Abstract

Purpose: Tadalafil’s exact analgesic mechanism is still unclear. The current study aimed to elucidate this mechanism in an inflammatory pain model. Methods: Computer-assisted simulation docking experiments were carried out to assess the binding of tadalafil to different ligands. The anti-inflammatory and analgesic effects of tadalafil were evaluated using formalin-induced paw edema and a von Frey filament test, respectively. The plantar paw of the mice was then dissected to quantify iNOS, nNOS, COX-2, TNFα, IL1, and IL10 gene expression levels using a real-time polymerase chain reaction. iNOS, TNFα, and COX-2 inhibition was reassessed in vitro using the ELISA technique. One-way analysis of variance followed by post hoc Tukey test or t-test was used to compare the means. Results: Docking analysis showed a superior binding score of tadalafil to COX-2, iNOS, IL-1, and TNF-α compared to that of indomethacin and morphine and a similar binding score to nNOS and IL-10 relative to that of indomethacin. In the in vivo study, tadalafil, after an hour of formalin administration, inhibited significantly paw edema, similar to indomethacin. Furthermore, it significantly increased the withdrawal force in the von Frey filament test as compared to the negative control, which was similar to the effect observed with indomethacin and morphine. The RT-PCR revealed that tadalafil reduced significantly the iNOS, COX-2, and TNF-α gene expressions but had no effect on nNOS, IL 1, and IL10. In vitro ELISA tests confirmed the inhibition of iNOS, COX-2, and TNF-α. Conclusion: Tadalafil probably exerts its analgesic effect through the simultaneous inhibition of iNOS, COX-2, and TNF-α, which is not the case with other nonsteroidal anti-inflammatory drugs. Nevertheless, further studies are required to confirm its mechanism.
1. Introduction
Pain is a normal physiological response of the body to injuries such as trauma, tissue damage, or inflammation.1 On the molecular level, cytokines such as tumor necrosis α (TNF-α) and interleukins (IL), eicosanoids formed through the action of cyclooxygenases and lipoxygenases, and histamine all play a key role in the pain sensation caused by inflammation.2 These inflammatory mediators activate cyclic adenosine 3,5-monophosphate/protein kinase A or protein kinase Cε pathways, which phosphorylate the voltage-gated ion channels and activate other channels. An inward electrical current, produced by these channels, depolarizes the neuronal membrane, leading to an increased probability of action potential generation. Upon the arrival of the action potential to the dorsal horn, neurotransmitters are released due to an increase in the intracellular calcium concentration. Neurotransmitters responsible for central sensitization include glutamate, substance P, and calcitonin gene-related peptide. Glutamate leads to the propagation of sharp, localized pain that results from activation of the N-methyl-d-aspartic acid (NMDA) receptors that generate nitric oxide (NO).1,3 Substance P binds to neurokinin 1, which leads to intracellular signaling that involves the activation of arachidonic acid pathways, NMDA receptors through activation of protein kinase C, and NO synthesis1,4 In fact, NO, concentrated in the dorsal horn of the spinal cord, is derived from diverse sources. Neuronal NO synthase (nNOS) is the predominant form of nitric oxide synthase in the dorsal horn and has a definite role in spinal cord circuits. The endothelial nitric oxide synthase (eNOS) is also found in some neuronal populations and blood vessels. Inducible nitric oxide synthase (iNOS) is expressed peripherally during inflammatory or neuropathic pain processes.1,3 NO has a dual role in nociception as observed by Kawabata et al. who found that a low dose of l-arginine enhanced nociception in the second phase of the formalin test, while a high dose suppressed this effect.5 Accordingly, NO donors have been considered analgesic drugs.1
Consequently, tadalafil, a phosphodiesterase 5 inhibitor, used to treat erectile dysfunction, has been investigated for its antinociceptive effect in various pain models.6,7 Clinically, tadalafil has been shown to significantly decrease the pain in cold complex regional pain syndrome patients.8 Investigation on rat arthritis models also supported the analgesic effect of tadalafil, which is suggested to be mediated by cGMP. Mehanna et al. proved that tadalafil exerts its analgesic effect through both the central and peripheral pain pathways.7 Furthermore, in the carrageenan-induced hyperplasia test, tadalafil was also proven to be a promising analgesic compound by inhibiting NO rather than increasing it.9 The effect of tadalafil is not reserved for male patients since Mayer et al. proved that phosphodiesterase inhibitors have some beneficial effects on female sexual disorders. Consequently, tadalafil may be a potential analgesic drug for males and females.10
Although all these studies have proven the efficacy of tadalafil as an analgesic agent, data are still insufficient to elucidate the exact mechanism of tadalafil’s analgesic effect to be approved clinically. Accordingly, the current study aimed to explicate the mechanism of tadalafil by investigating its effect on various mediators involved in inflammatory pain, namely cyclooxygenase-2 (COX2), interleukin-1 (Il-1), interleukin-10 (Il-10), nNOS, iNOS, and TNFα.
2. Materials and Methods
2.1. Animals
Male BALB/C mice (20–25 g) used in the in vivo experiments were retained under standard animal housing conditions including a temperature of 25 ± 2 °C, relative humidity of 40–70%, and dark–light cycles of 12/12 h. Animals had free access to water and standard laboratory chow. All procedures were done following the regulations and guidelines stipulated by the Institutional Animal Care and Use Guidelines at Beirut Arab University. Institutional Review Board approval was granted before the beginning of the procedures.
2.2. Chemicals
Pfizer, Sigma Chemical Co., and Renaudin were the sources of tadalafil, indomethacin, and morphine sulfate, respectively. Indomethacin served as a positive control for inflammation, while morphine sulfate was used for pain. Tadalafil and indomethacin were provided as a pure powder, which was dissolved in polyethene glycol 400 (PEG400) (Fluka analytica).11 Dilution of morphine sulfate was done using sterile water for injection. Indomethacin, morphine, and tadalafil were administered at a dose of 10, 5, and 1.5 mg/kg, respectively, according to a previous study done by Mehanna et al.7 All drugs were administered intraperitoneally (i.p.). Formalin prepared as 5% v/v was supplied by Sigma-Aldrich.
2.3. Molecular Docking and Mechanics Simulations
Molecular docking and mechanics simulations were carried out using Molecular Operating Environment (MOE) software. Interatomic interactions were modeled using the Merck Molecular force field (MMFF94X), where the total energy is given as12−16
EBij: bond stretching interaction; Kbij: harmonic force constrant; cs: cubic stretch constant; Δrij: deviation from the reference bond length between atoms i and j; EAijk: angle bending interaction; Kαijk: angular spring constant, cb: cubic constant; Δθijk: deviation from the reference bond angle between atoms i, j, and k; EBAijk:stretch bend interaction; EOOPijkl: out-of-plane bending; ETijkl: torsion interactions; EvdWij: van der Waals interactions; and EQij: electrostatic interactions.
The initial coordinates from the X-ray crystal structure of COX-2, iNOS, nNOS, IL-1, IL-10, and TNF-α used in the simulations were obtained from the Protein Data Bank (PDB ID: 5jw1, 2orr, 6pn4, 5r88, 3lqm, and 2az5, respectively). The ligand molecules were constructed using the builder molecule and were energy-minimized. The active sites of the receptors were generated using MOE-α Site Finder, and then, ligands were docked within this active site using MOE Dock. Consequently, molecular mechanics simulations were carried out using the enzyme parameters obtained from the crystallographic structure of the complex between COX-2 (PDB ID: 5jw1), iNOS (PDB ID: 2orr), nNOS (PDB ID: 6pn4), IL-1 (PDB ID: 5r88), IL-10 (PDB ID: 3lqm), and TNF-α (PDB ID: 2az5) with the cocrystallized ligands celecoxib, 4-(benzo[1,3]dioxol-5-yloxy)-2-(4-imidazol-1-yl-phenoxy)-pyrimidine, 7-(3-(aminomethyl)-4-(cyclopropylmethoxy)phenyl)-4-methylquinolin-2-amine, (S)-N-(4-carbamoylphenyl)-tetrahydrofuran-2-carboxamide, glycerol, and 6,7-dimethyl-3-[(methyl-{2-[methyl({1-[3-(trifluoromethyl)phenyl]-1H-indol-3 yl}methyl)amino]ethyl}amino)methyl]-4H-chromen-4-one, respectively.17−22 The lowest-energy conformation was selected, and the ligand interactions (hydrogen bonding, arene–H, and arene–arene interactions together with other hydrophobic interactions) with the receptors were recorded. Binding scores were calculated based on the strain energy between the two molecules based on the selected force field.
2.4. Experimental Design
2.4.1. Anti-inflammatory Activity of Tadalafil Using a Formalin-Induced Paw Edema Method
The acute anti-inflammatory activity of tadalafil was assessed using formalin-induced mice paw edema as described by Vogel.23 Indomethacin was used as a positive control. Mice were divided into 4 groups of 7–8 animals each. Outlier data were then omitted to retain five animals in each group. The first group received tadalafil at a dose of 1.5 mg/kg, being the ED50 as determined in a previous study.7 The second group received indomethacin at a dose of 10 mg/kg as a positive control, while the third and fourth groups received only the vehicle (PEG400) as a negative control group. After 1 h of injecting the compounds and the vehicle, 200 μL of a 5% v/v formalin solution was injected into the mice’s plantar side of the right hind paw except for the fourth group, which received a normal saline injection. The paw volume (Vpaw) was measured using the Ugo Basile 37140 plethysmometer immediately after the injection and at 1, 2, 3, and 4 h. The increase in paw volume (ΔVpaw) was calculated as
where Vpaw,t is the paw volume at the specific time t and Vpaw,injection is the paw volume immediately after formalin injection. The percentage increase in paw volume (%ΔVpaw) was then calculated as
where Vpaw,baseline is the paw volume at baseline. The percentage inhibition of paw edema was then determined as
where %ΔVpaw,control and %ΔVpaw,treated are the percentage increases in paw volume for the control and treated mice, respectively.
2.4.2. Assessment of Mechanical Nociception in Mice in Formalin-Induced Paw Edema Using Von Frey Filaments
The formalin-induced paw edema test is used to assess the nociceptive effect of tadalafil in two phases. The initial phase lasts for the first 5 min and is characterized by direct stimulation of the nociceptors. Accordingly, this stage is mainly inhibited by centrally-acting analgesic drugs. The second phase is between 15 and 30 min after the injection of formalin and is considered an inflammatory phase mediated by prostaglandins, NO, and others.24 The mechanical paw-withdrawal threshold measurement is done using von Frey filaments, according to the up-and-down model.25−27 Mice after receiving tadalafil or indomethacin or morphine or the vehicle are kept for 1 h to acclimatize in individual clear acrylic chambers fixed to an elevated wire mesh platform that allows access to the hind paw plantar surface. After 1 h of injecting each compound and the vehicle (PEG400), the mice are challenged with a 200 μL subcutaneous injection of 5% v/v formalin solution into the plantar side of the right hind paw. A second control group (PEG 400) received normal saline in the hind paw instead of formalin to determine the threshold without formalin-induced sensitization. Directly after injecting formalin or normal saline and after 30 min, von Frey filaments of 0.5–45.3 g were applied on the mice’s hind paw plantar surface. After 5 s, the absence of paw lifting led to the use of the next filament of increased weight, while paw lifting indicated a positive response that led to the use of the weakest filament. This paradigm continued until four consecutive negative or positive responses occurred. From the resulting scores, the mechanical paw-withdrawal threshold (mN) response was then calculated.28
2.4.3. In Vivo Investigation of Tadalafil’s Effect on Various Inflammatory Mediators Using the RT-PCR Technique
Mice were grouped into five groups of five mice each receiving intraperitoneally either the vehicle (polyethylene glycol 400) or indomethacin (10 mg/kg) or morphine (5 mg/kg) or tadalafil (1.5 mg/kg). After 1 h of the drug administration, 200 μL of 5%v/v formalin solution was injected into the right hind paw plantar surface; then, the mice were confined into a transparent chamber for 30 min. Afterward, the muscles of the hind paw were extracted after sacrificing the animals, and they were immediately frozen in liquid nitrogen. From the sacrificed animals’ tissues, total RNA was extracted using the TRIzol reagent. The total RNA yield and quality were spectrophotometrically determined using a NanoDrop (ND-1000, Wilmington, Delaware) at λ 260 and 260/280 nm, respectively. One mcg of the total RNA was reverse-transcribed, using a QuantiTect reverse transcription kit, into single-stranded complementary DNA (cDNA). Different inflammatory genes’ mRNA levels found in mouse tissues were determined using 5x FIREPol EvaGreen qPCR Mix, no ROX (Solis BioDyne, Tartu, Estonia). As a housekeeping gene and an internal control, mouse peptidylprolyl isomerase A (PPIA) was used. According to the nucleotide sequence obtained from the Gene Bank, gene-specific PCR primers (Table 1) were designed using Primer Express 3.0 (Applied Biosystems). Thermal cycling conditions used were as follows: initial activation step at 95 °C for 15 min followed by 40–50 cycles at 94 °C for 15 s, 56 °C for 20 s, and 72 °C for 30 s. Data were collected during the extension step using an automatic Rotor-Gene Q thermocycler (Qiagen). To verify the specificity and identity of the PCR products, a melting curve analysis was performed. The 2-ΔΔCt method was used to determine the mRNA relative expression levels of iNOS, nNOS, COX2, TNF-α, Il-1, and Il-10. Moreover, to confirm the PCR products, a 1.2% agarose gel electrophoresis run was performed.29
Table 1. Different Primers and Reverse Primers for Different Genes.
| genesa | primer | product size (bp) |
|---|---|---|
| iNOS | F:GGAATCTTGGAGCGAGTTGT | 99 |
| R:CCTCTTGTCTTTGACCCAGTAG | ||
| nNOS | F:GTGAGTGGACAGATGGAAGAAG | 101 |
| R:CCCTAGCACTGATAAGCAGAAG | ||
| COX-2 | F:CGGACTGGATTCTATGGTGAAA | 111 |
| R:CTTGAAGTGGGTCAGGATGTAG | ||
| TNFα | F:CTACCTTGTTGCCTCCTCTTT | 116 |
| R:GAGCAGAGGTTCAGTGATGTAG | ||
| IL1 | F:ATGGGCAACCACTTACCTATTT | 103 |
| R:GTTCTAGAGAGTGCTGCCTAATG | ||
| IL10 | F:TTGAATTCCCTGGGTGAGAAG | 95 |
| R:TCCACTGCCTTGCTCTTATTT | ||
| PPIA | F F:AATGCTGGACCAAACACAAA | 116 |
| R:TTCCACAATGTTCATGCCTT |
Inducible nitric oxide synthase (iNOS), neuronal nitric oxide synthase 1 (nNOS), cyclooxygenase-2 (COX2), tumor necrosis factor (TNFα), interleukin-1 (Il1), interleukin 10 (Il10), and peptidyl propyl isomerase A (PPIA).
2.4.4. In Vitro Investigation of Tadalafil’s Effect on iNOS, TNF-α, and COX-2 Using the ELISA Technique
iNOS inhibitory concentrations of tadalafil and the positive control were determined in vitro using an iNOS ELISA kit that applies the competitive enzyme immunoassay technique utilizing a polyclonal anti-iNOS antibody and an iNOS-HRP conjugate. After the reaction between the assay sample and the iNOS-HRS conjugate, the intensity of the blue-colored complex that appears was measured spectrophotometrically at 450 nm, which reflects the concentration of iNOS.
Using the TNF-α ELISA technique, the inhibitory concentrations of tadalafil and the positive control indomethacin were measured in vitro. The process was carried out in accordance with the ALPCO TNF-α ELISA kit, which makes use of a monoclonal antibody that is specific for Hu TNF-α. The absorbance was measured at 450 nm.
The in vitro colorimetric COX (ovine) inhibitor assay method, which makes use of the peroxidase component of cyclooxygenase, is used to conduct an in vitro COX-2 isoenzyme inhibition assay with tadalafil and the positive control indomethacin. By monitoring the appearance of oxidized N,N,N′,N′-tetramethyl-p-phenylenediamine, which is created during the reduction of PGG2 to PGH2, the peroxidase activity is measured colorimetrically at 590nm. The preparation of the reagent is carried out following the manufacturer’s instructions (catalog no. 560131; Cayman Chemicals Inc., AnnArbor, MI).
2.5. Statistical Analysis
Statistical Package for Social Science (SPSS) version 20 (IBM, New York-USA) was used to analyze the data, which were expressed as mean ± SD. One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test or t-test was used to assess the groups’ differences. Significance was considered at a p-value of less than 0.05.
3. Results
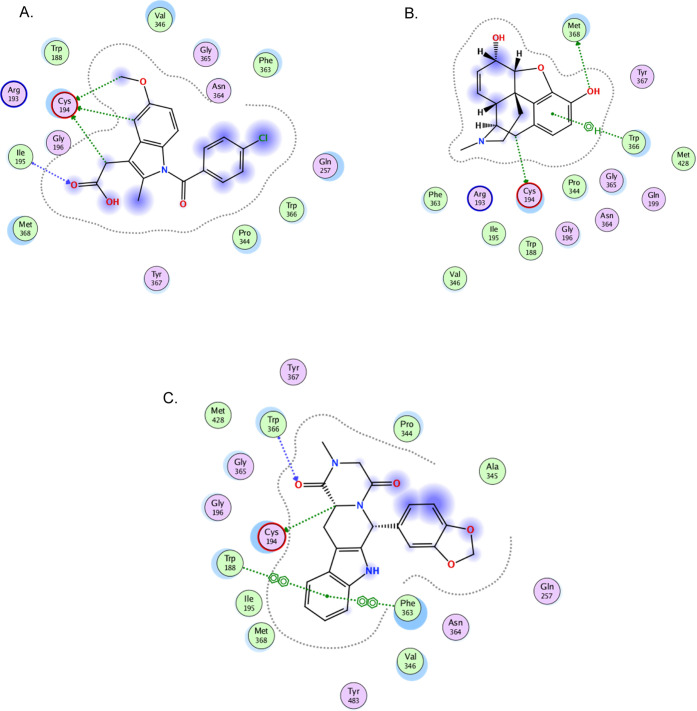
A docking study was used to assess the binding of tadalafil to different mediators involved in inflammatory pain. Comparing the binding modes of tadalafil to COX-2 versus the reference materials, celecoxib, indomethacin, and morphine, it was clear that celecoxib had superior binding interactions, where it formed four hydrogen bonds to different amino acids in the receptor. Morphine and tadalafil showed almost comparable bonding, where both formed one hydrogen bond with the receptor in addition to the hydrophobic interactions. Finally, indomethacin indicated the least binding, where it was only capable of hydrophobic binding to the receptor, even though it showed the best binding score (−7.7861). This indicated that indomethacin can reside within the pocket of the receptor without having the ability to form reasonable bonding with the different amino acid residues present in the active site (Table 2, Figure 1).
Table 2. Docking Scores of Tadalafil and the Positive Control Indomethacin and Morphine to the Binding Sites of COX2, iNOS, nNOS, IL-1, IL-10, and TNF-α.
| binding site | drug | docking score |
|---|---|---|
| COX-2 | indomethacin | –7.7861 |
| morphine | –5.1289 | |
| tadalafil | –8.1797 | |
| iNOS | indomethacin | –5.7651 |
| morphine | –5.4792 | |
| tadalafil | –7.5008 | |
| nNOS | indomethacin | –7.2830 |
| morphine | –5.6352 | |
| tadalafil | –7.2616 | |
| IL-1 | indomethacin | –5.8541 |
| morphine | –4.8657 | |
| tadalafil | –6.6988 | |
| IL-10 | indomethacin | –5.6242 |
| morphine | –4.3563 | |
| tadalafil | –5.9004 | |
| TNF-α | indomethacin | –5.0875 |
| morphine | –4.1433 | |
| tadalafil | –5.4228 |
Figure 1.
2D Binding mode of the inhibitor (celecoxib), references (indomethacin and morphine), and test drug tadalafil in the binding site of the COX-2 receptor (PDB ID: 5jw1) using MOE software. (A) 2D binding mode of the inhibitor celecoxib in the binding site of the COX-2 receptor (PDB ID: 5jw1) using MOE software; (B) 2D binding mode of the inhibitor indomethacin in the binding site of the COX-2 receptor (PDB ID: 5jw1) using MOE software; (C) 2D binding mode of the inhibitor morphine in the binding site of the COX-2 receptor (PDB ID: 5jw1) using MOE software; and (D) 2D binding mode of tadalafil docked and minimized in the binding site of the COX-2 receptor (PDB ID: 5jw1) using MOE software.
Concerning iNOS, the binding mode of tadalafil indicated a superior binding score of −7.5008 as compared to that of indomethacin and morphine with values of −5.7651 and −5.4792, respectively, implicating the need of a minimum amount of energy for tadalafil to reside within the binding site of the receptor. On the other hand, the binding modes of the three drugs tested, tadalafil, indomethacin, and morphine, were almost comparable, where all three drugs formed hydrogen bonds with the receptor in addition to arene–arene, arene–H, and other hydrophobic bonding (Table 2, Figure 2).
Figure 2.
2D binding mode of the references (indomethacin and morphine) and test drug tadalafil in the binding site of the iNOS receptor (PDB ID: 2orr) using MOE software. (A) 2D binding mode of the inhibitor indomethacin in the binding site of the iNOS receptor (PDB ID: 2orr) using MOE software; (B) 2D binding mode of the inhibitor morphine in the binding site of iNOS receptor (PDB ID: 2orr) using MOE software; and (C) 2D binding mode of tadalafil docked and minimized in the binding site of the iNOS receptor (PDB ID: 2orr) using MOE software.
The binding mode of tadalafil with nNOS as revealed by the molecular mechanics simulations of docking indicated a superior binding score of tadalafil (−7.2616) relative to that of morphine (−5.6352) and almost like that of indomethacin (−7.2830). Despite the favorable ability of indomethacin and tadalafil to reside in the binding site, the X-ray crystal structure revealed that both were incapable of forming hydrogen bonds and showed only several irrelevant hydrophobic bonding with the nNOS receptor. On the other hand, morphine was superior by showing a hydrogen bond between its phenolic OH and Glu592 residue in addition to the hydrophobic bonding to the receptor (Table 2, Figure 3).
Figure 3.
2D binding mode of the references (indomethacin and morphine) and the test drug tadalafil in the binding site of the nNOS receptor (PDB ID: 6pn4) using MOE software. (A) 2D binding mode of the inhibitor indomethacin in the binding site of the nNOS receptor (PDB ID: 6pn4) using MOE software; (B) 2D binding mode of the inhibitor morphine in the binding site of the nNOS receptor (PDB ID: 6pn4) using MOE software; and (C) 2D binding mode of tadalafil docked and minimized in the binding site of the nNOS receptor (PDB ID: 6pn4) using MOE software.
The enzyme parameters obtained from the crystallographic structure of IL-1 showed the binding mode of tadalafil, indicating a superior binding score of −6.6988 (thus, a lower amount of energy is needed), relative to that of both indomethacin and morphine (−5.8541 and −4.8657, respectively). On the other hand, the X-ray crystal structures of tadalafil and the two reference drugs with IL-1 indicated the superiority of morphine binding forming two hydrogen bonds compared to only one hydrogen bond in the case of both tadalafil and indomethacin (Table 2, Figure 4).
Figure 4.
2D binding mode of the references (indomethacin and morphine) and the test drug tadalafil in the binding site of the IL-1 receptor (PDB ID: 5r88) using MOE software. (A) 2D binding mode of the inhibitor indomethacin in the binding site of the IL-1 receptor (PDB ID: 5r88) using MOE software; (B) 2D binding mode of the inhibitor morphine in the binding site of the IL-1 receptor (PDB ID: 5r88) using MOE software; and (C) 2D binding mode of tadalafil docked and minimized in the binding site of the IL-1 receptor (PDB ID: 5r88) using MOE software.
The binding mode of tadalafil with IL-10 indicated by the molecular mechanics simulation revealed a superior binding score of −5.9004 relative to that of both indomethacin and morphine (−5.6242 and −4.3563, respectively). The X-ray crystal structure indicated comparable binding to the receptor, where all three drugs formed two hydrogen bonds to IL-10 in addition to several hydrophobic bonds (Table 2, Figure 5).
Figure 5.
2D binding mode of the references (indomethacin and morphine) and the test drug tadalafil in the binding site of the IL-10 receptor (PDB ID: 3lqm) using MOE software. (A) 2D binding mode of the inhibitor indomethacin in the binding site of the IL-10 receptor (PDB ID: 3lqm) using MOE software; (B) 2D binding mode of the inhibitor morphine in the binding site of IL-10 receptor (PDB ID: 3lqm) using MOE software; and (C) 2D binding mode of tadalafil docked and minimized in the binding site of the IL-10 receptor (PDB ID: 3lqm) using MOE software.
The docking study between the tadalafil and the reference drugs with TNF-α showed a binding score of −5.4228 for tadalafil, which is almost higher than that of both reference drugs, indomethacin and morphine (−5.0875 and −4.1433, respectively). The X-ray crystal structures revealed comparable binding of tadalafil and indomethacin to the receptor, where both drugs formed one hydrogen bond to the receptor in addition to several hydrophobic bonds. However, morphine showed only hydrophobic bonding with the receptor (Table 2, Figure 6).
Figure 6.
2D binding mode of the references (indomethacin and morphine) and the test drug tadalafil in the binding site of the TNF-α receptor (PDB ID: 2az5) using MOE software. (A) 2D binding mode of the inhibitor indomethacin in the binding site of the TNF-alpha receptor (PDB ID: 2az5) using MOE software; (B) 2D binding mode of the inhibitor morphine in the binding site of the TNF-alpha receptor (PDB ID: 2az5) using MOE software; and (C) 2D binding mode of tadalafil docked and minimized in the binding site of the TNF-alpha receptor (PDB ID: 2az5) using MOE software.
The in vivo anti-inflammatory effect of tadalafil was assessed using a formalin-induced paw edema test. Results showed a significantly lower edema volume in the tadalafil-treated mice group and indomethacin-treated one as compared to that of the control group. Moreover, the percentage inhibition of edema in the tadalafil group ranged from 40.94 to 51.47% throughout the 4 h intervals with a similar anti-inflammatory effect to that of indomethacin after 1 h of inflammation induction. On the other hand, indomethacin inhibited edema in a timely fashion, leading to a superior effect as compared to that of tadalafil after 3 and 4 h (Figure 7).
Figure 7.

Time course effect of tadalafil and the positive control indomethacin on the formalin-induced paw edema test. The ‘no formalin group’ refers to the group that received normal saline instead of formalin. Values are expressed as mean ± SD; (n = 5). *p < 0.05 compared to the negative control. **Edema volume = volume at t—volume at t0.
In addition to its anti-inflammatory effect, tadalafil has shown a significant nociceptive effect comparable to that of morphine and indomethacin in the inflammatory phase of the formalin-induced paw edema test. In fact, using the von Frey filament method, it was observed that the paw-withdrawal threshold in the tadalafil group, immediately after formalin induction, was higher than that of the vehicle but not significantly. In the inflammatory phase, after 30 min of formalin induction, tadalafil, morphine, and indomethacin groups showed significantly higher thresholds as compared to those of the vehicle groups. Moreover, the paw-withdrawal threshold in the vehicle group did not differ from the initial measurement and after 30 min, while it did for the other agents (Figure 8).
Figure 8.

Paw-withdrawal threshold of tadalafil in formalin-induced paw edema using the von Frey filament test as compared with the vehicle, indomethacin, and morphine. The ‘no formalin’ group received normal saline in the hind paw instead of formalin. Values are expressed as mean ± SD; (n = 5) *p < 0.05 as compared to the vehicle group (PEG 400) at t0. #p < 0.05 as compared to the vehicle group (PEG 400) at t30 min.
RT-PCR revealed that the iNOS expression was lower in the tadalafil group, which led to the decrease in the TNF-α expression with no effect on nNOS. Moreover, the tadalafil anti-inflammatory effect also may be mediated by COX-2 inhibition since the later expression was decreased with no effect on IL1 or IL10 (Table 3).
Table 3. Gene Expression of iNOS, Nnos, COX-2, IL-1, IL-10, and TNF-α Detected in the Mice Hind Paw Treated with Either Tadalafil, Indomethacin, Morphine, or the Vehiclea,b.
| vehicle | indomethacin | morphine | tadalafil | |
|---|---|---|---|---|
| iNOS | 0.200 ± 0.023 | 0.070 ± 0.018c | 0.365 ± 0.075c | 0.057 ± 0.005c |
| nNOS | 0.668 ± 0.117 | 0.503 ± 0.175 | 0.460 ± 0.160 | 0.688 ± 0.355 |
| COX-2 | 1.952 ± 0.355 | 0.170 ± 0.027c | 1.899 ± 0.889 | 0.190 ± 0.009c |
| IL-1 | 0.557 ± 0.055 | 0.410 ± 0.302 | 0.565 ± 0.259 | 0.228 ± 0.083 |
| IL-10 | 0.323 ± 0.015 | 0.500 ± 0.092 | 0.305 ± 0.087 | 0.36 ± 0.265 |
| TNF-α | 1.342 ± 0.120 | 1.060 ± 0.283 | 0.890 ± 0.167c | 0.594 ± 0.105c |
Relative mRNA expression levels were expressed as mean±SD (n = 5).
The ANOVA test was used to compare the means with the control (vehicle).
Denotes significant difference from the vehicle at p < 0.05.
In vitro ELISA tests confirmed the results of the RT-PCR by showing that tadalafil inhibits iNOS, TNF-α, and COX-2 significantly more than indomethacin with inhibitory concentrations of 0.80 ± 0.10, 9.50 ± 0.1, and 0.10 ± 0.01 for tadalafil versus 0.62 ± 0.02, 7.50 ± 0.20, and 0.08 ± 0.00 for indomethacin, respectively (Table 4).
Table 4. In Vitro iNOS, TNF-α, and COX-2 Enzyme Inhibitory Activitiesa.
| IC50e |
|||
|---|---|---|---|
| tested compound | iNOS | TNF-α | COX-2 |
| indomethacin | 0.62 ± 0.02 | 7.50 ± 0.20 | 0.08 ± 0.00 |
| tadalafil | 0.80 ± 0.10d | 9.50 ± 0.10b | 0.10 ± 0.01c |
t-test was done to compare between the tested compounds.
p = 0.000.
p = 0.003.
p = 0.042.
Values are expressed as mean ± SD in μm.
4. Discussion
NO has both beneficial and detrimental roles in inflammation. It has proinflammatory and immunosuppressive properties. It has been proven that NO is a vital component of the host’s adaptative response to noxious stimuli by increasing the expression of iNOS. NO generated during inflammation contributes to the erythema, edema, and increased vascular permeability detected during acute inflammation, by stimulating the synthesis of inflammatory prostaglandins through activation of COX-2. It is also proven that prolonged elevation of NO and iNOS contributes to the tissue damage detected in chronic inflammation.30,31 Accordingly, augmenting the effect of endogenous NO using phosphodiesterase inhibitors such as tadalafil can have a detrimental effect in the case of inflammation, accentuating the feeling of pain.30 On the other hand, several studies have proven the efficacy of tadalafil as an antinociceptive agent in inflammatory models. In the current study, docking analysis showed a superior binding score of tadalafil to COX-2, iNOS, IL-1, and TNF-α as compared to that of indomethacin and morphine and a similar binding score of nNOS and IL-10 relative to that of indomethacin. The X-ray crystal structure revealed good hydrogen bonding with COX-2 and TNF-α relative to that of indomethacin and a similar one with iNOS. Consequently, despite the high ability of tadalafil to reside in all receptors, it may exert its action on COX-2, iNOS, and TNF-α mainly by forming hydrogen bonds. Accordingly, the effect of tadalafil on iNOS highlights the probability that tadalafil exerts its action not by increasing the level of NO, as known, but by regulating its concentration, given that a low concentration of NO upregulates iNOS, while a high concentration has the opposite effect to prevent the overproduction of NO.31,32
Tadalafil’s anti-inflammatory effect was demonstrated by the formalin-induced paw edema test. Results showed that tadalafil possessed a significant anti-inflammatory effect as compared to that of the negative control and a similar effect to that of indomethacin within the first 2 h. A superior effect of indomethacin was detected after 3 and 4 h of edema induction relative to that of tadalafil. The effect of tadalafil was constant over the 4 h. The discrepancy in the effect of indomethacin and tadalafil may be due to the different pharmacokinetic and pharmacodynamic properties of both drugs.33
Tadalafil, in addition to its anti-inflammatory effect, exhibited a significant antinociceptive effect relative to that of the control and comparable to that of indomethacin and morphine in the paw induced edema von Frey filament test in the inflammatory phase only. This was also shown by Rocha et al., who reported that tadalafil exhibits a dose-dependent inhibition of hypernociception in zymosan-induced arthritis.6 On the other hand, Mehanna et al. showed that tadalafil had a significant antinociceptive effect in several tests, including the formalin one in both phases, through not only the nitric oxide pathway but also the involvement of opioid receptors.7 These controversies may be due to different methods being used since the formalin effect in Mehanna et al.’s study was assessed by recording the licking time.7
At the molecular level, Rocha et al. found that tadalafil decreased the TNF-α release but did not alter the IL-1 level in zymosan-induced arthritis.6 Equally, it was proven that sildenafil, another phosphodiesterase 5 inhibitor, exerts its anti-inflammatory effect by suppressing TNF-α production induced by lipopolysaccharide and by decreasing the level of NO instead of increasing it.34 In the current study, although docking showed the good ability of tadalafil to reside in all receptors studied and better hydrogen bonding to COX-2 and a similar one to iNOS, nNOS, TNF-α, IL-1, and IL-10, relative to that of indomethacin, the RT-PCR showed that tadalafil reduced iNOS, TNF-α, and COX-2 gene expressions with no effect on nNOS, IL-1, and IL-10. In vitro ELISA studies also confirmed the inhibition effect of tadalafil on the COX-2 enzyme, iNOS, and TNF-α. Consequently, the analgesic/anti-inflammatory effect of tadalafil is most probably exerted on COX-2, iNOS, and TNF-α. In fact, the effect of NO is regulated by its concentration, where micromolar ranges of NO are considered proinflammatory, while nanomolar concentrations are considered anti-inflammatory. Moreover, an increase in NO leads to the accumulation of cGMP, which has been linked to reduction in proinflammatory cytokines such as TNF-α and interleukins.35 Tadalafil is known to elevate the level of cGMP by inhibiting its breakdown by 5-phosphodiesterase, which explains the inhibition of TNF-α by tadalafil in the current study. The widespread expression of iNOS following inflammation has been linked to the host’s adaptative response to noxious stimuli and virulent pathogens, and it is regulated by the level of NO.31,32 Accordingly, in the current study, iNOS is inhibited, which is the direct effect of the increased level of NO caused by tadalafil, which also explains its anti-inflammatory effect. According to Cury et al., NO can induce peripheral hyperalgesia by regulating the expression and/or activity of cyclooxygenases.1 Consequently, moderation of NO can inhibit cyclooxygenases, which explains the fact that tadalafil has inhibited the COX-2 expression in the current study, as also demonstrated by the in vitro ELISA test. nNOS is the predominant NOS in the dorsal horn and has a definitive role in the central perception of pain, while iNOS is expressed peripherally during inflammation or neuropathic pain. iNOS participation in the central transmission of pain is doubtful.1 Therefore, since tadalafil inhibits the expression of iNOS and not nNOS, the probability that tadalafil exerts its action peripherally during the inflammation process arises.
5. Conclusions
Tadalafil exhibits a similar analgesic effect to that of morphine, implicating that it is a potentially potent analgesic agent. Moreover, its effect is mediated by the inhibition of several inflammatory mediators, including iNOS, COX-2, and TNF-α. Consequently, knowing that TNF-α is implicated in pain at both central and peripheral levels of sensitization and marketed nonsteroidal anti-inflammatory drugs increase TNF-α, tadalafil may represent a novel therapeutic approach to inflammatory pain management. Nevertheless, further studies are required to clarify its mechanism, especially its involvement in the central perception of pain.
The authors declare no competing financial interest.
References
- Cury Y.; Picolo G.; Gutierrez V. P.; Ferreira S. H. Pain and analgesia: The dual effect of nitric oxide in the nociceptive system. Nitric Oxide 2011, 25, 243–254. 10.1016/j.niox.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Linley J. E.; Rose K.; Ooi L.; Gamper N. Understanding inflammatory pain: ion channels contributing to acute and chronic nociception. Pflugers Arch. - Eur. J. Physiol. 2010, 459, 657–669. 10.1007/s00424-010-0784-6. [DOI] [PubMed] [Google Scholar]
- Freire M. A. M.; Guimarães J. S.; Leal W. G.; Pereira A. Pain Modulation by Nitric Oxide in the Spinal Cord. Front. Neurosci. 2009, 3, 175–181. 10.3389/neuro.01.024.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florentino I. F.; Silva D. P. B.; Silva D. M.; Cardoso C. S.; Moreira A. L. E.; Borges C. L.; Soares C. M. A.; Galdino P. M.; Liao L. M.; Ghedini P. C.; Menegatti R.; Costa E. A. Potential anti-inflammatory effect of LQFM-021 in carrageenan-induced inflammation: The role of nitric oxide. Nitric Oxide 2017, 69, 35–44. 10.1016/j.niox.2017.04.006. [DOI] [PubMed] [Google Scholar]
- Kawabata A.; Manabe S.; Manabe Y.; Takagi H. Effect of topical administration of l-arginine on formalin-induced nociception in the mouse: a dual role of peripherally formed NO in pain modulation. Br. J. Pharmacol. 1994, 112, 547–550. 10.1111/j.1476-5381.1994.tb13108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha F.A.C.; Silva F. Jr.; Silva F. S. Jr.; Leite A.C.R.M.; Leite A.; Girao V.C.C.; Girão V.; Castro R. R.; Castro R.; Cunha F. Q. Tadalafil analgesia in experimental arthritis involves suppression of intra-articular TNF release. Br. J. Pharmacol. 2011, 164, 828–835. 10.1111/j.1476-5381.2011.01469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehanna M. M.; Domiati S.; Nakkash Chmaisse H.; Mallah A. Antinociceptive effect of tadalafil in various pain models: Involvement of opioid receptors and nitric oxide cyclic GMP pathway. Toxicol. Appl. Pharmacol. 2018, 352, 170–175. 10.1016/j.taap.2018.05.013. [DOI] [PubMed] [Google Scholar]
- Groeneweg G.; Huygen F. J. P. M.; Niehof S. P.; Wesseldijk F.; Bussmann J. B. J.; Schasfoort F. C.; Stronks D. L.; Zijlstra F. J. Effect of tadalafil on blood flow, pain, and function in chronic cold Complex Regional Pain Syndrome: a randomized controlled trial. BMC Musculoskeletal Disord. 2008, 9, 143. 10.1186/1471-2474-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otari K. V.; Upasani C. D. Involvement of NO-Cgmp pathway in anti-hyperalgesic effect of PDE5 inhibitor tadalafil in experimental hyperalgesia. Inflammopharmacology 2015, 23, 187–194. 10.1007/s10787-015-0240-5. [DOI] [PubMed] [Google Scholar]
- Mayer M.; Stief C. G.; Truss M. C.; Uckert S. Phosphodiesterase inhibitors in female sexual dysfunction. World J. Urol. 2005, 23, 393–397. 10.1007/s00345-005-0015-5. [DOI] [PubMed] [Google Scholar]
- Shakeel F.; El-Badry M.; Haq N.; Alanazi F.; Alsarra I. Solution thermodynamics and solubility of tadalafil in PEG 400+water co-solvent mixtures at 298.15 to 333.15 K. J. Drug Delivery Sci. Technol. 2014, 24, 539–542. 10.1016/S1773-2247(14)50101-3. [DOI] [Google Scholar]
- Halgren T. A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. . [DOI] [Google Scholar]
- Halgren T. A. Merck molecular force field. II. MMFF94 van der Waals and electrostatic parameters for intermolecular interactions. J. Comput. Chem. 1996, 17, 520–552. . [DOI] [Google Scholar]
- Halgren T. A. Merck molecular force field. V. Extension of MMFF94 using experimental data, additional computational data, and empirical rules. J. Comput. Chem. 1996, 17, 616–641. . [DOI] [Google Scholar]
- Halgren T. A.; Nachbar R. B. Merck molecular force field. IV. Conformational energies and geometries for MMFF94. J. Comput. Chem. 1996, 17, 587–615. . [DOI] [Google Scholar]
- Halgren T. A. Merck molecular force field. III. Molecular geometries and vibrational frequencies for MMFF94. J. Comput. Chem. 1996, 17, 553–586. . [DOI] [Google Scholar]
- Orlando B. J.; Malkowski M. G. Substrate-selective inhibition of 27epuis27ygenase-2 by fenamic acid derivatives is dependent on peroxide tone. J. Biol. Chem. 2016, 291, 15069–15081. 10.1074/jbc.M116.725713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey D. D.; Adler M.; Amaiz D.; Eagen K.; Erickson S.; Guilford W.; Kenrock M.; Morrissey M.; Ohlmeyer M.; Pan G.; et al. Design, synthesis, and activity of 2-imidazol-1-ylpyrimidine derived inducible nitric oxide synthase dimerization inhibitors. J. Med. Chem. 2007, 50, 1146–1157. 10.1021/jm061319i. [DOI] [PubMed] [Google Scholar]
- Cinelli M. A.; Reidl C.; Li H.; Chreifi G.; Poulos T.; Silverman R. First Contact: 7-Phenyl-2-Aminoquinolines, Potent and Selective Neuronal Nitric Oxide Synthase Inhibitors That Target an Isoform-Specific Aspartate. J. Med. Chem. 2020, 63, 4528–4554. 10.1021/acs.jmedchem.9b01573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols C.; Ng J.; Keshu A.; Kelly G.; Conte M. R.; Marber M. S.; Fraternali F.; De Nicola G. F. Mining the PDB for Tractable Cases Where X-ray Crystallography Combined with Fragment Screens Can Be Used to Systematically Design Protein–Protein Inhibitors: Two Test Cases Illustrated by IL1β-IL1R and p38α–TAB1 Complexes. J. Med. Chem. 2020, 63, 7559–7568. 10.1021/acs.jmedchem.0c00403. [DOI] [PubMed] [Google Scholar]
- Yoon S.-i.; Jones B. C.; Logsdon N.; Harris B. D.; Deshpande A.; Radaeva S.; Halloran B.; Gao B.; Walter M. R. Structure and mechanism of receptor sharing by the IL-10R2 common chain. Structure 2010, 18, 638–648. 10.1016/j.str.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M. M.; Smith A. S.; Oslob J. D.; Flanagan W. M.; Braisted A. C.; Whitty A.; Cancilla M. T.; Wang J.; Lugovskoy A. A.; Yoburn J. C.; Fung A. D.; et al. Small-molecule inhibition of TNF-α. Science 2005, 310, 1022–1025. 10.1126/science.1116304. [DOI] [PubMed] [Google Scholar]
- Vogel GH E.Analgesic, Anti-inflammatory, and Anti-pyretic Activity. In Drug Discovery and Evaluation: Pharmacologic Assays, 3rd ed.; Springer: Berlin, Germany, 2008; pp 983–1116. [Google Scholar]
- Hunskaar S.; Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- Chaplan S. R.; Bach F. W.; Pogrel J. W.; Chung J. M.; Yaksh T. L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Tonello R.; Rigo F.; Gewehr C.; Trevisan G.; Pereira E.M.R.; Gomez M. V.; Ferreira J. Action of phα1β, a peptide from the venom of the spider phoneutria nigriventer, on the analgesic and adverse effects caused by morphine in mice. J. Pain 2014, 15, 619–631. 10.1016/j.jpain.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Deuis J. R.; Dvorakova L. S.; Vetter I. Methods Used to Evaluate Pain Behaviors in Rodents. Front. Mol. Neurosci. 2017, 10, 284. 10.3389/fnmol.2017.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon W. J. Efficient analysis of experimental observations. Annu. Rev. Pharmacol. Toxicol. 1980, 20, 441–462. 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Domiati S.; Mehanna M.; Ragab H.; Abdel Galil K.; Nakkash-Chmaisse H.; Mallah A. Elucidation of the molecular mechanism underlying the anti-inflammatory activity of an effective and safe bipyrazole-based compound. Inflammation Res. 2019, 68, 68. 10.1007/s00011-019-01225-z. [DOI] [PubMed] [Google Scholar]
- Jaffrey S. R.Nitric Oxide. In: Katzung B. G.; Vanderah T. W. Eds. Basic & Clinical Pharmacology, 15e. McGraw-Hill; Accessed April 24, 2021. https://accesspharmacy.mhmedical.com/content.aspx?bookid=2988§ionid=250597106. [Google Scholar]
- Sharma J. N.; Al-Omran A.; Parvathy S. S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. 10.1007/s10787-007-0013-x. [DOI] [PubMed] [Google Scholar]
- Tripathi P.; Tripathi P.; Kashyap L.; Singh V. The role of nitric oxide in inflammatory reactions. FEMS Immunol. Med. Microbiol. 2007, 51, 443–452. 10.1111/j.1574-695X.2007.00329.x. [DOI] [PubMed] [Google Scholar]
- Washington S. L.; Shindel A. W. A once-daily dose of tadalafil for erectile dysfunction: compliance and efficacy. Drug Des., Dev. Ther. 2010, 4, 159–171. 10.2147/dddt.s9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambriz-Tututi M.; Velazquez-Zamora D. A.; Urquiza-Marin H.; Granados-Soto V. Analysis of the mechanism underlying the peripheral antinociceptive action of sildenafil in the formalin test. Eur. J. Pharmacol. 2005, 512, 121–127. 10.1016/j.ejphar.2005.01.055. [DOI] [PubMed] [Google Scholar]
- Peixoto C. A.; Nunes A. K.; Garcia-Osta A. Phosphodiesterase-5 inhibitors: action on the signaling pathways of neuroinflammation, neurodegeneration, and cognition. Mediators Inflamm. 2015, 2015, 1–17. 10.1155/2015/940207. [DOI] [PMC free article] [PubMed] [Google Scholar]