Abstract
The transmembrane regulatory protein ToxR is required for expression of virulence factors in the human diarrheal pathogen Vibrio cholerae, including cholera toxin (CT) and the toxin coregulated pilus (TCP). ToxR is necessary for transcription of the gene encoding a second regulatory protein, ToxT, which is the direct transcriptional activator of CT and TCP genes. However, ToxR, independent of ToxT, directly activates and represses transcription of the outer membrane porins OmpU and OmpT, respectively. The genes encoding TCP and CT (and including ToxT) lie on horizontally acquired genetic elements, while the toxR, ompU, and ompT genes are apparently in the ancestral Vibrio chromosome. The contribution of ToxR-dependent modulation of outer membrane porins to cholera pathogenesis has remained unknown. We demonstrate that ToxR mediates enhanced bile resistance in a ToxT-independent manner. In both classical and El Tor biotypes of V. cholerae, a toxR mutant strain has a reduced minimum bactericidal concentration (MBC) of bile, the bile component deoxycholate (DC), and the anionic detergent sodium dodecyl sulfate (SDS) compared to both wild-type and toxT mutant strains. Classical and El Tor toxR mutant strains also exhibit reduced growth rates at subinhibitory concentrations of DC and SDS. Growth of either V. cholerae biotype in subinhibitory concentrations of bile or DC induces increased ToxR-dependent production of a major 38-kDa outer membrane protein, which was confirmed to be OmpU by Western blot. Measurement of transcription of a ompUp-lacZ fusion in both biotypes reveals stimulation (about two- to threefold) of ToxR-dependent ompU transcription by the presence of bile or DC, suggesting that ToxR may respond to the presence of bile. The toxR mutant strains of three additional human intestinal pathogenic Vibrio species, V. mimicus, V. fluvialis, and V. parahaemolyticus, display lower MBCs of bile, DC, and SDS and have altered outer membrane protein profiles compared to the parental wild-type strains. Our results demonstrate a conserved role for ToxR in the modulation of outer membrane proteins and bile resistance of pathogenic Vibrio species and suggest that these ToxR-dependent outer membrane proteins may mediate enhanced resistance to bile. We speculate that ToxR-mediated bile resistance was an early step in the evolution of V. cholerae as an intestinal pathogen.
The bacterium Vibrio cholerae causes the human diarrheal disease cholera. This organism colonizes the human small intestine, where it produces virulence factors that cause disease. Expression of a number of V. cholerae virulence factors, including the cholera toxin (CT) and the toxin coregulated pilus (TCP), is coordinately regulated by environmental signals resulting in high levels of expression within the host intestine but little to no expression outside the host (for a review, see reference 30). Coordinate expression of CT, TCP, and other virulence factors is controlled by a transmembrane DNA-binding protein, ToxR (27). ToxR requires another transmembrane transcriptional activator TcpP (13) to synergistically activate expression of toxT in response to specific laboratory conditions (14). ToxT is an AraC-like regulatory protein that directly activates transcription of several virulence genes, including ctx and tcp genes, which encode CT and TCP, respectively (8, 15).
ToxR, independent of TcpP and ToxT, also activates transcription of ompU (5), which encodes a major outer membrane porin (3) that has been suggested to be involved in adherence during pathogenesis (33). ToxR also represses the transcription of ompT, which encodes another outer membrane porin, in a TcpP- and ToxT-independent manner (V. DiRita, personal communication). These opposing activities of ToxR lead to virtually exclusive OmpU expression in wild-type strains and OmpT expression in toxR mutant strains, at least in vitro. Interestingly, both ToxR and OmpU homologues have been found in other Vibrio spp. (20, 29, 36), while toxT and the tcp and ctx genes are found on either a large pathogenicity island (17) or a lysogenic bacteriophage (35) only associated with epidemic V. cholerae strains. This suggests that toxT, tcp, and ctx genes were acquired relatively recently but toxR and ompU were present in the ancestral chromosome. The reasons for the ancestral regulatory protein ToxR gaining control over newly acquired virulence factor expression are unclear.
V. cholerae has the ability to cause global epidemics, or pandemics. It is believed that the first six cholera pandemics were caused by the classical V. cholerae biotype, while the seventh pandemic was caused by the El Tor biotype (1). These biotypes are differentiated in the laboratory by a number of characteristics, including different in vitro environmental signals which optimally induce virulence factor expression. Differential expression of virulence factors between the two biotypes of V. cholerae has been shown to be due to differential ToxR-dependent toxT expression (7). Presumably, ToxR-dependent transcription in both biotypes responds to common environmental signals within the host which have not yet been identified.
Bile is found at relatively high levels within the intestine, and resistance to bile is essential for enteric pathogens. Bile is composed primarily of bile salts, anionic detergents that not only aid in the digestion of fats but also are bacteriocidal due to their membrane solvent properties. The basic structure of the gram-negative bacteria provides some measure of resistance to bile by hiding the bile-sensitive cytoplasmic membrane beneath the relatively bile-resistant outer membrane (for a review, see reference 28). Lipopolysaccharide and outer membrane porins contribute to the resistance of Escherichia coli cells to bile (34). Additionally, efflux pumps have been identified which remove bile that reaches the cytoplasm of enteric bacteria (34). The inherent resistance of enteric bacteria to bile has been incorporated into their selective media, for example, thiosulfate-citrate-bile-sucrose (TCBS) for selection of Vibrio species. However, essentially nothing is known about V. cholerae resistance mechanisms to bile.
We demonstrate that ToxR mediates enhanced bile resistance in both biotypes of V. cholerae in a ToxT-independent manner in a way similar to the ToxR-dependent modulation of outer membrane porins. Moreover, ToxR modulates bile resistance and outer membrane protein expression in other pathogenic intestinal Vibrio species. Our results suggest that ToxR-dependent modulation of outer membrane proteins enhances bile resistance. Transcription of bile resistance gene(s) may have been one of the necessary prerequisites in the evolution of an ancestral transcriptional activator, ToxR, into the regulatory protein that controls virulence factor expression of an intestinal pathogen.
MATERIALS AND METHODS
Bacterial strains.
E. coli DH5α (12) was used for cloning experiments, and strain SM10λpir (26) was used to transfer plasmids to Vibrio strains by conjugation. All V. cholerae strains are isogenic with the classical strain O395 (23) or El Tor strain E7946 (24). V. cholerae KKV61, VJ740, and VJ739 have been described previously (4, 19). The ΔtoxR1 mutation was introduced into the chromosome of E7946 by plasmid pMD60, as previously described (19), to form strain KKV366. Strains O395, KKV61, VJ740, E7946, KKV366, and VJ739 were made phenotypically Lac− for β-galactosidase assays by the introduction of a chromosomal ΔlacZ mutation with plasmid pCG711, as described previously (9), to form strains KKV598, KKV62, KKV163, KKV557, KKV555, and KKV556, respectively.
The other Vibrio species used in these studies were ATCC strains 33809 (V. fluvialis), 33655 (V. mimicus), and 43996 (V. parahaemolyticus). Plasmids pKEK287, pKEK266, pKEK260, and pKEK273, containing internal “toxR” sequences from the various Vibrio species (see below), were mated into these strains to form the toxR mutant strains by plasmid cointegration (toxR::pGP704), as described previously (19). Correct insertion into the chromosomal toxR gene was confirmed by Southern blot.
Plasmid construction.
Amplification and cloning of toxR fragments using PCR with degenerate oligonucleotides has been described previously (29). These fragments were digested with SalI and EcoRI and then ligated into pGP704 (26) that had been similarly digested to form plasmids pKEK287 (V. cholerae toxR), pKEK266 (V. fluvialis toxR), pKEK260 (V. mimicus toxR), and pKEK273 (V. parahaemolyticus toxR). These plasmids were used to construct toxR Vibrio strains (see above).
The plasmids which express toxR from the arabinose-inducible promoter PBAD were constructed by first amplifying the toxR genes from both O395 and E7946 chromosomal DNA by PCR using oligonucleotides TOXR1 (5′-TTCGGATTAGGACACAACTCA-3′) and TOXR2 (5′-GCTCTAGATCTATTTTGCATAGCAAGATC-3′) (the XbaI site is underlined). The resulting fragments were digested with XbaI and ligated into pBAD24 (11) that had been digested with NcoI, blunt ended with Klenow fragment of DNA polymerase, and then digested with XbaI. This resulted in the construction of pKEK86 and pKEK150, which fuse the second codon of ToxR from a classical strain and an El Tor strain, respectively, to the initiating methionine of a translational PBAD fusion vector. There are four amino acid differences between the ToxR proteins of classical and El Tor strains (7).
The ompUp-lacZ transcriptional fusion plasmid pAL144, which contains the entire ompU promoter region from −675 to +22 with respect to the transcription startsite, has been described previously (5; the kind gift of V. DiRita).
Growth conditions and media.
Minimum bactericidal concentration (MBC) and growth rate assays were performed by growth in Luria broth (LB) containing various concentrations of bile (sodium choleate; Sigma), deoxycholate (Amersham), sodium dodecyl sulfate (SDS; lauryl sulfate; Sigma), or Triton X-100 (Sigma). V. cholerae strains containing PBAD vectors (e.g., in MBC and protein expression assays) were additionally grown in the presence of 0.05% arabinose and 50 μg of ampicillin per ml. V. cholerae strains were grown at 37°C, and the other Vibrio strains were grown at 30°C.
Strains were first grown 6 h to overnight in a roller drum in 1 ml of LB in 11-mm-diameter culture tubes at the appropriate temperature. For MBC, protein expression, and transcription assays, cultures were then diluted 1:100 in 0.15 M NaCl, and then 10 μl was used to inoculate 5 ml of LB into 16-mm-diameter culture tubes and was grown in a roller drum at either 30 or 37°C. For MBC assays, cultures were then plated on LB to enumerate viable bacteria; the MBC is that at which no viable bacteria were recovered. Results from three experiments performed independently gave the same MBC values as those reported in the tables. Growth rate assays were performed by diluting overnight cultures 1:100 into 20 ml of LB in a 125-ml Ehrlenmeyer flask, followed by growth in a shaking water bath at 37°C; the cell density was then determined by measuring the optical density at 600 nm (OD600). The relative growth rate was determined by measuring the slope of each exponential-phase growth curve and normalizing it to the exponential growth rate of the same strain grown in LB alone.
Transcription assays.
V. cholerae strains containing plasmids pAL144 or pTL61T were grown (see above) to stationary phase in LB alone or supplemented with 0.4% bile or 0.1% DC; these conditions were chosen to match those used for the detection of protein expression. Media also contained 100 μg of ampicillin per ml for the retention of plasmid. Samples were permeabilized with chloroform and SDS and assayed for β-galactosidase activity by the method of Miller (25).
Detection of protein expression.
Outer membrane fractions were prepared as described previously (22, 26). Proteins were separated by SDS–10% polyacrylamide gel electrophoresis (PAGE) prior to Western blotting with rabbit polyclonal antisera against V. cholerae OmpU (6; the kind gift of J. Peterson) utilizing an ECL detection system (Amersham).
RESULTS
toxR V. cholerae strains of both biotypes have reduced MBCs of bile.
The selective medium for Vibrio species, TCBS, contains a relatively high (0.8%) concentration of bile. We had observed a distinct growth defect of toxR V. cholerae strains relative to isogenic wild-type, toxT, or tcpP strains on this medium; the growth defect was not evident when the toxR strain was grown on the same medium lacking bile (data not shown). Based on this observation, experiments were designed to determine if ToxR plays a role in bile resistance of V. cholerae.
To determine the role of ToxR in bile resistance, wild-type V. cholerae strains of both classical and El Tor biotypes, and isogenic strains containing nonpolar chromosomal deletions of toxR (ΔtoxR) or toxT (ΔtoxT) were grown in various concentrations of bile, the individual bile component deoxycholate (DC), the anionic detergent SDS, or the nonionic detergent Triton X-100. For the wild-type and ΔtoxT strains, the MBCs of bile, DC, and SDS were identical but were higher for the El Tor biotype than for the classical biotype (Table 1). However, for the ΔtoxR mutant strains of both biotypes the MBCs of bile, DC, and SDS were lower than for the parental wild-type or ΔtoxT strains. Expression of ToxR from the PBAD promoter in the ΔtoxR strains restored the wild-type MBCs of bile, DC, and SDS. These results demonstrate a ToxR-dependent mechanism for enhanced bile resistance that is independent of ToxT. For all strains of both biotypes, including the ΔtoxR strains, the MBCs of the nonionic detergent Triton X-100 (45%) were identical, indicating that the ToxR-dependent resistance mechanism may be specific for anionic detergents.
TABLE 1.
ToxR is required for increased MBCs of bile, DC, and SDS in V. cholerae
| Biotype | Genotypea | Plasmidb | MBC
|
||
|---|---|---|---|---|---|
| % Bile | % DC | % SDS | |||
| Classical (O395) | Wild type | pBAD24 | 7.50 | 5.00 | 0.30 |
| pKEK86 (toxR+) | 7.50 | 5.00 | 0.30 | ||
| ΔtoxR | pBAD24 | 3.75 | 3.75 | 0.05 | |
| pKEK86 (toxR+) | 7.50 | 5.00 | 0.20 | ||
| ΔtoxT | pBAD24 | 7.50 | 5.00 | 0.30 | |
| pKEK86 (toxR+) | 7.50 | 5.00 | 0.30 | ||
| El Tor (E7946) | Wild type | pBAD24 | 15.00 | 7.50 | 0.50 |
| pKEK150 (toxR+) | 15.00 | 7.50 | 0.50 | ||
| ΔtoxR | pBAD24 | 7.50 | 5.00 | 0.10 | |
| pKEK150 (toxR+) | 15.00 | 7.50 | 0.40 | ||
| ΔtoxT | pBAD24 | 15.00 | 7.50 | 0.50 | |
| pKEK150 (toxR+) | 15.00 | 7.50 | 0.50 | ||
Actual strains used (see Materials and Methods): classical biotype strains O395 (wild type), KKV61 (ΔtoxR), and VJ740 (ΔtoxT), and El Tor biotype strains E7946 (wild type), KKV366 (ΔtoxR), and VJ739 (ΔtoxT).
Strains carry either the vector pBAD24 or plasmids pKEK86 and pKEK150, which express classical and El Tor biotype ToxR, respectively, from the PBAD promoter of pBAD24.
toxR mutant V. cholerae strains of both biotypes have reduced growth rates in the presence of anionic detergents.
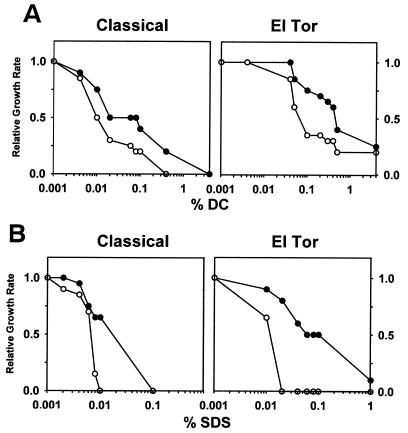
The MBCs for ΔtoxR strains of the anionic detergents (see above), while lower than those for isogenic wild-type strains, are still relatively high for these compounds, but the concentrations of individual bile salts within the intestine is probably lower. The bile salt concentration varies depending on the nutrition status, but it is estimated to be approximately 20 mM (∼1%) within the small intestine, where V. cholerae colonizes (16). In order to determine if ΔtoxR strains exhibit defects at lower bile salt concentrations, growth rates were determined for both wild-type and ΔtoxR strains of both biotypes over a wide range of DC concentrations (Fig. 1A). The growth rate at each DC concentration relative to the growth rate in the absence of DC was plotted as a function of DC concentration. Although the ΔtoxR and wild-type strains have identical growth rates in the absence of DC, at every concentration of DC supplemented to the medium of >2 orders of magnitude, the ΔtoxR strains had slower growth rates than did the wild-type strains (Fig. 1A). While the ΔtoxR strains of both biotypes exhibited reduced growth rates in the presence of a wide range of DC, isogenic ΔtoxT strains had growth rates identical to those for the wild-type strains at all DC concentrations (data not shown).
FIG. 1.
ΔtoxR mutant strains of classical and El Tor V. cholerae biotypes display slower growth rates in DC and SDS. (A) Relative growth rates of classical and El Tor V. cholerae strains in the presence of various concentrations of DC. Classical V. cholerae strains O395 (wt, ●) and KKV61 (ΔtoxR, ○) and El Tor V. cholerae strains E7946 (wt, ●) and KKV366 (ΔtoxR, ○) were grown in LB containing the DC concentrations indicated at 37°C (note the logarithmic scale for DC concentrations). Growth rates are shown relative to the growth rate in the absence of DC. (B) Relative growth rates of classical and El Tor V. cholerae strains in the presence of various concentrations of SDS. V. cholerae classical strains O395 (wt, ●) and KKV61 (ΔtoxR, ○) and El Tor strains E7946 (wt, ●) and KKV366 (ΔtoxR, ○) were grown in LB containing the SDS concentrations indicated at 37°C (note the logarithmic scale for SDS concentrations). Growth rates are shown relative to the growth rate in the absence of SDS.
The growth rates of ΔtoxR strains of both biotypes were even more noticeably reduced compared to the wild-type strains when grown in the presence of the anionic detergent SDS (Fig. 1B). The reduced growth rates of ΔtoxR strains were observed over a wide range (>2 orders of magnitude) of SDS concentrations. However, ΔtoxT strains exhibited growth rates identical to those of the wild-type strains over this range of SDS concentrations (not shown). These results are consistent with a ToxR-dependent mechanism for enhanced growth in the presence of bile and anionic detergents.
Bile induces ToxR-dependent expression of OmpU in both biotypes.
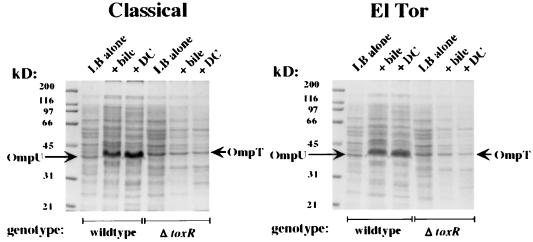
To identify ToxR-dependent factor(s) responsible for bile resistance that might be induced by the presence of bile or individual bile salts, wild-type and ΔtoxR strains of both biotypes were grown both in the absence or presence of bile and DC. Resolution of the total cellular proteins by SDS-PAGE revealed overexpression of a prominent ∼38-kDa protein in wild-type strains of either biotype grown in the presence of bile or DC (Fig. 2). Overexpression of this protein made an absolute determination of the molecular weight difficult, but further fractionation experiments (see below) revealed that this protein was the apparent size of the ToxR-dependent outer membrane porin OmpU. The ∼38-kDa protein was absent in ΔtoxR strains of either biotype grown either in the presence or in the absence of bile or DC; in these strains expression of the ToxR-repressed outer membrane porin OmpT (∼40 kDa) is apparent. Total protein patterns of ΔtoxT strains or ΔtoxR strains carrying a plasmid expressing ToxR (pKEK86 or pKEK150) of either biotype were indistinguishable from those of the parental wild-type strains (not shown).
FIG. 2.
A ToxR-dependent ∼38-kDa protein is overexpressed during growth of classical and El Tor V. cholerae strains in bile and DC. Whole-cell lysates of V. cholerae classical strains (left panel) O395 (wild type) and KKV61 (ΔtoxR) and El Tor strains E7946 (wild type) and KKV366 (ΔtoxR) grown in LB alone, LB plus 0.4% bile, or LB plus 0.1% DC, as indicated above lanes. Samples were matched by equivalent OD600 units, separated by SDS–10% PAGE, and stained with Coomassie blue. The first lane of each panel has molecular mass markers, which are noted in kilodaltons to the left. The known mobilities of OmpU and OmpT are indicated by arrows.
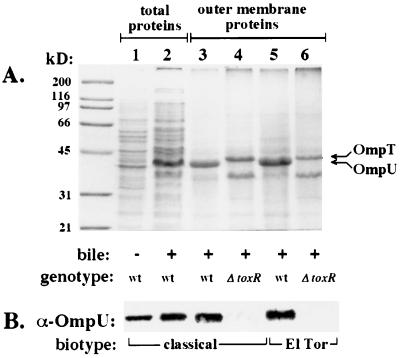
The only known ToxR-activated yet ToxT-independent factor is OmpU; therefore, we postulated that the ∼38-kDa ToxR-dependent factor overexpressed in the presence of bile was in fact OmpU. The ∼38-kDa protein was present in outer membrane fractions of wild-type cells of either biotype grown in bile but was absent from the outer membranes of ΔtoxR strains grown in bile, which instead had a prominent ∼40-kDa protein corresponding to the mobility of OmpT (Fig. 3A). Western blot with polyclonal antisera directed against OmpU confirmed that the ToxR-dependent ∼38-kDa outer membrane protein overexpressed in the presence of bile or DC was OmpU (Fig. 3B).
FIG. 3.
The ToxR-dependent ∼38-kDa protein localized in the outer membrane is OmpU. (A) Outer membrane fractions were prepared (22) of V. cholerae classical strains O395 (wt, lane 3) and KKV61 (ΔtoxR, lane 4) and El Tor strains E7946 (wt, lane 5) and KKV366 (ΔtoxR, lane 6) grown in LB with 0.4% bile (+bile); also shown are whole-cell lysates (total proteins) of strain O395 grown in LB alone (−bile, lane 1) or LB plus 0.4% bile (+bile, lane 2). Samples were matched by equivalent OD600 units and were separated by SDS–10% PAGE and stained with Coomassie blue. The left lane has molecular mass markers, which are noted in kilodaltons. The known mobilities of OmpU and OmpT are indicated by arrows. The identity of the ∼35-kDa outer membrane protein most apparent in ΔtoxR outer membranes is unknown. (B) The whole-cell and outer membrane protein samples above were subjected to Western analysis (see Materials and Methods) utilizing rabbit polyclonal antisera against OmpU.
ToxR-dependent transcription of ompU is stimulated by bile.
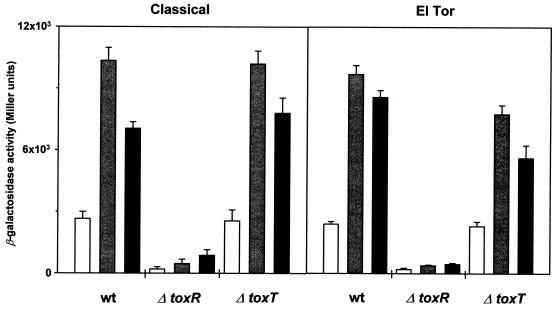
Because OmpU is increased in the presence of bile, we tested whether this is due to increased ToxR-dependent ompU transcription. Wild-type, ΔtoxR, and ΔtoxT strains of both biotypes containing a plasmid with a ompU promoter-lacZ transcriptional fusion were measured for β-galactosidase activity in the absence or the presence of bile and DC (Fig. 4). Only very low levels of ompU transcription were detected in ΔtoxR strains of either biotype under any growth condition, a finding consistent with the previous demonstration (5) that ToxR is required for high levels of ompU transcription. Relatively high levels of ompU transcription were detected in wild-type and ΔtoxT strains of both biotypes grown in LB alone. ompU transcription increased approximately two- to threefold when these strains were grown in the presence of either bile or DC. These results indicate that the overexpression of OmpU evident in toxR+ strains grown in bile or DC is apparently due at least in part to increased ToxR-dependent ompU transcription, suggesting that ToxR responds to bile.
FIG. 4.
ToxR-dependent ompU transcription is increased in the presence of bile or DC. V. cholerae classical biotype strains KKV598 (wild type [wt]), KKV62 (ΔtoxR), and KKV163 (ΔtoxT), and El Tor biotype strains KKV557 (wt), KKV555 (ΔtoxR), and KKV556 (ΔtoxT) carrying the ompUp-lacZ transcriptional fusion plasmid pAL144 were grown in LB (open bars) alone or supplemented with 0.4% bile (shaded bars) or 0.1% DC (solid bars) and then assayed for β-galactosidase as described in the text. Media also contained 100 μg of ampicillin per ml. Results are the average of three samples. The β-galactosidase activity of each strain harboring the vector pTL61T (21) alone grown under these conditions (ca. 500 Miller U), which can be considered background activity, has been subtracted out.
ToxR modulates outer membrane proteins and enhanced bile resistance in V. mimicus, V. fluvialis, and V. parahaemolyticus.
Because the toxR gene appears to be an ancestral Vibrio gene, we investigated whether ToxR-mediated outer membrane protein modulation and bile resistance were conserved among other Vibrio species and specifically in those that are intestinal pathogens. We previously identified toxR genes in two human intestinal pathogenic Vibrio species, V. mimicus and V. fluvialis (29), and toxR had been additionally identified in the intestinal pathogen V. parahaemolyticus (20). Insertional toxR mutant strains of V. mimicus and V. fluvialis, as well as of V. parahaemolyticus and V. cholerae were constructed as described (see Materials and Methods). The MBCs of bile, DC, and SDS were determined for wild-type and toxR mutant strains of V. mimicus, V. fluvialis, and V. parahaemolyticus. The toxR mutant strains of all three pathogenic Vibrio species exhibited lower MBCs for bile, DC, and SDS than the isogenic wild-type strains (Table 2).
TABLE 2.
ToxR is required for increased MBCs of bile, DC, and SDS in other Vibrio species
| Species | Genotypea | MBC
|
||
|---|---|---|---|---|
| % Bile | % DC | % SDS | ||
| V. fluvialis | Wild type | 25.00 | 7.50 | 0.50 |
| toxR | 7.50 | 5.00 | 0.30 | |
| V. mimicus | Wild type | 15.00 | 0.15 | 0.50 |
| toxR | 10.00 | 0.05 | 0.30 | |
| V. parahaemolyticus | Wild type | 10.00 | 0.25 | 0.30 |
| toxR | 7.50 | 0.05 | 0.10 | |
Actual strains used (see Materials and Methods): V. fluvialis strains ATCC 33809 (wild type) and 33809toxR, V. mimicus strains ATCC 33655 (wild type) and 33655toxR, and V. parahaemolyticus strains ATCC 43966 (wild type) and 43966toxR.
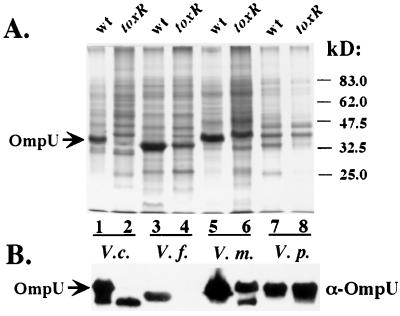
Outer membrane proteins from wild-type and toxR mutant strains of V. cholerae, V. fluvialis, V. mimicus, and V. parahaemolyticus grown in the presence of 0.4% bile were separated by SDS-PAGE (Fig. 5A). Differences in major outer membrane proteins were evident between the wild-type and toxR strains of V. fluvialis, V. mimicus, and V. parahaemolyticus, as had already been established for V. cholerae (lanes 1 and 2). V. fluvialis has a prominent outer membrane protein of ca. 36-kDa that is expressed at a lower level or possibly missing from the toxR V. fluvialis strain (and replaced by a protein of slightly higher molecular weight; lanes 3 and 4). V. mimicus, the most closely related Vibrio species to V. cholerae, expresses high levels of an outer membrane protein of the approximate molecular size of V. cholerae OmpU (∼38 kDa) that is reduced or missing in the toxR V. mimicus strain (lanes 5 and 6). The toxR V. mimicus strain expresses an outer membrane protein of the approximate size of V. cholerae OmpT (∼40 kDa) that is reduced or absent from the wild-type V. mimicus strain. Finally, V. parahaemolyticus expresses an outer membrane protein of ca. 36 kDa that is reduced or absent in the toxR V. parahaemolyticus strain (lanes 7 and 8).
FIG. 5.
Outer membrane proteins of V. mimicus, V. fluvialis, and V. parahaemolyticus are modulated by ToxR. (A) Outer membrane fractions were prepared as described earlier (26) of V. cholerae (V.c.) strains O395 (wild type [wt], lane 1) and O395toxR (toxR, lane 2), V. fluvialis (V.f.) strains 33809 (wt, lane 3) and 33809toxR (toxR, lane 4), V. mimicus (V.m.) strains 33655 (wt, lane 5) and 33655toxR (toxR, lane 6), and V. parahaemolyticus (V.p.) strains 43996 (wt, lane 7) and 43996toxR (toxR, lane 8). Strains were grown in LB supplemented with 0.4% bile. Samples were matched by equivalent OD600 units and separated by SDS–10% PAGE and stained with Coomassie blue. The mobility of molecular mass markers are noted in kilodaltons to the right. The known mobility of OmpU is indicated by arrow. (B) The outer membrane protein samples from wild-type and toxR mutant Vibrio strains (above) were subjected to Western analysis (see Materials and Methods) utilizing rabbit polyclonal antisera against OmpU; V. cholerae OmpU is indicated by an arrow.
The outer membrane fractions of these Vibrio strains were subjected to Western blot analysis with polyclonal antisera against V. cholerae OmpU (Fig. 5B). The high levels of OmpU present in the outer membrane of the V. cholerae wild-type strain were detected with the OmpU antisera, and very little OmpU could be detected in the V. cholerae toxR strain outer membrane (lanes 1 and 2; this antisera cross-reacts with a ∼35-kDa V. cholerae outer membrane protein that is not ToxR regulated). The V. fluvialis ∼36-kDa outer membrane protein positively regulated by ToxR cross-reacts with OmpU antisera and cannot be detected in the V. fluvialis toxR mutant strain (lanes 3 and 4). The V. mimicus ∼38-kDa outer membrane protein cross-reacted with the OmpU antisera, and the toxR V. mimicus strain clearly expressed less of this protein, or possibly a distinct but antigenically related protein of higher mobility (lanes 5 and 6). Interestingly, another outer membrane protein of ∼35 kDa that cross-reacts with the OmpU antisera is apparent in the toxR V. mimicus strain but not in the wild-type strain, suggesting that this protein is negatively regulated by ToxR. A V. parahaemolyticus ∼38-kDa outer membrane protein cross-reacted with the OmpU antisera but appears to be expressed at the same level in both wild-type and toxR V. parahaemolyticus strains. Our results demonstrate that ToxR modulates bile resistance and outer membrane proteins in other pathogenic Vibrio species. Furthermore, it appears that an OmpU homologue is positively regulated by ToxR in V. fluvialis and V. mimicus. The outer membrane protein of V. parahaemolyticus that is positively regulated by ToxR (∼36 kDa) does not cross-react with the V. cholerae OmpU antisera and may therefore not be an OmpU homologue.
DISCUSSION
The transmembrane protein ToxR is the master regulator of V. cholerae pathogenesis. ToxR is required for expression of the major virulence factors CT and TCP (27). However, ToxR is not the direct activator of TCP genes and apparently also not the direct activator of CT genes (4). Instead, ToxR, together with another transmembrane protein, TcpP, activates the toxT gene under inducing environmental conditions (8, 13). ToxT, an AraC-like transcriptional activator, then directly activates the genes encoding CT and TCP (4). The environmental signals that stimulate toxT transcription were originally thought to be sensed and responded to by ToxR (7) but now appear to be inducing conditions for the expression of tcpP (2, 31). Thus, TcpP, once made, appears to “coerce” ToxR into activating toxT transcription, something ToxR apparently does not normally do in the absence of TcpP (13). The tcp genes (including tcpP and toxT) are on a large pathogenicity island that may in fact be a filamentous bacteriophage (18), like bacteriophage CTXφ, which encodes the ctx genes (35). These horizontally transferable elements are found only in epidemic strains of V. cholerae, but toxR has been found in other bacteria within the genera Vibrio and Photobacterium (20, 29, 36). This leads to the question of what the original role of ToxR was in V. cholerae prior to acquisition of the cholera-specific virulence genes.
The original role of ToxR appears to be as a regulator of outer membrane proteins. In V. cholerae, ToxR, independent of ToxT and TcpP, activates transcription of ompU, which encodes a major outer membrane porin (5), and also represses transcription of ompT, which encodes another major outer membrane porin (V. DiRita, personal communication). In Photobacterium profundum ToxR likewise activates expression of a “porin-like” outer membrane protein OmpL while repressing expression of another outer membrane protein, OmpH (36). In this study we have demonstrated that ToxR modulates the expression of outer membrane proteins in V. mimicus, V. fluvialis, and V. parahaemolyticus, some of which are OmpU homologues (and therefore likely porins). These results suggest that the ancestral role of ToxR was as a modulator of outer membrane proteins, but then why would this protein be usurped as the regulator of virulence factor expression in V. cholerae?
The present study has uncovered a previously unknown role for ToxR as a modulator of enhanced bile resistance. ToxR, independent of ToxT, is required for enhanced survival and the growth of both V. cholerae biotypes in the presence of bile salts and anionic detergents. Moreover, ToxR is required for enhanced survival of the intestinal pathogens V. mimicus, V. fluvialis, and V. parahaemolyticus in the presence of bile salts and anionic detergents. All of these Vibrio species must be resistant to bile in order to persist within the intestine and cause disease. Perhaps this ancestral role in bile resistance led to the evolution of ToxR as the regulator of recently acquired virulence genes in V. cholerae that are expressed within the intestine.
Several lines of evidence suggest that the ToxR-regulated outer membrane proteins are involved in bile resistance. (i) OmpU and OmpT are the only known ToxR-dependent but ToxT- and TcpP-independent factors in V. cholerae, as with ToxR-dependent bile resistance. (ii) ToxR modulates both bile resistance and outer membrane proteins in other Vibrio species. (iii) OmpU is overexpressed when V. cholerae is grown in the presence of bile, apparently in part a result of increased ToxR-dependent ompU transcription. A previous study by Gupta and Chowdhury (10) failed to identify OmpU overexpression during V. cholerae growth in bile; in that study only outer membrane preparations were compared, rather than whole-cell lysates and ompU transcription, which may explain this discrepancy. One mechanism for ToxR-mediated enhanced bile resistance in V. cholerae would be inhibited influx of anionic detergents through the OmpU porin channel in comparison to the OmpT porin channel. This mechanism of bile resistance is seen in E. coli, where a strain expressing only the OmpF porin exhibits slower growth kinetics in the presence of DC compared to a strain expressing only the OmpC porin (34). However, no direct proof of OmpU and OmpT involvement in bile resistance exists yet in V. cholerae. An ompU mutant strain would be predicted to be more sensitive to bile, if OmpU has a protective role in the presence of bile, but we have not yet succeeded in our attempts to create such a mutant strain. Other laboratories have also noted failure in attempts to create a ompU V. cholerae strain, and this has been attributed to a possible essential role for OmpU (32).
ToxR transcribes high levels of ompU even in the absence of bile or DC, but in their presence ompU transcription increases, suggesting that ToxR transcriptional activity may be modulated by the presence of bile salts. The transcriptional activity of ToxR from P. profundum is modulated by pressure and also by local anesthetics such as procaine (36). Welch and Bartlett (36) postulate that both pressure and anesthetics change the membrane structure and that ToxR, which resides within the cytoplasmic membrane, actually responds to these membrane changes. Procaine has also been shown to modulate expression of the ToxR regulon in V. cholerae (13). Our studies indicate that ToxR may respond to another class of membrane-disruptive agents, namely, bile salts. A conserved mechanism of ToxR sensing and responding to membrane disruption in Vibrio species is an attractive hypothesis that awaits verification. The presence of bile salts in the environment signifies entry into the intestinal tract and would be a possible signal to stimulate ToxR-dependent transcription not only of bile resistance mechanisms but also of the ToxT-dependent virulence cascade.
ACKNOWLEDGMENTS
We thank Victor DiRita for kindly providing strains and plasmids and Johnny Peterson for providing OmpU antisera.
This study was supported by an Institutional new faculty award of the Howard Hughes Medical Institute to K.E.K. and National Institutes of Health Microbial Pathogenesis training grant AI07271-15 to D.P.
REFERENCES
- 1.Blake P A. Historical perspectives on pandemic cholera. In: Wachsmuth I K, Blake P A, Olsvik O, editors. Vibrio cholerae and cholera: molecular to global perspectives. Washington, D.C.: American Society for Microbiology; 1994. pp. 293–295. [Google Scholar]
- 2.Carroll P A, Tashima K T, Rogers M B, DiRita V J, Calderwood S B. Phase variation in tcpH modulates expression of the ToxR regulon in Vibrio cholerae. Mol Microbiol. 1997;25:1099–1111. doi: 10.1046/j.1365-2958.1997.5371901.x. [DOI] [PubMed] [Google Scholar]
- 3.Chakrabarti S R, Chaudhuri K, Sen K, Das J. Porins of Vibrio cholerae: purification and characterization of OmpU. J Bacteriol. 1996;178:524–530. doi: 10.1128/jb.178.2.524-530.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Champion G A, Neely M N, Brennan M A, DiRita V J. A branch in the ToxR regulatory cascade of Vibrio cholerae revealed by characterization of toxT mutant strains. Mol Microbiol. 1997;23:323–331. doi: 10.1046/j.1365-2958.1997.2191585.x. [DOI] [PubMed] [Google Scholar]
- 5.Crawford J A, Kaper J B, DiRita V J. Analysis of ToxR-dependent transcription activation of ompU, the gene encoding a major envelope protein in Vibrio cholerae. Mol Microbiol. 1998;29:235–246. doi: 10.1046/j.1365-2958.1998.00925.x. [DOI] [PubMed] [Google Scholar]
- 6.Das M, Chopra A K, Cantu J M, Peterson J W. Antisera to selected outer membrane proteins of Vibrio cholerae protect against challenge with homologous and heterologous strains of V. cholerae. FEMS Immun Med Microbiol. 1998;22:303–308. doi: 10.1111/j.1574-695X.1998.tb01219.x. [DOI] [PubMed] [Google Scholar]
- 7.DiRita V J, Neely M, Taylor R K, Bruss P M. Differential expression of the ToxR regulon in classical and El Tor biotypes of Vibrio cholerae is due to biotype-specific control over toxT expression. Proc Natl Acad Sci USA. 1996;93:7991–7995. doi: 10.1073/pnas.93.15.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiRita V J, Parsot C, Jander G, Mekalanos J J. Regulatory cascade controls virulence in Vibrio cholerae. Proc Natl Acad Sci USA. 1991;88:5403–5407. doi: 10.1073/pnas.88.12.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardel C L, Mekalanos J J. Alterations in Vibrio cholerae motility phenotypes correlate with changes in virulence factor expression. Infect Immun. 1996;64:2246–2255. doi: 10.1128/iai.64.6.2246-2255.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta S, Chowdhury R. Bile affects production of virulence factors and motility of Vibrio cholerae. Infect Immun. 1997;65:1131–1134. doi: 10.1128/iai.65.3.1131-1134.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guzman L-M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:577–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 13.Hase C C, Mekalanos J J. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci USA. 1998;95:730–734. doi: 10.1073/pnas.95.2.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins D E, DiRita V J. Transcriptional control of toxT, a regulatory gene in the ToxR regulon of Vibrio cholerae. Mol Microbiol. 1994;14:17–29. doi: 10.1111/j.1365-2958.1994.tb01263.x. [DOI] [PubMed] [Google Scholar]
- 15.Higgins D E, Nazareno E, DiRita V J. The virulence gene activator ToxT from Vibrio cholerae is a member of the AraC family of transcriptional activators. J Bacteriol. 1992;174:6974–6980. doi: 10.1128/jb.174.21.6974-6980.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofmann A F. Bile secretion and the enterohepatic circulation of bile acids. In: Feldman M, Scharschmidt B F, Sleisenger M H, editors. Gastrointestinal and liver disease. W. B. Philadelphia, Pa: Saunders Co.; 1998. pp. 937–948. [Google Scholar]
- 17.Karaolis D K, Johnson J A, Bailey C C, Boedeker E C, Kaper J B, Reeves P R. A Vibrio cholerae pathogenicity island associated with epidemic and pandemic strains. Proc Natl Acad Sci USA. 1998;95:3134–3139. doi: 10.1073/pnas.95.6.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karaolis D K, Somara S, Maneval D R J, Johnson J A, Kaper J B. A bacteriophage encoding a pathogenicity island, a type-IV pilus and a phage receptor in cholera bacteria. Nature. 1999;399:375–379. doi: 10.1038/20715. [DOI] [PubMed] [Google Scholar]
- 19.Klose K E, Mekalanos J J. Differential regulation of multiple flagellins in V. cholerae. J Bacteriol. 1998;180:303–316. doi: 10.1128/jb.180.2.303-316.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin Z, Kumagai K, Baba K, Mekalanos J J, Nishibuchi M. Vibrio parahaemolyticus has a homolog of the Vibrio cholerae toxRS operon that mediates environmentally induced regulation of the thermostable direct hemolysin gene. J Bacteriol. 1993;175:3844–3855. doi: 10.1128/jb.175.12.3844-3855.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linn T, Pierre R S. Improved vector system for constructing transcriptional fusions that ensure independent translation of lacZ. J Bacteriol. 1990;172:1077–1084. doi: 10.1128/jb.172.2.1077-1084.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lohia A, Chatterjee A N, Das J. Lysis of Vibrio cholerae cells: direct isolation of the outer membrane from whole cells by treatment with urea. J Gen Microbiol. 1984;130:2027–2033. doi: 10.1099/00221287-130-8-2027. [DOI] [PubMed] [Google Scholar]
- 23.Mekalanos J J, Collier R J, Romig W R. Enzymic activity of cholera toxin. II. Relationships to proteolytic processing, disulfide bond reduction, and subunit composition. J Biol Chem. 1979;254:5855–5861. [PubMed] [Google Scholar]
- 24.Mekalanos J J, Swartz D J, Pearson G D, Harford N, Groyne F, de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983;306:551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- 25.Miller J H. A short course in bacterial genetics. 2nd ed. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 26.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller V L, Taylor R K, Mekalanos J J. Cholera toxin transcriptional activator ToxR is a transmembrane DNA binding protein. Cell. 1987;48:271–279. doi: 10.1016/0092-8674(87)90430-2. [DOI] [PubMed] [Google Scholar]
- 28.Nikaido H. Outer membrane. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B Jr, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 29–47. [Google Scholar]
- 29.Osorio C R, Klose K E. A region of the transmembrane regulatory protein ToxR that tethers the transcriptional activation domain to the cytoplasmic membrane displays wide divergence among Vibrio species. J Bacteriol. 2000;182:526–528. doi: 10.1128/jb.182.2.526-528.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skorupski K, Taylor R K. Control of the ToxR virulence regulon in Vibrio cholerae by environmental stimuli. Mol Microbiol. 1997;25:1003–1009. doi: 10.1046/j.1365-2958.1997.5481909.x. [DOI] [PubMed] [Google Scholar]
- 31.Skorupski K, Taylor R K. A new level in the Vibrio cholerae ToxR virulence cascade: AphA is required for transcriptional activation of the tcpPH operon. Mol Microbiol. 1999;31:763–771. doi: 10.1046/j.1365-2958.1999.01215.x. [DOI] [PubMed] [Google Scholar]
- 32.Sperandio V, Bailey C, Giron J A, DiRita V J, Silveira W D, Vettore A L, Kaper J B. Cloning and characterization of the gene encoding the OmpU outer membrane protein of Vibrio cholerae. Infect Immun. 1996;64:5406–5409. doi: 10.1128/iai.64.12.5406-5409.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sperandio V, Giron J A, Silveira W D, Kaper J B. The OmpU outer membrane protein, a potential adherence factor of Vibrio cholerae. Infect Immun. 1995;63:4433–4438. doi: 10.1128/iai.63.11.4433-4438.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thanassi D G, Cheng L W, Nikaido H. Active efflux of bile salts by Escherichia coli. J Bacteriol. 1997;179:2512–2518. doi: 10.1128/jb.179.8.2512-2518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waldor M K, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 36.Welch T J, Bartlett D H. Identification of a regulatory protein required for pressure-responsive gene expression in the deep-sea bacterium Photobacterium species strain SS9. Mol Microbiol. 1998;27:977–985. doi: 10.1046/j.1365-2958.1998.00742.x. [DOI] [PubMed] [Google Scholar]