Abstract
Vibrio mimicus differs from Vibrio cholerae in a number of genotypic and phenotypic traits but like V. cholerae can give rise to diarrheal disease. We examined clinical isolates of V. mimicus for the presence of CTXΦ, the lysogenic filamentous bacteriophage that carries the cholera toxin genes in epidemic V. cholerae strains. Four V. mimicus isolates were found to contain complete copies of CTXΦ. Southern blot analyses revealed that V. mimicus strain PT5 contains two CTX prophages integrated at different sites within the V. mimicus genome whereas V. mimicus strains PT48, 523-80, and 9583 each contain tandemly arranged copies of CTXΦ. We detected the replicative form of CTXΦ, pCTX, in all four of these V. mimicus isolates. The CTX prophage in strain PT5 was found to produce infectious CTXΦ particles. The nucleotide sequences of CTXΦ genes orfU and zot from V. mimicus strain PT5 and V. cholerae strain N16961 were identical, indicating contemporary horizontal transfer of CTXΦ between these two species. The receptor for CTXΦ, the toxin-coregulated pilus, which is encoded by another lysogenic filamentous bacteriophage, VPIΦ, was also present in the CTXΦ-positive V. mimicus isolates. The nucleotide sequences of VPIΦ genes aldA and toxT from V. mimicus strain PT5 and V. cholerae N16961 were identical, suggesting recent horizontal transfer of this phage between V. mimicus and V. cholerae. In V. mimicus, the vibrio pathogenicity island prophage was integrated in the same chromosomal attachment site as in V. cholerae. These results suggest that V. mimicus may be a significant reservoir for both CTXΦ and VPIΦ and may play an important role in the emergence of new toxigenic V. cholerae isolates.
Cholera toxin (CT) is encoded by the ctxAB operon, which resides in the genome of CTXΦ, a filamentous bacteriophage that specifically infects Vibrio cholerae (40). CTXΦ is found in all epidemic V. cholerae isolates but is rarely recovered from non-O1/non-O139 V. cholerae environmental isolates (12). The CTXΦ genome can integrate into the V. cholerae genome to form a stable prophage or it can replicate as a plasmid in isolates lacking an appropriate integration site. Of the nearly 200 recognized serogroups of V. cholerae only the O1 and O139 serogroups are associated with epidemics of cholera (2). In classical biotype strains of V. cholerae serogroup O1 there is a CTX prophage on each of the two V. cholerae chromosomes (24, 38), whereas in El Tor biotype strains of V. cholerae serogroup O1 the CTX prophages are tandemly arranged on the larger of the two chromosomes (26, 30). CTXΦ has a 6.9-kb genome made up of two functionally diverse regions: the core and RS2 regions. The core region encodes CT and contains the genes involved in phage morphogenesis, including genes that are thought to encode the major and minor phage coat proteins and a protein required for CTXΦ assembly (40). The RS2 region contains genes required for replication, integration, and regulation of CTXΦ (20, 43). In El Tor V. cholerae isolates, the CTX prophage genome is often flanked by an additional 2.7-kb region, designated RS1, that is similar to RS2 but that contains an additional gene (15, 26, 43).
Uptake of CTXΦ into V. cholerae is dependent upon the toxin-coregulated pilus (TCP), a bundle-forming pilus that is also an essential intestinal colonization factor (36). Initially, it was shown that the genes encoding TCP resided on a pathogenicity island known as the TCP island or vibrio pathogenicity island (VPI), which is integrated near the ssrA gene (18, 21). However, TCP has recently been proposed to be encoded on a novel filamentous phage named VPIΦ (19). Thus, the filamentous phage VPIΦ encodes TCP, an important virulence factor, and is the receptor for another temperate filamentous phage, CTXΦ, which itself encodes a virulence factor, CT.
The species Vibrio mimicus was first proposed to encompass biochemically atypical non-O1 V. cholerae isolates. V. mimicus is phenotypically and genotypically distinct from V. cholerae in several respects, and it can be readily differentiated from V. cholerae on the basis of a number of biochemical reactions (10). Thus, unlike V. cholerae, V. mimicus is negative in sucrose, Voges-Proskauer, corn oil, and Jordan tartrate reactions (10). Sequence analysis of the mdh (malate dehydrogenase) gene from a V. mimicus strain revealed that the average pairwise divergence between typical V. cholerae isolates and V. mimicus was 10.5% (6). The natural habitat of V. mimicus, like that of V. cholerae, is the aquatic ecosystem, where it has been found both as a free-living bacterium and in association with phytoplankton and crustaceans (1, 7). Consumption of V. mimicus-contaminated shellfish has been linked to the development of gastroenteritis (1). However, unlike V. cholerae, V. mimicus has not been associated with epidemics of diarrhea. The virulence determinants of V. mimicus have not been well characterized. The ability of V. mimicus strains to produce various toxins has been studied (7, 8, 31, 33–35). Several V. mimicus strains isolated from clinical and environmental sources were shown to produce multiple toxins, including a hemolysin, zonula occludens toxin, and a heat-stable enterotoxin, as well as a CT-like toxin (7, 8, 31, 33–35). To date, the presence of CTXΦ in V. mimicus has not been reported.
The distribution of CTXΦ and VPIΦ outside of V. cholerae is unknown, as is the extent to which V. mimicus and V. cholerae share pools of bacteriophages. We therefore examined clinical isolates of V. mimicus to determine if this species contains the genes composing CTXΦ and/or VPIΦ and consequently whether V. mimicus can serve as a reservoir for these important bacteriophages. We found V. mimicus isolates that contained both the CTX and VPI prophages. Nucleotide sequence identity of genes from both these prophages with the respective V. cholerae prophages suggests contemporary horizontal transfer of bacteriophages between these two Vibrio species.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains used in this study are shown in Table 1. Bacterial strains were stored at −70°C in Luria-Bertani (LB) broth containing 20% glycerol. The antibiotic streptomycin was used at 200 μg/ml.
TABLE 1.
Bacterial strains used in this study
| Strain | Serogroup (biotype) | Isolation
|
Reference | |
|---|---|---|---|---|
| Country | Yr | |||
| V. mimicus | ||||
| PT5 | O115 | Bangladesh | 1985 | 33 |
| PT48 | O115 | Bangladesh | 1985 | 33 |
| 523-80 | O115 | United States | 1980 | 33 |
| 9583 | O115 | United States | 1980 | 33 |
| 9581 | O41 | India | 1990 | This study |
| 9582 | Rough | India | 1990 | This study |
| 92-81 | O8 | United States | 1980 | This study |
| 531-90 | O41 | Japan | 1990 | This study |
| 546-80 | Untypeable | United States | 1980 | This study |
| V. cholerae | ||||
| N16961 | O1 (El Tor) | Bangladesh | 1975 | 17 |
| SM115 | O1 (El Tor) | Bahrain | 1978 | 15 |
| O395 | O1 (classical) | India | 1964 | 26 |
| LAC-1 | O1 (classical) | India | 1964 | 39 |
Southern hybridization analyses.
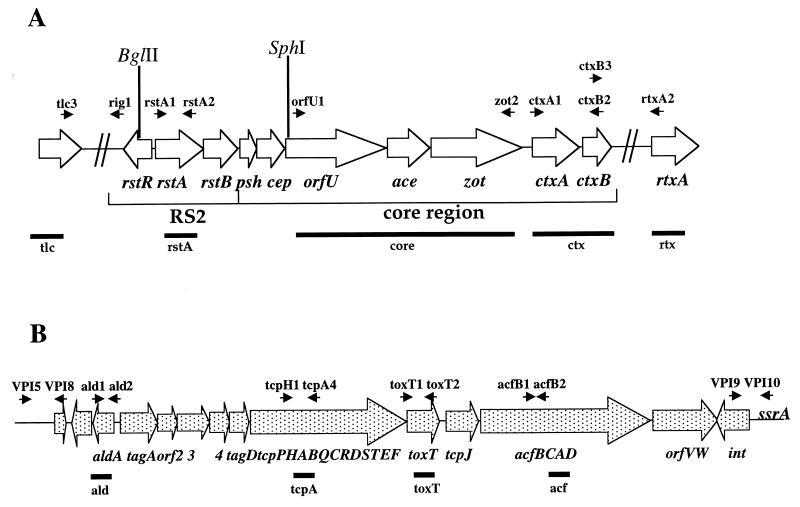
V. mimicus DNA was extracted and purified using the G-nome DNA isolation kit from BIO101 (Vista, Calif.). DNA was digested with several restriction enzymes (New England Biolabs, Beverley, Mass.), and the fragments were separated by electrophoresis in 0.6% agarose. The fragments were then transferred to nylon membranes for hybridization. Three nonradioactive DNA probes to detect the presence of CTXΦ were produced by PCR amplification using V. cholerae strain SM115 (15) as a template (Table 2 and Fig. 1). DNA probes were labeled with fluorescein-conjugated nucleotides and, after hybridization, were detected by the ECL system according to the manufacturer's protocol (Amersham; Arlington Heights, Ill.). The rstA probe (5) assays for the presence of the RS2 region, whereas the core (5) and ctx probes assay for the presence of the core region of the CTXΦ genome. Southern blot analyses to detect the presence of DNA sequences that flank the CTX prophage in El Tor V. cholerae, the TLC element and an RTX cluster, in V. mimicus were performed using the DNA probes tlc (5) and rtx (5).
TABLE 2.
Primers and probes used in this study
| Phage and primer | Forward or reverse primer sequence (5′–3′) | PCR product size (bp) | Probe | Reference |
|---|---|---|---|---|
| CTXΦ | ||||
| rstA1 | ACTCGATACAAACGCTTCTC | 1,009 | rstA | 43 |
| rstA2 | AGAATCTGGAAGGTTGAGTG | |||
| orfU1 | CGTCACACCAGTTACTTTTCG | 2,536 | core | 37 |
| zot2 | AACCCCGTTTCACTTCTAC | 14 | ||
| ctxA1 | AGTCAGGTGGTCTTATGCC | 1,037 | ctx | WSb |
| ctxB2 | TTGCCATACTAATTGCGG | |||
| tlc3 | GGGAATGTTGAGTTCTCAGTG | 1,548 | tlc | 32 |
| tlc4 | GTTGCGAAGTGGATTTTGTG | |||
| rtxA1 | CACTCATTCCGATAACCAC | 1,366 | rtxA | 23 |
| rtxA2 | GCGATTCTCAAAGAGATGC | |||
| rig1 | CACGCTACGTCGCTTATGT | NAa | NA | 30 |
| ctxB3 | CCGCAATTAGTATGGCAA | NA | NA | WS |
| VPIΦ | ||||
| ald1 | ATTCTTCTGAGGATTGCTGAT | 884 | ald | 29 |
| ald2 | TTTTCTTGATTGTTAGGATGC | |||
| tcpH1 | AGCCGCCTAGATAGTCTGTG | 1,200 | tcpA | 28 |
| tcpA4 | TCGCCTCCAATAATCCGAC | |||
| toxT1 | AGGAGATGGAAGTGGTGTG | 1,055 | toxT | 16 |
| toxT2 | CTTGGTGCTACATTCATGG | |||
| acfB1 | GATGAAAGAACAGGAGAGA | 1,180 | acf | 21 |
| acfB2 | CAGCAACCACAGCAAAACC | |||
| VPI5 | GTGAATCTTGATGAGACGC | 529 | VPIL | 18 |
| VPI8 | GCCATTGGGTAAGTAGC | |||
| VPI9 | CCAATCCTTTGTGACGTTC | 705 | VPIR | 18 |
| VPI10 | GGAAATCAGGAAGGTCAAAC |
NA, not applicable.
FIG. 1.
(A) Schematic representation of the CTX prophage (∼7 kb) and surrounding sequences in V. cholerae. The open reading frames (ORFs) are shown as arrows. Black arrowheads, locations of the PCR primers listed in Table 2; vertical lines, restriction enzyme sites; horizontal bars, positions of the three DNA probes used for hybridization. (B) Schematic representation of the VPI prophage (∼40 kb) in V. cholerae. The ORFs and gene clusters are shown as dotted arrows. Black arrowheads, locations of the PCR primers listed in Table 2; horizontal bars, locations of the four DNA probes used for hybridization.
To determine the distribution of VPIΦ among V. mimicus isolates, four DNA probes which span the genome of VPIΦ, ald, tcpA, toxT, and acf (Fig. 1 and Table 2), were utilized. The ald probe includes the aldA gene found in the 5′ region of the VPI prophage. The tcpA probe encompasses the tcpA gene in the 5′ region of the tcp operon, whereas the toxT probe hybridizes to the 3′ region of this operon. The acf probe is limited to the acfB gene of the acf gene cluster in the 3′ region of the VPI prophage. All probes were made by PCR amplification from V. cholerae strain SM115 and purified using the Qiaquick PCR purification kit (Qiagen, Valencia, Calif.).
PCR analyses.
To determine whether the chromosomal DNA sequences flanking the 5′ and 3′ ends of the CTX prophage in V. mimicus were identical to those of El Tor V. cholerae, we carried out a PCR assay with primer pairs rig1 and tlc3 and ctxB3 and rtxA2 (Table 2; Fig. 1A), which are located within the CTX prophage and the known flanking sequences of V. cholerae El Tor strain N16961.
PCR assays were also used to determine whether the chromosomal regions flanking the 5′ and 3′ ends of the VPI prophage in V. mimicus were identical to those in V. cholerae. Primer pairs VPI5 and VPI8 and VPI9 and VPI10, designed from the 5′ and 3′ junction sequences of the VPI prophage from V. cholerae strain N16961, were used for these assays. Primer VPI5 is derived from a sequence in the 5′ chromosomal flanking region of the VPI prophage integration site in V. cholerae, and primer VPI8 is located within the 5′ region of the VPI prophage genome. To amplify the VPI prophage 3′ chromosomal junction, primer VPI9, which lies within the putative integrase gene of VPIΦ, and primer VPI10, which is located within the 3′ flanking chromosomal DNA of V. cholerae strain N16961, were used (Table 2, Fig. 1B).
Detection of the replicative form of CTXΦ.
To determine whether the V. mimicus strains that were found to contain the CTX prophage also harbored the replicative form of CTXΦ, Qiagen plasmid spin kits were used to isolate plasmid DNA from 5-ml overnight cultures. These plasmid DNA preparations were tested for the presence of the ∼7-kb pCTX plasmid band by Southern blot analysis using the CTXΦ core region DNA probe (Table 2, Fig. 1A).
Recovery of infectious CTXΦ particles.
We assayed filter-sterilized supernatants from each of the CTXΦ-positive V. mimicus strains for the presence of infectious CTXΦ particles as previously described (9, 22). Briefly, 1.5 ml of sterile supernatant from 2-ml overnight cultures was mixed with 1 μl of agglutinated classical strain O395 for 20 min. After addition of 1.5 ml of LB broth, the cultures were incubated overnight at 30°C. Qiagen plasmid spin kits were then used to isolate plasmid DNA from these overnight cultures. These plasmid DNA preparations were digested with SphI, which linearizes the CTXΦ genome, separated on agarose gels, and assayed for the presence of pCTX DNA by Southern analysis.
Nucleotide sequencing and analysis.
The orfU and zot genes from the CTXΦ in V. mimicus strain PT5 were sequenced directly from the products of PCR. PCR primers were designed from the published sequences of these genes in V. cholerae (14, 37) (Table 2). The aldA and toxT genes of VPIΦ from PT5 were also sequenced directly from PCR products with primers designed from the published V. cholerae sequences (18, 21) (Table 2 and Fig. 1). The mdh gene sequence was also determined for V. mimicus strain PT5 using primers derived from a V. cholerae El Tor strain N16961 sequence (http://www.tigr.org/tigr_home/tdb/mdb/mdb.html). Sequencing was performed with an Applied Biosystems model 373A automated DNA sequencing system at the Tufts University School of Medicine Core Facility using a DyeDeoxy terminator cycle sequencing kit. The sequences were determined in both orientations with additional internal primers, and the overlapping sequences were assembled and edited with the MacVector program. Comparisons of the V. mimicus mdh, orfU, aldA, and toxT gene sequences with the respective V. cholerae sequences were carried out using MacVector.
Mouse colonization assay.
A competition assay between either O395 or a lacZ deletion mutant of O395, LAC-1 (42), and spontaneous Smr derivatives of three of the four VPIΦ-positive V. mimicus strains was done essentially as described previously (42). PT5 is lacZ positive, whereas 9583 and 523-80 are lacZ negative. The LAC-1 strain has been shown to colonize the suckling mouse intestine as well as O395 does. In this assay LAC-1 and O395 served as standards for comparative analysis of the intestinal colonization properties of the different test strains. Three- to five-day-old suckling mice (Charles River) were inoculated orally with a 1:1 mixture of V. cholerae O395 or LAC-1 and a V. mimicus test strain and then sacrificed 24 h later. Viable-cell counts were obtained by plating dilutions of the intestinal homogenates on LB agar containing streptomycin and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (40 μg/ml). Three animals were used per group. The relative colonization efficiencies of the two strains were calculated by comparing the ratios of blue (lacZ+) colonies to white (ΔlacZ) colonies in the intestinal homogenates and the inocula.
Nucleotide sequence accession numbers.
The nucleotide sequences obtained during this study have been deposited in GenBank under accession no. AF207856 to AF207858.
RESULTS AND DISCUSSION
Presence of CTXΦ genes in V. mimicus.
A recent report by Shi et al. (33) demonstrated the presence of ctxA-related sequences in four V. mimicus clinical isolates. Since we previously found that the ctxAB genes are part of the genome of CTXΦ, we investigated whether these four strains contained a CTX prophage(s). We also tested five additional V. mimicus clinical isolates that were previously found not to contain the ctxA gene for CTXΦ-related sequences. We found by PCR and DNA hybridization analyses that the four V. mimicus isolates previously reported to contain ctxA-related sequences, PT5, PT48, 523-80, and 9583, each contained a complete CTXΦ (Table 3). Remarkably, although these four V. mimicus strains all belonged to the same serogroup (O115), they were recovered at different times and from different continents (Table 1). The five additional V. mimicus isolates examined did not show homology to any of the CTXΦ probes, indicating the absence of CTXΦ in these isolates.
TABLE 3.
Distribution of CTXΦ and VPIΦ genes in V. mimicus strains
| Strain | Presence of indicated gene or DNA segment of:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CTXΦ
|
VPIΦ
|
||||||||||
| TLC | rstA | orfU-zot | ctxAB | RTX | 5′ junction | ald | tcpA | toxT | acfB | 3′ junction | |
| PT5 | − | + | + | + | − | + | + | + | + | + | + |
| PT48 | − | + | + | + | − | + | + | + | + | + | + |
| 523-80 | − | + | + | + | − | + | + | + | + | + | + |
| 9583 | − | + | + | + | − | + | + | + | + | + | + |
| 9581 | − | − | − | − | − | − | − | − | − | − | − |
| 9582 | − | − | − | − | − | − | − | − | − | − | − |
| 92-81 | − | − | − | − | − | − | − | − | − | − | − |
| 531-90 | − | − | − | − | − | − | − | − | − | − | − |
| 546-80 | − | − | − | − | − | − | − | − | − | − | − |
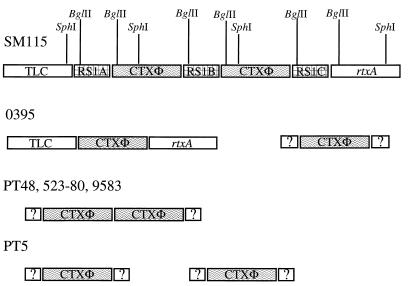
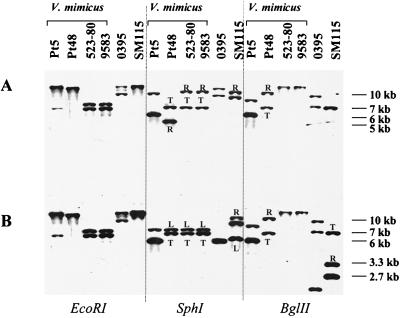
We further analyzed the CTXΦ genes in the four strains containing ctxAB by Southern blotting, to determine if the CTXΦ genome was integrated into the V. mimicus chromosome (as is observed in V. cholerae) and to assess the copy number and arrangement of the CTX prophages. Southern hybridization analyses were carried out on chromosomal DNA prepared from each V. mimicus strain digested with restriction enzymes that cut either at no sites (EcoRI) or at one site (SphI, BglII) in the CTX prophage genomes in classical and El Tor V. cholerae strains (30). Similar to analyses of classical strains of V. cholerae, these analyses of strain PT5 indicate the presence of two CTX prophages at different locations in the genome (Fig. 2). Thus, when strain PT5 chromosomal DNA was digested with EcoRI and hybridized with either the core region or rstA probes, two bands were observed (Fig. 3A and B). The presence of two unlinked CTX prophages in PT5 was confirmed in blots of SphI- and BglII-digested DNA. In these digests, with either the core or rstA probes, two bands were also observed (Fig. 3).
FIG. 2.
Chromosomal arrangement of CTX prophages in V. cholerae strains SM115 and O395 and in V. mimicus strains PT5, PT48, 523-80, and 9583 as determined by Southern blot and PCR analyses. Vertical lines indicate restriction enzyme sites. Boxes with question marks adjacent to the CTX prophages represent those cases where the phage-chromosome junction sequences are unknown.
FIG. 3.
Southern blot analyses of the integrated copies of CTXΦ in V. mimicus strains PT5, PT48, 583-80, and 9583 and V. cholerae classical strain O395 and El Tor strain SM115. Equal amounts of chromosomal DNAs from the indicated strains were digested with EcoRI, SphI, and BglII, electrophoresed, transferred to nitrocellulose, and probed with CTXΦ core (A) and rstA (B) probes. R and L, right and left junction fragments, respectively; T, tandem arrangements of integrated CTXΦ.
In V. mimicus strain PT48, a clinical isolate from Bangladesh, Southern blot analyses demonstrated the presence of tandemly arranged CTX prophages, similar to the arrangement present in El Tor V. cholerae strains (Fig. 2). Southern hybridization of EcoRI-digested PT48 DNA probed with the core or rstA probes revealed only a single hybridizing band >12 kb (Fig. 3A and B). However, PT48 DNA digested with SphI and hybridized with the core probe revealed two hybridization species: a 5-kb band and a 7-kb band (Fig. 3A). The 5-kb band represents the right junction fragment of CTXΦ in the chromosome, and the 7-kb band corresponds to the tandemly integrated copies of CTXΦ. Hybridization with the rstA probe also identified two bands, a 7-kb tandem band and an 8-kb left junction fragment (Fig. 3B). The fact that the size of the tandem band in the SphI digests of PT48 probed with the core and rstA probes is 7 kb and not ∼10 kb, as seen with the El Tor strain SM115, suggests that there is no intervening RS1 sequence between the CTX prophages in PT48. BglII digests of PT48 DNA hybridized with the core and rstA probes revealed two bands, confirming the tandem arrangement of CTX prophages in PT48.
V. mimicus clinical isolates 523-80 and 9583, which were recovered from patients in the United States, also contained tandemly arranged CTX prophages (Fig. 2). The strains gave identical banding patterns when digested with EcoRI, SphI, or BglII, and probed with the core region or the rstA probe. Thus, two fragments of EcoRI-digested 523-80 DNA hybridized with the core probe: a 7-kb band and an 8-kb band (Fig. 3A). An identical pattern was found with the rstA probe, suggesting the presence of two CTX prophages at different map positions. However, Southern blots of SphI-digested DNA probed with the core probe gave two bands: a 7-kb band and a 5-kb band; similarly, hybridization with the rstA probe gave a 7-kb band and an 8-kb band, suggesting a tandem arrangement of CTX prophages similar to the pattern seen with strain PT48. Taken together, these results suggest that the CTX prophages in 523-80 and 9583 contain an EcoRI site not present in the PT5, PT48, or V. cholerae serogroup O1 CTX prophages, explaining the two bands seen with this enzyme (Fig. 3A and B). Digestion of 523-80 and 9583 DNA with BglII and hybridization with core and rstA probes also suggested DNA sequence polymorphisms in the CTX prophages in these strains, as both probes identified a single >12-kb band, indicating the absence of a BglII site in rstR. These analyses of the arrangements of the CTX prophages in these four V. mimicus isolates demonstrate that, as for V. cholerae, the CTX prophages in V. mimicus occur in multiple copies and have alternative arrangements in the genome.
Analysis of CTX prophage flanking DNA.
V. cholerae serogroup O1 strains contain tandemly arranged copies of an integrated toxin-linked cryptic plasmid, pTLC, adjacent to the 5′ end of the CTX prophages (32). Adjacent to the region 3′ of the CTX prophages there is a recently described RTX toxin gene cluster (23). We used PCR analyses to investigate whether the TLC element and the RTX cluster are present in the CTX prophage-positive and -negative V. mimicus strains. The primer pairs for these PCRs, rig1 and tlc3 and ctxB3 and rtxA2 (Fig. 1), were designed to amplify TLC sequences 5′ of the CTX prophage and RTX sequences 3′ of the CTX prophage. No PCR products were detected in the nine strains tested using these primer pairs (Table 3). To further investigate whether there are sequences homologous to TLC and RTX in these V. mimicus strains, we performed Southern blot analyses with a 1.5-kb tlc probe and a 1.3-kb rtxA probe. No hybridization bands were obtained. Therefore, V. mimicus, unlike V. cholerae, for which previous studies have demonstrated the co-occurrence of the CTX prophage and the TLC element, has no TLC element.
Detection of CTXΦ RF and infectious virions.
To determine whether the four V. mimicus strains that harbor the CTX prophage also contained the ∼7-kb plasmid replicative form (RF) of CTXΦ, pCTX, plasmid DNA preparations from these strains were probed with a CTXΦ core region probe in a Southern blot. The plasmid preparations from all four strains yielded hybridizing DNA of the appropriate size (data not shown), indicating that these strains harbored the CTXΦ RF in addition to the CTX prophage.
We then tested whether these strains produced infectious CTXΦ particles. To accomplish this, filtered sterile supernatants from overnight cultures of V. mimicus strains were used to infect agglutinated (TCP+) classical strain O395 with CTXΦ (27), according to an established protocol (9, 22, 40). Cell-free supernatant from one strain, PT5, was found to transfer CTXΦ DNA to O395, thus demonstrating the ability of this strain to produce infectious CTX virions (data not shown). This is the first report of the isolation of CTXΦ outside of V. cholerae. This finding indicates that CTXΦ has more than one host species and hence that V. mimicus may represent an important reservoir of CTXΦ in the natural environment. For reasons which are not yet known, we were unable to detect transfer of CTXΦ DNA from supernatants derived from PT48, 523-80, and 9583. It is possible that our inability to detect transfer of CTXΦ from these three V. mimicus strains into O395 is a reflection of phage immunity. We have found that the CTXΦ repressor, RstR, functions as an effector of phage immunity by repressing transcription of rstA, a gene required for CTXΦ replication (9, 20). If rstA in the CTXΦ derived from V. mimicus strains PT48, 523-80, and 9583 is repressed by the RstRclassical repressor in O395, then these three V. mimicus-derived CTXΦs would not replicate in this strain.
Comparison of V. mimicus and V. cholerae CTXΦ nucleotide sequences.
There is a sporadic distribution of CTXΦ in V. cholerae and V. mimicus strains. Among V. cholerae isolates, CTXΦ is confined to epidemic strains and is rarely recovered from non-O1/non-O139 isolates. This sporadic distribution of CTXΦ in V. cholerae and V. mimicus populations is most likely the result of horizontal transfer of CTXΦ between Vibrio species. However, it is also possible that the CTX prophage was present in the most recent common ancestor of V. cholerae and V. mimicus, with subsequent loss of this phage from most isolates of these two species. To distinguish between these two possibilities and to begin to elucidate the evolutionary history of CTXΦ, we sequenced two genes from the core region of the CTXΦ derived from V. mimicus strain PT5 and compared these sequences with sequences of the same region from El Tor V. cholerae strain N16961 from the TIGR database (http://www.tigr.org/tigr_home/tdb/mdb/mdb.html). We also sequenced the chromosomally encoded malate dehydrogenase gene (mdh), which is a basic metabolic housekeeping gene found in all Vibrio species and therefore is ancestral to the species. Comparison of the mdh sequences from V. mimicus and V. cholerae provides a measure of the divergence of the two species. Of the 640 bp of mdh sequenced from V. mimicus strain PT5, there were 64 polymorphic sites, compared with the V. cholerae mdh from strain N16961. This indicates that the level of nucleotide sequence divergence between the two species is approximately 10%, similar to values from previous reports (6). This level of nucleotide sequence divergence is similar to the level of divergence between Escherichia coli and Salmonella enterica (3, 4). In striking contrast, the 992 bp of the orfU gene and the 1,036 bp of the zot gene derived from V. mimicus strain PT5 and V. cholerae strain N16961 contained no polymorphic nucleotide sites. This nucleotide sequence identity of the CTXΦ genes within otherwise divergent chromosomal backgrounds strongly argues against the possibility that CTXΦ was inherited from a common ancestor of V. cholerae and V. mimicus. Rather, the CTXΦ sequence identity between these two species suggests that there was relatively recent horizontal transfer of this phage between these two species. The site of this phage transfer could be their shared ecological niche: the estuarine environment. However, since the V. mimicus isolates examined are clinical isolates, we cannot formally rule out the possibility that CTXΦ transfer between these two species may have occurred in the human host.
Presence of VPIΦ genes in V. mimicus.
The receptor for CTXΦ in V. cholerae is TCP. The genes encoding TCP have recently been proposed to be an integral part of the genome of the newly described novel filamentous phage VPIΦ, found in pathogenic isolates of V. cholerae (19). Since TCP plays a critical role in the uptake of CTXΦ into V. cholerae, we tested whether VPI prophage sequences were present in the four toxigenic and five nontoxigenic clinical isolates of V. mimicus. All four CTXΦ+ V. mimicus isolates yielded PCR products with four sets of primer pairs that span the VPIΦ genome (Table 3; Fig. 1). In contrast, PCR products were not amplified from the five V. mimicus strains that did not harbor a CTX prophage (Table 3). Southern analyses confirmed that these five strains lacked sequence homology to VPIΦ. In V. cholerae the VPI prophage is found adjacent to the ssrA locus (19). Interestingly, by PCR analyses we found that the VPI prophage was integrated at the identical chromosomal sites in the four VPI prophage-positive V. mimicus isolates (Table 3), and we were able to amplify by PCR the chromosomal junction fragments of the VPI prophage integration site of V. cholerae from all V. mimicus toxigenic isolates.
Since TCP is required for CTXΦ infection of V. cholerae, it was suggested that there were two critical sequential steps in the evolution of pathogenic V. cholerae (5, 12, 13, 25, 40, 41). First, strains had to acquire TCP (presumably via infection with VPIΦ), and second, having acquired the CTXΦ receptor, these TCP+ strains were then infected with and lysogenized by CTXΦ. Our finding that all four V. mimicus isolates that contained CTX prophages also contained VPI prophages supports the notion that TCP is required for acquisition of CTXΦ. The co-occurrence of these two bacteriophages in two different species is striking from an evolutionary perspective, as it would appear that these two unrelated bacteriophages have similar host ranges and that their acquisition and potentially their function are intimately interconnected.
Comparison of V. mimicus and V. cholerae VPI prophage nucleotide sequences.
To begin to address the evolutionary history of VPIΦ in V. cholerae and V. mimicus, we sequenced two genes carried by VPIΦ, aldA and toxT from V. mimicus strain PT5, and compared these sequences with the aldA and toxT sequences from V. cholerae strain N16961. One of the sequenced genes, aldA, codes for an aldehyde dehydrogenase (29), and the other, toxT, codes for a transcriptional activator for virulence gene expression (11). The aldA and toxT sequences from PT5 and N16961 were identical. The identity of these VPI prophage sequences from V. mimicus and V. cholerae suggests recent horizontal transfer of VPIΦ between V. mimicus and V. cholerae and lends additional support to the idea that the VPI is a mobile genetic element.
Mouse colonization assay.
The identification of V. mimicus strains containing the CTX and VPI prophages, whose genomes encode many of the known virulence factors of V. cholerae, raised the question of whether these V. mimicus isolates are as pathogenic as V. cholerae isolates that contain these prophages. To begin to experimentally assess the virulence properties of three of the V. mimicus strains containing both prophages (PT5, 523-80, and 9583), we performed mouse colonization assays. Twenty four hours after 1:1 mixtures of O395 with each of these three strains were intragastrically inoculated into suckling mice, the ratio of V. mimicus cells to O395 cells in intestinal homogenates was found to be less than 1:1,000 for all three V. mimicus strains. Thus, compared to V. cholerae, these V. mimicus isolates appear to be extremely attenuated for intestinal colonization. The molecular basis for this attenuation is not known. If TCP is properly expressed in vivo by these V. mimicus strains containing the VPI prophage, these data indicate that other colonization factors, such as the serogroup antigen, are critical for intestinal colonization.
Conclusions.
The extent to which V. cholerae and V. mimicus share gene pools is unknown, as is the ancestry of bacteriophages in these species. The data presented in this report indicate that V. cholerae is not the only host of both CTXΦ and VPIΦ. The finding of nucleotide sequence identity of genes from both CTXΦ and VPIΦ derived from V. mimicus and V. cholerae strongly suggests that contemporary horizontal transfer of bacteriophages between these species has occurred. However, we cannot conclude from this sequence identity whether these phage genomes were transferred from V. cholerae to V. mimicus or vice versa. Also, although it seems likely that these two phage genomes were transferred between these Vibrio species via transduction, we cannot strictly come to this conclusion from our data. Regardless of this limitation, V. mimicus may represent an important environmental reservoir for both bacteriophages and, therefore, may play a significant role in the emergence of new toxigenic V. cholerae isolates.
ACKNOWLEDGMENTS
We thank our colleagues Brigid Davis and Bianca Hochhut for critically reading the manuscript. We are most grateful to Anne Kane and the NEMC GRASP Center (grant P30DK-34928) for providing us with culture media.
This work was supported by NIH grant AI-42347 to M.K.W. E.F.B. was supported by NIH training grant T32 AI-07329. M.K.W. is a PEW Scholar of Biomedical Research.
REFERENCES
- 1.Acuna M T, Diaz G, Bolanos H, Barquero C, Sanchez O, Sanchez L M, Mora G, Chaves A, Campos E. Sources of Vibrio mimicus contamination of turtle eggs. Appl Environ Microbiol. 1999;65:336–338. doi: 10.1128/aem.65.1.336-338.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert M J. Epidemiology & molecular biology of Vibrio cholerae O139 Bengal. Indian J Med Res. 1996;104:14–27. [PubMed] [Google Scholar]
- 3.Boyd E F, Li J, Ochman H, Selander R K. Comparative genetics of the inv/spa invasion gene complex of Salmonella enterica. J Bacteriol. 1997;179:1985–1991. doi: 10.1128/jb.179.6.1985-1991.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd E F, Nelson K, Wang F-S, Whittam T S, Selander R K. Molecular genetic basis of allelic polymorphism in malate dehydrogenase (mdh) in natural populations of Escherichia coli and Salmonella enterica. Proc Natl Acad Sci USA. 1994;91:1280–1284. doi: 10.1073/pnas.91.4.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyd E F, Waldor M K. Alternative mechanism of cholera toxin acquisition by Vibrio cholerae: generalized transduction of CTXΦ by bacteriophage CP-T1. Infect Immun. 1999;67:5898–5905. doi: 10.1128/iai.67.11.5898-5905.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byun R, Elbourne L D, Lan R, Reeves P R. Evolutionary relationships of pathogenic clones of Vibrio cholerae by sequence analysis of four housekeeping genes. Infect Immun. 1999;67:1116–1124. doi: 10.1128/iai.67.3.1116-1124.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campos E, Bolanos H, Acuna M T, Diaz G, Matamoros M C, Raventos H, Sanchez L M, Sanchez O, Barquero C. Vibrio mimicus diarrhea following ingestion of raw turtle eggs. Appl Environ Microbiol. 1996;62:1141–1144. doi: 10.1128/aem.62.4.1141-1144.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chowdhury M A, Hill R T, Colwell R R. A gene for the enterotoxin zonula occludens toxin is present in Vibrio mimicus and Vibrio cholerae O139. FEMS Microbiol Lett. 1994;119:377–380. doi: 10.1111/j.1574-6968.1994.tb06916.x. [DOI] [PubMed] [Google Scholar]
- 9.Davis B M, Kimsey H H, Chang W, Waldor M K. The Vibrio cholerae O139 Calcutta CTXΦ is infectious and encodes a novel repressor. J Bacteriol. 1999;181:6779–6787. doi: 10.1128/jb.181.21.6779-6787.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis B R, Fanning G R, Madden J M, Steigerwalt A G, Bradford H B, Jr, Smith H L, Jr, Brenner D J. Characterization of biochemically atypical Vibrio cholerae strains and designation of a new pathogenic species, Vibrio mimicus. J Clin Microbiol. 1981;14:631–639. doi: 10.1128/jcm.14.6.631-639.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiRita V J, Parsot C, Jander G, Mekalanos J J. Regulatory cascade controls virulence in Vibrio cholerae. Proc Natl Acad Sci USA. 1991;88:5403–5407. doi: 10.1073/pnas.88.12.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faruque S M, Albert M J, Mekalanos J J. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol Mol Biol Rev. 1998;62:1301–1314. doi: 10.1128/mmbr.62.4.1301-1314.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faruque S M, Asadulghani, Saha M N, Alim A R, Albert M J, Islam K M, Mekalanos J J. Analysis of clinical and environmental strains of nontoxigenic Vibrio cholerae for susceptibility to CTXΦ: molecular basis for origination of new strains with epidemic potential. Infect Immun. 1998;66:5819–5825. doi: 10.1128/iai.66.12.5819-5825.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fasano A, Baudry B, Pumplin D W, Wasserman S S, Tall B D, Ketley J M, Kaper J B. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc Natl Acad Sci USA. 1991;88:5242–5246. doi: 10.1073/pnas.88.12.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg I, Mekalanos J J. Cloning of the Vibrio cholerae recA gene and construction of a Vibrio cholerae recA mutant. J Bacteriol. 1986;165:715–722. doi: 10.1128/jb.165.3.715-722.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins D E, Nazareno E, DiRita V J. The virulence gene activator ToxT from Vibrio cholerae is a member of the AraC family of transcriptional activators. J Bacteriol. 1992;174:6974–6980. doi: 10.1128/jb.174.21.6974-6980.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaper J B, Lockman H, Baldini M M, Levine M M. Recombinant nontoxigenic Vibrio cholerae strains as attenuated cholera vaccine candidates. Nature. 1984;308:655–658. doi: 10.1038/308655a0. [DOI] [PubMed] [Google Scholar]
- 18.Karaolis D K, Johnson J A, Bailey C C, Boedeker E C, Kaper J B, Reeves P R. A Vibrio cholerae pathogenicity island associated with epidemic and pandemic strains. Proc Natl Acad Sci USA. 1998;95:3134–3139. doi: 10.1073/pnas.95.6.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karaolis D K, Somara S, Maneval D R, Jr, Johnson J A, Kaper J B. A bacteriophage encoding a pathogenicity island, a type-IV pilus and a phage receptor in cholera bacteria. Nature. 1999;399:375–379. doi: 10.1038/20715. [DOI] [PubMed] [Google Scholar]
- 20.Kimsey H H, Waldor M K. CTXΦ immunity: application in the development of cholera vaccines. Proc Natl Acad Sci USA. 1998;95:7035–7039. doi: 10.1073/pnas.95.12.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovach M E, Shaffer M D, Peterson K M. A putative integrase gene defines the distal end of a large cluster of ToxR-regulated colonization genes in Vibrio cholerae. Microbiology. 1996;142:2165–2174. doi: 10.1099/13500872-142-8-2165. [DOI] [PubMed] [Google Scholar]
- 22.Lazar S, Waldor M K. ToxR-independent expression of cholera toxin from the replicative form of CTXΦ. Infect Immun. 1998;66:394–397. doi: 10.1128/iai.66.1.394-397.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin W, Fullner K J, Clayton R, Sexton J A, Rogers M B, Calia K E, Calderwood S B, Fraser C, Mekalanos J J. Identification of a Vibrio cholerae RTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc Natl Acad Sci USA. 1999;96:1071–1076. doi: 10.1073/pnas.96.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mekalanos J J. Duplication and amplification of toxin genes in Vibrio cholerae. Cell. 1983;35:253–263. doi: 10.1016/0092-8674(83)90228-3. [DOI] [PubMed] [Google Scholar]
- 25.Mekalanos J J, Rubin E J, Waldor M K. Cholera: molecular basis for emergence and pathogenesis. FEMS Immunol Med Microbiol. 1997;18:241–248. doi: 10.1111/j.1574-695X.1997.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 26.Mekalanos J J, Swartz D J, Pearson G D, Harford N, Groyne F, de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983;306:551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- 27.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogierman M A, Voss E, Meaney C, Faast R, Attridge S R, Manning P A. Comparison of the promoter proximal regions of the toxin-co-regulated tcp gene cluster in classical and El Tor strains of Vibrio cholerae O1. Gene. 1996;170:9–16. doi: 10.1016/0378-1119(95)00744-x. [DOI] [PubMed] [Google Scholar]
- 29.Parsot C, Mekalanos J J. Expression of the Vibrio cholerae gene encoding aldehyde dehydrogenase is under control of ToxR, the cholera toxin transcriptional activator. J Bacteriol. 1991;173:2842–2851. doi: 10.1128/jb.173.9.2842-2851.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearson G D, Woods A, Chiang S L, Mekalanos J J. CTX genetic element encodes a site-specific recombination system and an intestinal colonization factor. Proc Natl Acad Sci USA. 1993;90:3750–3754. doi: 10.1073/pnas.90.8.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramamurthy T, Albert M J, Huq A, Colwell R R, Takeda Y, Takeda T, Shimada T, Mandal B K, Nair G B. Vibrio mimicus with multiple toxin types isolated from human and environmental sources. J Med Microbiol. 1994;40:194–196. doi: 10.1099/00222615-40-3-194. [DOI] [PubMed] [Google Scholar]
- 32.Rubin E J, Lin W, Mekalanos J J, Waldor M K. Replication and integration of a Vibrio cholerae cryptic plasmid linked to the CTX prophage. Mol Microbiol. 1998;28:1247–1254. doi: 10.1046/j.1365-2958.1998.00889.x. [DOI] [PubMed] [Google Scholar]
- 33.Shi L, Miyoshi S, Hiura M, Tomochika K, Shimada T, Shinoda S. Detection of genes encoding cholera toxin (CT), zonula occludens toxin (ZOT), accessory cholera enterotoxin (ACE) and heat-stable enterotoxin (ST) in Vibrio mimicus clinical strains. Microbiol Immunol. 1998;42:823–828. doi: 10.1111/j.1348-0421.1998.tb02357.x. [DOI] [PubMed] [Google Scholar]
- 34.Spira W M, Fedorka-Cray P J. Production of cholera toxin-like toxin by Vibrio mimicus and non-O1 Vibrio cholerae: batch culture conditions for optimum yields and isolation of hypertoxigenic lincomycin-resistant mutants. Infect Immun. 1983;42:501–509. doi: 10.1128/iai.42.2.501-509.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spira W M, Fedorka-Cray P J. Purification of enterotoxins from Vibrio mimicus that appear to be identical to cholera toxin. Infect Immun. 1984;45:679–684. doi: 10.1128/iai.45.3.679-684.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor R K, Miller V L, Furlong D B, Mekalanos J J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci USA. 1987;84:2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trucksis M, Galen J E, Michalski J, Fasano A, Kaper J B. Accessory cholera enterotoxin (Ace), the third toxin of a Vibrio cholerae virulence cassette. Proc Natl Acad Sci USA. 1993;90:5267–5271. doi: 10.1073/pnas.90.11.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trucksis M, Michalski J, Deng Y K, Kaper J B. The Vibrio cholerae genome contains two unique circular chromosomes. Proc Natl Acad Sci USA. 1998;95:14464–14469. doi: 10.1073/pnas.95.24.14464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waldor M K, Colwell R, Mekalanos J J. The Vibrio cholerae O139 serogroup antigen includes an O-antigen capsule and lipopolysaccharide virulence determinants. Proc Natl Acad Sci USA. 1994;91:11388–11392. doi: 10.1073/pnas.91.24.11388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waldor M K, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 41.Waldor M K, Mekalanos J J. Progress toward live-attenuated cholera vaccines. In: Kiyono H, Ogra P L, McGhee J R, editors. Mucosal vaccines. New York, N.Y: Academic Press; 1996. pp. 229–240. [Google Scholar]
- 42.Waldor M K, Mekalanos J J. ToxR regulates virulence gene expression in non-O1 strains of Vibrio cholerae that cause epidemic cholera. Infect Immun. 1994;62:72–78. doi: 10.1128/iai.62.1.72-78.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waldor M K, Rubin E J, Pearson G D, Kimsey H, Mekalanos J J. Regulation, replication, and integration functions of the Vibrio cholerae CTXΦ are encoded by region RS2. Mol Microbiol. 1997;24:917–926. doi: 10.1046/j.1365-2958.1997.3911758.x. [DOI] [PubMed] [Google Scholar]