of ruthenium with equilibration time. With the increase in two-phase extraction time, there is an increase in the extraction of Ru which reaches a maximum at 20 min. Further increase in extraction time does not alter the % extraction of Ru much with only few marginal variations. This may be due to the statistical uncertainty in analyses or error in sample aliquoting which altogether contributes 5–10% variation in results and admissible similar to any standard radiometric analysis. The result clearly indicates that the optimized reaction time can be considered as suitable from a practical application point of view for any solvent extraction method. Therefore, 30 min was set as the equilibrium/mixing time for all subsequent stages of experiments for Ru extraction from the alkaline medium using Aliquat 336.
Figure 2.
Kinetics of ruthenium extraction from an alkaline medium; feed: [Ru] = 135 ppm, pH = 13, [citric acid] = 0.48 M, organic phase composition = 10% Aliquat 336 + 10% IDA + 80% n-dodecane, O/A = 1:1 (v/v).
3.3. Effect of Citric Acid Concentration
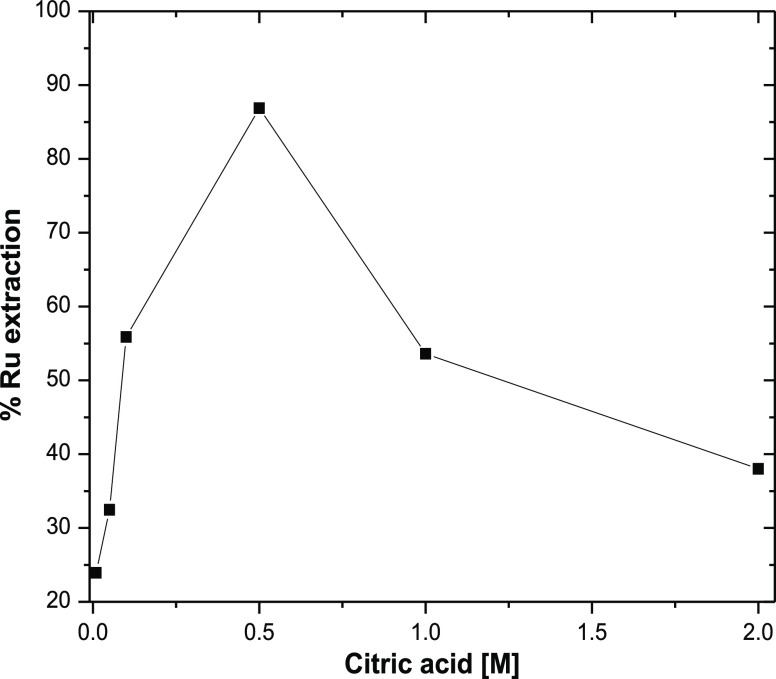
Sometimes the addition of an aqueous phase modifier or complexing agent alters the metal speciation within the feed solution which in other way can change the properties and extent of metal ion extraction. The effect of citric acid in the aqueous phase on the extraction of ruthenium (139 ppm) from alkaline medium (pH = 13) has been studied using 50% Aliquat 336 and 10% IDA in dodecane as the organic solvent. Figure 3 shows the results of % extraction of ruthenium with the variation of citric acid concentration from 0.01 to 2 M at room temperature with 25 min of contact time. With the increase in citric acid concentration from 0.01 to 2 M there is an increase in ruthenium extraction initially which reaches a maximum at 0.5 M, beyond which there is a continuous reduction in ruthenium extraction up to 2 M citric acid concentration. This result suggests that the citric acid in the alkaline medium gets dissociated to form citrate anions which form stable anionic complexes with ruthenium ion of higher charges (vide infra). At pH = 13, all the carboxylic acid groups of citric acid molecules are ionized (pKa1 = 5.2, pKa2 = 9.3, and pKa3 = 12.1) and act as a tridentate chelating ligand.42 The citrate anion at pH = 13 can replace the nitroso and nitrate groups from the primary coordination of Ru3+ and form [Ru(NO)(citrate)x(OH)y]n− type of complexes (vide infra) of varying stoichiometry (1:1, 1:2, etc.). The reduction in extraction above 0.5 M citrate ion concentration is may be due to the competition between the anionic metal complexes with anionic citrate which comes into play above this concentration. Therefore, further experiments were carried out with 0.5 M citric acid concentration to optimize the other extraction parameters.
Figure 3.
Effect of citric acid concentration on the extraction of ruthenium from an alkaline medium; feed: [Ru] = 139 ppm, pH = 13; organic phase composition = 50% Aliquat 336 + 10% IDA + 40% dodecane, O/A = 1:1 (v/v).
3.4. Effect of Aliquat 336 and IDA Concentration
In the present study, Aliquat 336 and IDA are used as the ruthenium extractant and organic phase modifier, respectively. The organic solubility (n-dodecane as the diluent) of Aliquat 336 is very poor; therefore, some organic soluble additives should be added which have similar properties (like polarity) as the extractant used. This will increase the solubility of the extractant in the diluent as well as reduce the chances of third phase formation after metal extraction. In order to determine the effect of extractant as well as phase modifier (IDA) concentration in the solvent, we have systematically varied their concentrations and carried out the liquid–liquid extraction experiments. Figure 4 shows the % variation of ruthenium extraction upon the concentration of Aliquat 336 (with 10% IDA) and IDA (with 50% Aliquat). From the experiments, it can be noticed that there is a continuous increase in ruthenium extraction when Aliquat 336 concentration in the solvent was increased and reaches a maximum at around 50% (v/v) which became constant afterward. This observation is quite normal in the case of solvent extraction studies where at the lower extractant concentration there is a presence of free metal ions into the solution, which get reduced as the extractant concentration increases and after a certain concentration no free metal ions exist in the solution.
Figure 4.
Effect of Aliquat 336 and IDA concentration on ruthenium extraction from an alkaline medium; feed: [Ru] = 135 ppm, pH = 13, [critic acid] = 0.48 M, organic phase composition = 10–60% Aliquat 336 + 10–50% IDA + rest n-dodecane, O/A = 1:1 (v/v).
Similarly, IDA concentration is also increased systematically and conducted extraction experiments. The result indicates that with the increase in IDA concentration, there is a continuous decrease in ruthenium extraction in the organic phase. This may due to the higher aqueous solubility of IDA (polar molecule and may alter the metal speciation) at higher concentrations or difficulty in phase separation. Therefore, 50% Aliquat 336 and 10% IDA concentration was considered as the optimum concentration of the organic phase in the further studies.
3.5. Back Extraction
Back extraction is a very important property for any liquid–liquid extraction process because the complete recovery of the valuable species is possible after a successful back extraction in the aqueous phase. The effectiveness of a back extractant/stripping agent depends upon the nature of the species that gets extracted into the organic phase as well as the nature of the organic phase involved. The stripping of ruthenium from a loaded organic phase (50% Aliquat 336 and 10% IDA in dodecane) was studied to achieve the maximum recovery of Ru from the organic phase to aqueous phase with minimum quantity of stripping agent added to minimize secondary waste volume. Figure 5 represents the % back extraction (S) of ruthenium when two chemical agents viz. HCl and HNO3 are used at two different concentrations. These are very common reagents and most suitable chemicals for further processing point of view with minimum interference in the process. The result indicates that between HCl and HNO3, nitric acid acts as a more powerful reagent to back extract ruthenium from organic phase to aqueous phase. The more effectiveness of nitric acid in the back extraction of ruthenium may be due to its more oxidizing power than hydrochloric acid. Moreover, with the increase in acid concentration for both the acids from 4 to 8 M, there is an increase in back extraction of ruthenium observed from 17 to 23% for HCl and 49 to 73% for HNO3. With 8 M nitric acid, ∼75% of Ru gets back extracted into the aqueous phase with a single contact.
Figure 5.
Back extraction of ruthenium from a loaded organic phase; extraction: [Ru] = 157 ppm, pH = 13, organic phase composition = 50% Aliquat 336 + 10% IDA + 40% dodecane, [citric acid] = 0.5 M, stripping contact time = 30 min.
3.6. Experiments with Active Tracer (106Ru)
Active experiments were carried out to verify the performance of Aliquat 336-based organic solvents toward separation of ruthenium in the presence of active tracer 106Ru. 106Ru was added into 130 ppm inactive ruthenium solution in the form of ruthenium nitrosyl nitrate. 106Ru was determined in the solution with the help of a HPGe detector fitted with a 4K multichannel analyzer. γ-energies of 511.7 and 621.6 keV were selected as two major γ energies of 106Rh (always in radioactive equilibrium because of very short half-life of 30.1 s), the daughter product of 106Ru. The γ abundance values of these two γ rays are 20.2 and 9.93, respectively.
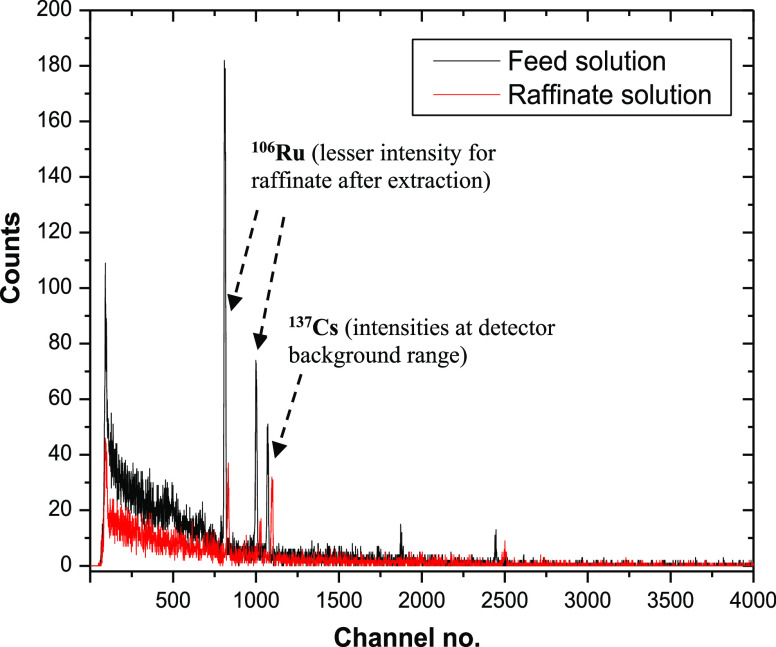
In an experiment in the presence of 106Ru radiotracer in the feed solution, the extraction by the Aliquat 336-based solvent showed that 106Ru was extracted significantly into the organic phase with the optimized conditions. It can be seen from Figure 6, which exhibits the γ-spectrum of the feed and raffinate solution together. The photo peaks of 106Ru/106Rh at 511.71 and 621.64 keV were reduced to ∼20% of the initial intensity, while the 661.6 keV peak of 137Cs remains almost unaltered which is in the detector background range. This clearly signifies the extraction of 106Ru from the solution to the organic phase. Therefore, active experiments are also in line with the inactive experiments and validate the efficacy of the Aliquat 336-based solvent toward ruthenium extraction.
Figure 6.
γ-spectra of feed and raffinate solution in the active study; feed: [Ru] = 135 ppm, 106Ru added as a radiotracer, pH = 13, [critic acid] = 0.48 M, organic phase composition = 50% Aliquat 336 + 10% IDA + 40% n-dodecane, O/A = 1:1 (v/v).
3.7. Ru Separation from Actual Waste Solution
The actual waste solution contains 137Cs, 90Sr, 106Ru, and 125Sb as the major radionuclides as indicated in Table 1. The initial activity of 137Cs was 2.2 mCi/L which is much higher than 106Ru present (order of 10–2 mCi/L) in the waste. 106Ru estimation by HPGe in the presence of that much high concentration of 137Cs was not possible due to the small γ peaks of 106Rh (equilibrium with 106Ru) at 511 and 621 keV was suppressed by the high Compton continuum due to 137Cs. Therefore, 137Cs was removed from the solution initially by passing it through a glass column filled with RF polycondensate resin preconditioned with 0.1 M NaOH solution. After passing the solution through the column, the 137Cs content was reduced to 0.12 mCi/L. Then, after adding pure 106Ru tracer, the solution was subjected to solvent extraction using 10–50% Aliquat 336 and 10% IDA in dodecane. The result (in Figure 7) indicates that with the increase in Aliquat 336 concentration, the % Ru extraction is continuously increased and reaches a maximum at 50% Aliquat concentration. It is also very interesting to note that the coextraction of 137Cs and 125Sb was always below 5% throughout the experiments, which indicates the selective separation of 106Ru from this complex nature of waste solution. This result establishes the practical utility of the present liquid–liquid extraction method of Ru from alkaline solution.
Figure 7.
Effect of Ru separation from actual alkaline waste; feed: [106Ru] = 0.25 mCi/L, pH = 13, [critic acid] = 0.5 M, organic phase composition = 10–50% Aliquat 336 + 10% IDA + rest n-dodecane, contact time = 30 min, O/A = 1:1 (v/v).
3.8. FTIR and Raman Analysis
FT-IR spectroscopy is a valuable and common spectroscopic technique to identify various functional groups in organic compounds. The FTIR spectra of pure Aliquat 336 (spectra a), 50% Aliquat 336 diluted in 10% isodecanol and n-dodecane (spectra b), and organic phase after ruthenium extraction (spectra c) have been recorded for comparison (Figure 8). The main vibrational stretching frequencies for different functional groups have been tabulated in Table 2.
Figure 8.
FTIR spectra of pure Aliquat 336 (a), 50% Aliquat 336 diluted with IDA and n-dodecane (b), and organic phase after the extraction of ruthenium (c).
Table 2. FTIR Vibrational Stretching Frequencies of Aliquat 336, Aliquat 336 Diluted with Isodecanol and n-Dodecane, and Organic Phase after the Extraction of Ruthenium.
| vibration frequency, cm–1 | vibration mode |
|---|---|
| 3380 | O–H stretching frequency of water molecule |
| 2922 | CH3anti-symmetric stretching vibration band |
| 2853 | CH3anti-symmetric stretching vibration band |
| 1722 | asymmetric stretching of COO– in citric acid |
| 1636 | symmetric stretching of COO– in citric acid |
| 1627 | O–H bending vibration |
| 1465 | CH3 bending mode |
The broad vibrational band at 3380 cm–1 may be assigned to the O–H stretching of water molecules present in all the FTIR spectra (spectra a, b, and c). This indicates that water molecules are present in Aliquat 336 and IDA as impurities. It can also be observed that, the intensity of the band is higher for c than both a and b. This signifies that, after contacting the Ru-containing aqueous solution with the organic phase it absorbs moisture. The intense and sharp peaks at 2922 and 2853 cm–1 are due to the CH3 anti-symmetric stretching vibration. The peak at 1627 cm–1 is responsible for the O–H symmetric bending (scissoring) mode of vibration. In the case of spectrum of c, the additional peaks at 1722 and 1636 cm–1 can be assigned to the asymmetric and symmetric stretching of the C=O (COO–) bond in citrate molecule.43 The asymmetric stretching is due to the carboxylic group of the citrate bonded to the Ru metal ion. This observation supports the bonding of Ru with citrate molecules in the complexes which get extracted upon contact with the organic phase through ion-exchange. On the other hand, the fully ionized free carboxylate group of citric acid is responsible for symmetric stretching, which is quite possible above pH 8 of the solution. The peaks at 1465 and 1378 cm–1 can be assigned to the CH3 bending modes. All the peaks below 1378 and up to 500 cm–1 are due to the wagging and rocking modes of CH2 groups, symmetric and anti-symmetric deformation, and rocking vibrational mode of CH3 present in Aliquat 336, n-dodecane, and isodecanol molecules. This is very important to notice that there is no difference or shift in peak positions of the organic phase before and after the extraction of ruthenium. It clearly inferred that the ruthenium species are not covalently bonded with Aliquat 336 or isodecanol during ruthenium extraction and therefore a pure ion-exchange mechanism (anion exchange) is responsible for such extraction.
Figure 9 represents the Raman spectra of the aqueous feed, pure, and Ru-loaded solvents, and raffinate solution (a–e). The absorbance peak at ∼3430 cm–1 is due to the symmetrical and asymmetrical stretching vibration of water molecules present in the solvent (as impurities) as well as in the aqueous medium. As expected, this peak is more intense in the case of the feed and raffinate and Ru-loaded organic solvent contacted with aqueous feed. The strong bands between 2800 and 3100 cm–1 can be assigned as combined bands for the −CH2 symmetric stretching, −CH3 symmetric stretching, and −CH3 antisymmetric stretching vibration of long chain alkyl groups. Unambiguously, these peaks are more eminent in the case of the organic phase which is made up with long chain alkyl groups than the aqueous phase. The overtone peaks of CH2 and CH3 scissors can be found at 2723 cm–1 in the Raman spectra. The stretching frequency of C=O observed at 1720 cm–1 is due to the presence of citric acid in the aqueous medium of the feed and raffinate solution. The Raman active peaks at 1447, 1302, 1120, and 1075 cm–1 are due to the CH2 and CH3 bending, CH2 twisting, and symmetric C–C stretching mode, respectively, which are very common for linear alkanes. The broad bands between 800 and 900 cm–1 can be designated to the several bands due to C1–C2 stretching, CH3 rocking, and C–O stretching. No significant peak due to any Ru complex was observed either in the Ru feed and raffinate solution or the Ru-extracted organic phase. Therefore, we cannot anticipate any Ru complex at least from Raman spectra except ruling out some common Ru-specific peaks. The absence of peaks at 810–825 cm–1 (RuO sym stretch ν1), 848 cm–1 (ν3), and 770–780 cm–1 (RuO asym stretch) can eliminate the presence of RuO42– in aqueous solutions at pH = 13 in the presence of citrate.44,45 Similarly, the presence of RuO4 also can be excluded in the Ru bearing solution from the absence of peaks at 883 cm–1 (ν1) and 918 cm–1 (ν3).46 The absence of peaks at 1933–1893 cm–1 (nitrosyl stretch), 1530 cm–1 (nitrate asym stretch), 1420 cm–1 (nitrite asym stretch), 1336, 1318 cm–1 (nitrite sym stretch), 1280 cm–1 (nitrate sym stretch) is a direct indicative of exclusion of ruthenium(III) nitrosyl nitrate complexes in aqueous solution,25 which is possible in the highly alkaline medium and presence of citrate (vide infra).
Figure 9.
Raman spectra of ruthenium feed (a), Aliquat 336 pure (b), solvent (50% Aliquat 336 + 10% IDA + 40% dodecane) (c), solvent after Ru extraction (d), and Ru raffinate solution (e).
3.9. Speciation Studies
Speciation studies helps in understanding the strength and kind of interaction by a metal ion with a specified chelating agent. Aqueous speciation of ruthenium in the presence of citric acid was probed by absorption spectrophotometry. Nitrosyl–nitrate-bonded ruthenium species of the form [RuNO(NO3)x(OH)y(H2O)z] (where x + y + z = 5) are formed on the dissolution of spent nuclear fuel in nitric acid.47 The addition of NaOH (increase in pH) alters the ruthenium coordination sphere by replacing the nitrato and aqua moieties by hydroxide ions and eventually results in the formation of [Ru(NO)(OH)5]2– species.39−41 Literature reports showed that the broad absorption peak centered at 350 nm is an indicator for the existence of [Ru(NO)(OH)5]2– species. The present titration, where citrate is added to Ru at 0.1 M NaOH is carried out to understand the interaction of citrate with [Ru(NO)(OH)5]2– species. Citrate is a tribasic acid with three pKa values centered successively at 5.2, 9.3, and 12.1, respectively.42 The present studies employed at 0.1 M NaOH (pH ∼ 13) allow the citric acid to get completely dissociated to be available as the triply negative charged anion form. Thus, the complexation is dominated by the replacement of OH or NO ions in [Ru(NO)(OH)5]2– by citrate ions. The absorption spectrum in the 300–600 nm range was recorded for each incremental addition of 10–50 μL of citrate into a fixed concentration of Ru at 0.1 M NaOH medium (Figure 10). Hypspec programming is used to analyze the absorption data. The analysis of absorbance data showed the formation of 1:1 and 1:2 species by citrate with Ru, the latter being more thermodynamically stable (log β = 3.79 ± 0.07) than the former (log β = 1.21 ± 0.04). Thus, the addition of citrate results in the formation of 1:2 species preferably.
Figure 10.
Variation in the absorption profile of ruthenium on incremental additions of citric acid; concentrations: [Ru] = 9.894 × 10–4 M and [citrate] = 0.1 M; initial volume of Ru is 1.5 mL.
3.10. DFT Analyses
DFT-based calculations were carried out using Turbomole programming48 to understand the speciation in detail at a molecular level. An hybrid functional B3LYP at the TZVP level of theory49,50 was used to reveal the most stable geometries by calculating the binding energies for the formation of [Ru(NO)(OH)5]2–, citrate, [Ru(NO)(citrate)(OH)2]2–, and [Ru(citrate)2]3– (Figure 11). The formation of [Ru(NO)(citrate)(OH)2]2– and [Ru(citrate)2]3– from spectrophotometric titration guided us to optimize their structure and calculate for binding energies. The optimized geometries showed that citrate acts as a tridentate chelator. The two oxygens from carboxylate and an oxygen from the hydroxyl group are involved in bonding with ruthenium, resulting in [Ru(NO)(citrate)(OH)2]2– and [Ru(citrate)2]3– species, while the oxygen from the third carboxylate of citric acid at terminal carbon remains uncoordinated. Initially, the absolute thermodynamic functions (enthalpy as H, and entropy as S) for formation of all species (NO, OH, H2O, [Ru(NO)(OH)5]2–, citrate, [Ru(NO)(citrate)(OH)2]2–, and [Ru(citrate)2]3–) were calculated using Turbomole. From which the free energy of formation (G) for each species is calculated as G = H – TS. From this, the changes in Gibbs free energy of formation for [Ru(NO)(citrate)(OH)2]2– and [Ru(citrate)2]3– are calculated by the below relations
 |
3 |
 |
4 |
Figure 11.
Optimized geometries for most stable formations of citrate, [Ru(NO)(OH)5]2–, [Ru(NO)(citrate)(OH)2]2–, and [Ru(citrate)2]3– species; color code of atoms: Ru—cyan; N—violet; O—red; C—gray; and H—white.
The binding and Gibb’s free energy of formation (Table 3) also indicate higher strength of 1:2 complexes than 1:1 complexes, corroborating the experimental observation. The shorter Ru–O bond distances (Table 3) indicate a stronger interaction, and these values are relatively shorter for 1:2 complexes than 1:1 complexes. Thus, the DFT-estimated binding energies and bond distances further support the preferential 1:2 complex formation in the Ru–citrate system at 0.1 M NaOH.
Table 3. Theoretically Estimated Binding and Gibb’s Free Energy of Formation for Ru–Citrate Species from [Ru(NO)(OH)5]2– and the Bond Distance (in Å) between Ru and the Atoms Directly Coordinated to It.
| species | BE/(kJ/mol) | ΔG/(kJ/mol) | Ru–N | Ru–O(OH) | N–O | Ru–O(citrate) |
|---|---|---|---|---|---|---|
| [Ru(NO)(OH)5]2– | 1.73 | 2.05, 2.02, 2.11, 2.05, 2.12 | 1.19 | |||
| [Ru(NO)(citrate)(OH)2]2– | –359.91 | –459.99 | 1.72 | 2.05, 1.98 | 1.17 | 2.10, 2.08, 2.14 |
| [Ru(citrate)2]3– | –387.45 | –517.22 | 2.09, 2.10, 2.04, 2.04, 2.10, 2.09 |
4. Conclusions
Extraction of ruthenium from an alkaline medium has been studied through a liquid–liquid extraction method using Aliquat 336-based anion exchanger, IDA as an organic phase modifier, and n-dodecane as a diluent. 50% Aliquat 336 and 10% IDA in dodecane were found to be as an optimized solvent system. The maximum Ru extraction was possible with pH = 13 as aqueous phase pH and 20 min of equilibration time in the presence of 0.5 M citric acid which acted as an aqueous complexing agent or phase modifier. HNO3 acts as an effective stripping agent which can back extract ∼75% loaded ruthenium in the aqueous phase in a single contact. Speciation studies using UV–vis, FTIR, and Raman spectroscopy indicate the formation of [Ru(NO)(citrate)(OH)2]2– and [Ru(citrate)2]3– in the aqueous solution when citric acid is added into the feed solution. These complexes are found to be responsible for the anion exchange mechanism with the anionic liquid exchanger Aliquat 336. DFT-based quantum mechanical calculation inferred the preference of [Ru(citrate)2]3– complexes in the feed solution than [Ru(NO)(citrate)(OH)2]2–. The Gibb’s free energy change for the formation of both the complexes is negative and exhibits their spontaneous formation. The above solvent system selectively separates ruthenium from an actual alkaline ILLW in the presence of 137Cs, 90Sr, and 125Sb.
Acknowledgments
Sincere thanks are due to Computer Centre, BARC for providing ANUPAM parallel computational facility. D.D. would like to acknowledge Dr. Amar Kumar, Shri Anand Gangadharan, and Dr. C.P. Kaushik, WMD, BARC for their continuous support and encouragement for the work. D.D. is thankful to Dr. Sk. Musharaf Ali, ChED, BARC and his laboratory members/staff for providing analytical help at different stages of the studies.
The authors declare no competing financial interest.
References
- Lefebvre C.; Dumas T.; Tamain C.; Ducres T.; Solari P. L.; Charbonnel M. C. Addressing Ruthenium speciation in tri-n-butyl-phosphate solvent extraction process by Fourier transform infrared, extended X-ray absorption fine structure, and single crystal X-ray diffraction. Ind. Eng. Chem. Res. 2017, 56, 11292–11301. 10.1021/acs.iecr.7b02973. [DOI] [Google Scholar]
- Croff A.; Alexander C.. Decay Characteristics of Once-through LWR and LMFBR Spent Fuels, High-level Wastes and Fuel-assembly Structural Material Wastes; ORNL: TN (USA), 1980; pp 1–212. [Google Scholar]
- Swain P.; Annapoorani S.; Srinivasan R.; Mallika C.; Mudali U. K.; Natarajan R. Separation of ruthenium from simulated nuclear waste in nitric acid medium using n-paraffin hydrocarbon. Sep. Sci. Technol. 2014, 49, 112–120. 10.1080/01496395.2013.815629. [DOI] [Google Scholar]
- Bush R. P. Recovery of platinum group metals from high level radioactive waste. Platin. Met. Rev. 1991, 35, 202–208. [Google Scholar]
- Patel N.Speciation and Separation of Fission Product Rhodium; Loughborough University, 1985; pp 1–256. [Google Scholar]
- Smith F.; Mc Duffie H. M. Recovery of nonradioactive palladium and rhodium from radioactive waste. Sep. Sci. Technol. 1981, 16, 1071–1079. 10.1080/01496398108057600. [DOI] [Google Scholar]
- Wiley W.Pacific Northwest Laboratory Annual Report for 1978 to the DOE Assistant Secretary for Environment; Part 1; Biomedical Sciences, Battelle Pacific Northwest Labs.: Richland, WA (USA), 1979; pp 1–135.
- Mun C.; Cantrel L.; Madic C. Review of literature on ruthenium behavior in nuclear power plant severe accidents. Nucl. Technol. 2006, 156, 332–346. 10.13182/nt156-332. [DOI] [Google Scholar]
- Frazier M.; Andrews T.; Thompson B.; Wincek M.. Evaluation of Toxic Effects of Heavy Metals and Chelating Agents in VERO Cells; PNNL, Annual Report, 1977. [Google Scholar]
- Cotton F. A.; Wilkinson G.; Murillo C. A.; Bochmann M.; Grimes R.. Advanced Inorganic Chemistry; Wiley: New York, 1988; pp 1–841. [Google Scholar]
- Seddon E. A.; Seddon K. R.. The Chemistry of Ruthenium; Elsevier, 2013; pp 1–1373. [Google Scholar]
- Seddon K. R. 3. Ruthenium. Coord. Chem. Rev. 1985, 67, 171–242. 10.1016/0010-8545(85)85014-1. [DOI] [Google Scholar]
- Boswell G. G. J.; Soentono S. Ruthenium nitrosyl complexes in nitric acid solutions. J. Inorg. Nucl. Chem. 1981, 43, 1625–1632. 10.1016/0022-1902(81)80350-8. [DOI] [Google Scholar]
- Fletcher J.; Brown P.; Gardner E.; Hardy C.; Wain A.; Woodhead J. Nitrosylruthenium nitrato complexes in aqueous nitric acid. J. Inorg. Nucl. Chem. 1959, 12, 154–173. 10.1016/0022-1902(59)80106-8. [DOI] [Google Scholar]
- Pruett D. The Solvent Extraction Behavior of Ruthenium I - The Nitric Acid-Tri-n-Butyl Phosphate System. Radiochimi. Acta 1980, 27, 115–120. 10.1524/ract.1980.27.2.115. [DOI] [Google Scholar]
- Taylor R.Reprocessing and Recycling of Spent Nuclear Fuel; W Publishing, Ed.; Elsevier, 2015; Vol. 79, pp 1–641. [Google Scholar]
- Abonneau E.; Baron P.; Berthon C.; Berthon L.; Béziat A.; Bisel I.; Bonin L.; Bossé E.; Boullis B.; Broudic J. C.; et al. Treatment and Recycling of Spent Nuclear Fuel; CEA: Paris, 2008; pp 1–176. [Google Scholar]
- Schulz W. W.; Navratil J. D.; Science and Technology of Tributyl Phosphate. Vol. I: Synthesis, Properties, Reactions and Analysis; CRC Press, 1984; pp 1–352. [Google Scholar]
- Swain P.; Mallika C.; Srinivasan R.; Mudali K. U.; Natarajan R. Separation and recovery of ruthenium: A review. J. Radioanal. Nucl. Chem. 2013, 298, 781–796. 10.1007/s10967-013-2536-5. [DOI] [Google Scholar]
- Mun C.; Cantrel L.; Madic C. A literature review on Ruthenium behaviour in nuclear power plant severe accidents. Nucl. Technol. 2006, 156, 1–39. 10.13182/NT156-332. [DOI] [Google Scholar]
- Grehl M.; Meyer H.; Schafer D.. Method for the separation of ruthenium from noble metal solutions. U.S. Patent 6,475,448 B2, 2002.
- Haas M.; Weuta P.; Wolf A.; Schlueter O. K.. Separation and recovery of ruthenium: a review. U.S. Patent 0,287,282 A1, 2008.
- Meier H.; Bosche D.; Zimmerhackl E.; Albrecht W.; Hecker W.; Menge P.; Ruckdeschel A.; Unger E.; Zeitler G. Solvent extraction of ruthenium from aqueous hydrochloric and hydrobromic acid solutions. Mikrochim. Acta 1969, 57, 1083–1096. 10.1007/bf01219253. [DOI] [Google Scholar]
- Lingen J.; Shiyan L.; Guangcheng L.; Jingxuan S.; Manchang R. A study of the separation and refining of Ru from a mixture of platinum metals with a tertiary amine. Solvent Extr. Ion Exch. 1989, 7, 613–624. 10.1080/07360298908962327. [DOI] [Google Scholar]
- Dirks T.; Dumas T.; Solari P. L.; Charbonnel M. C. Ruthenium Nitrosyl Structure in Solvent Extraction Systems: A Comparison of Tributyl Phosphate, Tetrabutyl Urea, N-Methyl, N-Octyl Ethylhexanamide, and N,N,N′,N′-Tetraoctyl Diglycolamide. Ind. Eng. Chem. Res. 2019, 58, 14938–14946. 10.1021/acs.iecr.9b02555. [DOI] [Google Scholar]
- Sharma S.; Ghosh S. K.; Sharma J. N. Dialkylmethyl-2-(N,N-diisobutyl)acetamidoammonium iodide as a ruthenium selective ligand from nitric acid medium. J. Hazard. Mat. 2015, 295, 17–21. 10.1016/j.jhazmat.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Grummitt W. E.; Hardwick W. H.. Recovery of ruthenium values. U.S. Patent 2,967,209 A, 1961.
- Sonar N.; Mishra P.; Kore S.; Sonavane M.; Kulkarni Y.; Raj K.; Manchanda V. Treatment of106Ru Present In Intermediate Level Radioactive Liquid Waste With Nickel Sulphide. Sep. Sci. Technol. 2009, 44, 506–515. 10.1080/01496390802286546. [DOI] [Google Scholar]
- McIsaac L.; Baker J.; Krupa J.; LaPointe R.; Meikrantz D.; Schroeder N.. Study of Bidentate Compounds for Separation of Actinides from Commercial LWR Reprocessing Wastes; INL: Idaho FallsID (United States), 1979; pp 1–109. [Google Scholar]
- Ansari S. A.; Mohapatra P. K. A review on solid phase extraction of actinides and lanthanides with amide based extractants. J. Chromatogr. A 2017, 1499, 1–20. 10.1016/j.chroma.2017.03.035. [DOI] [PubMed] [Google Scholar]
- Rard J. A.Thermodynamic Data Bases for Multivalent Elements: An Example for Ruthenium; Lawrence Livermore National Laboratory: CA (USA), 1987; pp 1–28. [Google Scholar]
- Rard J. A. Chemistry and thermodynamics of ruthenium and some of its inorganic compounds and aqueous species. Chem. Rev. 1985, 85, 1–39. 10.1021/cr00065a001. [DOI] [Google Scholar]
- Rard J. A. Correction - Chemistry and Thermodynamics of Ruthenium and Some of Its Inorganic Compounds and Aqueous Species. Chem. Rev. 1986, 86, 731. 10.1021/cr00074a600. [DOI] [Google Scholar]
- Osman J. R.; Crayston J. A.; Richens D. T. Structure of Tetrameric Aqua Ruthenium(IV): an Investigation by Ruthenium K Edge EXAFS. Inorg. Chem. 1998, 37, 1665–1668. 10.1021/ic9711271. [DOI] [Google Scholar]
- Schulz W. W.; Metcalf S. G.; Barney G. S.. Radiochemistry of Ruthenium; Rockwell International Corp.: Richland, WA (USA), Rockwell Hanford Operations, 1984; pp 1–185. [Google Scholar]
- Verma P. K.; Mohapatra P. K. Ruthenium recovery from alkaline radioactive feeds using an extraction chromatography resin containing Aliquat 336. Sep. Purif. Technol. 2021, 259, 118099. 10.1016/j.seppur.2020.118099. [DOI] [Google Scholar]
- Sonar N.; Sonavane M.; Valsala T.; Kulkarni Y.; Raj K.; Manchanda V. Use of Nickel Sulphide-PMMA Composite Beads for Removal of106Ru from Alkaline Radioactive Liquid Waste. Sep. Sci. Technol. 2009, 44, 3753–3769. 10.1080/01496390903182529. [DOI] [Google Scholar]
- Blasius E.; Glatz J. -P.; Neumann W. Ruthenium Nitrosyl Complexes in Radioactive Waste Solutions of Reprocessing Plants. Radiochim. Acta 1981, 29, 159–166. 10.1524/ract.1981.29.23.159. [DOI] [Google Scholar]
- Blasius E.; Luxenburger H. J.; Neumann W. Ruthenium Nitrosyl Complexes in Radioactive Waste Solutions of Reprocessing Plants. Radiochim. Acta 1984, 36, 149–154. 10.1524/ract.1984.36.3.149. [DOI] [Google Scholar]
- Leroy A. F.; Morris J. C. The kinetics of hydrolysis of rutheniumnitrosyltrichloride. J. Inorg. Nucl. Chem. 1971, 33, 3437–3453. 10.1016/0022-1902(71)80666-8. [DOI] [Google Scholar]
- Gans P.; Sabatini A.; Vacca A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. 10.1016/0039-9140(96)01958-3. [DOI] [PubMed] [Google Scholar]
- Brown M. A.; Kropf A. J.; Paulenova A.; Gelis A. V. Aqueous complexation of citrate with neodymium(iii) and americium(iii): a study by potentiometry, absorption spectrophotometry, microcalorimetry, and XAFS. Dalton Trans. 2014, 43, 6446–6454. 10.1039/c4dt00343h. [DOI] [PubMed] [Google Scholar]
- Sritham E.; Gunasekaran S. FTIR spectroscopic evaluation of sucrose-maltodextrin-sodium citrate bioglass. Food Hydrocolloids 2017, 70, 371–382. 10.1016/j.foodhyd.2017.04.023. [DOI] [Google Scholar]
- Naji M.; Di Lemma F. D.; Kovács A.; Beneš O.; Manara D.; Colle J.-Y.; Pagliosa G.; Raison P.; Konings R. J. M. Joint Raman spectroscopic and quantum chemical analysis of the vibrational features of Cs2RuO4. J. Raman Spectrosc. 2015, 46, 661–668. 10.1002/jrs.4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith W. P. Raman spectra of ruthenium tetroxide and related species. J. Chem. Soc. A 1968, 1663–1664. 10.1039/j19680001663. [DOI] [Google Scholar]
- Levin I. W.; Abramowitz S. Vibrational Spectra and Force Field of Ruthenium Tetroxide. J. Chem. Phys. 1969, 50, 4860–4865. 10.1063/1.1670981. [DOI] [PubMed] [Google Scholar]
- Fletcher J.; Jenkins I.; Lever F.; Martin F.; Powell A.; Todd R. Nitrato and nitro complexes of nitrosylruthenium. J. Inorg. Nucl. Chem. 1955, 1, 378–401. 10.1016/0022-1902(55)80048-6. [DOI] [Google Scholar]
- TurbomoleV6, 3 A development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH; TURBOMOLE GmbH, 1989–2007. since 2007. http://www.turbomole.com.
- Becke A. D. A new mixing of Hartree-Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. 10.1063/1.464304. [DOI] [Google Scholar]
- Lee C.; Yang W.; Parr R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B: Condens. Matter Mater. Phys. 1988, 37, 785–789. 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]