Abstract
Background
Disordered Treg counts and function have been observed in patients with SARS-Cov-2 and are thought to contribute to disease severity. In hemodialysis patients, scarce data are available on the Treg response to SARS-CoV-2 or its relation to the clinical presentation.
Methods
A cross-sectional study included one hundred patients divided into three groups, thirty SARS-CoV-2-infected hemodialysis patients (COV-HD), and thirty confirmed SARSCoV-2 infected patients (COV), and forty non-infected hemodialysis patients (HD). Flow cytometric analysis of CD4, CD25, FoxP3, and CD39+ Tregs was done for all patients and tested for correlation to in-hospital mortality, clinical, radiological severity indices.
Results
COV-HD and COV patients had significantly lower Treg cell count than HD patients (Median value of 0.016 cell/ μl vs 0.28 cell/ μl, respectively- P: 0.001). COV-HD patients had higher CD39+ Tregs (median value of 0.006 cell/ μl vs 0.002 cell/ μl, respectively- P: 0.04). COV-HD patients had significantly lower hospital stay (median value of 3 vs 13 days, P:0.001), ICU admission rates (26.5% vs 46.7%, P:0.005) and in-hospital mortality (20.7% versus 43.3%, P:0.003) than COV patients. Treg and CD39 expressing Treg counts were not correlated to severity indices in both groups. A high neutrophil to lymphocyte ratio is strongly correlated to disease severity in COV-HD patients.
Conclusions
This study provides evidence of T-cell, particularly T-regulatory cell decline in SARS-CoV-2 and suggests that hemodialysis per se does not distinctively impact the T-cell response. COV-HD patients exhibited a higher CD39+ Treg count and a better clinical profile, however, larger studies are needed to extrapolate on these findings.
Keywords: CD39+ Tregs- COVID 19- Hemodialysis- T regulatory cells- SARS-CoV-2
Background
The host response to viral infection, particularly the cellular immune response is mandatory to control viral replication and enhance viral clearance. In patients with Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, low levels of T-cells have been reported in several studies, this lymphopenia is thought to result from the recruitment of T cells to the site of infection [1]. As a subset of CD4+ T cells, T regulatory cells (Tregs) are responsible for counteracting the overactivation of the immune response which is a hallmark of SARS-CoV-2 [2]. Altered absolute and relative numbers of Treg counts have been observed in patients with severe SARS-CoV-2 and are thought to contribute to the pathogenesis of immune hyperreactivity [3]. This controversy as regard the number and function of Tregs in SARS-CoV-2 patients is due to the the different identification methods of Tregs which vary between the phenotypic and functional assessment [4]. Scarce data on the immune response to SARS-CoV-2 in hemodialysis patients are currently available. Basically, the accumulation of uremic toxins was reported to be associated with impaired or dysregulated immune response, both innate and adaptive immunity [5, 6]. A lower thymic output and premature aging of the T-lymphocytes have been already reported [5–7], which in turn increases the susceptibility to infections including pulmonary infections. Earlier studies have reported a 15-fold increase in mortality in hemodialysis patients due to respiratory tract infections [8]. In the era of SARS-CoV-2, hemodialysis patients exhibit greater risks for SARS-CoV-2 infection as well as increasing disease severity. The mortality rates of infected HD patients were expected also to be high. Conversely, a study from china reported that HD patients had comparable mortality and fewer ICU admissions when hospitalized with SARS-CoV-2 [9]. This may be related to the extracorporeal therapies that have been studied as potential treatments for the removal of cytokines in critically ill patients with SARS-CoV-2 [10, 11]. Also, it was hypothesized that constant repeated antigenic stimulation of T lymphocytes in patients undergoing hemodialysis may accelerate the aging of the immune system, all of which could affect the immune response and hence the clinical outcomes in hemodialysis patients with SARS-CoV-2 [12]. Tregs, the key mediators of immune homeostasis, are of great functionality in end stage kidney disease (ESKD) patients and be remarkably decreased in hemodialysis patients before the pandemic of SARS-CoV-2 [5]. Notably, Tregs served as a protective measure against progressive renal fibrosis in chronic kidney disease by suppressing the production of proinflammatory cytokines and decreasing the effector phenotype [8, 10–12]. Tregs express CD25 and FOXP3 which is a transcriptional regulator that controls the development and function of Tregs and is expressed by almost all inhibitory Tregs [13]. CD39 is predominantly expressed on human CD4+ Foxp3+ T cells. It has revealed a number of neoteric functions in close relation with Tregs and may be an indispensable chip to identify Tregs [14]. CD39 is considered a functional Treg marker as it directly contributes to the suppressive capacity of Tregs. Some studies have reported that CD39+ Tregs have more immunosuppressive effects than CD39- Tregs [15]. Currently, the influence of renal insufficiency and subsequent hemodialysis on the expression and differentiation of Tregs in SARS-CoV-2 infected hemodialysis patients is a non-settled issue. This study aims to evaluate the T lymphocytes’ expression patterns especially Tregs and the level of CD39 expressing Tregs in SARS-CoV-2 infected hemodialysis patients. Also, testing their relation to in-hospital mortality, clinical and radiological severity indices.
Materials and methods
This is a cross-sectional study carried out at Mansoura University Hospitals, Mansoura University, during the period between April and December 2021.
Patient selection
The study population included one hundred patients divided into three groups for comparison. Thirty hemodialysis patients with confirmed SARS-CoV-2 (COV-HD group), thirty confirmed SARS-CoV-2 infected patients (COV Group), and 40 non-infected hemodialysis patients (HD Group). The three groups were compared regarding clinical, laboratory, radiological, and outcome data. All patients were consented for participation in the study.
Sample size calculation
Sample size was calculated by PASS software for Windows, version 11.0.8. PASS 11. NCSS, LLC. Kaysville, Utah, USA. (www.ncss.com). Calculation relied upon a previous study that characterized Tregs in HD patients [16]. Group sample sizes of 30 and 30 achieve 85% power to reject the null hypothesis of zero effect size when the population effect size is 0.8 and the significance level (alpha) is 0.050 using a two-sided two-sample equal-variance t-test. Another control group of non-infected HD patients was included for comparison.
Inclusion and exclusion criteria
SARS-COV-2 infected patients were included based on a confirmed real-time reverse transcription-polymerase chain reaction (rRT-PCR) test for the qualitative detection of the nucleic acid in a nasopharyngeal swab. The exclusion criteria were restricted to the following: Patients who refused the enrolment in the study, patients with a history of autoimmune disease, active malignancy, and patients on current immune-modulatory drugs.
Data collection
Data of COV-HD and COV patients were collected from cases admitted to Mansoura University Hospital- Isolation Section. Data from HD patients were collected from Mansoura Nephrology and Dialysis, Mansoura University, Egypt. The time of data collection was 8 months.
Demographic and clinical data: Epidemiological characteristics, comorbidities, coagulation function markers on admission, and disease severity, were obtained from patients’ medical records. The SARS-CoV-2 severity was judged according to the need for hospitalization, duration of hospital stays, oxygen saturation at time of admission, need for oxygen supplementation, and in-hospital mortality.
Blood sampling and laboratory
For HD patients, blood samples were collected from the arteriovenous fistula just before starting the HD session. Routine laboratory tests were performed within the days of blood sampling using an automated analyser, complete blood picture, differential leucocytic count, and D-dimer test.
Flow cytometric T-reg cell analysis
Flow cytometry was performed on a FACSCanto II flow cytometer (BD Biosciences, USA).
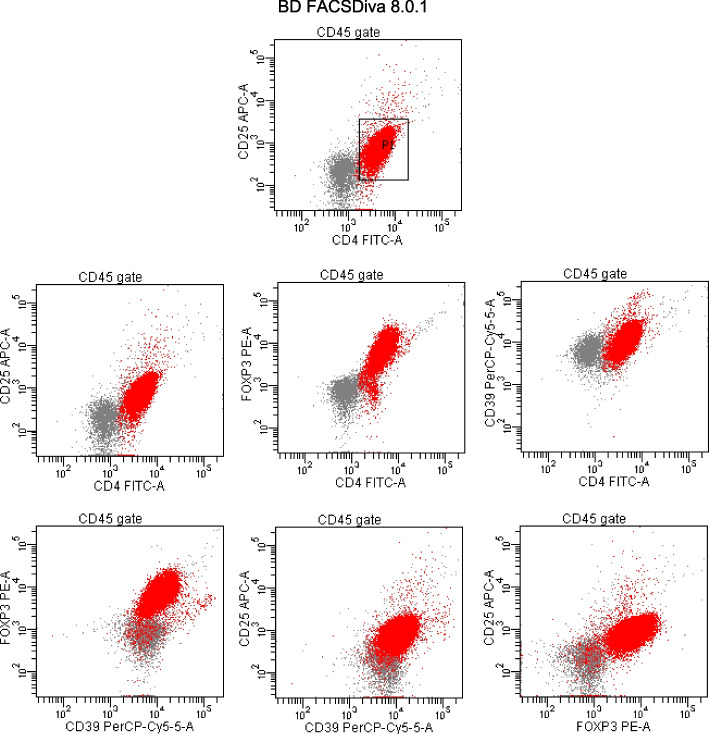
Briefly, 50 μl of fresh whole blood was incubated with the appropriate amounts of fluorochrome-labelled monoclonal antibodies CD45 Krome Orange (clone J33), CD4 FITC (clone 13B8.2), CD25 APC (clone IHT44H3) and CD39 PC5.5 (clone BA54) from Beckman Coulter, France. Incubation was done at room temperature in the dark for 15 min using appropriate mouse immunoglobulin isotypes as a control. Following incubation, 1 ml erythrocyte lysing solution (VersaLyse, Beckman Coulter) was added to the samples and incubated under the same conditions for 20 min. Then, cells were fixed and permeabilized (using intraprep permeabilization reagent, Beckman Coulter), followed by intracellular staining with anti-FoxP3-PE (clone 259D, Beckman Coulter, France) for 30 min. Cells were resuspended in PBS and analysed. Finally, the cells were characterized by flow cytometry analysis using BD FACEDiva Software. Analysis performed with CD4 + CD25 + CD39+ FOXP3+ T-reg cells expressed as a percentage of the whole CD4 subset (Fig. 1).
Fig. 1.
A representative flowcytometric plot demonstrating FoxP3 versus CD25 and CD39 expression on CD4+ T cells
Chest radiography
A Spiral CT scan was done for all patients from the root of the neck to the level of the upper pole of the kidneys during a single-breath hold using a 1 mm slice thickness. Images were reconstructed in axial, coronal, and sagittal reformats with standard pulmonary filtering. Several studies have been published reporting chest CT findings in SARS-CoV-2. in our study, we used total severity score (TSS).
Total severity score [17]
In this score, five lobes of the lungs were assessed for ground-glass opacities, mixed ground-glass opacities, or consolidation. Each lobe given 0 to 4 points, depending on the percentage of the involved lobe: 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%), or 4 (76–100%). The total severity score (TSS) is calculated by summing the points from each of the five lobes. The TSS cut-off for identifying the severe-critical type of 7.5.
Quantitative analysis of ground glass percentage
All digital imaging and communications in medicine (DICOM) data of thin cut CT chest were analyzed using Synapse 3D Fujifilm Medical Systems version 3.5 on specific workstations for automated analysis calculate ground-glass opacities (GGOs).
The data sets include 4 groups of density ranges: red color representing emphysema: From − 1024 to − 950 HU, Yellow color representing normal lung: From − 949 to − 750 HU, Blue color representing GGOs: From − 749 to − 300 HU, and violet color representing consolidation: From − 299 to + 40 HU.
Additionally, CT was assessed for the presence of other CT findings associated with COVID-19 such as consolidations, crazy-paving, subpleural bands, vascular dilatation, and reverse halo signs as well as atypical features of COVID-19 such as pulmonary nodules, mediastinal lymphadenopathy, and pleural effusion with mention of laterality, lobar affection (upper or lower), and distribution pattern (peripheral, central, or diffuse).
Figures 2 and 3 show the radiological differentiation of disease burden between mild and severe cases.
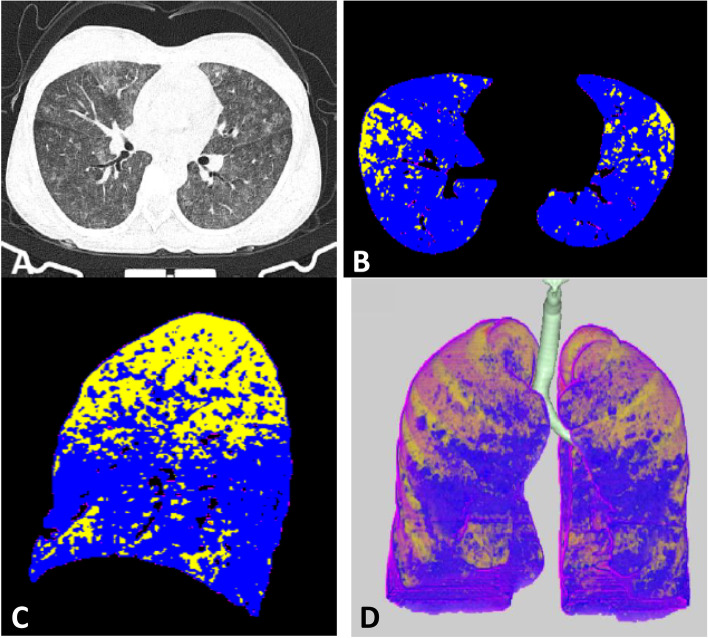
Fig. 2.
CT findings of female patient with mild disease, Total severity score (TSS) equal 4. A Axial CT image shows right lower lobe ground glass density. B and C Axial and sagittal colored analysis of the lung density revealed mild ground glass percentage (20%) in blue color compared to normal lung density which is represented with yellow color. D 3D image analysis of the lung with different densities
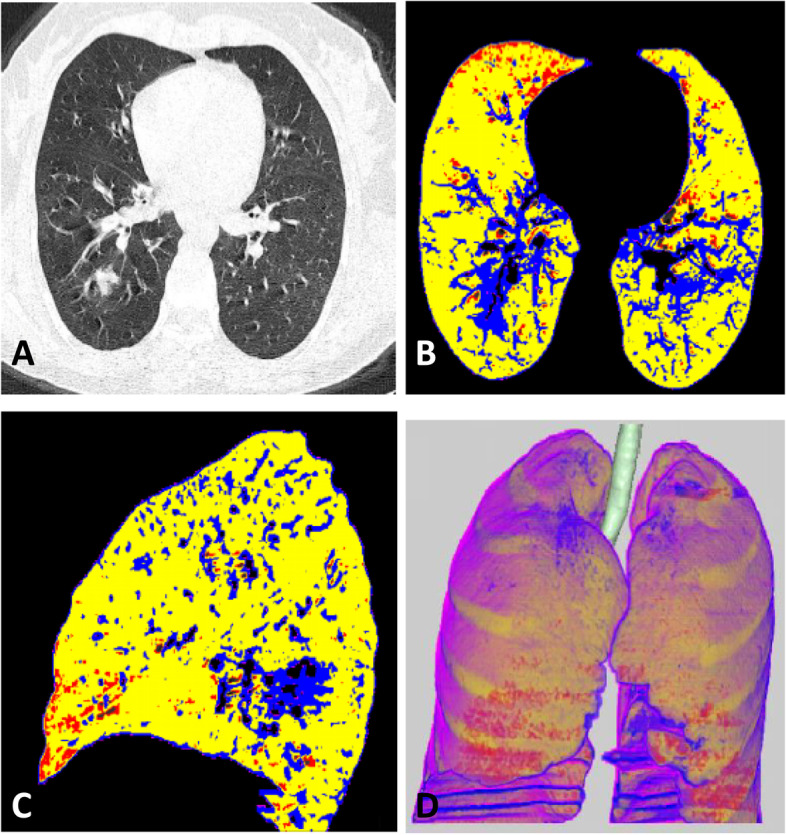
Fig. 3.
CT findings of Male patient with marked disease, Total severity score (TSS) equal 18. A Axial CT image shows multiple ground glass densities seen scattered in the scanned lungs. B and C Axial and sagittal colored analysis of the lung density revealed marked ground glass percentage (70%) in blue color compared to normal lung density which is represented with yellow color. D 3D image analysis of the lung with different densities
Statistical analysis
The collected data were coded, processed & analysed using the Statistical Package for Social Science (SPSS) version 25 for Windows on personal computers. Qualitative information was described as percentages and numbers. While quantitative information was described as means [± standard deviation (SD)] for normally distributed variables or medians (interquartile range; minimum-maximum), for non-normally distributed data. To assess the normality of the distribution of variables, the Shapiro-Wilk test was used. For comparing the groups, the t-test was used for normally distributed data of the variables of two groups, while the Mann-Whitney test was used for non-normally distributed data of variables of two groups. A Chi-square test was done for comparing qualitative variables’ data between the study groups. The correlation statistics were done by Spearman’s rank test. The level of significance will be considered at 5% (P ≤ 0.05).
Results
SARS-CoV-2 infected patients (COV-HD+ COV groups) in comparison to the HD group
SARS-CoV-2 infected patients had significantly lower WBCs total and differential counts than the HD group (Table 1). CD4+ T cells and T regulatory cells were also significantly lower in all COV cases whether they are on hemodialysis or not than non-infected HD group [45.1 cell/ μl (1.9–397.6) versus 125.2 cell/ μl (7.3–1162.9), P 0.001, and 0.016 cell/ μl (0–5.77) versus 0.28 cell/ μl (0–140.9), P < 0.001, respectively]. Neutrophil to lymphocyte ratio was significantly higher in SARS-Cov2 patients [3.44 (1.44–21.7) versus 1.88 (0.7–4.99) in HD patients, P < 0.001] (Table 1).
Table 1.
Comparing demographic and laboratory data between all Cases of COVID (COV-HD+ COV groups) and control (HD group)
| Case (COV-HD+ COV groups) | Control (HD group) | P Value | |
|---|---|---|---|
| Number | 60 | 40 | |
| Gender | |||
| Male | 30 (50) | 17 (42.5%) | 0.54 a |
| Female | 30 (50) | 23 (57.5%) | |
| CBC differential counts | |||
| Hemoglobin (mg/dl) | 10.3 (6.5–17.6) | 10.1 (7.7–14.8) | 0.28 a |
| Total WBCs (cell/ μl) | 7600 (3200–22,500) | 6350 (2300–10,200) | 0.02 a |
| Neutrophil (cell/ μl) | 5402 (2080–18,225) | 3270 (782–6796) | < 0.001 a |
| Lymphocyte (cell/ μl) | 1137 (480–5000) | 1722 (665–3276) | 0.002 a |
| NL ratio (%) | 3.44 (1.44–21.7) | 1.88 (0.7–4.99) | < 0.001 a |
| Flowcytometry counts | |||
| CD4+ T cells (cell/ μl) | 45.1 (1.9–397.6) | 125.2 (7.36–1162.9) | 0.001 a |
| Tregs (cell/ μl) | 0.016 (0–5.77) | 0.28 (0–140.9) | < 0.001 a |
| CD39+ Tregs (cell/ μl) | 0.0031 (0–5.6) | 0.049 (0–135.3) | 0.001 a |
Non-parametric data: Median (25–75%)
Nominal data: Number (Percentage)
aMann-Whitney U test
SARS-Cov2 infected hemodialysis (COV-HD group) in comparison to infected patients, not on regular hemodialysis (COV group)
COV-HD and COV groups were comparable in their WBCs total and differential counts, CD4+ T cells, and T regulatory cells, however, there was a significantly higher CD39 expression on T regulatory cells in COV-HD patients than in COV patients [0.006 cell/ μl (0–5.6) versus 0.002 (0–0.14), P 0.04]. COV-HD patients had significantly shorter hospital stay [3 (0–12) days versus 15 (3–33) days, P 0.001], better oxygen saturation at admission [89% (72–96%) versus 88% (60–99%)], less ICU admission (26.5% versus 46.7%, P 0.009) and in-hospital mortality rates (23.5% versus 43.3%, P 0.005) than COV group. D-dimer levels were significantly higher in COV-HD than in COV patients (Table 2).
Table 2.
Comparing demographic and laboratory data between COV and COV-HD patients
| COV group (n = 30) | COV-HD group (n = 30) | P Value | |
|---|---|---|---|
| Demographic and Clinical data | |||
| Age | 60.6 (13.6) | 54 (8.9) | 0.115 a |
| Gender | |||
| Male | 9 (30) | 21 (70) | 0.006 |
| Female | 21 (70) | 9 (30) | |
| Diabetes | 14 (46.7) | 13 (43) | 0.5 |
| Number of days of hospital admission | 15 (3–33) | 3 (0–12) | 0.001 a |
| O2 Saturation at admission | 88 (60–99) | 89 (72–96) | 0.008 a |
| Place of stay (OPC, Ward, ICU) | |||
| OPC | 2 (6.7) | 12 (40) | 0.009c |
| Ward | 14 (46.7) | 10 (33.5) | |
| ICU | 14 (46.7) | 8 (26.5) | |
| Outcome | |||
| OPC | 1 (3.3) | 11 (36.5) | 0.005c |
| Discharge | 16 (53.3) | 12 (40) | |
| Death | 13 (43.3) | 7 (23.5%) | |
| Radiological Data | |||
| GGO percentage% | 50 (0–86) | 45 (0–69) | 0.19 a |
| Total severity score in CT (TSS) (out of 20) | 7.5 (2–15) | 7 (2–15) | 0.12 a |
| TSS (Severe > 7.5) | 16 (53%) | 10 (33%) | 0.08 a |
| Laboratory data | |||
| CBC and Coagulation | |||
| Hemoglobin (mg/dl) | 11.25 (6.5–17.6) | 9.5 (7.8–12.7) | < 0.001 a |
| Total WBCs (cell/ μl) | 8616 (3200–20,800) | 7300 (3560–22,500) | 0.423a |
| Neutrophil (cell/ μl) | 6790 (2080–14,890) | 4346 (2242–18,225) | 0.152 a |
| Lymphocyte (cell/ μl) | 1100 (500–3600) | 1204 (480–5000) | 0.9 a |
| NL ratio | 5.6 (1.81–13.22) | 2.8 (1.84–21.75) | 0.132 a |
| D-dimer (ng/ml) | |||
| Less than 200 | 19 (63.3%) | 13 (43.3) | 0.04 a |
| 200–500 | 8 (26.7%) | 4 (13.3) | |
| More than 500 | 3 (10%) | 13 (43.3) | |
| Flowcytometry Data | |||
| CD4+ T cells (cell/ μl) | 33.8 (2.2–359.1) | 52.16 (1.92–399.6) | 0.4 a |
| Tregs (cell/ μl) | 0.011 (0–0.33) | 0.028 (0–5.77) | 0.1 a |
| CD39 expressing Tregs (cell/ μl) | 0.002 (0–0.14) | 0.006 (0–5.6) | 0.04 a |
Non-parametric data: Median (25–75%)
Nominal data: Number (Percentage)
a Mann-Whitney U test
C chi square test
Correlation statistics of different T cell population counts with the clinical and radiological severity criteria
In COV-HD group
Lymphopenia is significantly correlated to degree of hypoxemia (R = 0.5, P 0.004), higher rates of ICU admission (R = − 0.36, P 0.04), radiological severity in CT chest score (R = − 0.41, P 0.02- R = − 0.45, P 0.01). Neutrophil to lymphocyte ratio (NLR) is significantly correlated positively to all radiological and clinical severity parameters including the in-hospital mortality (Table 3).
Table 3.
Correlation statistics of different T cell population counts with the clinical and radiological severity criteria in COV-HD and COV patients
| Clinical Severity Parameters | Radiological Severity Parameters | ||||||
|---|---|---|---|---|---|---|---|
| Number of days of admission | Hypoxemia | ICU Admission | In-Hospital Mortality | TSS CTa | Percentage of GGOa | ||
| COV-HD | Lymphocytes |
R = − 0.33s P 0.07s |
R = − 0.5s P 0.004sa |
R = − 0.36s P 0.04sa |
R = − 0.3s P 0.09s |
R = − 0.41s P 0.02sa |
R = − 0.45s P 0.01sa |
| NLRa |
R = 0.4s P 0.02sa |
R = 0.54s P 0.002sa |
R = 0.64s P < 0.001sa |
R = 0.71s P < 0.001sa |
R = 0.61s P < 0.001sa |
R = 0.63s P < 0.001s |
|
| CD4+ T cells |
R = − 0.14s P 0.4s |
R = 0.26s P 0.1s |
R = 0.18s P 0.3s |
R = − 0.1s P 0.3s |
R = − 0.25s P 0.1s |
R = − 0.2s P 0.2s |
|
| Tregs |
R = 0.1s P 0.4s |
R = 0.1s P 0.4s |
R = 0.1s P 0.4s |
R = 0.1s P 0.4s |
R = 0.1s P 0.3s |
R = 0.2s P 0.2s |
|
| CD39 Expressing Tregs |
R = 0.09s P 0.6s |
R = 0.05s P 0.08s |
R = 0.1s P 0.5s |
R = 0.1s P 0.3s |
R = 0.1s P 0.5s |
R = 0.1s P 0.3s |
|
| COV | Lymphocytes |
R = 0.04 s P 0.85 s |
R = − 0.06 s P 0.7 s |
R = 0.02 s P 0.8 s |
R = 0.004 s P 0.9 s |
R = 0.18s P 0.3s |
R = 0.31s P 0.08s |
| NLR |
R = 0.1 s P 0.5 s |
R = 0.06 s P 0.7 s |
R = 0.29 s P 0.1 s |
R = 0.13 s P 0.4 s |
R = 0.06 s P 0.7 s |
R = −.09 s P 0.6 s |
|
| CD4+ T cells |
R = − 0.4s P 0.03sa |
R = − 0.1s P 0.5s |
R = − 0.2s P 0.2s |
R = − 0.54s P 0.003sa |
R = − 0.53s P 0.003sa |
R = − 0.01s P 0.9s |
|
| Tregs |
R = 0.09s P 0.6s |
R = − 0.05s P 0.7s |
R = 0.1s P 0.4s |
R = − 0.3s P 0.06s |
R = − 0.2s P 0.3s |
R = − 0.06s P 0.7s |
|
| CD39 Expressing Tregs |
R = −0.01s P 0.9s |
R = − 0.06s P 0.7s |
R = 0.3s P 0.08s |
R = − 0.1s P 0.5s |
R = − 0.14s P 0.4s |
R = − 0.07s P 0.7s |
|
R: correlation coefficient (S: spearman rank test)
NLR Neutrophil to lymphocytes ratio
TSS CT Total severity score in chest computed tomography
GGO Ground Glass Opacity
P P value
asignificant correlations
In COV group
CD4+ T cell counts are correlated negatively to the length of hospital stay (R = − 0.4, P 0.03), total severity CT chest score (R = − 0.53, P 0.003), and in-hospital mortality (R = − 0.54, P 0.003). lymphopenia, T reg, and CD- 39 expressing Treg counts are not correlated to any parameter of the clinical and radiological severity (Table 3).
Discussion
Patients on maintenance hemodialysis are particularly susceptible to SARS-CoV-2 infection due to uremia-related immune dysfunction, enhanced comorbidity burden, and the risk of cross-contamination in hemodialysis units. It is unknown whether hemodialysis patients represent a distinctive group of patients with a distinct immune response to SARS-CoV-2. It is recognized that the T- lymphocyte count is considerably decreased in patients with SARS-CoV-2 as well as in HD patients on an individual basis [18, 19]. This lymphopenia appears more manifest in patients with severe disease [18–20]. In a case series of five hemodialysis patients who contracted SARS-CoV-2, lymphopenia was reported in all cases with pulmonary ground-glass opacities as the most common radiologic findings [21]. Notably, earlier studies have found that decreased lymphocyte count is a clinical predictor of mortality due to SARS-CoV-2 infection [22, 23]. This observation was further confirmed in the study of Wang et al., who revealed that increased counts of circulating lymphocytes in elderly patients with SARS-CoV-2 were predictive of a better outcome [24]. Similarly, The NLR has been considered an independent biomarker for predicting poor clinical outcomes [25]. Comparably the present study reported significantly reduced lymphocyte count in COV-HD and COV patients. The NLR was significantly higher in COV-HD and COV patients compared to patients in the HD group. The NLR positively correlated to the TSS score and percent of ground-glass opacities while negatively correlated to the oxygen saturation, hospital stay, ICU need, and in-hospital mortality in COV-HD patients, this was not applied to COV patients. Tregs alterations have been largely observed in patients with SARS-CoV-2, recent studies have demonstrated a significant reduction in Treg numbers in SARS-CoV-2 patients, which has been associated with increasing disease severity and increased risk of respiratory failure [26, 27]. Meckiff et al. presented a single-cell transcriptomic analysis of more than 100,000 viral antigen-reactive CD4 + T cells from 40 patients with SARS-CoV-2 and found a decreased proportion of SARS-CoV-2-reactive regulatory T cells [26]. A notable reduction in the frequency of Tregs as well as the expression levels of correlated factors FoxP3, transforming growth factor-β [TGF-β], and IL-10) in the SARS-CoV-2 ICU patients has been reported [27]. On the other side, many studies confirmed increased Treg counts in mild, moderate [28], and severe [29] forms of SARS-CoV-2 infection. Another report recorded elevated levels of activated Tregs in early asymptomatic SARS-CoV-2 infection despite the normal total number of Tregs [30]. The present study showed a significantly reduced total number of CD4+ lymphocytes and Tregs in patients with SARS-CoV-2 (COV-HD + COV) than in non-infected HD patients suggesting that the observed alterations in CD4 lymphocyte and neutrophil counts resulted from a SARS-CoV-2 effect rather than an HD effect. Moreover, it is noteworthy to point out an unusual phenotype of Tregs which has been recently identified in patients with severe SARS-CoV-2 disease and is largely similar to that identified in tumor-infiltrating Tregs which expresses proinflammatory mediators and is mostly associated with poor outcomes [3, 31] but unfortunately, our study did not permit further investigation on this phenotype. CD 39 has an important role in the anti-inflammatory effect of Treg [15]. A previous study demonstrated that CD 39+ cell count was significantly higher in SARS-CoV-2 infected adult patients versus a healthy control group, an observation that is not applied to a juvenile cohort in the same study [32]. In our study, CD 39+ Tregs were significantly lower in SARS-CoV-2 infected groups than in the non-infected ones, although they were found to be significantly higher in COV-HD patients in a subgroup analysis. Being applied to the general population with healthy controls, it is difficult to compare our findings on HD patients with their results. Another area of confusion is the mortality risk in HD patients with SARS-CoV-2 infection. Some previously published reports showed high mortality rates among maintenance hemodialysis patients hospitalized for SARS-CoV-2 [33–36]. Some other studies demonstrated that chronic inflammation, a salient feature in HD patients, might protect those patients from severe COVID-19-related symptoms, ICU admission, and mortality [9, 16]. These discordant results are accepted because of the limitations known in the observational studies and multiple confounders which can affect the patient prognosis like age [37], associated comorbidities, and selection bias. The present study revealed a better survival rate, less severe hypoxemia indicating hospital or ICU admission in COV-HD than COV patients despite the comparable age, comorbidity, radiological severity criteria. This may be attributed to many factors: the fact that HD patients are more monitored compared to non-HD leading to early identification and management of infection, lessened severity indices of patients included in the COV-HD group, or perhaps the consequence of more frequent cytokine clearance in regular HD sessions. Whether or not CD 39+ Tregs contributed to a better clinical profile in our COV-HD patients, this still needs furher exploration on larger sized samples. A larger sample size is needed for performing multivariate analyses as well. We admit other three important limitations: (1) the study did not include a helathy control group from the general population for comparison; (2) the data of the drug therapy was not analyzed; (3) suppression of T cell proliferation is needed for a better evaluation of Treg.
Conclusions
This study was carried out to evaluate the effect of hemodialysis on T-regulatory cells in SARS-COV-2 infected patients, and provide evidence of T-cell, particularly T-regulatory cell decline in hemodialysis patients with SARS-COV-2 and suggest that hemodialysis per se does not distinctively impact the T-cell response in patients with SARS-CoV-2. Therefore, the T-cell targeted therapies for SARS-CoV-2 in the general population may be effectively used in hemodialysis patients. The role of CD39 expressing Tregs and Treg phenotyping should be further studied on a wider scale in hemodialysis patients.
Acknowledgments
The authors would like to express their special thanks of gratitude to the Institutional Research Board, Faculty of Medicine, Mansoura University for approaving this work.
Abbreviations
- CD
Cluster of differentiation
- ESKD
End Stage Kidney Disease
- rRT-PCR
Real-Time Reverse Transcription-Polymerase Chain Reaction
- SARS-CoV-2
Severe Acute Respiratory Syndrome coronaVirus 2
- Treg
T regulator lymphocytes
Authors’ contributions
Emad Samaan, Ragy Nader, Ahmed Gomaa, and Rasha Sheimes have given substantial contributions to the conception and the design of the manuscript, Marwa Omar and Tamer Gaber to acquisition, analysis, and interpretation of the data. Doaa Khedr and Hend Gamal did the radiological evaluation. Nashwa AbouSamra and Doaa Shahin did the laboratory assessment and Flow cytometry analysis. All authors have participated to drafting the manuscript. All authors contributed equally to the manuscript and read and approved the final version of the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors report no involvement in the research by the sponsor that could have influenced the outcome of this work.
Availability of data and materials
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by Institutional Research Board, Faculty of Medicine, Mansoura University (IRB ID: R.21.02.1206) and all the data and blood sampling procedures were under its guidelines and regulations. Written informed consent for participation was obtained from the study participants.
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose. They have no competing interests to declare that are relevant to the content of this article.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stasi A, Castellano G, Ranieri E, Infante B, Stallone G, Gesualdo L, et al. SARS-CoV-2 and viral sepsis: immune dysfunction and implications in kidney failure. J Clin Med. 2020;9(12):4057. doi: 10.3390/jcm9124057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palm NW, Medzhitov R. Not so fast: adaptive suppression of innate immunity. Nat Med. 2007;13(10):1142–1144. doi: 10.1038/nm1007-1142b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galván-Peña S, Leon J, Chowdhary K, Michelson DA, Vijaykumar B, Yang L, et al. Profound Treg perturbations correlate with COVID-19 severity. Proc Natl Acad Sci. 2021;118(37):e2111315118. doi: 10.1073/pnas.2111315118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Zheng J, Islam MS, Yang Y, Hu Y, Chen X. The role of CD4(+)FoxP3(+) regulatory T cells in the immunopathogenesis of COVID-19: implications for treatment. Int J Biol Sci. 2021;17(6):1507–1520. doi: 10.7150/ijbs.59534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaier M, Leick A, Uhlmann L, Kälble F, Morath C, Eckstein V, et al. End-stage renal disease, dialysis, kidney transplantation and their impact on cd 4+ t-cell differentiation. Immunology. 2018;155(2):211–224. doi: 10.1111/imm.12947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meijers RW, Litjens NH, de Wit EA, Langerak AW, van der Spek A, Baan CC, et al. Uremia causes premature ageing of the T cell compartment in end-stage renal disease patients. Immun Ageing. 2012;9(1):1–8. doi: 10.1186/1742-4933-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Betjes MG, Langerak AW, Van Der Spek A, De Wit EA, Litjens NH. Premature aging of circulating T cells in patients with end-stage renal disease. Kidney Int. 2011;80(2):208–217. doi: 10.1038/ki.2011.110. [DOI] [PubMed] [Google Scholar]
- 8.Sarnak MJ, Jaber BLJC. Pulmonary infectious mortality among patients with end-stage renal disease. Chest. 2001;120(6):1883–1887. doi: 10.1378/chest.120.6.1883. [DOI] [PubMed] [Google Scholar]
- 9.Xiong F, Tang H, Liu L, Tu C, Tian J-B, Lei C-T, et al. Clinical characteristics of and medical interventions for COVID-19 in hemodialysis patients in Wuhan, China. J Am Soc Nephrol. 2020;31(7):1387–1397. doi: 10.1681/ASN.2020030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheashaa H, Nagy E, Younis D, Shemies RS. COVID-19 in nephrologist practice: A review of current knowledge. Kuwait Med J. 2021;232–8.
- 11.Alharthy A, Faqihi F, Memish ZA, Balhamar A, Nasim N, Shahzad A, et al. Continuous renal replacement therapy with the addition of CytoSorb cartridge in critically ill patients with COVID-19 plus acute kidney injury: a case-series. Artif Organs. 2021;45(5):E101–EE12. doi: 10.1111/aor.13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nongnuch A, Ngampongpan W, Srichatrapimuk S, Wongsa A, Thongpraphai S, Boonarkart C, et al. Immune response to influenza vaccination in ESRD patients undergoing hemodialysis vs. hemodiafiltration. PLoS One. 2020;15(2):e0227719. doi: 10.1371/journal.pone.0227719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan YY, Flavell RAJN. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445(7129):766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 14.Zhao H, Bo C, Kang Y, Li H. What else can CD39 tell us? Front Immunol. 2017;8:727. doi: 10.3389/fimmu.2017.00727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bastid J, Cottalorda-Regairaz A, Alberici G, Bonnefoy N, Eliaou JF, Bensussan A. ENTPD1/CD39 is a promising therapeutic target in oncology. Oncogene. 2013;32(14):1743–1751. doi: 10.1038/onc.2012.269. [DOI] [PubMed] [Google Scholar]
- 16.Prietl B, Odler B, Kirsch AH, Artinger K, Eigner M, Schmaldienst S, et al. Chronic Inflammation Might Protect Hemodialysis Patients From Severe COVID-19. Front Immunol. 2022;13:821818. doi: 10.3389/fimmu.2022.821818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li K, Fang Y, Li W, Pan C, Qin P, Zhong Y, et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur Radiol. 2020;30(8):4407–4416. doi: 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang W, Berube J, McNamara M, Saksena S, Hartman M, Arshad T, et al. Lymphocyte subset counts in COVID-19 patients: a meta-analysis. Cytometry A. 2020;97(8):772–776. doi: 10.1002/cyto.a.24172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu R, Wang Y, Li J, Han H, Xia Z, Liu F, et al. Decreased T cell populations contribute to the increased severity of COVID-19. Clin Chim Acta. 2020;508:110–114. doi: 10.1016/j.cca.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li R, Tian J, Yang F, Lv L, Yu J, Sun G, et al. Clinical characteristics of 225 patients with COVID-19 in a tertiary Hospital near Wuhan, China. J Clin Virol. 2020;127:104363. doi: 10.1016/j.jcv.2020.104363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang R, Liao C, He H, Hu C, Wei Z, Hong Z, et al. COVID-19 in hemodialysis patients: a report of 5 cases. Am J Kidney Dis. 2020;76(1):141–143. doi: 10.1053/j.ajkd.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Candia P, Prattichizzo F, Garavelli S, Matarese G. T cells: warriors of SARS-CoV-2 infection. Trends Immunol. 2021;42(1):18–30. doi: 10.1016/j.it.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, He W, Yu X, Hu D, Bao M, Liu H, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80(6):639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang A-P, Liu J-p, Tao W-q, H-M L. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84:106504. doi: 10.1016/j.intimp.2020.106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meckiff BJ, Ramírez-Suástegui C, Fajardo V, Chee SJ, Kusnadi A, Simon H, et al. Imbalance of regulatory and cytotoxic SARS-CoV-2-reactive CD4+ T cells in COVID-19. Cell. 2020;183(5):1340–1353. doi: 10.1016/j.cell.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadeghi A, Tahmasebi S, Mahmood A, Kuznetsova M, Valizadeh H, Taghizadieh A, et al. Th17 and Treg cells function in SARS-CoV2 patients compared with healthy controls. J Cell Physiol. 2021;236(4):2829–2839. doi: 10.1002/jcp.30047. [DOI] [PubMed] [Google Scholar]
- 28.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, Huang J, Huang Y, Chen J, Huang Y, Jiang X, et al. Characteristics of immune cells and cytokines in patients with coronavirus disease 2019 in Guangzhou, China. Hum Immunol. 2020;81(12):702–708. doi: 10.1016/j.humimm.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Zhang E, Zhong M, Yang Q, Hong K, Shu T, et al. Impaired T cell functions along with elevated activated Tregs at the early stage of asymptomatic SARS-CoV-2 infection. 2020. [Google Scholar]
- 31.Neumann J, Prezzemolo T, Vanderbeke L, Roca CP, Gerbaux M, Janssens S, et al. An open resource for T cell phenotype changes in COVID-19 identifies IL-10-producing regulatory T cells as characteristic of severe cases. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simsek A, Kizmaz MA, Cagan E, Dombaz F, Tezcan G, Asan A, et al. Assessment of CD39 expression in regulatory T-cell subsets by disease severity in adult and juvenile COVID-19 cases. J Med Virol. 2022;94(5):2089–2101. doi: 10.1002/jmv.27593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stefan G, Mehedinti AM, Andreiana I, Zugravu AD, Cinca S, Busuioc R, et al. Clinical features and outcome of maintenance hemodialysis patients with COVID-19 from a tertiary nephrology care center in Romania. Ren Fail. 2021;43(1):49–57. doi: 10.1080/0886022X.2020.1853571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hilbrands LB, Duivenvoorden R, Vart P, Franssen CF, Hemmelder MH, Jager KJ, et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35(11):1973–1983. doi: 10.1093/ndt/gfaa261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu CM, Weiner DE, Aweh G, Miskulin DC, Manley HJ, Stewart C, et al. COVID-19 among US dialysis patients: Risk factors and outcomes from a national dialysis provider. Am J Kidney Dis. 2021;77(5):748–756. doi: 10.1053/j.ajkd.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jager KJ, Kramer A, Chesnaye NC, Couchoud C, Sánchez-Álvarez JE, Garneata L, et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across. Europe. 2020;98(6):1540–1548. doi: 10.1016/j.kint.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.La Porta E, Baiardi P, Fassina L, Faragli A, Perna S, Tovagliari F, et al. The role of kidney dysfunction in COVID-19 and the influence of age. Sci Rep. 2022;12(1):8650. doi: 10.1038/s41598-022-12652-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.