Abstract
Background and Aim
Helicobacter pylori (H. pylori) eradication has become popular as it prevents the development of gastric cancer. There have been no comprehensive studies on advanced gastric cancer (AGC) after eradication; thus, the clinical characteristics remain unclear. This study aimed to compare the characteristics of AGC after eradication and with current H. pylori infection and evaluate the esophagogastroduodenoscopy (EGD) follow‐up after eradication.
Methods
This single‐center, retrospective study included 261 consecutive patients diagnosed with AGC through EGD. The patients were grouped based on their H. pylori status: eradication (n = 48) and infection (n = 213) groups. Univariate analysis was conducted to compare clinicopathological characteristics between groups. The clinical course of the eradication group was analyzed by dividing the patients into three groups according to the interval from the last EGD until AGC detection: short‐interval (<1 year), intermediate‐interval (2–3 years), and long‐interval (4–5 years) groups.
Results
The radical resection (R0) rate was higher in the eradication group. In surgical cases, the median tumor diameter was shorter in the eradication group. Analysis of EGD surveillance after eradication in 36 available cases showed that 24 (66.7%) were detected within 5 years after eradication, and 3 (8.3%) were diagnosed as AGC > 20 years after eradication. The R0 rates in the short‐, intermediate‐, and long‐interval groups were 83.3%, 71.4%, and 60%, respectively.
Conclusions
AGC after eradication was more often detected at the phase in which R0 resection was possible. EGD follow‐up with tight intervals of at least 5 years after eradication is advisable.
Keywords: advanced gastric cancer, endoscopic surveillance, eradication, Helicobacter pylori
Introduction
Helicobacter pylori (H. pylori) infection is a risk factor for gastric cancer (GC). 1 , 2 , 3 Fukase et al. 4 showed that H. pylori eradication reduces the risk of GC development in patients who underwent endoscopic submucosal dissection for managing early GC (EGC). Based on these results, H. pylori eradication treatment has become common. Accordingly, several studies have reported on the clinicopathological characteristics of GC after H. pylori eradication, 5 , 6 , 7 most of which focus on EGC. In Japan, the 2018 statistics showed that GC is the third leading cause of death among all cancerous lesions, due to advanced GC (AGC). In routine esophagogastroduodenoscopy (EGD), we occasionally encounter AGC that has progressed despite H. pylori eradication. There have been no comprehensive studies on the characteristics of AGC after H. pylori eradication, and its characteristics remain unclear.
Therefore, this study aimed to clarify the clinicopathological characteristics of AGC after H. pylori eradication in comparison with AGC with current H. pylori infection. Furthermore, to explore the optimal strategy for EGD follow‐up after eradication, we analyzed the number of years after eradication and the intervals of EGD surveillance in cases with eradication.
Methods
Patients
This retrospective, single‐center study included 538 consecutive patients who were clinically diagnosed with AGC through EGD between January 2018 and December 2019. Among the 538 patients, 463 were selected after excluding those who had not been examined for H. pylori status (n = 75). The 463 patients, according to the criteria outlined below, were grouped into the eradication group and infection group (Fig. 1).
Figure 1.
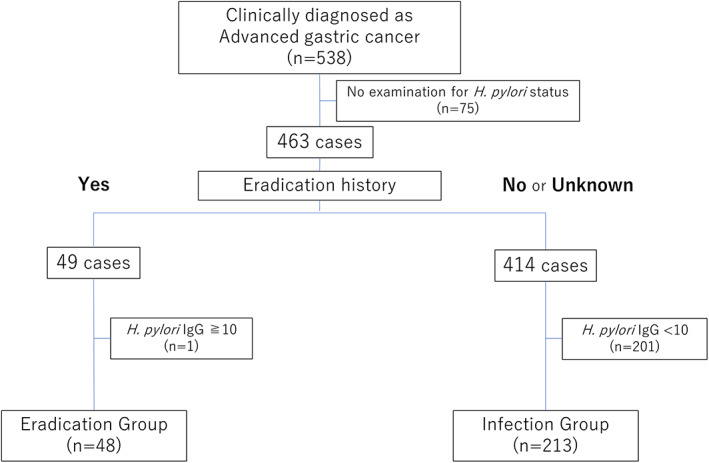
Flowchart of patient enrollment
The eradication group included patients (1) with a history of H. pylori eradication and (2) negative for H. pylori on laboratory examinations (i.e., H. pylori immunoglobulin G [IgG] antibody [H. pylori antibody II, EIKEN Co. Ltd.] level < 10 U/mL or negative urea breath test result). Patients with no eradication history or unknown eradication history were excluded from the eradication group. A representative endoscopic image of the eradication group is shown in Fig. 2a.
Figure 2.
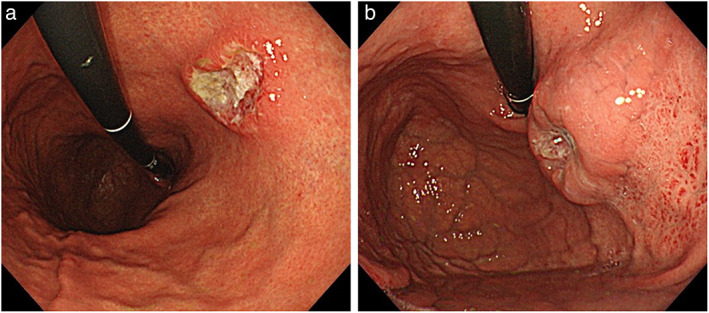
Endoscopic images of advanced gastric cancer. (a) Advanced gastric cancer (AGC) after Helicobacter pylori (H. pylori) eradication. Type 2 lesion located at the lesser curvature of the middle body. The background mucosa shows atrophic gastritis, consistent with after H. pylori eradication. (b) AGC with current H. pylori infection. Type 2 lesion located at the anterior wall of the upper body. Diffuse redness is exhibited, suggesting H. pylori current infection.
The infection group included patients (1) with no history of H. pylori eradication and (2) positive for H. pylori on laboratory examinations (i.e., H. pylori IgG antibody level ≥ 10 U/mL or positive urea breath test result). Patients with a history of eradication were excluded from the infection group. A representative endoscopic image of the infection group is shown in Fig. 2b.
This study was approved by the Institutional Review Board of the Cancer Institute Hospital (Institutional Review Board no. 2020–1179).
Clinicopathological assessment
According to the Japanese Classification of Gastric Carcinoma, 8 AGC comprises pT2, pT3, or pT4 tumors irrespective of lymph node metastasis. The location and macroscopic type of GC were classified according to the Japanese Classification of Gastric Carcinoma. The histological type was classified into differentiated and undifferentiated types according to the definition by Nakamura et al. 9 According to the Japanese Classification of Gastric Carcinoma, the differentiated type consists of tumors corresponding to papillary adenocarcinoma (pap), well‐differentiated tubular adenocarcinoma (tub1), or moderately differentiated tubular adenocarcinoma (tub2), and the undifferentiated type consists of tumors corresponding to poorly differentiated tubular adenocarcinoma (por) or signet‐ring cell carcinoma (sig). In this study, differentiated‐type predominant adenocarcinoma and undifferentiated‐type predominant adenocarcinoma were considered as differentiated type and undifferentiated type, respectively. Other histological types, such as neuroendocrine carcinoma, squamous cell carcinoma, and hepatoid adenocarcinoma, were defined as special types.
In the present analysis, we considered the family history of GC in first‐degree relatives only, that is, parents, siblings, and children. The Brinkman index (BI) is the product of the number of cigarettes smoked per day multiplied by the duration of use (days). BI was calculated based on the data recorded during patient interviews.
Comparative analysis of the eradication group and infection group
Patients' characteristics were summarized by subgroups of the eradication group and infection group, and all cases in both groups were compared using univariate analysis to clarify the clinical characteristics of the eradication group. The clinical characteristics included age, sex, family history of GC, comorbidities requiring medication (hypertension, dyslipidemia, and type 2 diabetes mellitus), smoking history (BI), tumor location, morphological type, radical resection (R0) rate, and clinical stage.
Comparative analysis confined to surgical cases
Furthermore, cases in which surgery was performed as initial treatment in both groups were compared using univariate analysis to clarify the pathological characteristics of the eradication group. Pathological characteristics included tumor diameter, histological type, invasive depth, lymphovascular invasion, and pathological stage. Cases in which chemotherapy was administered before surgery were excluded from surgical cases.
Clinical course after H. pylori eradication
We analyzed the clinical course after H. pylori eradication relative to the number of years after eradication and the interval of EGD surveillance. Patients in the eradication group were classified into three groups according to the interval from the last EGD until AGC was detected, short‐interval group (within 1 year), intermediate‐interval group (within 2–3 years), and long‐interval group (within 4–5 years). Patients who were uncertain about when they had their last EGD were excluded.
Statistical analysis
All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria, version 3.4.1). 10 Categorical parameters were compared using the χ2‐test and continuous parameters using the Mann–Whitney U test. In all analyses, a P value <0.05 indicated statistical significance.
Results
Forty‐eight patients in the eradication group and 213 patients in the infection group (28 patients in the eradication group and 96 patients in the infection group underwent surgical treatment as initial treatment) were selected.
Comparison of clinicopathological characteristics between the eradication and infection groups in all cases in this study
A comparison of characteristics between the eradication and infection groups is shown in Table 1. The ratio of smoking history was significantly higher (P < 0.05) in the eradication group (n = 35, 72.9%) than in the infection group (n = 109, 51.2%), while the BI showed no difference between the two groups (P = 0.18). The R0 rate was significantly higher in the eradication group (n = 35, 72.9%) than in the infection group (n = 117, 54.9%) (P < 0.05). There were no significant differences in age, sex, family history of GC, hypertension, dyslipidemia, type 2 diabetes mellitus, tumor location, macroscopic types, treatment methods, and clinical stage.
Table 1.
Comparison of characteristics between the eradication and infection groups
| Eradication group | Infection group | ||
|---|---|---|---|
| (n = 48) | (n = 213) | P value | |
| Age, years | 66 ± 13.3 | 66 ± 12.5 | ns |
| Sex, male, n (%) | 34 (70.8) | 136 (63.8) | ns |
| Family history of gastric cancer, n (%) | 12 (25.0) | 53 (24.9) | ns |
| Hypertension, n (%) | 16 (33.3) | 67 (31.5) | ns |
| Dyslipidemia, n (%) | 12 (25.0) | 29 (13.6) | ns |
| Type 2 diabetes mellitus, n (%) | 8 (16.7) | 32 (15.0) | ns |
| Smoking history, n (%) | 35 (72.9) | 109 (51.2) | <0.05 |
| Brinkman index | 280 (0–492.5) [0–1520] | 70 (0–640) [0–3300] | ns |
| Location, n (%) | ns | ||
| Upper region | 15 (31.3) | 52 (24.4) | |
| Middle region | 22 (45.8) | 91 (42.7) | |
| Lower region | 11 (22.9) | 69 (32.4) | |
| Macroscopic type, n (%) | ns | ||
| Type 0 | 4 (8.3) | 22 (10.3) | |
| Type 1 | 2 (4.2) | 7 (3.3) | |
| Type 2 | 11 (22.9) | 42 (19.7) | |
| Type 3 | 15 (31.3) | 99 (46.5) | |
| Type 4 | 16 (33.3) | 38 (17.8) | |
| Type 5 | 1 (2.1) | 5 (2.3) | |
| Treatment method, n (%) | ns | ||
| Surgery | 28 (58.3) | 96 (45.1) | |
| Conversion surgery | 6 (12.5) | 31 (14.5) | |
| Chemotherapy | 14 (29.2) | 77 (36.2) | |
| Radical resection, n (%) | 35 (72.9) | 117 (54.9) | <0.05 |
| Clinical stage, n (%) | ns | ||
| IB | 6 (12.5) | 15 (7.0) | |
| II | 11 (22.9) | 41 (19.2) | |
| III | 16 (33.3) | 56 (26.3) | |
| IV | 15 (31.2) | 101 (47.4) |
Age is expressed as mean ± SD. Brinkman index is expressed as median (interquartile range). Eradication group: advanced gastric cancer after Helicobacter pylori (H. pylori) eradication; infection group: advanced gastric cancer with current H. pylori infection; ns, not significant.
Comparison of clinicopathological characteristics between the eradication and infection groups in surgical cases in this study
A comparison of the characteristics of surgical cases with initial treatment between the eradication and infection groups is shown in Table 2. The median tumor diameter of the eradication group tended to be smaller than that of infection group (37.5 ± 56.4 mm vs. 50.0 ± 36.6 mm, P = 0.056). No significant differences were found between groups in age, sex, and histological type, invasion depth, lymphovascular invasion, and pathological stage. In both groups, the histological type of more than half of the patients was undifferentiated‐type adenocarcinoma, and there was no significant difference between the two groups.
Table 2.
Comparison of characteristics in surgical cases between the eradication and infection groups
| Eradication group | Infection group | ||
|---|---|---|---|
| (n = 28) | (n = 96) | P value | |
| Age, years | 65 ± 13.0 | 64 ± 13.1 | ns |
| Sex, male, n (%) | 19 (67.9) | 57 (59.4) | ns |
| Tumor diameter, mm | 37.5 ± 56.4 [15–200] | 50 ± 36.6 [18–203] | 0.056 |
| Histological type, n (%) | ns | ||
| Differentiated type | 9 (32.1) | 29 (30.2) | |
| Undifferentiated type | 17 (60.7) | 66 (68.8) | |
| Special type | 2 (7.1) | 1 (1.0) | |
| Invasion depth, n (%) | ns | ||
| pT2 | 8 (28.6) | 24 (25.0) | |
| pT3 | 11 (39.2) | 26 (27.1) | |
| pT4a | 7 (25.0) | 44 (45.8) | |
| pT4b | 2 (7.1) | 2 (2.1) | |
| Lymphovascular invasion, n (%) | ns | ||
| Ly0 and V0 | 4 (14.3) | 10 (10.4) | |
| Ly1 or V1 | 7 (25.0) | 41 (42.7) | |
| Ly1 and V1 | 17 (60.7) | 45 (46.9) | |
| Pathological stage, n (%) | ns | ||
| IB | 6 (21.4) | 16 (16.7) | |
| II | 8 (28.6) | 37 (38.5) | |
| III | 14 (50.0) | 34 (35.4) | |
| IV | 0 | 9 (9.3) |
Age is expressed as mean ± SD. Tumor diameter is expressed as median ± SD [range]. Eradication group: advanced gastric cancer after Helicobacter pylori (H. pylori) eradication; infection group: advanced gastric cancer with current HH. pylori infection; ns, not significant; Ly0 and Ly1, negative and positive for lymphatic invasion, respectively; V0 and V1, negative and positive for venous invasion, respectively.
Analysis of the clinical course in the eradication group
Of the 48 cases in the eradication group, 36 (75%) had information on the period between the time of their eradication therapy and the time when AGCs were detected. Their distribution is shown in Figure 3. The median interval period was 4.5 years; 24 cases (66.7%) were detected in <5 years after eradication and 3 cases were detected >20 years after eradication. Twenty‐four patients (50%) were able to identify the interval from the last EGD until AGC was detected. There were 12, 7, and 5 patients in the short‐, intermediate‐, and long‐interval groups, respectively. The R0 rates for the short‐, intermediate‐, and long‐interval groups were 83.3% (10/12), 71.4% (5/7), and 60% (3/5), respectively (P = 0.53).
Figure 3.
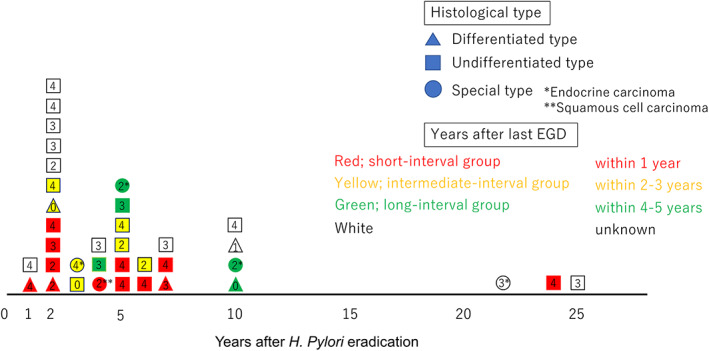
Distribution of years elapsed after Helicobacter pylori (H. pylori) eradication. Thirty‐six cases (75%) had information on the period between the time of their eradication therapy and the time when advanced gastric cancers were detected. Histological types are indicated by the different shapes, and groups based on years after the last esophagogastroduodenoscopy are indicated by the different colors. The numbers in the diagrams indicate the morphological type.
Discussion
We evaluated the clinicopathological characteristics of AGC after H. pylori eradication by comparison with currently H. pylori‐infected AGC as the control group. The results of this study showed that AGC after eradication was significantly more often detected at the phase in which R0 resection was possible. The analysis of the clinical course and the interval between EGDs after eradication suggest the optimal strategy for EGD follow‐up after eradication. To our knowledge, no comprehensive study on the characteristics of AGC after eradication has been conducted because eradication therapy has not been widely used for a long time. Therefore, the present study is considered valuable because a relatively large number of patients with AGC were examined based on the H. pylori infection status.
In recent years, there have been several reports 5 , 6 , 7 on the characteristics of EGC after H. pylori eradication. EGC after H. pylori eradication is a smaller size than EGC with current H. pylori infection. Regarding AGC in the present study, in the eradication group, the tumor diameter of AGC in the eradication group tended to be smaller than that in the infection group. In a pathological study by Yamamoto et al., 5 GC detected after H. pylori eradication was smaller and had a lower Ki‐67 labeling index than H. pylori‐positive GCs, speculating that GC cell proliferation was suppressed by H. pylori eradication. Yamamoto et al.'s study was confined to EGC; furthermore, most GCs after eradication were differentiated‐type adenocarcinoma (17/18). Therefore, the result of the previous study cannot be generally applied to our study, because more than half of the AGCs after eradication we examined were undifferentiated‐type adenocarcinoma. However, in the present study, the R0 rate was significantly higher, and the tumor size tended to be smaller in the eradication group. Therefore, H. pylori eradication may have some inhibitory effects on cell proliferation in AGC as well as EGC, but these need to be investigated in the future. It is not clear whether AGC after H. pylori eradication has the same suppressed tumor proliferative ability as EGC, or our result might be because AGC in the eradication group was detected at a relatively earlier stage than that in the infection group, simply due to the tight interval between EGD surveillance.
Reportedly, most EGCs after H. pylori eradication are detected within a short period. 6 In the present study, 66.7% (24/36) of the cases were detected within 5 years after H. pylori eradication. In addition, 91.7% (11/12) in the short‐interval group were detected within 7 years after eradication. Therefore, EGD follow‐up with tight intervals of at least 5 years after eradication is advisable. In cases where AGC was detected 10 years after eradication, at least half of the patients did not undergo endoscopic follow‐up 4 or 5 years prior, resulting in GC being diagnosed in the advanced stage. Furthermore, there were three cases of AGC detected 20 years after H. pylori eradication, and two of them were undifferentiated‐type adenocarcinomas, which are known to have a poorer prognosis than the differentiated type. 11 , 12 , 13 The possibility of GC development should be considered even after long periods after H. pylori eradication. By contrast, the present study included two cases of AGC that developed within 1 year after eradication therapy. They were both type 4 AGC, which is known to progress rapidly and show poor prognosis. 14 , 15
In the present study, the proportion of patients with smoking history was significantly higher in the eradication group than in the infection group (72.9% vs. 51.2%, P < 0.05), while the BI showed no difference between the two groups (P = 0.18). Two previous meta‐analyses have reported on smoking and GC and concluded that smoking is associated with GC 16 , 17 ; however, no association has been proven for the amount or duration of smoking. Horiuchi et al. compared patient characteristics, such as medical history and alcohol and tobacco use, between patients with H. pylori‐uninfected undifferentiated‐type EGC and patients with H. pylori‐positive undifferentiated‐type EGC. The investigation of clinical factors identified smoking history as possibly contributing to the pathogenesis of H. pylori‐uninfected undifferentiated‐type EGC. 18 In the eradication group, the effects of chronic inflammation due to H. pylori infection may be weak, if any, especially in patients who have been eradicated for a long time. 19 In fact, in this study, the smoking rate was as high as 85.7% (6 of 7) among the patients who had been eradicated for more than 10 years. Therefore, the results of the present study may indicate a correlation between smoking and carcinogenesis after H. pylori eradication.
In the short‐interval group, the R0 rate was 83.3% (10 of 12), while in the long‐interval group, the R0 rate was 60% (3 of 5). A previous report 20 regarding the interval of endoscopic screening for GC showed that the survival rates were significantly higher in patients with an endoscopic screening history 1 and 2 years before diagnosis than in patients with endoscopic screening history 3 years before diagnosis. The present study indicates that an extended interval of endoscopic screening may be a potential risk factor for non‐curative GC, even in patients who had H. pylori eradication.
The present study has some limitations. First, this was a retrospective, single‐center study. Second, the present study did not accumulate enough AGC cases after H. pylori eradication for analysis. Third, the interval of EGD surveillance may differ between groups; this might have affected the characteristics of AGC after H. pylori eradication. Last, because our institution is a cancer‐specialized hospital, there might have been a selection bias when our study's prevalence rate is compared with the general prevalence rate, considering the frequency of patient referrals. However, this study included all patients with AGC who underwent EGD, and the bias is likely to be minimal in this single‐center study.
In conclusion, the R0 rate in the eradication group was significantly higher than that in infection group, and the tumor diameter in the eradication group tended to be smaller. The clinical characteristics of the eradication group might indicate a correlation between smoking and carcinogenesis after H. pylori eradication. Analysis of EGD surveillance methods in the eradication group suggested that EGD surveillance should be uninterrupted, and the interval between EGDs should not be extended.
Declaration of conflict of interest: None.
Financial support: The authors have no funding sources.
References
- 1. Parsonnet J, Friedman GD, Vandersteen DP et al. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 1991; 325: 1127–231. [DOI] [PubMed] [Google Scholar]
- 2. Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta‐analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998; 114: 1169–79. [DOI] [PubMed] [Google Scholar]
- 3. Uemura N, Okamoto S, Yamamoto S et al. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001; 345: 784–9. [DOI] [PubMed] [Google Scholar]
- 4. Fukase K, Kato M, Kikuchi S et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open‐label, randomized controlled trial. Lancet. 2008; 372: 392–7. [DOI] [PubMed] [Google Scholar]
- 5. Yamamoto K, Kato M, Takahashi M et al. Clinicopathological analysis of early‐stage gastric cancers detected after successful eradication of Helicobacter pylori . Helicobacter. 2011; 16: 210–16. [DOI] [PubMed] [Google Scholar]
- 6. Kamada T, Hata J, Sugiu K et al. Clinical features of gastric cancer discovered after successful eradication of Helicobacter pylori: results from a 9‐year prospective follow‐up study in Japan. Aliment. Pharmacol. Ther. 2005; 21: 1121–6. [DOI] [PubMed] [Google Scholar]
- 7. Mori G, Nakajima T, Asada K et al. Incidence of and risk factors for metachronous gastric cancer after endoscopic resection and successful Helicobacter pylori eradication: results of a large‐scale, multicenter cohort study in Japan. Gastric Cancer. 2016; 19: 911–18. [DOI] [PubMed] [Google Scholar]
- 8. Japanese Gastric Cancer Association . Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011; 14: 101–12. [DOI] [PubMed] [Google Scholar]
- 9. Nakamura K, Sugano H, Takagi K. Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gann. 1965; 59: 251–8. [PubMed] [Google Scholar]
- 10. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013; 48: 452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ribeiro MM, Sarmento JA, Sobrinho Simões MA, Bastos J. Prognostic significance of Lauren and Ming classifications and other pathologic parameters in gastric carcinoma. Cancer. 1981; 47: 780–4. [DOI] [PubMed] [Google Scholar]
- 12. Adachi Y, Yasuda K, Inomata M, Sato K, Shiraishi N, Kitano S. Pathology and prognosis of gastric carcinoma: well versus poorly differentiated type. Cancer. 2000; 89: 1418–24. [PubMed] [Google Scholar]
- 13. Lee T, Tanaka H, Ohira M et al. Clinical impact of the extent of lymph node micrometastasis in undifferentiated‐type early gastric cancer. Oncology. 2014; 86: 244–52. [DOI] [PubMed] [Google Scholar]
- 14. Chen CY, Wu CW, Lo SS, Hsieh MC, Lui WY, Shen KH. Peritoneal carcinomatosis and lymph node metastasis are prognostic indicators in patients with Borrmann type IV gastric carcinoma. Hepato‐Gastroenterology. 2002; 49: 874–7. [PubMed] [Google Scholar]
- 15. An JY, Kang TH, Choi MG, Noh JH, Sohn TS, Kim S. Borrmann type IV: an independent prognostic factor for survival in gastric cancer. J. Gastrointest. Surg. 2008; 12: 1364–9. [DOI] [PubMed] [Google Scholar]
- 16. Trédaniel J, Boffetta P, Buiatti E, Saracci R, Hirsch A. Tobacco smoking and gastric cancer: review and meta‐analysis. Int. J. Cancer. 1997; 72: 565–73. [DOI] [PubMed] [Google Scholar]
- 17. Ladeiras‐Lopes R, Pereira AK, Nogueira A et al. Smoking and gastric cancer: systematic review and meta‐analysis of cohort studies. Cancer Causes Control. 2008; 19: 689–701. [DOI] [PubMed] [Google Scholar]
- 18. Horiuchi Y, Fujisaki J, Ishizuka N et al. Study on clinical factors involved in Helicobacter pylori‐uninfected, undifferentiated‐type early gastric cancer. Digestion. 2017; 96: 213–19. [DOI] [PubMed] [Google Scholar]
- 19. Forbes GM, Warren JR, Glaser ME, Cullen DJ, Marshall BJ, Collins BJ. Long‐term follow‐up of gastric histology after Helicobacter pylori eradication. J. Gastroenterol. Hepatol. 1996; 11: 670–3. [DOI] [PubMed] [Google Scholar]
- 20. Hamashima C, Narisawa R, Ogoshi K, Kato T, Fujita K. Optimal interval of endoscopic screening based on stage distributions of detected gastric cancers. BMC Cancer. 2017; 17: 740–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


