Abstract
Background and Aim
Steroids have long been used in inducing remission of inflammatory bowel disease (IBD). Chronic use, defined as therapy greater than 3 months, has been implicated in complications including increased hospital length of stay (LOS), infections, and even death. In our retrospective study, we aim to identify the complications of chronic steroid use in patients with IBD.
Methods
The fourth quarter of 2015–2019 National Inpatient Sample (NIS) was used in this study. International Classification of Diseases (ICD‐10) codes were used to identify patients with a diagnosis of IBD and chronic steroid use. Adverse outcomes of chronic steroid use in IBD patients were analyzed, such as osteoporosis, opportunistic infections, mortality rate, and LOS. Cohorts were weighted using an algorithm provided by the NIS allowing for accurate national estimates.
Results
A total of 283 970 patients had a diagnosis of IBD. Of those, 18 030 patients had concurrent chronic steroid use. Racial disparities existed, with 77.4% White, 12.7% Black, and 6.0% Hispanic. Patients with a history of IBD and chronic steroid use were found to have higher odds of developing osteoporosis, opportunistic infections, and acute thromboembolic events but did not have higher odds of mortality.
Conclusion
There is much controversy about whether IBD patients should be on chronic steroids for maintenance therapy and this study highlights the importance of this decision as patients on chronic steroid use had higher odds of developing adverse effects. These results stress the importance of monitoring patients on steroids and avoiding chronic use.
Keywords: corticosteroids, inflammatory bowel disease, steroid, steroid celiac disease, ulcerative colitis
Steroids have long been used in inducing remission of inflammatory bowel disease (IBD), but there is controversy about whether IBD patients should be on chronic steroids for maintenance therapy. In this retrospective study, we aim to identify the complications of chronic steroid use in patients with IBD. Our results highlight the importance of monitoring patients on steroids and avoiding chronic use as patients on chronic steroid use had higher odds of developing adverse effects, such as osteoporosis, opportunistic infections, and acute thromboembolic events.

Introduction
Inflammatory bowel disease (IBD) is a chronic progressive subset of diseases involving the gastrointestinal (GI) tract that encompasses both ulcerative colitis (UC) and Crohn's disease (CD). UC causes inflammation and ulcers in the large intestine and rectum, while CD can affect any portion of the GI tract. Since the 1950s, corticosteroids have been used to induce remission in both UC and CD and are currently still used despite advances in medical treatments. 1 , 2
There is no doubt that corticosteroids are highly effective agents for inducing remission of IBD; however, they are often linked to an abundance of adverse effects. These include but are not limited to increased risk of infection, Cushing's syndrome, osteoporosis, avascular necrosis, hyperglycemia, type 2 diabetes mellitus, amenorrhea, adrenocortical atrophy, hypertension, cataracts, and peptic ulcers. 3 Liechtenstein et al. found that when compared with biologic therapy and immunomodulators, prolonged use of steroids had an increased risk of both morbidity and mortality in IBD patients. 4 Targownik et al. reviewed data from 1994 to 2008 and found that despite the advances in biologic therapies, patient exposure to steroids remained 50% among patients with IBD in the first 5 years from initial diagnosis and increased to 62% in the first 10 years. 5
Initiatives to reduce steroid use are taking place in the United Kingdom and Europe. GI societies in the United Kingdom have found “steroid free clinical remission” significantly improved the quality of life for patients. 6 In the United Kingdom, the guidelines for standard of care among IBD patients state that steroid use be monitored and that patients with chronic steroid use be discussed on a multidisciplinary level to improve patient care and quality of life. 7 In light of what is known about chronic steroid use in patients with IBD, our aim was to assess the overall effect of chronic steroid use in patients with IBD.
Methods
Data source
In this study, we used the National Inpatient Sample (NIS) 2015 quarter 4 through 2019. The NIS is a publicly available database by the healthcare cost and utilization project and encompasses data from over 40 states using the International Classification of Diseases (ICD) coding in the inpatient setting. 8 It is one of the largest available databases in the United States, with approximately 20% discharge samples. Discharge weights are provided by the NIS, which allows for estimates of hospitalizations on a national level. 8 The NIS does not include any patient data for the outpatient setting. Since the information is publicly available, Institutional Review Board approval was not necessary.
Study population
Our study included all patients with a diagnosis of IBD using ICD 10 coding (Crohn's disease: K50.X; Ulcerative Colitis: K51.X) between 2015 quarter 4 through 2019. Patients who were on chronic oral steroids (ICD 10: Z79.52), defined as therapy greater than 3 months, or had a personal history of systemic steroid therapy (ICD 10: Z92.241), were compared with those who were not on chronic oral steroids or had a personal history of systemic steroid therapy. Primary endpoints were adverse outcomes associated with chronic steroid use among IBD patients such as mortality, length of stay (LOS), opportunistic infections (such as pneumocystis pneumonia, cytomegalovirus, mycoplasma infection, herpes zoster infection, toxoplasma, or cryptococcal infection), osteoporosis, sepsis (defined by ICD 10 as meeting two or more systemic inflammatory response syndrome criteria plus a bacterial source of infection), other bone disorders (i.e. stress fracture, pathological fracture, nontraumatic fracture not previously described), diabetes mellitus type 2, other metabolic syndrome related disorders (i.e. hypertension, hyperlipidemia, and obesity), mood disorder (i.e. manic episodes, bipolar disorder, depression, persistent mood disorder, and unspecified mood disorder), Cushing's syndrome, cerebrovascular accident (CVA), transient ischemic attack (TIA), acute thromboembolic event, myocardial infarction (MI), adrenal insufficiency, acute pancreatitis, and drug‐induced cataracts.
Statistical analysis
Pearson chi‐square test and Student's t‐test were used for evaluating categorical and continuous variables, respectively. After adjusting for baseline characteristics and comorbidities such as payer status, age, gender, race, hospital bed size, Charlson comorbidity index (CCI), day of admission, hospital region, and median income quartile based on zip code, a two‐step hierarchical multivariate regression model was utilized to estimate the risk of various adverse events related to chronic steroid use. All data represented were adjusted according to NIS guidelines and all results were represented as weighted using an algorithm provided by the NIS to allow for national estimates. 8 All analyses were performed using Stata version 17 (StataCorp LLC).
Results
A total of 283 970 patients from the fourth quarter of 2015 to 2019 had a documented diagnosis of IBD. Of those, 18 030 (6.35%) patients had concurrent chronic steroid use or a personal history of systemic steroid use. Majority of IBD patients without and with chronic steroid use were White (78.41% vs 77.46% respectively; P < 0.001) and female (56.31% vs 52.90% respectively; P < 0.001). The mean age of the patients with IBD and chronic steroid use was 49 years with a standard deviation of 19 years (Table 1). In addition, IBD patients not on chronic steroid use had higher baseline CCI compared with those on chronic steroid use (1.42 ± 1.91 vs 1.25 ± 1.72 respectively; P < 0.001).
Table 1.
Baseline characteristics of patients with underlying inflammatory bowel disease with chronic corticosteroid therapy compared with those not on chronic corticosteroid therapy
| Not on chronic corticosteroid therapy (n = 268 940; 93.65%) | Chronic corticosteroid therapy (n = 18 030; 6.35%) | P‐value | |
|---|---|---|---|
| Mean age (±SD) | 52.67 (±20.14) | 49.70 (±19.94) | <0.001 |
| Charlson comorbidity index (±SD) | 1.42 (±1.91) | 1.25 (±1.72) | <0.001 |
| Gender | <0.001 | ||
| Male | 117 500 (43.69%) | 8492 (47.10%) | |
| Female | 151 440 (56.31%) | 9538 (52.90%) | |
| Race | <0.001 | ||
| White | 210 876 (78.41%) | 13 966 (77.46%) | |
| Black | 30 148 (11.21%) | 2299 (12.75%) | |
| Hispanic | 17 158 (6.38%) | 1084 (6.01%) | |
| Asian or Pacific Islander | 3362 (1.25%) | 234 (1.30%) | |
| Native American | 1049 (0.39%) | 49 (0.27%) | |
| Other | 6374 (2.37%) | 400 (2.22%) | |
| Length of stay (day) † | Reference | 0.26 | <0.001 |
Mean difference adjusted for payer status, age, gender, race, hospital bed size, Charlson comorbidity index (CCI), day of admission, hospital region, and median income quartile based on zip code.
The cohort with chronic steroid use had a 1.9‐fold increase in developing osteoporosis compared with those without chronic steroid use (P < 0.001; CI 1.77–2.05). Similarly, patients with chronic steroid use had a 2.1‐fold increase in opportunistic infections (P < 0.001; CI 1.80–2.41) and a 1.5‐fold increase in developing acute thrombotic events (P < 0.001; CI 1.34–1.64) (Fig. 1). They also had a 10‐fold increase in developing Cushing's syndrome (P < 0.001; CI 7.29–14.67). Lastly, patients on chronic steroids had a significantly increased odds, nearly nine‐fold, of developing adrenal insufficiency (P < 0.0001; CI 6.96–10.62).
Figure 1.
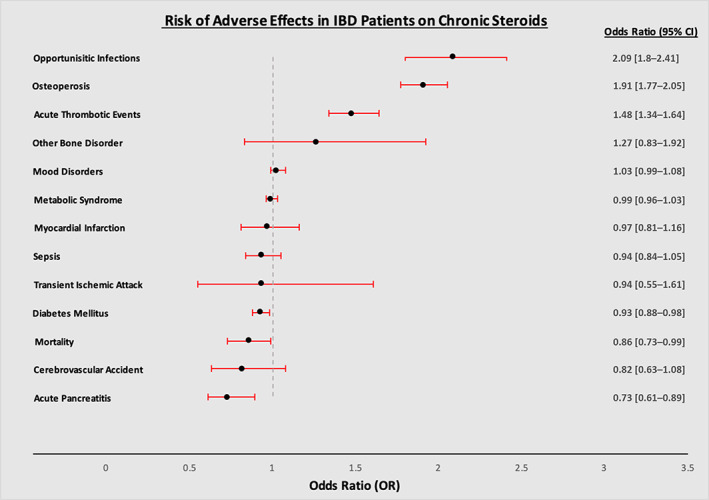
Adjusted odd ratio of various adverse events in patients with inflammatory bowel disease and concurrent chronic corticosteroid therapy compared with those not on chronic corticosteroid therapy.
There were no statistically significant differences in the risk of developing sepsis, TIA, CVA, MI, other bone disorders, other metabolic syndrome related disorders, drug‐induced cataracts, or mood disorders among the two cohorts (Table 2). However, patients within the chronic steroid cohort were found to have a longer LOS (adjusted mean difference 0.26 days, P < 0.001) than those not on steroids and did not have higher odds of mortality (aOR 0.86, P = 0.049).
Table 2.
Adjusted odds ratio of various adverse outcomes in patients with inflammatory bowel disease on chronic corticosteroid therapy compared with those not on chronic corticosteroid therapy
| Patients with inflammatory bowel disease and steroid use (n = 18 030) | |||
|---|---|---|---|
| Side effects | Odds ratio | P‐value | Confidence interval |
| Osteoporosis | 1.91 | <0.001 | 1.77–2.05 |
| Other bone disorders | 1.27 | 0.271 | 0.83–1.92 |
| Opportunistic infections | 2.09 | <0.001 | 1.80–2.41 |
| Sepsis | 0.94 | 0.252 | 0.84–1.05 |
| Acute thrombotic event | 1.48 | <0.001 | 1.34–1.64 |
| Transient ischemic attack | 0.94 | 0.830 | 0.55–1.61 |
| Cerebrovascular accident | 0.82 | 0.156 | 0.63–1.08 |
| Myocardial infarction | 0.97 | 0.719 | 0.81–1.16 |
| Diabetes mellitus | 0.93 | 0.007 | 0.88–0.98 |
| Cushing's syndrome | 10.35 | <0.001 | 7.29–14.67 |
| Adrenal insufficiency | 8.59 | <0.001 | 6.96–10.62 |
| Metabolic syndrome | 0.99 | 0.958 | 0.96–1.03 |
| Acute pancreatitis | 0.73 | 0.002 | 0.61–0.89 |
| Mood disorders | 1.03 | 0.129 | 0.99–1.08 |
| Drug‐induced cataract | 2.98 | 0.349 | 0.30–29.34 |
| Mortality | 0.86 | 0.049 | 0.74–0.99 |
Discussion
The prolonged use of corticosteroids in IBD patients poses an immense risk for increased morbidity when compared against immunomodulators and biologic therapies. 8 Despite this fact, it has been documented that one in eight patients with UC and one in four patients with CD in the United Kingdom experience chronic steroid use of greater than 6 months. This was also found to be true in Europe, Canada, and the United States within the first 5 years of diagnosis. 9 Our study revealed that patients with chronic steroid use had an increased risk of thrombotic events, osteoporosis, and opportunistic infections.
The cause for the prothrombotic state in IBD appears to be due to local or systemic inflammation and is dependent on disease severity. 10 Spina et al. searched for possible inherited causes of thrombosis in 47 IBD patients with either arterial or venous thrombus sites. Among all patients, the genetic causes of thrombosis were significantly less frequent, suggesting acquired risk factors as the root cause. Twig et al. discussed possible mechanisms and believe that the intestinal inflammation in IBD activates the coagulation pathway, which causes the increased release of prothrombotic factors. 11 Another study suggests that upregulation of cytokines such as interleukin‐6 (IL‐6) and tumor necrosis factor (TNF) leads to the prothrombotic state in IBD patients. 12
In patients with IBD there are several risk factors that contribute to the increased risk of osteoporosis, such as chronic inflammation, steroid use, nutritional deficiencies, and bowel resection. 13 Osteoporosis has been found in approximately 20–50% of both male and female patients with IBD. 14 Our study strengthens this finding in that IBD patients with chronic steroid use had nearly two‐fold higher odds of osteoporosis. One study found that the majority of patients with IBD between the age of 20 and 40 years had a significant reduction in the bone mineral density of their lumbar spines, with 67% of patients with UC and 23–55% of patients with CD having osteopenia. 14 Reinshagen suggested that the steroid use and chronic inflammatory process leads to bone loss through TNF‐alpha mediated inhibition of the osteoprotegerin (OPG) system. TNF‐alpha is released by T cells that are activated by the inflammation and increase the binding of RANKL and RANK, which in turn increase bone resorption and risk for bone erosion. 15 Osteoprotegerin on the other hand is secreted by osteoblasts and protects the skeleton from excessive resorption by binding to the RANKL and inhibiting RANK from binding to it.
The increased use of immunosuppressive drugs in the treatment of IBD has raised concerns about infection risks. Data from Janssen Biotech in Pennsylvania found that 1.81 of every 1000 patients developed opportunistic infections after chronic steroid use. 16 Our study further supports this finding as IBD patients with chronic steroid use had a two‐fold higher odds of developing opportunistic infections. In addition, Irving et al. conducted a matched cohort study from 2014 to 2019 where they evaluated the risk of common infections such as upper respiratory tract infections (URTI), acute bronchitis, GI infections, skin infections, herpes zoster, urinary tract infection, and pneumonias in IBD patients compared with matched controls. They found that patients with IBD had an increased risk of developing common infections, 46% with IBD compared with 36% without IBD. 17 Irving et al. went one step further and found that IBD patients on oral steroids and other immunotherapies also had increased risk of infection compared with those only on 5‐ASA.
Alternatives to steroids should always be considered when prescribing medications for patients with IBD. Current guidelines recommend 5‐aminosalicylic acid (5‐ASA) as a first‐line treatment option, before corticosteroids, in mild to moderate UC. Several therapies offer a possible steroid sparing option such as thiopurines, anti‐TNF agents, vedolizumab, ustekinumab, and tofacitinib. 9 Barriers to specialist advice, timely escalation, and patient education likely contribute to high rates of excess prescription steroids in a primary care setting. Selinger et al. conducted a study across 20 hospitals and implemented quality improvement initiatives and found that the 11 hospitals that made these changes were less likely to have used excess prescriptions of steroids. 18
The strengths of this study include the large sample size, which increases the power of the study, and the availability of detailed data the NIS provided us, such as differences among races. More importantly, this study was conducted using the NIS database and did not allow for any selection bias. Another strength that our study poses is that it raises awareness to a very common problem that arises in patients with IBD. Despite these strengths, several limitations existed in our study. One limitation is the administrative nature of the dataset because patient encounters were not able to be traced longitudinally and information regarding readmission and whether the patient had undergone surgery was not assessed. Also, we were unable to determine whether the patients had ≥2 diagnoses of IBD (UC or Crohn's) so misclassification could have been possible in some cases. Another limitation is that the NIS does not provide the severity of the disease process and establishing a timeline regarding the disease process is difficult. This is particularly important as patients who had severe disease would theoretically be at a higher risk for developing adverse events than those with mild or moderate disease. Lastly, smoking history was not able to be accounted for, which poses a potential confounding variable as smoking has been linked to disease severity in IBD.
Conclusion
Corticosteroids remain an essential tool in treating patients with IBD. Their use and misuse is a key factor in the quality of life of patients with IBD and warrants further monitoring. It is imperative that physicians consider an immunomodulator or anti‐TNF agent in patients with IBD who are on steroids, as long‐term use of steroids can have various adverse outcomes, as found in our study. Further research is warranted to determine whether chronic steroid use should be included in the maintenance management of IBD and whether the benefits outweigh the risks.
Declaration of conflict of interest: The authors declare that there is no actual or potential conflict of interest with financial support or competing interests.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its tables and figures. The National Inpatient Sample (NIS) fourth quarter of 2015–2019, a publicly available data sample, was used in this study.
References
- 1. Jones JH, Lennard‐Jones JE. Corticosteroids and corticotrophin in the treatment of Crohn's disease. Gut. 1966; 7: 181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Truelove SC, Witts LJ. Cortisone in ulcerative colitis: preliminary report on a therapeutic trial. Br. Med. J. 1954; 2: 375–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Irving PM, Gearry RB, Sparrow MP, Gibson PR. Review article: appropriate use of corticosteroids in Crohn's disease [published correction appears in Aliment Pharmacol Ther. 2008 Mar 15;27(6):528‐9]. Aliment. Pharmacol. Ther. 2007; 26: 313–29. [DOI] [PubMed] [Google Scholar]
- 4. Lichtenstein GR, Feagan BG, Cohen RD et al. Serious infection and mortality in patients with Crohn's disease: more than 5 years of follow‐up in the TREAT™ registry. Am. J. Gastroenterol. 2012; 107: 1409–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Targownik LE, Nugent Z, Singh H, Bernstein CN. Prevalence of and outcomes associated with corticosteroid prescription in inflammatory bowel disease. Inflamm. Bowel Dis. 2014; 20: 622–30. [DOI] [PubMed] [Google Scholar]
- 6. Melmed GY, Siegel CA. Quality improvement in inflammatory bowel disease. Gastroenterol. Hepatol. 2013; 9: 286–92. [PMC free article] [PubMed] [Google Scholar]
- 7. Selinger CP, Parkes GC, Bassi A et al. A multi‐centre audit of excess steroid use in 1176 patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017; 46: 964–73. [DOI] [PubMed] [Google Scholar]
- 8. National Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP) . Agency for Healthcare Research and Quality, Rockville Cited 15 Feb 2022. Available from URL: https://www.hcup-us.ahrq.gov/nisoverview.jsp.
- 9. Blackwell J, Selinger C, Raine T, Parkes G, Smith MA, Pollok R. Steroid use and misuse: a key performance indicator in the management of IBD. Frontline Gastroenterol. 2020; 12: 207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spina L, Saibeni S, Battaglioli T, Peyvandi F, de Franchis R, Vecchi M. Thrombosis in inflammatory bowel diseases: role of inherited thrombophilia. Am. J. Gastroenterol. 2005; 100: 2036–41. [DOI] [PubMed] [Google Scholar]
- 11. Twig G, Zandman‐Goddard G, Szyper‐Kravitz M, Shoenfeld Y. Systemic thromboembolism in inflammatory bowel disease: mechanisms and clinical applications. Ann. N. Y. Acad. Sci. 2005; 1051: 166–73. [DOI] [PubMed] [Google Scholar]
- 12. Esmon CT. Inflammation and thrombosis. J. Thromb. Haemost. 2003; 1: 1343–8. [DOI] [PubMed] [Google Scholar]
- 13. Annese V. A Review of Extraintestinal Manifestations and Complications of Inflammatory Bowel Disease. Saudi J. Med. Med. Sci. 2019; 7: 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reinshagen M. Osteoporosis in inflammatory bowel disease. J. Crohns Colitis. 2008; 2: 202–7. [DOI] [PubMed] [Google Scholar]
- 15. Saidenberg Kermanac'h N, Bessis N, Cohen‐Solal M, De Vernejoul MC, Boissier MC. Osteoprotegerin and inflammation. Eur. Cytokine Netw. 2002; 13: 144–53. [PubMed] [Google Scholar]
- 16. Veereman‐Wauters G, de Ridder L, Veres G et al. Risk of infection and prevention in pediatric patients with IBD: ESPGHAN IBD Porto Group commentary. J. Pediatr. Gastroenterol. Nutr. 2012; 54: 830–7. [DOI] [PubMed] [Google Scholar]
- 17. Irving PM, de Lusignan S, Tang D, Nijher M, Barrett K. Risk of common infections in people with inflammatory bowel disease in primary care: a population‐based cohort study. BMJ Open Gastroenterol. 2021; 8: e000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Selinger CP, Parkes GC, Bassi A et al. Assessment of steroid use as a key performance indicator in inflammatory bowel disease‐analysis of data from 2385 UK patients. Aliment. Pharmacol. Ther. 2019; 50: 1009–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its tables and figures. The National Inpatient Sample (NIS) fourth quarter of 2015–2019, a publicly available data sample, was used in this study.


