Abstract
The contamination of soil with organic pollutants has been accelerated by agricultural and industrial development and poses a major threat to global ecosystems and human health. Various chemical and physical techniques have been developed to remediate soils contaminated with organic pollutants, but challenges related to cost, efficacy, and toxic byproducts often limit their sustainability. Fortunately, phytoremediation, achieved through the use of plants and associated microbiomes, has shown great promise for tackling environmental pollution; this technology has been tested both in the laboratory and in the field. Plant–microbe interactions further promote the efficacy of phytoremediation, with plant growth-promoting bacteria (PGPB) often used to assist the remediation of organic pollutants. However, the efficiency of microbe-assisted phytoremediation can be impeded by (i) high concentrations of secondary toxins, (ii) the absence of a suitable sink for these toxins, (iii) nutrient limitations, (iv) the lack of continued release of microbial inocula, and (v) the lack of shelter or porous habitats for planktonic organisms. In this regard, biochar affords unparalleled positive attributes that make it a suitable bacterial carrier and soil health enhancer. We propose that several barriers can be overcome by integrating plants, PGPB, and biochar for the remediation of organic pollutants in soil. Here, we explore the mechanisms by which biochar and PGPB can assist plants in the remediation of organic pollutants in soils, and thereby improve soil health. We analyze the cost-effectiveness, feasibility, life cycle, and practicality of this integration for sustainable restoration and management of soil.
Keywords: biochar, organic pollutants, phytoremediation, plant growth-promoting bacteria, soil pollution
1. Introduction
The extensive use of pesticides, organic solvents, pharmaceuticals, and other chemicals has resulted in the distribution of organic pollutants throughout global ecosystems.1,2 Organic pollutants are mainly the products and byproducts of anthropogenic processes. They include polychlorinated biphenyls,3 poly- and per-fluoroalkyl substances,2 pesticides,4 polycyclic aromatic hydrocarbons (PAHs), petroleum-based hydrocarbons,5 polybrominated diphenyl ethers,6 dibenzofurans, dioxins,7 and pharmaceuticals and personal care products.8 These toxicants can pass from prey to predator through the food chain. Their unique physical and chemical properties enable them to persist in the environment and travel long distances from the point of release.9−11 Exposure to organic pollutants can result in significant health risks for humans, including cancer, congenital abnormalities, obesity, damage to the central and peripheral nervous system, disruption of immune, endocrine, and reproductive systems, diabetes, allergies, and increased vulnerability to infections.12−14
Many techniques (including chemical, physical, electrical, biological, electrochemical, and physicochemical) have been developed to remediate organic pollutants in soils that may require sophisticated infrastructure and/or result in incomplete removal or generate hazardous secondary wastes. For example, in situ chemical oxidation is thought to be a quick and effective way to remove organic pollutants from contaminated environments,4,15 but it is costly and often results in undesirable harmful oxidation products that can further damage the environment. The application of strong oxidizing agents also poses serious health risks to the people handling them. Thus, alternatives or complementary techniques that are less “hands on”, more affordable, safe, and sustainable and provide more complete remediation are desirable. Such alternatives should promote the development of the circular economy and support biodiversity and functioning of the surrounding ecosystems. Moreover, as the world strives to resolve the climate change crisis, the use of renewable resources for remediating contaminated soils in a climate-smart manner should also be emphasized. Fortunately, the possibility of using natural renewable resources and/or their products for pollutant remediation is in line with these requirements. For this reason, the application of plants, microorganisms, or biochar to contain, transform, and/or degrade organic pollutants has received more attention in recent years.10,16−18 However, each renewable resource (i.e., plants, microorganisms, and biochar) has its own set of limitations when used separately to remediate contaminated soils, necessitating comprehensive research of the potential of their combined use for pollutant remediation.
Various authors have reviewed the potential use of these renewable resources for remediating organic pollutants in contaminated environments, focusing only on biochar,19 microorganisms,20 plants,21 or a combination of two of them such as biochar and microorganisms,22 plants and biochar,23 and plants and microorganisms.24 However, biochar–bacterium–plant partnerships for the remediation of organic pollutants in soils have received little attention. The complementarity that exists among the multifunctional values of biochar, plants, and bacteria for environmental management deserves closer attention as a strategy for controlling soil pollution, improving soil health, and maximizing ecological sustainability. In this review, we discuss both direct and indirect mechanisms by which biochar and plant growth-promoting bacteria (PGPB) can promote phytoremediation of organic pollutants in soils and analyze the social, economic, and environmental implications of this integration.
2. Phytoremediation of Organic Pollutants: Mechanisms and Limitations
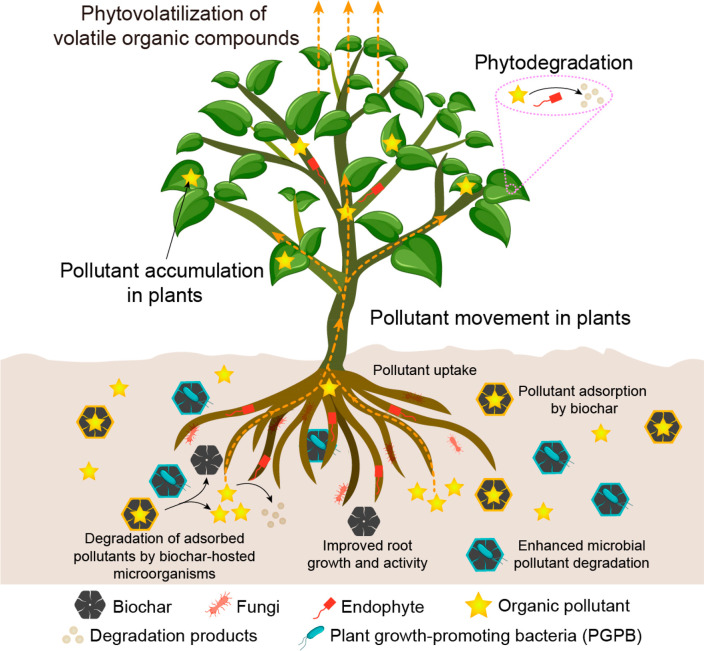
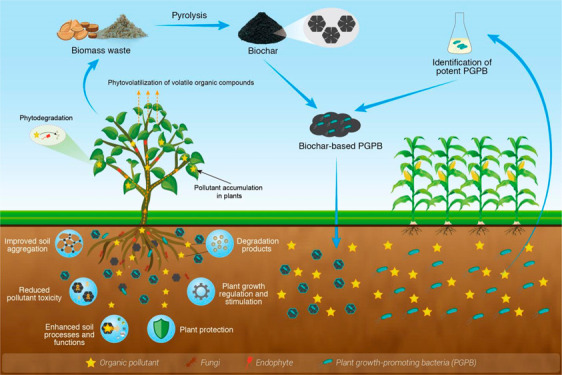
Phytoremediation uses various types of plants for the removal, transfer, degradation, and containment of hazardous pollutants in soils, surface waters, and groundwater.10,25 Plants normally take up organic pollutants from the soil via their root system, often only in small amounts, and transport them into different tissues, where they can be stored, stabilized, degraded, or volatilized.26 The root cell walls filter organic compounds, preventing compounds over a certain size from entering or leaving the roots.27 Organic compounds with a molecular mass of <1000 g mol–1 can be readily absorbed by plants.28 However, damage to roots can result in the uptake of organics with molecular masses of ≤20 000 g mol–1.29,30 The phytoremediation of organic pollutants can be classified mainly into (1) phytoextraction (or phytoaccumulation), in which plants extract pollutants from the soil via roots and translocate them to their harvestable parts, (2) phytodegradation, also known as phytotransformation, in which organic pollutants are broken down into nontoxic products through metabolic processes within the plants, and (3) phytovolatilization, which is a process by which plants absorb organic pollutants from soils and release them into the atmosphere through transpiration in volatile forms (Figure 4). It should be noted that phytoaccumulation approaches require a plan for the safe disposal and/or valorization of potentially contaminated biomass.
Figure 4.
Proposed mechanisms of synergistic contributions of biochar, PGPB, and plants for remediation of organic pollutants in soils. (1) Various functional groups, a porous structure, and a large surface area of biochar promote the sorption of organic pollutants, thereby reducing the toxicity of organic pollutants to microbes and plants in the soil. (2) Biochar-resident PGPB may further degrade the adsorbed organic pollutants, while plants provide C as root exudates facilitating microbial co-metabolism. (3) PGPB and biochar can improve root growth and activity, which is beneficial for the absorption of pollutants by plants. (4) Once in the plant endosphere, pollutants can accumulate in various plant tissues, be degraded by plant enzymes and endophytic microorganisms, or be released into the atmosphere through volatilization.
In addition to the phytoremediation mechanisms described above, phytoremediation processes may be mediated by plant roots outside plant tissues. Roots can secrete a range of enzymes capable of degrading organic pollutants directly17 and/or exude carbon-rich rhizodeposits, which facilitate root colonization and sustain communities of beneficial microorganisms capable of transforming organic pollutants, in a process termed phytoremediation ex planta.31 These rhizodeposits consist of low-molecular weight organic compounds, including amino acids, organic acids, sugars, and phenolics that are used as an energy source for rhizosphere microbial communities, including those with plant growth-promoting and pollutant-degrading activities.32 Meanwhile, high-molecular weight organic compounds, such as mucilage and proteins, may alter the chemical and physical properties of soil, which enhance root–soil interactions and facilitate the movement of roots through the soil.32,33
Knowledge about a specific contaminated site is vital for the successful phytoremediation of organic pollutants. The pollutant’s physicochemical properties such as volatility, solubility in water, the octanol–water and octanol–air partition coefficients, and specific toxicities are more obvious primary concerns that interact with plant characteristics, including morphology, lipid content, and physiology of root and shoot systems.34,35 Factors that influence pollutant uptake and root interactions are also vital, including soil pH, porosity, temperature, bulk density, hydrodynamics, the richness, composition, structure, interactions, and function of microbial communities within soil patches, mineral quality, and organic matter availability. However, phytoremediation is subject to the following constraints. (1) Xenobiotic organic compounds need to be in the proximity of the root zone of plants used for phytoremediation, and (2) high concentrations of bioavailable toxins in soil inhibit plant growth. The abilities of several plant species to decontaminate organic pollutants have been explored. Plants known to absorb and accumulate organic pollutants include maize (Zea mays L.), sunflower (Helianthus annuus),36 switchgrass (Panicum virgatum),37 hybrid poplar trees (Populus tremula × Populus alba),38 tobacco (Nicotiana tabacum),6 and rice (Oryza sativa) among others, as reviewed by Afzal et al.17 Others have been found to take up, translocate, and volatilize organic pollutants, including willow (Salix viminalis), alfalfa (Medicago sativa L.), bald cypress (Taxodium distichum), weeping willow (Salix babylonica), and common reed (Phragmites australis).39
Whether hyperaccumulation or rhizodegradation is the target, an extensive root system is key.40 Root biomass and root length strongly influence the efficiency of the phytoremediation process and have previously been found to be positively correlated with the ability of a plant to transport and concentrate pollutants from the soil into the harvestable aboveground shoots.41 However, some studies have shown that most organic pollutants impair root growth and the development of plants.42−44 Plants do not achieve high biomass yields in heavily polluted soils, which affects the bioaccumulation of pollutants and the economic return of a phytoremediation project. In addition, toxic chemicals can interfere with the availability of essential plant nutrients in the soil, again restricting plant growth.45 Previously pristine soils that become contaminated are typically low in other nutrients; hence, external input of bioavailable nutrients will help remove limits to phytoremediation but should be well contained within the contaminated area. Alternatively, plants with established symbioses with nutrient-acquiring bacteria can make up for this nutrient deficit.
3. PGPB-Assisted Phytoremediation of Organic Pollutants
PGPB with pollutant-degrading activities can be especially useful for revitalizing organic pollutant-contaminated soils.46 Many bacterial species in genera such as Rhizobium,5Klebsiella,47Pseudomonas,48Acinetobacter and Alcaligenes,46 and Bacillus(49) are known to improve plant growth and health and enable plants to withstand and remediate environmental pollutants. For instance, Singha et al.46 observed a 19.1% increase in shoot length in rice seedlings treated with Pseudomonas aeruginosa, while rice seedling root length increased by 26.5% upon treatment with Klebsiella pneumoniae in pyrene-contaminated soil.
In addition to their beneficial impacts on plant growth and development (as discussed in section 7), many PGPB can transform organic pollutants, such as petroleum hydrocarbons,50 polychlorinated biphenyls,3 PAHs,46 and pesticides,51,52 into nontoxic products or minerals. Petroleum-degrading rhizospheric bacteria, such as Bacillus thurigiensis, Bacillus pumilus, and Rhodococcus hoagii, isolated from the rhizosphere of Panicum aquaticum Poir53 also exhibit plant growth-promoting activities.54−57 As observed in different laboratory and field studies, plant–bacterium partnerships may provide promising approaches for the remediation of organic pollutants and restoration of ecological functions of contaminated environments (Table S2).
The primary factor promoting microbial colonization and interaction with plants in the rhizosphere is the sustained release of root exudates, providing labile carbon and signaling cues that trigger plant–microbe interactions.58,59 However, toxic chemicals under soil and plant growth conditions can alter the composition and quantity of root exudates, thereby affecting the colonization and activity of microorganisms in the rhizosphere.60,61 Additionally, when introduced into the soil from the external environment, microorganisms frequently fail to exert their beneficial effects, possibly due to a lack of favorable habitat for their growth and the poor fitness of laboratory-grown inoculants to compete with indigenous microbial communities in polluted soils.62,63 Therefore, while it is vital to select robust and competitive PGPB on a site-specific basis, there is a real need to develop a microbial carrier capable of providing a suitable habitat for microbial growth and propagules. Furthermore, one that reduces pollutant mobility and toxicity and improves soil quality (enhancing the growth and metabolic activities of plants) would be highly beneficial for the remediation of organic pollutants in soils.
4. Identifying PGPB for Soil Remediation
To establish a robust and functional microbial inoculant for soil remediation, it is important to understand how microorganisms interact. While many studies of PGPB-assisted phytoremediation are often based on culture-dependent monocultures (Table S3), this is not reflective of the condition that prevails in nature, where taxonomically and metabolically diverse microorganisms live in multispecies biofilms,64 either cooperating or competing with one another for resources.65 Another drawback is that only <1% of microorganisms in the natural environment are thought to be culturable.66,67
Previous studies indicated that microbial strains with high pollutant degrading activity under controlled laboratory conditions might have low efficiencies and survival rates in field-scale bioremediations.67,68 Su et al.69 further confirmed the presence of viable but nonculturable bacteria in contaminated natural environments. In alignment with this, the identification and selection of functional “culturable” organisms that integrate well with non-culturable communities have been performed at the site to be remediated. With recent advances in high-throughput molecular technologies, the development of culture-independent methods provides a platform for linking microbial community structure, interactions, and system function that may help in designing “robust” and “predictable” multispecies PGPB-based biofilms for better plant growth and soil remediation.70
A logical progression in the development of microbial consortia that are sympathetic to the receiving ecosystem is to become more accommodating of the microbial diversity already present at the site needing remediation. This will involve using site-specific metagenomics and other omics technologies to gain a holistic view of microbial diversity and metabolic potential in the affected environment, helping to better understand how inocula can better complement the preexisting community. In addition to increasing the chances of creating an effective, cooperative, and stable biofilm, this approach will minimize unforeseen impacts on local ecosystems but maximize the sustainability of PGPB-assisted phytoremediation through cooperation.
5. Biochar for the Delivery of PGPB
Traditional methods for delivering specific microbiota into soils often compromise inocula, mainly due to desiccation before microbes can fully colonize the contaminated soil.71 Therefore, numerous novel carrier systems have been developed to improve the survival and distribution of microbial inoculants, many of which are rhizobia.72−74 Although currently available carriers are highly effective in improving the survival and effectiveness of inoculants, some of them are costly and not available worldwide. Peat and vermiculite, for example, are not available worldwide, and their extraction, manufacture, and transport harm the environment by accelerating the return of terrestrially sequestered carbon into the atmosphere. The Wildlife Trusts recently found that the use of peat in UK horticulture alone may have accelerated the emission (by hundreds to thousands of years) of up to 31 million tons of CO2 into the atmosphere since 1990.75 However, this figure excludes the even more concerning emissions from fossil fuels (formerly stable for millions of years) used in bagging and distribution. In this regard, a more affordable material with properties similar or superior to those of existing carriers would be advantageous. One that can reverse the trend of increasing atmospheric concentrations of CO2 would be even better.
Biochar has been lauded as a carrier material to deliver both nutrients (organic and/or chemical fertilizers) and microbial inoculants to agricultural soils.76 Biochar is a porous carbonaceous material produced from the pyrolysis of biomass at high temperatures ranging from 300 to 900 °C under oxygen-limited or anoxic conditions.77 First used as a soil amendment to promote carbon sequestration,78 biochar has since been shown to be beneficial for a variety of other purposes, including the enhancement of soil fertility, microbial growth and activities, and pollution remediation.79 The mechanisms through which biochar supports microbial growth and activities are diverse and are driven by its unique physical and chemical properties, as reviewed by Zhu et al.80 Briefly, the provision of a microbial habitat with biochar can be attributed to its large volume of pores and surface area. Biochar is found to have a total pore volume and a pore surface area that are larger than those of most soils, with its total surface area being ∼5 times larger than that of the average soil.81 A large surface area of biochar can contribute to the improvement of water retention in soil, allowing biochar to retain moisture in micro- and mesopore spaces, which in turn keeps microorganisms hydrated in drying soil.82 Biochar can also positively affect the growth and activities of microorganisms by increasing the soil pH and improving aeration.83 Through the sorption of nutrient cations and anions with its surface functional groups (cation and anion exchange capacity),84 biochar can retain and supply nutrients for microbial growth in the soil.
On the basis of these constructive features, many studies suggest that biochar is suitable for boosting the effectiveness and shelf life of microbial inoculants in soil.85−87 For example, when comparing pine bark-derived biochar and sewage sludge-derived biochar with poultry litter and perlite as the carrier of Bradyrhizobium strains, Araujo et al.85 found that pine bark-derived biochar was an optimal carrier, extending the shelf life of bacteria up to one year. Sashidhar et al.76 and Ajeng et al.86 compiled and discussed studies of various types of biochar used for inoculant deployment, some of which also reported enhanced remediation functions.88−90 However, not all biochars are suitable for use as microbial carriers or soil conditioners. For instance, biochar with a very high pH, an excessive amount of ash, and inorganic or organic pollutants can be toxic and/or have no benefit for halophobic or acid-loving plants and bacteria.
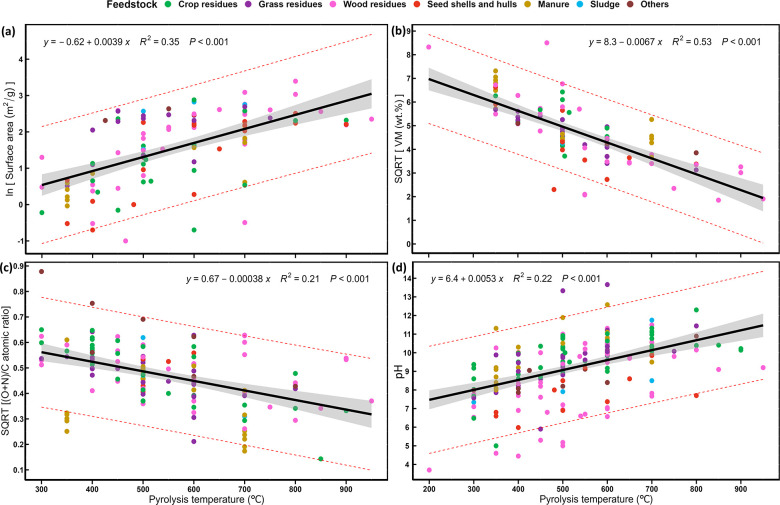
To better understand the influence of pyrolysis temperature and feedstock on the physicochemical properties of biochar and its function as a bacterial carrier and soil conditioner, we collected data for 249 paired observations from 38 peer-reviewed studies. The results were used to quantify the linear (or nonlinear) relationships of biochar surface area, volatile matter content, pH, and other properties with pyrolysis temperature and feedstock type (Figure 1). Biochar derived from lignocellulosic biomass (LB) (e.g., crop residues and wood residues) showed an increased surface area and porosity that are beneficial to house soil microbes. In the analysis of reported data, the average specific surface area of biochars from wood residues (275.21 m2 g–1) was >4 times that of biochars from manure (56.03 m2 g–1) (Table S1). However, biochars from LB suffer from mineral nutrient deficiencies compared to biochars from nonlignocellulosic biomass (NLB) (e.g., animal manure and sewage sludge). This may reduce the competitiveness of LB-derived biochar in soil that lacks mineral nutrients to support plant and microbial growth.19 Among the feedstocks analyzed, wood-derived biochar had the lowest average nitrogen (1.037%) and sulfur (0.078%) content, much lower than those of manure-derived biochar (3.16% N and 7.35% S). Nonetheless, fertilizers (chemical or organic) can be added to biochar after the pyrolysis process.
Figure 1.
Dependence of biochar properties on feedstock type and pyrolysis temperature: (a) specific surface area (SSA), (b) volatile matter, (c) (oxygen + nitrogen)/carbon ratio, and (d) pH. We analyzed data for normality. If the data did not conform to the normal distribution, we used a natural logarithm (ln) or square root (SQRT) to transform the data. Different colored circles indicate different feedstock types, and the black line is a general linear regression between biochar properties and pyrolysis temperatures. Panels a and d show an increasing pyrolysis temperature is linked to an increasing specific surface area and pH, while panels b and c show a negative relationship between the (oxygen + nitrogen)/carbon ratio and volatile matter content with pyrolysis temperature. Data are from Table S1.
The drawbacks of biochars from NLB are exacerbated by pore filling with a large amount of volatile organic matter (40–80%), which can prevent microbial colonization.91 The large amount of volatile organic compounds (VOCs) and environmentally persistent free radicals of biochars from NLB can be toxic to soil microbiota.80 In contrast to lower pyrolysis temperatures (<400 °C), higher pyrolysis temperatures (>500 °C) result in biochars with a larger surface area and higher porosity, carbon content, and pH, but lower nitrogen, hydrogen, oxygen, and surface functional group content, which can reduce the cation exchange capacity and nutrient sorption for microbial growth (Figure 1).92 In this regard, determining the proper feedstock type and pyrolysis temperature requires a balanced and comprehensive understanding of which combination (high or low temperature and NLB or LB) is more favorable to produce biochar with desirable properties to sustain which kinds of microorganisms and for what applications.
Postpyrolysis modifications of biochar benefit microbial inoculants through chemical and physical modifications or supplementation with minerals.93 When using biochar as a carrier for PGPB, consideration should also be given to the properties of the soil to which the biochar-based bacterial inoculant will be applied, as well as the properties of the bacterial surface and plant growth conditions.94,95 Therefore, characterizations of the soil, plants, PGPB, and biochar are important steps for specific agricultural and environmental applications.76 Given the variability of soil properties and growth requirements for microorganisms and plants, there are no universally accepted standards for the application of biochar; however, as minimum requirements, biochar must have the following physicochemical characteristics to be effective as a soil amendment and inoculant carrier: black carbon, >15% C; surface area, >100 m2 g–1; O/C ratio, <0.4; H/C ratio, <0.6.96 To prevent additional contamination, the levels of pollutants (both organic and inorganic) in biochar must be lower than those found in contaminated soils and below the limits set by national and international standards. This may open up opportunities for the use of biochars (in remediation) that fail to meet the standards required for use in uncontaminated soils. In addition, before using biochar to immobilize microbial inoculants, it is very important to perform toxicity, growth, and metabolism tests to determine what type of and at what rate biochar is compatible with particular microorganisms.93 In some cases, biochar may contain contaminants such as heavy metals, most of which are introduced from biomass feedstock sources (e.g., plants can absorb heavy metals from the soil). Some organic contaminants (such as PAHs, dioxins, and furans) and environmentally persistent free radicals can be generated during the pyrolysis process.97 The presence of these contaminants in biochar can cause toxicity to microorganisms.80,98 As with any other soil amendment or microbial carrier, the rate at which biochar is applied must be determined by comprehensive field and microbiological compatibility testing.86,99 At present, numerous studies of the bioformulation of biochar as a potential inoculant carrier for soil application have been undertaken in laboratory settings,76,86 and there is insufficient field information to recommend the rate at which biochar should be used as a microbial carrier for different soil types and plants.
6. Biochar-Mediated Soil Remediation
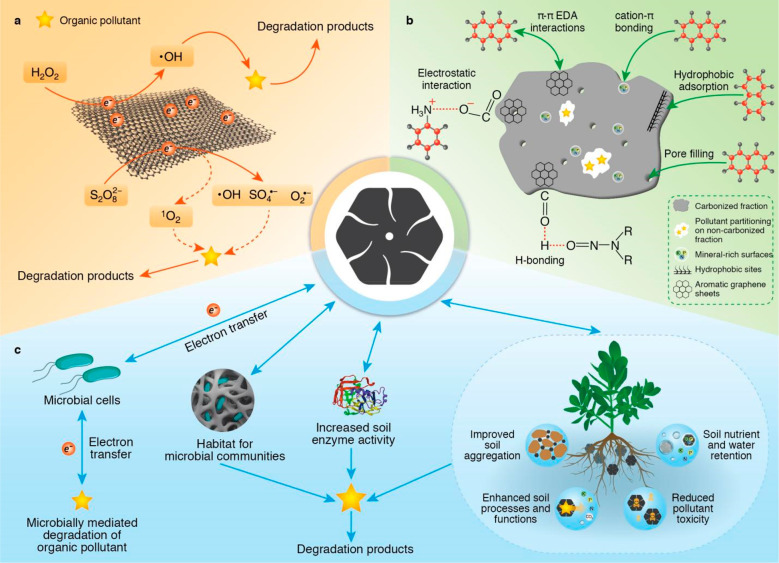
Biochar has sparked a great deal of interest in the remediation of soils contaminated with different environmental pollutants (organics and inorganics) because of its multifunctional value as a soil amendment in impoverished soils.19,75 Biochar is involved in the remediation of organic pollutants in soils through a variety of processes, either directly through the immobilization of pollutants or indirectly by influencing soil metabolic activities and degradation (Figure 2). Direct sorption of organic pollutants to biochar reduces the mobility, bioavailability, and toxicity of pollutants in soil.100 Organic pollutants can be immobilized in the soil through a number of interactions with biochar, including pore filling, electrostatic interaction, partition, electron donor and acceptor interaction, hydrophobic interaction, and π–π electron donor–acceptor interaction (Figure 2b).79,101 The carbonized fraction of biochar mediates adsorption (electrostatic attraction and nonpolar biochar–pollutant interactions), while the uncarbonized fraction of biochar mediates partition.80 Both sorption mechanisms are mainly dependent on the pyrolysis temperature, with partition predominating at low pyrolysis temperatures (<400 °C) and adsorption predominating at high pyrolysis temperatures (>500 °C).102,103
Figure 2.
Proposed mechanisms by which biochar mediates the remediation of organic pollutants in soil. (a) Free radicals from biochar can react with O2 to produce •OH and/or activate S2O82– or H2O2 to produce reactive oxygen species (•OH, SO4•–, and O2•–), which facilitate the oxidation/degradation of organic pollutants. (b) Organic pollutants can be immobilized in the soil through several interactions with biochar, including pore filling, electrostatic interaction, partition, electron donor and acceptor interaction, hydrophobic interaction, and π–π electron donor–acceptor interaction.79,101 The carbonized fraction of biochar mediates adsorption (electrostatic attraction and nonpolar biochar–pollutant interactions), while the uncarbonized faction of biochar mediates partition.80 (c) With its porous surface, biochar can accommodate soil microbial communities, immobilize and release enzymes, improve soil processes and functions, and transfer electrons to microorganisms and pollutants, hence influencing plant and microbial metabolism of organic pollutants in soil.
In general, the sorption effectiveness of biochar is determined by its properties (e.g., porosity, aromaticity, basicity, specific surface area, and surface functional groups), as well as those of the organic pollutants (e.g., molecular size and hydrophobicity) and soil (e.g., organic matter, clay content, microbial community structure, pH, and texture) in addition to other environmental factors that govern pollutant mobility.104−106 The surface area, porosity, aromaticity, and polarity of biochar are regarded to be the most critical properties that determine the sorption of organic pollutants and are influenced by the feedstock type, pyrolytic condition, and postproduction treatment processes. Increasing the pyrolysis temperature increases the surface area and the number of micropores of biochar and improves the sorption of organic pollutants by pore filling and hydrophobic interaction.92 This trend is related to the rate of decomposition of feedstocks and the amount of volatile matter released during the carbonization process. For example, pore filling occurs as a result of the presence of mesopores and micropores on the biochar surface; volatile compounds filling the micropores are released at higher pyrolysis temperatures, leaving internal pores available to both house soil microbiota and sorb organic pollutants.19,107
In contrast to biochar made from lignocellulosic biomass (woods and crop residues), those made from NLB (e.g., sewage sludge, animal litter, hair, and algae) have smaller surface areas, lower carbon contents, and higher cation exchange capacities, even at higher pyrolysis temperatures. These differences may be related to the content of cellulose, hemicellulose, and lignin, as well as the level of moisture in the biomass,108 but the amount of internal air space in the materials at the onset of pyrolysis is pivotal and contributes to the creation of pores through partial combustion and providing a physical route for VOCs to escape. In this regard, it is crucial to have a balanced and holistic understanding to produce and apply biochar with desirable properties for soil remediation.
Electrostatic interaction is critical for biochar’s adsorption of ionic organic pollutants. In addition to hydrophobic moieties, many biochar surface moieties are also negatively charged, which may assist the electrostatic binding of cationic organic pollutants.109,110 The electrostatic interaction depends on the functional groups of the biochar. Low pyrolysis temperatures have been shown to produce biochar with more functional groups than biochar produced at high temperatures.104 Additionally, the hydrophobicity of biochar is critical for the adsorption of both neutral and hydrophobic organic contaminants. Biochar with a low degree of surface oxidation is often hydrophobic and capable of immobilizing hydrophobic organics through both partitioning and hydrophobic adsorption.19,111 The aromaticity of both biochar and pollutants is considered to greatly influence π–π electron donor–acceptor interactions between biochar and adsorbates, which in turn may affect the adsorption process.112 The π-electron cloud of biochar aromatic fragments can noncovalently interact with anions, cations, proton donor functional groups, and the π-electron cloud of target aromatic pollutants; therefore, π–π electron donor–acceptor interaction is thought to be a dominant mechanism in the sorption of aromatic organics by biochar.113 Although sorption of organic pollutants to biochar is a promising strategy for immobilizing organic pollutants in soil and minimizing their detrimental effects on ecological functioning, it is not a definitive solution for soil remediation because adsorbed pollutants may remain in the soil with possible long-term toxic effects, which requires other strategies for removing immobilized organic pollutants from the soil.114 Functional biochar can be prepared by chemical and physical modifications of the raw biochar or by impregnating it with minerals or (nano)composites, or by supplementing it with specific microorganisms to increase its sorption or remediation capacity with respect to specific organic pollutants.79,90,115−117
Direct mechanisms of the removal of pollutants by biochar may also occur via the degradation of pollutants by the biochar itself or by biochar-supported (nano)composite materials.115,116 Basically, free radicals generated by biochar can react with oxygen (O2) to produce hydroxyl radicals (•OH) and/or activate S2O82– or H2O2 to produce reactive oxygen species [•OH, sulfate radicals (SO4•–), and superoxide radical (O2•–)], which then mediate the oxidation/degradation of organic pollutants (Figure 2a).118−120 In the presence of external oxidants, the redox-active part of the biochar can act as electron carriers, interacting with oxidants and enhancing the formation of strongly oxidizing free radicals, which are critical for the degradation of organic pollutants.121 Different types of biochar, produced from different feedstocks, pyrolyzed at different temperatures, and/or modified with different methods, have been used to treat organic pollutants in various types of contaminated soil, and their application rate, dominant mechanisms of action, and efficiencies are summarized in Table S3. The indirect mechanisms by which biochar contributes to the degradation of organic pollutants in soil are illustrated in Figure 2c.
7. Biochar-Based PGPB: Key Considerations for Phytoremediation
7.1. Biochar-Based PGPB for Mitigation of Nutrient Deficiency
Microorganisms and biochar improve soil health and nutrient status in the rhizosphere via different mechanisms (Figure 3). Of all plant nutrients, nitrogen (N) typically limits plant growth and development because plant demand is high, but available forms of N are rapidly denitrified to N2 and N2O under waterlogged conditions and easily leached (as NO3–) under more dynamic conditions.122 However, many bacteria can fix N2 to NH4+ through biological nitrogen fixation. Several nitrogen-fixing bacteria were isolated from organic pollutant-contaminated sites and were proven to promote the growth of legumes in contaminated soils.5,46−49 In addition to providing suitable habitats for organisms involved in nitrogen cycling within the biochar,123 it can also influence functional genes at a distance of several millimeters from the particles themselves. In this way, a “functional asymmetry” can be created, for example, with bacterial amoA becoming more dominant as the distance between biochar and bulk soil is reduced.124 Accordingly, biochar can either decrease or increase nitrogen use efficiency, for example, by increasing pH (causing ammonia volatilization) or decreasing the rate of volatilization, promoting biological nitrification, or directly reacting with the carboxyl groups of biochar to produce NH4+ or an amide radical/group.126 Nitrous oxide (N2O) emissions are typically decreased after incorporation of biochar,125,126 with a meta-analysis showing decreases of 54% are typical.127 Biochar applied with chemical nitrogen fertilizers increased the nitrogen use efficiency by 43.1% compared with that of fertilizer nitrogen application alone,128 and urea plus biochar application increased the maize yield by 26% compared with that of urea application alone in tropical soils.125 Both the improved nitrogen use efficiency and crop yield were attributed to the lower N release rates of the biochar-based nitrogen fertilizers.125 Biochar application has also been proven to increase crop yields by enhancing biological nitrogen fixation by rhizobia that live in symbiosis with legumes.129 However, raw biochar can immobilize nitrogen in the early months following application, and external nitrogen is typically added to overcome this. Other corrections may also be required to control other deleterious factors that can adversely affect the structure and activity of the biochar microbiome.80
Figure 3.
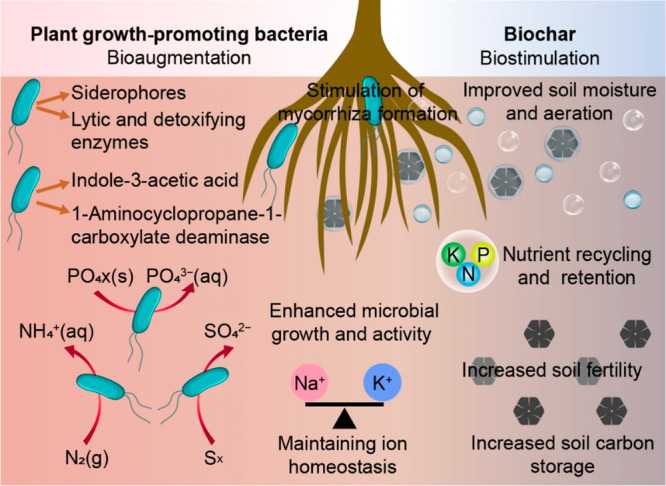
Bioaugmentation by PGPB and biostimulation by biochar are the two main strategies to complement phytoremediation and improve soil qualities. PGPB can (1) fix nitrogen, solubilize phosphate and potassium, and oxidize sulfur (ruby arrows);155 (2) stimulate mycorrhiza formation, which can be beneficial for both plant protection and nutrient availability; and (3) produce allelochemicals (e.g., siderophores, lytic and detoxifying enzymes, indole-3-acetic acid, and ACC deaminase) (gold arrows), regulate plant growth and development, and protect plants from pathogens. (4) With its porous structure, biochar can serve as a habitat for PGPB and other soil microbiomes,80 contributing to their growth, survival, and activity. (5) Due to its ability to retain and release nutrients slowly, biochar may act as a source of nutrients for both microbes and plants for an extended period of time in soil. (6) Biochar can also improve soil aeration, pH, and water and carbon content and thus alter the growth of soil microbiomes and plants and their interactions.129 (7) By reducing sodium uptake and increasing potassium uptake by roots, biochar can maintain ion homeostasis in plants.173,174
Some PGPB can increase the level of plant-available phosphorus (P) by solubilizing insoluble phosphorus compounds in the soil. The most common and prevalent pathway for PGPB to dissolve inorganic phosphorus compounds is to produce organic acids (such as acetic acid, malic acid, citric acid, succinic acid, oxalic acid, tartaric acid, 2-ketogluconic acid, and lactic acid), with gluconic acid playing a prominent role in phosphate solubilization.130 The released organic acids decrease the local soil pH and facilitate the dissolution of inorganic phosphates complexed with calcium, iron, and aluminum. PGPB may also solubilize inorganic phosphates through proton extrusion as well as the production of inorganic acids, exopolysaccharides, and siderophores. Meanwhile, organic phosphates can be solubilized by enzymes, including nonspecific phosphatases, phytases, and C–P lyases, released by PGPB.131 The genus Bacillus has the most species capable of solubilizing otherwise insoluble phosphorus compounds (including Bacillus subtilis, Bacillus megaterium, Bacillus coagulans, and Bacillus circulans), followed by Pseudomonas.131−133 In addition, some PGPB are “mycorrhizal helpers”, including Streptomyces sp. and Pseudomonas sp., which assist the development of both ectomycorrhiza and arbuscular mycorrhizae. In turn, these mycorrhizal fungi help improve the availability of phosphorus to plants.134,135
Biochar can also improve the uptake of P by plants in agricultural soils through the direct contribution of plant-available phosphorus, alteration of the soil pH, and/or stimulation of plant roots to access soil P.136 Biochars produced from biowastes, such as animal manure and sewage sludge, are rich in P and can increase its availability in soil.137 Moreover, biochar can be combined with synthetic phosphate fertilizers to form a slow release P fertilizer138 or used as an inoculum carrier for delivering phosphate-solubilizing bacteria into agricultural soils.139 When used as a carrier for P fertilizer or P-mobilizing inocula, biochar releases P slowly and steadily over a longer period, promoting P recycling while reducing its losses from soil. However, the impact of biochar on plant-available P in soils largely depends on the biochar feedstock type, pyrolysis temperature, application rate, and soil properties.136,140
Potassium (K) is another important macronutrient needed in large amounts for adequate plant growth and development. However, approximately 90–98% of soil K is in forms that are often considered “nonexchangeable” limiting the availability of K for plant use.141 PGPB can solubilize this K by releasing organic acids and other extracellular polymeric substances that contribute to displacing K+ from K-bearing minerals. These potassium-solubilizing bacteria, including members of genera like Bacillus, Pseudomonas, Burkholderia, Acidothiobacillus, Paenibacillus, and Rhizobium, and their mechanisms of action have been discussed by Sattar et al.142 Biochar can greatly increase soil K content and increase the availability of water-soluble and exchangeable K in soil.143 Soils rich in K-bearing minerals tend to extend biochar impacts on plant K uptake.144 This is because biochar can improve the survival and functioning of K-solubilizing microbial communities in soils rich in K-bearing minerals, which in turn can contribute to the weathering of K minerals in soils.144,145
Sulfur (S) is also an essential nutrient for plants. However, elemental sulfur (S0) and reduced sulfur compounds found in soil organic matter and some fertilizers are not readily available for plants. Sulfur-oxidizing bacteria, such as Acidithiobacillus sp. and Thiobacillus sp., can oxidize reduced sulfur to sulfate, which is plant-available.146,147 Biochar can also enhance the plant availability of S through direct S contribution and/or supporting microbial S oxidizing activities. The speciation and content of sulfur in biochar may vary depending on the feedstock type and production condition. Cheah et al.148 produced biochar from oak and corn stover under pyrolysis and gasification conditions and observed the following outcomes. (1) Oak biochar contained 160 mg of S L–1, while corn stover biochar had 600–800 mg of S L–1. (2) Both oak biochar and corn stover biochar pyrolyzed at 500–600 °C contained organosulfur, sulfate, and sulfide. (3) Biochar generated through gasification at 850 °C had a large amount of organosulfur.
PGPB-chelating agents also increase the availability of iron, manganese, zinc, and copper to plants.143 Almost all known PGPB release siderophores (high-affinity iron-chelating compounds) that bind and solubilize iron from mineral surfaces, thus facilitating the availability and uptake of iron by plants. These siderophore-producing rhizobacteria and their mechanisms of action, as well as different types of microbial siderophores, have been described and discussed elsewhere.149,150
In addition, PGPB can enhance the production of root exudates that form soluble complexes with the micronutrients (e.g., iron, manganese, zinc, and copper), making them available for plant uptake. Though micronutrients are required for optimum plant growth and development, their presence at high concentrations can be harmful or even toxic to plants. Excess bioaccumulation of micronutrients is usually accompanied by plant growth inhibition and impairment of important plant physiological processes, which may lead to chlorosis, premature death of cells in plant tissues (necrosis), and an imbalance of different reactive oxygen species.151,152 In this regard, biochar can alleviate phytotoxicity by increasing soil pH, which can reduce metal bioavailability in soils,153 or adsorbing bioavailable metals directly into the biochar itself.154
7.2. Effects of Biochar and PGPB on the Regulation of Plant Growth and Development
Indole-3-acetic acid (IAA) is the most common plant auxin and one of the key factors for enhancing the phytoremediation of soil contaminated with organic pollutants. In a study by Li et al.,156 exogenous IAA treatment induced soil peroxidase production, improved soil microbial biomass, and alleviated abiotic stress, ultimately promoting the removal of phenanthrene from soil. In addition to plants, many PGPB (and ∼80% of rhizobacteria) can produce IAA, as a competent signaling molecule for communication between plants and soil bacteria. IAA-producing bacteria span a wide range of genera, including Bacillus, Serratia, Azospirillum, Acinetobacter, Pseudomonas, Rhizobium, and Streptomyces.155 Although IAA helps plants to withstand environmental stresses, very high concentrations of IAA may inhibit root and shoot growth or cause other growth abnormalities.157 Therefore, IAA close attention is required to avoid inhibitory effects from excess dosage. On the contrary, there is a degree of tolerance in the system as plants may control excess IAA through neutralization mechanisms, including inactivation of IAA by direct oxidation of IAA to OxIAA and by conjugation with sugars, amino acids, or peptides.158 In addition to IAA, PGPB can produce other important plant hormones, such as gibberellins, cytokinins, and abscisic acid, which influence the hormonal balance in plants and their response to the external environment.159 The mechanisms of the action of bacterial phytohormones in plant growth regulation and their development have been reviewed by Kudoyarova et al.160 Some studies have indicated that the application of biochar partly boosts plant growth and development by activating the gibberellin pathway161 and enhancing the transcription of auxin- and brassinosteroid-related genes, which play a key role in inducing systemic stress tolerance in plants.162
Another central regulator of plant development and the stress response is ethylene gas, which controls diverse physiological processes throughout the plant life cycle, including the growth of roots, leaves, flowers, and fruits, rhizobial nodulation in legumes, rooting of tissue cultures, and the interaction between plant and beneficial microorganisms.143 Plants respond to biotic and abiotic stresses by producing ethylene from 1-aminocyclopropane 1-carboxylate (ACC).163 It has been shown that organic pollutants can induce an increased level of production of ethylene in plants.164,165 However, ethylene can be harmful to plants when in abundance, causing plant growth inhibition and promoting premature senescence.166,167
Of the different mechanisms used by PGPB to promote the growth of plants, the production of the enzyme ACC deaminase is greatly important for improving the performance of plants grown in contaminated soils.168 This enzyme can decrease plant ethylene levels by converting ACC, an immediate precursor of ethylene in plants, into NH3 and α-ketobutyrate, promoting plant growth and survival under adverse stress conditions such as drought, salinity, and organic and inorganic contamination (Figure 3). It has been shown that plants inoculated with bacteria that have ACC deaminase activity or transgenic plants expressing the bacterial enzyme ACC deaminase accumulate greater amounts of heavy metal pollutants within the plant tissues and produce longer roots and greater root density.169,170 Microorganisms with ACC deaminase activity can promote the growth of plants involved in the phytoremediation of organic pollutants. Some ACC deaminase-producing bacteria can degrade organic contaminants and include representatives from Rhizobium,(5)Klebsiella,(47)Pseudomonas,(48)Acinetobacter and Alcaligenes,(46)Bacillus,49 etc. To better understand how these ACC deaminase-producing bacteria promote plant growth and help plants remove organic pollutants from soils, the reader is referred to the reviews of Glick168 and Arshad et al.169
7.3. Effects of Biochar and PGPB on Plant Protection
Apart from improving soil fertility and stimulating plant growth, PGPB can also act as biocontrol agents by protecting the plants from phytopathogens (i.e., fungi, bacteria, nematodes, and viruses) using a wide range of defense mechanisms. The generally understood biocontrol mechanisms mediated by PGPB include the production of inhibitory allelochemicals, such as siderophores, secondary antibiotic metabolites, biocidal volatiles, lytic enzymes, detoxifying enzymes, and induction of systemic resistance in plants.168,171,172
Iron is an essential micronutrient for all soil organisms, including plant pathogens. PGPB can limit the availability of iron in the soil by competitive removal. Iron–siderophore complexes are readily available to organisms with specific receptors, such as ferrireductases and adjacent iron(II) transporters, on their outer cell membrane.169 However, pathogens without receptors for siderophore–iron complexes will suffer from iron deficiency and face the risk of local extinction. Because plants can access iron chelated with siderophores, bacterial siderophores improve plant iron nutrition and protect plants from some pathogens.170,171 In addition, some PGPB can produce a variety of antibiotics. These PGPB include some species of genera such as Bacillus, Streptomyces, Stenotrophomonas (which can produce oligomycin A, kanosamine, zwittermicin A, and xanthobaccin), and Pseudomonas (which can produce 2,4-diacetylphloroglucinol, amphisin, hydrogen cyanide, pyoluteorin, cyclic lipopeptides, tensin, and phenazines).168 Some of these antibiotics produced by PGPB have proved to be effective in inhibiting the growth of phytopathogenic microorganisms and interestingly are finding new applications in human medicine.168,172 PGPB can also exert their biocontrol abilities by producing extracellular enzymes, including lipase, β-1,3-glucanase, laminarinase, chitinase, and protease, which have the potential to suppress plant pathogens. For instance, chitinase and laminarinase produced by Pseudomonas stutzeri YPL-1 degraded mycelia of Fusarium solani (a fungal pathogen causing root rot with severe losses in many agriculturally valuable crops);173 a chitinase- and protease-producing Serratia plymuthica was found to protect cucumber from Botrytis cinerea and Sclerotinia sclerotiorum diseases,174 and β-1,3 glucanase produced by Pseudomonas cepacia destroyed the cell walls of Rhizoctonia solani, Sclerotium rolfsii, and Pythium ultimwn, hence decreasing the incidence of diseases caused by phytopathogenic fungi.175
Some bacteria have the ability to detoxify and/or degrade phytotoxins. For instance, Pantoea dispersa strain SB1403 showed a strong capacity for enzymatic detoxification of albicidins (a family of highly potent antibiotics and phytotoxins produced by Xanthomonas albilineans), which are the main pathogenesis drivers of sugar cane leaf scald disease.176 In addition to the direct mechanisms of biocontrol mediated by PGPB described above, some rhizosphere bacteria and mycorrhizal fungi can also induce resistance in plants. To date, two main forms of induced resistance have been described in plants: systemic acquired resistance (SAR) and induced systemic resistance (ISR). The latter is attributed to beneficial PGPB and fungi and depends on the signaling pathways of salicylic acid, ethylene, and jasmonic acid.177 In particular, PGPB can mediate ISR by releasing bioactive compounds that elicit specific immune responses in plants.178 Ryu et al.179 were the first to indicate that VOCs, such as 2,3-butanediol and acetoin, produced by Bacillus amyloliquefaciens IN937a and B. subtilis GB03, reduced the severity of disease caused by Erwinia carotovora subsp. carotovora in Arabidopsis thaliana via the ethylene signaling pathway. Later, other studies have suggested that ISR can be elicited by bacterial lipopolysaccharides,180 siderophores,181 biosurfactants,182 antibiotics,183 and flagella.184 The PGPB-mediated ISR has been shown to suppress plant diseases caused by various fungal, bacterial, and viral pathogens and, in some instances, even by insects and nematodes, under both greenhouse and field conditions.185,186 The discussion of ISR by PGPB is beyond the scope of this review; however, other excellent reviews cover this topic further.177,186,187
Biochar can also significantly reduce the risk of plant disease by (1) mediating systemic resistance to pathogens (i.e., upregulating pathways and genes associated with plant defense),188 (2) stimulating soil microbial activities, and (3) reshaping the soil microbiome, which can positively affect microbially mediated ISR.189,190 However, some studies show that while lower application rates (≤1%) of biochar reduce the severity of several diseases, higher application rates (≥3%) are ineffective or can induce plant diseases.191 In addition, biochar properties, such as water retention capacity, nutrient content, redox ability, sorption potential, pH, and concentration of toxic and/or hormone-like compounds, can greatly influence a soil–rhizosphere–pathogen–plant system and consequently impact the severity of plant diseases.192 Therefore, optimization of biochar feedstock type, production condition, and application rate is of utmost importance for the effective use of biochar to suppress plant diseases.
7.4. Dividing the Carbon and Sharing the Load
Many PGPB have the ability to degrade organic substances, such as pesticides, polychlorinated biphenyls, and hydrocarbon contaminants.5,193−195 In general, these bacteria use some or all of the organic pollutants as a carbon or nutrient source for growth, which may lead to a complete degradation (mineralization) of the organic pollutants.195,196 For instance, Ma et al.197 isolated Pseudomonas sp. JM2, a bacterial strain capable of using phenanthrene as its sole carbon source. In another study, Burkholderia fungorum DBT1, a bacterial strain isolated from an oil refinery discharge, could use dibenzothiophene, phenanthrene, fluorene, and naphthalene as sole sources of carbon and energy in addition to showing plant growth-promoting activities.195 However, some organic pollutants, especially polychlorinated biphenyls, cannot be used as carbon and energy sources for PGPB and may limit microbial activities in the rhizosphere. In this case, plants may come to the rescue by releasing secondary metabolites in their root exudates, which can be used as a microbial nutrient source to facilitate bacterial degradation of organic pollutants in soil in a process known as co-metabolism.198 These plant-derived secondary metabolites attract beneficial and pollutant-degrading microbes to the plant rhizosphere, thereby enhancing microbial metabolism for the degradation of recalcitrant organic pollutants.199 Furthermore, plants may release harmless organic pollutant analogues, which lead to the production of organic pollutant-degrading enzymes by indigenous microflora.200 For instance, plant-derived salicylate was found to stimulate PAH degradation by Pseudomonas saccharophila P15.201 Another study showed that salicylate from Salix alaxensis (felt-leaf willow) promoted polychlorinated biphenyl degradation from 25% to 40% and increased microbial community diversity and metabolic activity in the soil.202 Plant terpenes, coumarins, resin acids, and flavonoids, which can act as growth substrates of microbes and/or inducers of microbial activities, have also been associated with the degradation of different organic pollutants.16,194 Additionally, some xenobiotic organic compounds exhibit hydrophobicity due to the presence of reduced aliphatic hydrocarbon fragments, which may inhibit their bioavailability for microbial degradation.203 The bioavailability and extractability of organic pollutants decrease with increasing contact time with soil and other organic matter (e.g., biochar).204 This process has been termed “aging”, which can reduce the biodegradability of organic pollutants by soil microbes and uptake by plants, posing major barriers to biological remediation.205 However, some microorganisms in the soil can produce biosurfactants that increase the bioavailability of water-insoluble organic pollutants.206 These biosurfactants are amphiphilic, meaning they have both hydrophilic and hydrophobic moieties, allowing them to bridge aqueous and non-aqueous phases.207 They disperse hydrophobic organic pollutants into small droplets, reducing their surface and interfacial tension as well as enhancing their availability for microbial degradation.208 Among the most renowned biosurfactants are the surface-active glycolipids (rhamnolipids) produced by Pseudomonas aeruginosa,209 surfactin, iturin, and fengycin lipopeptides produced by B. subtilis,210 and phospholipids produced by Corynebacterium lepus.211 In addition to helping microbial cells catabolize water-insoluble organic pollutants, biosurfactants also drive a variety of other properties. These include (1) selective biocontrol activity against microbial competitors,210 (2) enhanced (patchy) hydrophobicity of the cell surface to improve the affinity between the cell and substrate,212 (3) reduced surface and interfacial tensions to facilitate gas and water exchange, (4) promoting the formation of microcolonies in the early phases of biofilm formation, (5) facilitating the dispersal of cells from mature biofilms,209,213,214 (6) reducing the hydrophobicity of biochar in the soil, thereby making organic matter and minerals in biochar bioavailable for microbial use, and (7) enhancing the desorption of organic pollutants from biochar, thereby making the pollutants available for microbial degradation. Importantly, the desorption and subsequent degradation of organic pollutants from biochar can create new adsorption sites in the biochar, which can help the biochar adsorb more pollutants. Therefore, screening and application of biosurfactant-producing microorganisms can facilitate remediation of soil contaminated with persistent or immiscible organic compounds.
Coming to the aid of PGPB overloaded with organic pollutants, biochar can mitigate the toxicity of these organic pollutants by reducing their mobility and bioavailability through surface adsorption, partition, and sequestration (Figure 2). As a cost-effective and eco-friendly adsorbent, biochar has been widely applied for the remediation of organic pollutants, such as malachite green,215 fluorinated herbicides,216 simazine,217 bisphenol A, 17α-ethinyl estradiol, phenanthrene,218 methyl violet,110 and diuron.219 In summary, biochar reduced the mobility of the treated pollutants and reduced the contamination of local environments. The presence of different active functional groups (such as pyridinium, carboxyl, oxonium, nitrile, hydroxyl, amine, phenolic hydroxyl, amide, and carbonyl groups, among others) and aromatic carbons on biochar surfaces may have binary effects on accepting and donating electrons, which plays a key role in the adsorption of chemical pollutants.220−222 Biochar with a large surface area and high porosity offers an appropriate habitat for microorganisms responsible for the degradation of organic pollutants.223,224 Microorganisms hosted in biochar can then degrade the adsorbed pollutants, replenishing active microbial sites for further adsorption and degradation of pollutants.224 Biochar and PGPB were combined for the remediation of petroleum hydrocarbons. Zhang et al.50 isolated a petroleum hydrocarbon-degrading bacterial strain, Corynebacterium variabile HRJ4, and immobilized C. variabile HRJ4 on biochar. They reported the following results. (1) Biochar-immobilized C. variabile HRJ4 could tolerate and degrade petroleum hydrocarbons under saline-alkaline conditions. (2) Bacterially loaded biochar exhibited effective degradation of an n-alkane and polycyclic aromatic hydrocarbon mixture. (3) Biochar-immobilized C. variabile HRJ4 enhanced degradation of total petroleum hydrocarbons by ≤78.9% after incubation for 7 days compared to C. variabile HRJ4 alone. In another study by Ma et al.,225 the combined application of biochar and Klebsiella sp. PCX accelerated biodegradation of polyacrylamide in soil by enhancing the metabolism of Klebsiella sp. PCX itself and stimulating the activities of indigenous polyacrylamide-degrading microorganisms. These authors concluded that the combined use of biochar and bacteria may enhance organic pollutant biodegradation through both bioaugmentation and biostimulation strategies. Because biochar can overcome many of the environmental constraints that impair the performance of remedial microorganisms, it follows that phytoremediation also has much to gain.
8. Biochar–Bacterium–Plant Synergy in Remediating Organic Pollutants
Biochar, bacteria, and plants are all composed of widely available, natural, and renewable resources. In addition, they can be engineered to enhance their efficiencies in environmental remediation.31,226,227 We now address the question of why their partnership is crucial for the sustainable remediation of organic pollutants in soils. Phytoremediation alone is inhibited by the presence of high concentrations of pollutants. Biochar mainly does not remove organic pollutants by itself but immobilizes pollutants in the environment. PGPB alone cannot survive in soil indefinitely without plants, and their survival and activity are greatly improved by the presence of a sorbent (e.g., biochar) to ameliorate the abiotic stresses caused by the contaminant.
All of the components of a biochar–bacterium–plant synergy mentioned above are further enhanced by the matrix in which they find themselves, the soil. A “healthy” soil in the context of phytoremediation is one that supports optimal and resilient bioremediation. Biochar and plants both provide suboptimal soils with much-needed (non-organic pollutant) carbon. Biochar provides a recalcitrant and immediately stable physical carbon framework to improve gas exchange and reduce the burdens of the pollutant (both physical and chemical), while plants provide fresh and labile photosynthate C to assist microbes and soil fauna in maintaining the physical stability of soil structure against destructive forces, and ultimately helping to transition a soil from being one that is supporting bioremediation activity to one that is supporting the ecosystem.
There are further interactions that take place over time. For example, bacteria that can colonize internal biochar surfaces may further metabolize the adsorbed pollutants (Figure 4),224 and/or root exudates from plants may stimulate desorption of pollutants from biochar, making pollutants susceptible to microbial degradation in the soil.228 Biochar can increase microbial population size and enzyme activities associated with pollutant degradation and nutrient cycling in the rhizosphere, depending on its properties. Plants also release enzymes, such as peroxidases and phenol oxidases, in root exudates, which can degrade partly oxidized pollutant metabolites (produced via microbial oxidation of original organic pollutants).229 In a study by Hussain et al.,230 it was found that biochar, bacteria, and plants can interact successfully to bring about improved remediation of petroleum hydrocarbons. The synergistic effects of biochar, plants, and bacteria on remediation of heavy metals and PAHs were further demonstrated using six plant species and a number of PAH-degrading bacterial strains, Acinetobacter junii HS29, Enterobacter cloacae HS32, Enterobacter aerogenes HS39, Enterobacter asburiae HS22, Brevibacillus reuszeri HS37, and Stenotrophomonas sp. HS16, from oil-contaminated soil.231 The combined approach improved plant antioxidative defenses and helped to remove heavy metals and PAHs from the soil. It is clear that improving our understanding of the potential of this trinity with a view to the remediation of environmental pollutants holds promise for the sustainable management of suboptimal soils.
9. Sustainability Analysis of the Biochar–Bacterium–Plant System
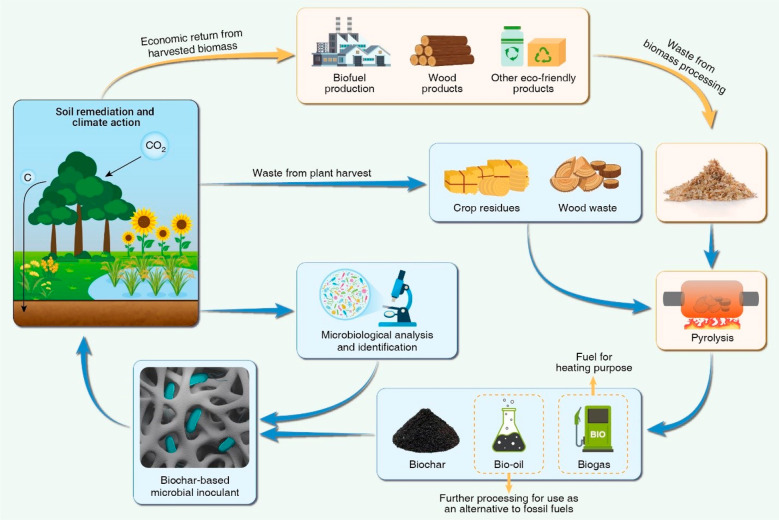
“A blueprint to achieve a better and more sustainable future for all people and the world by 2030” was established as the mission statement of the Sustainable Development Goals (SDGs) by the United Nations (UN) in 2015.232 Soil plays a vital role in sustaining life on Earth and is critical to achieving the UN SDGs. Given the global problem of pollution of soil by various anthropogenic pollutants, it is imperative to intensify the search for sustainable soil restoration and management techniques that are feasible and practical on a global scale.233 Preference should be given to techniques that are cost-effective. Additional value is derived when techniques are in line with the SDGs (such as action to combat climate change and the promotion of life on land), with positive social, economic, and environmental impacts. The integration of plants, biochar, and microbes for remediating polluted soils is well suited as it is based on widely available renewable resources and feasible approaches with the potential to remove pollutants and improve soil health, reverse the trend of increasing atmospheric CO2 levels, and promote a circular economy, thereby generating social and economic benefits (Figure 5). In our view, developing such a sustainable and functional system that can address key agricultural, horticultural, and environmental issues while engaging and benefiting local communities deserves research prioritization.
Figure 5.
Example of a sustainable model that maximizes the cyclicality and profitability of biochar–bacterium–plant systems for organic pollutant remediation and climate change mitigation.
Because no studies have analyzed the cost of combining biochar, microorganisms, and plants for soil remediation, we will analyze the cost based on the reported cost of phytoremediation, bioremediation, and biochar, as well as analyze possible economic returns and additional benefits. Phytoremediation, the use of plants and their associated microbiomes to remediate contaminated soils, is one of the sustainable techniques for controlling soil pollution and improving soil health with good public acceptance, offering the lowest cost compared to other techniques.10,234 For instance, the incineration of contaminated soils can cost up to $100/m3, yet soils contaminated with explosives and hazardous substances, especially chlorinated hydrocarbons, dioxins, and polychlorinated biphenyls, require even more intensive management techniques that can cost well beyond $1047–1540/m3.235 In comparison, the cost of phytoremediation can range between $0.02 and $1.00/m3 per year, which is much cheaper than the cost of physical and chemical methods, but of variable efficacy.236,237 It has been claimed that phytoremediation can save between 50% and 99% of the cost of standard treatment or that its economic return may even exceed the cost, depending on the types of plants and contaminants treated.238 However, phytoremediation takes much longer than other remediation techniques because the plants require time to grow and remove pollutants from contaminated soils.10 The cost of phytoremediation is strongly dependent on the local situation, such as the location and depth of contamination, pollutant type, resource availability, soil conditions, processing, utilization, or economic value of biomass. While investigating the possible most expensive phytoremediation procedure based on the local price rate in China, Wan et al.234 found that the cost of actual field applications of phytoremediation was $37.7/m3, with initial and operational costs making up 46.02% and 53.98% of the total cost, respectively. Among the initial costs were pollution analysis, development of a remediation plan, soil preparation, construction or purchase of nursery equipment, an irrigation system, a temporary storage facility, waste incineration equipment, etc. Transporting the materials to the remediation site required building roads, bridges, and culverts. The cost of personnel and supplies, the use of major machinery, and any other direct or indirect expenses were all included in the operational cost. On the basis of their observations, the authors suggested that the costs of the expensive phytoremediation project could be offset by the benefits in fewer than seven years. Via promotion of the phytoremediation of organic pollutants, it may be possible to rehabilitate contaminated soils and provide economic assistance and clean air to local communities. Unlike inorganic pollutants (such as heavy metals), organic pollutants can be completely degraded to relatively nontoxic components either in soil or in plants by the action of plant enzymes and/or plant-associated microbiomes. This may facilitate treatment and multipurpose use of biomass produced from phytoremediation.239,240 However, before deciding on the usage of biomass, one must carry out a toxicological analysis to determine whether the degradation of organic pollutants in plants has resulted in the formation of toxic byproducts.241 In addition, planning for the efficient use of plant biomass after harvest can make phytoremediation even more cost-effective and sustainable. In this regard, exploiting the possibility of producing valuable materials (e.g., ornaments, paper, timber, bioenergy, and other eco-friendly products) in addition to producing biochar from harvested plants and their residues can negate the cost of the process, making phytoremediation more profitable and sustainable.240
The cost for enhanced bioremediation (microbial decomposition without plants) of contaminants was reported to be $13.4/m3242 but can be higher, ranging from $30 to $100/m3 depending on the soil properties, type and degree of contamination, and quantity and type of amendments applied.243 These expenditures include preliminary microbiological investigation, site preparation, microbial inoculation, laboratory analysis, and monitoring and supervision. However, the cost can be expected to be reduced by combining bioremediation with phytoremediation and biochar as a functional supplement.
The promotion of biochar as a carbon-negative tool for soil and environmental restoration, global climate change mitigation, energy production, and a circular bioeconomy is not new; however, the feasibility of using biochar from its source and production to end use needs to be refined to maximize sustainability. In fact, no technology can be considered sustainable unless it can translate into long-term financial success.244 Despite extensive national and international advocacy and research on the use of biochar in agricultural production and environmental remediation in recent years,78,143 large-scale applications of biochar remain limited.245 In many cases, the high costs associated with biochar production, such as purchasing equipment for the pyrolysis operation, make widespread agricultural use of biochar in the field an unrealistic option.246 For example, producing, transporting, and applying biochar in the field have been estimated to cost between $222 and $584 per ton (an underestimate by today’s standards), which greatly exceeds the comparable $96.13 per ton profit increase from using biochar as an agronomic soil amendment.247 We propose that the biochar–plant–microbe synergy can provide a greater value return when the biomass products are used to produce more biochar for the purpose of remediating suboptimal soils (Figure 5).
10. Environmental Implications and Future Perspectives
Phytoremediation is an effective biological means to remediate chemical pollutants, including organic pollutants. This technology benefits from the interactions between plants and microorganisms (indigenous and exogenous). The use of bacteria with both pollutant-degrading and plant growth-promoting activities is one of the most viable bioremediation strategies for improving pollutant removal efficiency. Multiple roles of PGPB, ranging from improving soil fertility (biofertilization) and protecting plants from pathogens (biocontrol) to regulating and enhancing plant growth (biostimulation) and breaking down pollutants (biodegradation), promise to improve the efficiency of phytoremediation of organic pollutants. However, the growth, multiplication, and activities of the inoculated PGPB are affected by the soil’s nutrient content, porosity, and organic matter content. If inoculated without a proper medium, the remedial PGPB frequently fail to develop their beneficial effects, often due to inefficient colonization of the rhizosphere or poor vertical transport into the soil. In addition, high concentrations of bioavailable pollutants can negatively affect the growth and activities of microorganisms and plants involved in phytoremediation. In this regard, biochar can enhance phytoremediation by providing habitats for microorganisms, promoting plant growth, reducing the toxicity of pollutants to microorganisms, remediating pollutants, and alleviating various biotic and abiotic stresses in plants. The use of biochar as a carrier of PGPB is a very promising approach for improving the efficiency of the phytoremediation of organic pollutants, especially in highly contaminated soils. Simultaneously, wide-scale use of biochar as a vector for the sequestration of carbon from the atmosphere has been slow to take off because the application of raw biochar has limited benefits. The synergies proposed here are of higher ultimate value and may therefore help to increase commercial opportunities and implementation. When carefully designed and used, the biochar–bacterium–plant system may provide a viable and sustainable solution for the remediation, revegetation, and restoration of soils contaminated with organic pollutants.
On the basis of the current understanding of synergy among biochar, PGPB, and plants for remediation, we recommend the following for further research: (1) explore all facets of biochar–bacterium–plant associations in remediating organic contamination in soils, (2) explore the results of tailoring biochar, bacteria, and plants using cutting-edge engineering strategies in biotechnology and nanotechnology to enhance the functionality of the biochar–bacterium–plant systems in remediating organic pollutant contamination in soils, (3) investigate the remediation of soils co-contaminated with organic and metal pollutants, and (4) analyze how native microbial communities and soil functional genes adapt and incorporate genes and species delivered in biochar-based inocula.
In conclusion, the development of the biochar–bacterium–plant systems in soil remediation requires intensive research, not only on understanding their biochemical and molecular mechanisms but also on the evaluation of their applicability in different soil types. Identification of potent PGPB, biochar, plants, and genes encoding enzymes and other polymeric substances that contribute to the remediation of organic pollutants and the development of novel omics and nanotechnology-based approaches represent key areas of research to improve the biochar–PGPB–plant interactions for the sustainable treatment of organic pollutant-contaminated soils.
Acknowledgments
This work was funded by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA28030501), the National Key Research and Development Program of China (2020YFC1807000, 2019YFC1804203, and 2018YFC1800400), the National Natural Science Foundation of China (41977137, 41991333, and 42007145), the Outstanding Youth Fund of Natural Science Foundation of Jiangsu, China (BK20150050), the Key Program of Frontier Sciences, Chinese Academy of Sciences (QYZDJ-SSW-DQC035), and the Center for Health Impacts of Agriculture (CHIA) of Michigan State University. F.W. was supported by a fellowship from the Alexander von Humboldt Foundation for experienced researchers. J.D.H. acknowledges ANSO Scholarship for Young Talents in China.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.2c02976.
Dependence of the properties and functions of biochar on the temperature of the feedstock and pyrolysis (Table S1), some of the bacteria that stimulate plant growth and their effect on the phytoremediation of organic pollutants (Table S2), and effects of different types of biochar on organic pollutants in different types of contaminated soil (Table S3) (PDF)
Author Contributions
# L.X. and J.D.H. contributed equally to this work and they are co-first authors.
The authors declare no competing financial interest.
Supplementary Material
References
- Kumar M.; Bolan N. S.; Hoang S. A.; Sawarkar A. D.; Jasemizad T.; Gao B.; Keerthanan S.; Padhye L. P.; Singh L.; Kumar S.; Vithanage M.; Li Y.; Zhang M.; Kirkham M. B.; Vinu A.; Rinklebe J. Remediation of Soils and Sediments Polluted with Polycyclic Aromatic Hydrocarbons: To Immobilize, Mobilize, or Degrade?. Journal of Hazardous Materials 2021, 420, 126534. 10.1016/j.jhazmat.2021.126534. [DOI] [PubMed] [Google Scholar]
- Bolan N.; Sarkar B.; Vithanage M.; Singh G.; Tsang D. C. W.; Mukhopadhyay R.; Ramadass K.; Vinu A.; Sun Y.; Ramanayaka S.; Hoang S. A.; Yan Y.; Li Y.; Rinklebe J.; Li H.; Kirkham M. B. Distribution, Behaviour, Bioavailability and Remediation of Poly- and per-Fluoroalkyl Substances (PFAS) in Solid Biowastes and Biowaste-Treated Soil. Environ. Int. 2021, 155, 106600. 10.1016/j.envint.2021.106600. [DOI] [PubMed] [Google Scholar]
- Damaj M.; Ahmad D. Biodegradation of Polychlorinated Biphenyls by Rhizobia: A Novel Finding. Biochem. Biophys. Res. Commun. 1996, 218 (3), 908–915. 10.1006/bbrc.1996.0161. [DOI] [PubMed] [Google Scholar]
- Suanon F.; Tang L.; Sheng H.; Fu Y.; Xiang L.; Wang Z.; Shao X.; Mama D.; Jiang X.; Wang F. Organochlorine Pesticides Contaminated Soil Decontamination Using TritonX-100-Enhanced Advanced Oxidation under Electrokinetic Remediation. Journal of Hazardous Materials 2020, 393, 122388. 10.1016/j.jhazmat.2020.122388. [DOI] [PubMed] [Google Scholar]
- Teng Y.; Shen Y.; Luo Y.; Sun X.; Sun M.; Fu D.; Li Z.; Christie P. Influence of Rhizobium Meliloti on Phytoremediation of Polycyclic Aromatic Hydrocarbons by Alfalfa in an Aged Contaminated Soil. Journal of Hazardous Materials 2011, 186 (2), 1271–1276. 10.1016/j.jhazmat.2010.11.126. [DOI] [PubMed] [Google Scholar]
- Vrkoslavová J.; Demnerová K.; Macková M.; Zemanová T.; Macek T.; Hajšlová J.; Pulkrabová J.; Hrádková P.; Stiborová H. Absorption and Translocation of Polybrominated Diphenyl Ethers (PBDEs) by Plants from Contaminated Sewage Sludge. Chemosphere 2010, 81 (3), 381–386. 10.1016/j.chemosphere.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Wagrowski D. M.; Hites R. A. Insights into the Global Distribution of Polychlorinated Dibenzo-p-Dioxins and Dibenzofurans. Environ. Sci. Technol. 2000, 34 (14), 2952–2958. 10.1021/es991138o. [DOI] [Google Scholar]
- Ferreira A. R.; Guedes P.; Mateus E. P.; Ribeiro A. B.; Couto N. Emerging Organic Contaminants in Soil Irrigated with Effluent: Electrochemical Technology as a Remediation Strategy. Science of The Total Environment 2020, 743, 140544. 10.1016/j.scitotenv.2020.140544. [DOI] [PubMed] [Google Scholar]
- Persistent Organic Pollutants: A Global Issue, a Global Response; U.S. Environmental Protection Agency, 2012. [Google Scholar]
- Gatheru Waigi M.; Sun K.; Gao Y. Sphingomonads in Microbe-Assisted Phytoremediation: Tackling Soil Pollution. Trends Biotechnol. 2017, 35 (9), 883–899. 10.1016/j.tibtech.2017.06.014. [DOI] [PubMed] [Google Scholar]
- Kelly B. C.; Ikonomou M. G.; Blair J. D.; Morin A. E.; Gobas F. A. P. C. Food Web-Specific Biomagnification of Persistent Organic Pollutants. Science 2007, 317, 236. 10.1126/science.1138275. [DOI] [PubMed] [Google Scholar]
- Ennour-Idrissi K.; Ayotte P.; Diorio C. Persistent Organic Pollutants and Breast Cancer: A Systematic Review and Critical Appraisal of the Literature. Cancers 2019, 11 (8), 1063. 10.3390/cancers11081063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S.; Bowers W. J.; Nakai J. S.; Yagminas A.; Mueller R.; Pulido O. Effects of Environmentally Relevant Mixtures of Persistent Organic Pollutants on the Developmental Neurobiology in Rats. Toxicologic Pathology 2013, 41 (1), 38–47. 10.1177/0192623312451370. [DOI] [PubMed] [Google Scholar]
- Lee D.-H.; Porta M.; Jacobs D. R. Jr.; Vandenberg L. N. Chlorinated Persistent Organic Pollutants, Obesity, and Type 2 Diabetes. Endocrine Reviews 2014, 35 (4), 557–601. 10.1210/er.2013-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas J. M.; Vicente F.; Santos A.; Romero A. Soil Remediation Using Soil Washing Followed by Fenton Oxidation. Chemical Engineering Journal 2013, 220, 125–132. 10.1016/j.cej.2012.11.137. [DOI] [Google Scholar]
- Arslan M.; Imran A.; Khan Q. M.; Afzal M. Plant–Bacteria Partnerships for the Remediation of Persistent Organic Pollutants. Environmental Science and Pollution Research 2017, 24 (5), 4322–4336. 10.1007/s11356-015-4935-3. [DOI] [PubMed] [Google Scholar]
- Afzal M.; Khan Q. M.; Sessitsch A. Endophytic Bacteria: Prospects and Applications for the Phytoremediation of Organic Pollutants. Chemosphere 2014, 117, 232–242. 10.1016/j.chemosphere.2014.06.078. [DOI] [PubMed] [Google Scholar]
- Ni N.; Kong D.; Wu W.; He J.; Shan Z.; Li J.; Dou Y.; Zhang Y.; Song Y.; Jiang X. The Role of Biochar in Reducing the Bioavailability and Migration of Persistent Organic Pollutants in Soil–Plant Systems: A Review. Bull. Environ. Contam. Toxicol. 2020, 104 (2), 157–165. 10.1007/s00128-019-02779-8. [DOI] [PubMed] [Google Scholar]
- Ji M.; Wang X.; Usman M.; Liu F.; Dan Y.; Zhou L.; Campanaro S.; Luo G.; Sang W. Effects of Different Feedstocks-Based Biochar on Soil Remediation: A Review. Environ. Pollut. 2022, 294, 118655. 10.1016/j.envpol.2021.118655. [DOI] [PubMed] [Google Scholar]
- Jabbar N. M.; Alardhi S. M.; Mohammed A. K.; Salih I. K.; Albayati T. M. Challenges in the Implementation of Bioremediation Processes in Petroleum-Contaminated Soils: A Review. Environmental Nanotechnology, Monitoring & Management 2022, 18, 100694. 10.1016/j.enmm.2022.100694. [DOI] [Google Scholar]
- Roe R. A. L.; MacFarlane G. R. The Potential of Saltmarsh Halophytes for Phytoremediation of Metals and Persistent Organic Pollutants: An Australian Perspective. Mar. Pollut. Bull. 2022, 180, 113811. 10.1016/j.marpolbul.2022.113811. [DOI] [PubMed] [Google Scholar]
- Saeed M.; Ilyas N.; Jayachandran K.; Shabir S.; Akhtar N.; Shahzad A.; Sayyed R. Z.; Bano A. Advances in Biochar and PGPR Engineering System for Hydrocarbon Degradation: A Promising Strategy for Environmental Remediation. Environ. Pollut. 2022, 305, 119282. 10.1016/j.envpol.2022.119282. [DOI] [PubMed] [Google Scholar]
- Sarma H.; Narayan M.; Peralta-Videa J. R.; Lam S. S. Exploring the Significance of Nanomaterials and Organic Amendments — Prospect for Phytoremediation of Contaminated Agroecosystem. Environ. Pollut. 2022, 308, 119601. 10.1016/j.envpol.2022.119601. [DOI] [PubMed] [Google Scholar]
- Sarma H.; Nava A. R.; Prasad M. N. V. Mechanistic Understanding and Future Prospect of Microbe-Enhanced Phytoremediation of Polycyclic Aromatic Hydrocarbons in Soil. Environmental Technology & Innovation 2019, 13, 318–330. 10.1016/j.eti.2018.12.004. [DOI] [Google Scholar]
- Feng J.; Shen X.; Chen J.; Shi J.; Xu J.; Tang C.; Brookes P. C.; He Y. Improved Rhizoremediation for Decabromodiphenyl Ether (BDE-209) in E-Waste Contaminated Soils. Soil Ecology Letters 2019, 1 (3), 157–173. 10.1007/s42832-019-0007-9. [DOI] [Google Scholar]
- Bolan N. S.; Park J. H.; Robinson B.; Naidu R.; Huh K. Y. In Chapter Four - Phytostabilization: A Green Approach to Contaminant Containment; Academic Press, 2011; Vol. 112, pp 145–204. 10.1016/B978-0-12-385538-1.00004-4 [DOI] [Google Scholar]
- Zhang C.; Feng Y.; Liu Y.-W.; Chang H.-Q.; Li Z.-J.; Xue J.-M. Uptake and Translocation of Organic Pollutants in Plants: A Review. Journal of Integrative Agriculture 2017, 16 (8), 1659–1668. 10.1016/S2095-3119(16)61590-3. [DOI] [Google Scholar]
- Kvesitadze G.; Khatisashvili G.; Sadunishvili T.; Kvesitadze E. In Plants for Remediation: Uptake, Translocation and Transformation of Organic Pollutants BT - Plants, Pollutants and Remediation; Öztürk M., Ashraf M., Aksoy A., Ahmad M. S. A., Hakeem K. R., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp 241–308. 10.1007/978-94-017-7194-8_12 [DOI] [Google Scholar]
- Lawlor D. W. Absorption of Polyethylene Glycols by Plants and Their Effects on Plant Growth. New Phytologist 1970, 69 (2), 501–513. 10.1111/j.1469-8137.1970.tb02446.x. [DOI] [Google Scholar]
- Janes B. E. The Effect of Molecular Size, Concentration in Nutrient Solution, and Exposure Time on the Amount and Distribution of Polyethylene Glycol in Pepper Plants. Plant Physiology 1974, 54 (3), 226–230. 10.1104/pp.54.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.-D.; Li Q.-J.; Luo B.; Chen X.-Y. Ex Planta Phytoremediation of Trichlorophenol and Phenolic Allelochemicals via an Engineered Secretory Laccase. Nat. Biotechnol. 2004, 22 (7), 893–897. 10.1038/nbt982. [DOI] [PubMed] [Google Scholar]
- Bertin C.; Yang X.; Weston L. A. The Role of Root Exudates and Allelochemicals in the Rhizosphere. Plant and Soil 2003, 256 (1), 67–83. 10.1023/A:1026290508166. [DOI] [Google Scholar]
- Hassan M. K.; McInroy J. A.; Kloepper J. W. The Interactions of Rhizodeposits with Plant Growth-Promoting Rhizobacteria in the Rhizosphere: A Review. Agriculture 2019, 9, 142. 10.3390/agriculture9070142. [DOI] [Google Scholar]
- Paterson S.; Mackay D.; Tam D.; Shiu W. Y. Uptake of Organic Chemicals by Plants: A Review of Processes, Correlations and Models. Chemosphere 1990, 21 (3), 297–331. 10.1016/0045-6535(90)90002-B. [DOI] [Google Scholar]
- Ren W.; Wang Y.; Huang Y.; Liu F.; Teng Y. Uptake, Translocation and Metabolism of Di-n-Butyl Phthalate in Alfalfa (Medicago Sativa). Science of The Total Environment 2020, 731, 138974. 10.1016/j.scitotenv.2020.138974. [DOI] [PubMed] [Google Scholar]
- Kacálková L.; Tlustoš P. The Uptake of Persistent Organic Pollutants by Plants. Cent. Eur. J. Biol. 2011, 6 (2), 223–235. 10.2478/s11535-010-0116-z. [DOI] [Google Scholar]
- Phouthavong-Murphy J. C.; Merrill A. K.; Zamule S.; Giacherio D.; Brown B.; Roote C.; Das P. Phytoremediation Potential of Switchgrass (Panicum Virgatum), Two United States Native Varieties, to Remove Bisphenol-A (BPA) from Aqueous Media. Sci. Rep. 2020, 10 (1), 835. 10.1038/s41598-019-56655-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty S. L.; James C. A.; Moore A. L.; Vajzovic A.; Singleton G. L.; Ma C.; Khan Z.; Xin G.; Kang J. W.; Park J. Y.; Meilan R.; Strauss S. H.; Wilkerson J.; Farin F.; Strand S. E. Enhanced Phytoremediation of Volatile Environmental Pollutants with Transgenic Trees. Proc. Natl. Acad. Sci. U. S. A. 2007, 104 (43), 16816–16821. 10.1073/pnas.0703276104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limmer M.; Burken J. Phytovolatilization of Organic Contaminants. Environ. Sci. Technol. 2016, 50 (13), 6632–6643. 10.1021/acs.est.5b04113. [DOI] [PubMed] [Google Scholar]
- Cheng L.; Zhou Q.; Yu B. Responses and Roles of Roots, Microbes, and Degrading Genes in Rhizosphere during Phytoremediation of Petroleum Hydrocarbons Contaminated Soil. International Journal of Phytoremediation 2019, 21 (12), 1161–1169. 10.1080/15226514.2019.1612841. [DOI] [PubMed] [Google Scholar]
- Gleba D.; Borisjuk N. V.; Borisjuk L. G.; Kneer R.; Poulev A.; Skarzhinskaya M.; Dushenkov S.; Logendra S.; Gleba Y. Y.; Raskin I. Use of Plant Roots for Phytoremediation and Molecular Farming. Proc. Natl. Acad. Sci. U. S. A. 1999, 96 (11), 5973–5977. 10.1073/pnas.96.11.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiyagi S. A.; Ajaz S.; Azam M. F. Effect of Some Pesticides on Plant Growth, Root Nodulation and Chlorophyll Content of Chickpea. Archives of Agronomy and Soil Science 2004, 50 (6), 529–533. 10.1080/03650340410001729735. [DOI] [Google Scholar]
- Srivastava A. K.; Singh D. Assessment of Malathion Toxicity on Cytophysiological Activity, DNA Damage and Antioxidant Enzymes in Root of Allium Cepa Model. Sci. Rep. 2020, 10 (1), 886. 10.1038/s41598-020-57840-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.; Rengel Z.; Meney K. Polynuclear Aromatic Hydrocarbons (PAHs) Differentially Influence Growth of Various Emergent Wetland Species. Journal of Hazardous Materials 2010, 182 (1), 689–695. 10.1016/j.jhazmat.2010.06.087. [DOI] [PubMed] [Google Scholar]
- Weissengruber L.; Möller K.; Puschenreiter M.; Friedel J. K. Long-Term Soil Accumulation of Potentially Toxic Elements and Selected Organic Pollutants through Application of Recycled Phosphorus Fertilizers for Organic Farming Conditions. Nutrient Cycling in Agroecosystems 2018, 110 (3), 427–449. 10.1007/s10705-018-9907-9. [DOI] [Google Scholar]
- Singha L. P.; Sinha N.; Pandey P. Rhizoremediation Prospects of Polyaromatic Hydrocarbon Degrading Rhizobacteria, That Facilitate Glutathione and Glutathione-S-Transferase Mediated Stress Response, and Enhance Growth of Rice Plants in Pyrene Contaminated Soil. Ecotoxicology and Environmental Safety 2018, 164, 579–588. 10.1016/j.ecoenv.2018.08.069. [DOI] [PubMed] [Google Scholar]
- Rajkumari J.; Paikhomba Singha L.; Pandey P. Genomic Insights of Aromatic Hydrocarbon Degrading Klebsiella Pneumoniae AWD5 with Plant Growth Promoting Attributes: A Paradigm of Soil Isolate with Elements of Biodegradation. 3 Biotech 2018, 8 (2), 118. 10.1007/s13205-018-1134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T.; Xu J.; Xie W.; Yao Z.; Yang H.; Sun C.; Li X. Pseudomonas Aeruginosa L10: A Hydrocarbon-Degrading, Biosurfactant-Producing, and Plant-Growth-Promoting Endophytic Bacterium Isolated From a Reed (Phragmites Australis). Frontiers in microbiology 2018, 9, 1087. 10.3389/fmicb.2018.01087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudzai Z.; Treesubsuntorn C.; Thiravetyan P. Inoculated Clitoria Ternatea with Bacillus Cereus ERBP for Enhancing Gaseous Ethylbenzene Phytoremediation: Plant Metabolites and Expression of Ethylbenzene Degradation Genes. Ecotoxicology and Environmental Safety 2018, 164, 50–60. 10.1016/j.ecoenv.2018.07.121. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Tang J.; Wang L.; Liu J.; Gurav R. G.; Sun K. A Novel Bioremediation Strategy for Petroleum Hydrocarbon Pollutants Using Salt Tolerant Corynebacterium Variabile HRJ4 and Biochar. Journal of Environmental Sciences 2016, 47, 7–13. 10.1016/j.jes.2015.12.023. [DOI] [PubMed] [Google Scholar]
- Wang B.; Wang Q.; Liu W.; Liu X.; Hou J.; Teng Y.; Luo Y.; Christie P. Biosurfactant-Producing Microorganism Pseudomonas Sp. SB Assists the Phytoremediation of DDT-Contaminated Soil by Two Grass Species. Chemosphere 2017, 182, 137–142. 10.1016/j.chemosphere.2017.04.123. [DOI] [PubMed] [Google Scholar]
- Nakamura T.; Motoyama T.; Suzuki Y.; Yamaguchi I. Biotransformation of Pentachlorophenol by Chinese Chive and a Recombinant Derivative of Its Rhizosphere-Competent Microorganism, Pseudomonas Gladioli M-2196. Soil Biology and Biochemistry 2004, 36 (5), 787–795. 10.1016/j.soilbio.2004.01.006. [DOI] [Google Scholar]
- Viesser J. A.; Sugai-Guerios M. H.; Malucelli L. C.; Pincerati M. R.; Karp S. G.; Maranho L. T. Petroleum-Tolerant Rhizospheric Bacteria: Isolation, Characterization and Bioremediation Potential. Sci. Rep. 2020, 10 (1), 2060. 10.1038/s41598-020-59029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N.; Bano A.; Rahman M. A.; Guo J.; Kang Z.; Babar M. A. Comparative Physiological and Metabolic Analysis Reveals a Complex Mechanism Involved in Drought Tolerance in Chickpea (Cicer Arietinum L.) Induced by PGPR and PGRs. Sci. Rep. 2019, 9 (1), 2097. 10.1038/s41598-019-38702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masood S.; Zhao X. Q.; Shen R. F. Bacillus Pumilus Promotes the Growth and Nitrogen Uptake of Tomato Plants under Nitrogen Fertilization. Scientia Horticulturae 2020, 272, 109581. 10.1016/j.scienta.2020.109581. [DOI] [Google Scholar]
- Jinal H. N.; Gopi K.; Prittesh P.; Kartik V. P.; Amaresan N. Phytoextraction of Iron from Contaminated Soils by Inoculation of Iron-Tolerant Plant Growth-Promoting Bacteria in Brassica Juncea L. Czern. Environmental Science and Pollution Research 2019, 26 (32), 32815–32823. 10.1007/s11356-019-06394-2. [DOI] [PubMed] [Google Scholar]
- Hoang S. A.; Lamb D.; Seshadri B.; Sarkar B.; Choppala G.; Kirkham M. B.; Bolan N. S. Rhizoremediation as a Green Technology for the Remediation of Petroleum Hydrocarbon-Contaminated Soils. Journal of Hazardous Materials 2021, 401, 123282. 10.1016/j.jhazmat.2020.123282. [DOI] [PubMed] [Google Scholar]
- Bais H. P.; Weir T. L.; Perry L. G.; Gilroy S.; Vivanco J. M. THE ROLE OF ROOT EXUDATES IN RHIZOSPHERE INTERACTIONS WITH PLANTS AND OTHER ORGANISMS. Annual Review of Plant Biology 2006, 57 (1), 233–266. 10.1146/annurev.arplant.57.032905.105159. [DOI] [PubMed] [Google Scholar]
- Korenblum E.; Dong Y.; Szymanski J.; Panda S.; Jozwiak A.; Massalha H.; Meir S.; Rogachev I.; Aharoni A. Rhizosphere Microbiome Mediates Systemic Root Metabolite Exudation by Root-to-Root Signaling. Proc. Natl. Acad. Sci. U. S. A. 2020, 117 (7), 3874–3883. 10.1073/pnas.1912130117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montiel-Rozas M. M.; Madejón E.; Madejón P. Effect of Heavy Metals and Organic Matter on Root Exudates (Low Molecular Weight Organic Acids) of Herbaceous Species: An Assessment in Sand and Soil Conditions under Different Levels of Contamination. Environ. Pollut. 2016, 216, 273–281. 10.1016/j.envpol.2016.05.080. [DOI] [PubMed] [Google Scholar]
- Badri D. V.; Vivanco J. M. Regulation and Function of Root Exudates. Plant, Cell & Environment 2009, 32 (6), 666–681. 10.1111/j.1365-3040.2009.01926.x. [DOI] [PubMed] [Google Scholar]
- Radwan S. S.; Al-Mailem D. M.; Kansour M. K. Bioaugmentation Failed to Enhance Oil Bioremediation in Three Soil Samples from Three Different Continents. Sci. Rep. 2019, 9 (1), 19508. 10.1038/s41598-019-56099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchez; Patureau; Dabert; Juretschko; Doré; Delgenès; Moletta; Wagner Ecological Study of a Bioaugmentation Failure. Environmental Microbiology 2000, 2 (2), 179–190. 10.1046/j.1462-2920.2000.00091.x. [DOI] [PubMed] [Google Scholar]
- Flemming H.-C.; Wingender J.; Szewzyk U.; Steinberg P.; Rice S. A.; Kjelleberg S. Biofilms: An Emergent Form of Bacterial Life. Nature Reviews Microbiology 2016, 14 (9), 563–575. 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- Zelezniak A.; Andrejev S.; Ponomarova O.; Mende D. R.; Bork P.; Patil K. R. Metabolic Dependencies Drive Species Co-Occurrence in Diverse Microbial Communities. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 (20), 6449–6454. 10.1073/pnas.1421834112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmont T. O.; Robe P.; Cecillon S.; Clark I. M.; Constancias F.; Simonet P.; Hirsch P. R.; Vogel T. M. Accessing the Soil Metagenome for Studies of Microbial Diversity. Appl. Environ. Microbiol. 2011, 77 (4), 1315–1324. 10.1128/AEM.01526-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodor A.; Bounedjoum N.; Vincze G. E.; Erdeiné Kis Á.; Laczi K.; Bende G.; Szilágyi Á.; Kovács T.; Perei K.; Rákhely G. Challenges of Unculturable Bacteria: Environmental Perspectives. Reviews in Environmental Science and Bio/Technology 2020, 19 (1), 1–22. 10.1007/s11157-020-09522-4. [DOI] [Google Scholar]
- Megharaj M.; Ramakrishnan B.; Venkateswarlu K.; Sethunathan N.; Naidu R. Bioremediation Approaches for Organic Pollutants: A Critical Perspective. Environ. Int. 2011, 37 (8), 1362–1375. 10.1016/j.envint.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Su X.; Sun F.; Wang Y.; Hashmi M. Z.; Guo L.; Ding L.; Shen C. Identification, Characterization and Molecular Analysis of the Viable but Nonculturable Rhodococcus Biphenylivorans. Sci. Rep. 2015, 5 (1), 18590. 10.1038/srep18590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahlali R.; Ibrahim D. S. S.; Belabess Z.; Kadir Roni M. Z.; Radouane N.; Vicente C. S. L.; Menéndez E.; Mokrini F.; Barka E. A.; Galvão de Melo e Mota M.; Peng G. High-Throughput Molecular Technologies for Unraveling the Mystery of Soil Microbial Community: Challenges and Future Prospects. Heliyon 2021, 7 (10), e08142. 10.1016/j.heliyon.2021.e08142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald A.; Lane S. R.. Biochar as a Microbial Carrier; 2017. [Google Scholar]
- Stephens J. H. G.; Rask H. M. Inoculant Production and Formulation. Field Crops Research 2000, 65, 249. 10.1016/S0378-4290(99)00090-8. [DOI] [Google Scholar]
- Albareda M.; Rodríguez-Navarro D. N.; Camacho M.; Temprano F. J. Alternatives to Peat as a Carrier for Rhizobia Inoculants: Solid and Liquid Formulations. Soil Biol. Biochem. 2008, 40, 2771. 10.1016/j.soilbio.2008.07.021. [DOI] [Google Scholar]
- Abd El-Fattah D. A.; Eweda W. E.; Zayed M. S.; Hassanein M. K. Effect of Carrier Materials, Sterilization Method, and Storage Temperature on Survival and Biological Activities of Azotobacter Chroococcum Inoculant. Ann. Agric. Sci. 2013, 58, 111. 10.1016/j.aoas.2013.07.001. [DOI] [Google Scholar]
- Doar N.Analysis of the Failure of Voluntary Measures to Halt Peat Use in UK Horticulture between 1990 and 2020. The Wildlife Trusts: Newark-on-Trent, U.K., 2022.
- Sashidhar P.; Kochar M.; Singh B.; Gupta M.; Cahill D.; Adholeya A.; Dubey M. Biochar for Delivery of Agri-Inputs: Current Status and Future Perspectives. Science of The Total Environment 2020, 703, 134892. 10.1016/j.scitotenv.2019.134892. [DOI] [PubMed] [Google Scholar]
- Wang F.; Harindintwali J. D.; Yuan Z.; Wang M.; Wang F.; Li S.; Yin Z.; Huang L.; Fu Y.; Li L.; Chang S. X.; Zhang L.; Rinklebe J.; Yuan Z.; Zhu Q.; Xiang L.; Tsang D. C. W.; Xu L.; Jiang X.; Liu J.; Wei N.; Kästner M.; Zou Y.; Ok Y. S.; Shen J.; Peng D.; Zhang W.; Barcelo D.; Zhou Y.; Bai Z.; Li B.; Zhang B.; Wei K.; Cao H.; Tan Z.; Zhao L.; He X.; Zheng J.; Bolan N.; Liu X.; Huang C.; Dietmann S.; Luo M.; Sun N.; Gong J.; Gong Y.; Brahushi F.; Zhang T.; Xiao C.; Li X.; Chen W.; Jiao N.; Lehmann J.; Zhu Y.-G.; Jin H.; Schaeffer A.; Tiedje J. M.; Chen J. M. Technologies and Perspectives for Achieving Carbon Neutrality. Innovation 2021, 100180. 10.1016/j.xinn.2021.100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf D.; Amonette J. E.; Street-Perrott F. A.; Lehmann J.; Joseph S. Sustainable Biochar to Mitigate Global Climate Change. Nat. Commun. 2010, 1, 56. 10.1038/ncomms1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolan N.; Hoang S. A.; Beiyuan J.; Gupta S.; Hou D.; Karakoti A.; Joseph S.; Jung S.; Kim K.-H.; Kirkham M. B.; Kua H. W.; Kumar M.; Kwon E. E.; Ok Y. S.; Perera V.; Rinklebe J.; Shaheen S. M.; Sarkar B.; Sarmah A. K.; Singh B. P.; Singh G.; Tsang D. C. W.; Vikrant K.; Vithanage M.; Vinu A.; Wang H.; Wijesekara H.; Yan Y.; Younis S. A.; Van Zwieten L. Multifunctional Applications of Biochar beyond Carbon Storage. Int. Mater. Rev. 2021, 1–51. 10.1080/09506608.2021.1922047. [DOI] [Google Scholar]
- Zhu X.; Chen B.; Zhu L.; Xing B. Effects and Mechanisms of Biochar-Microbe Interactions in Soil Improvement and Pollution Remediation: A Review. Environ. Pollut. 2017, 227, 98–115. 10.1016/j.envpol.2017.04.032. [DOI] [PubMed] [Google Scholar]
- Quilliam R. S.; Glanville H. C.; Wade S. C.; Jones D. L. Life in the ‘Charosphere’ – Does Biochar in Agricultural Soil Provide a Significant Habitat for Microorganisms?. Soil Biology and Biochemistry 2013, 65, 287–293. 10.1016/j.soilbio.2013.06.004. [DOI] [Google Scholar]
- Glaser B.; Lehmann J.; Zech W. Ameliorating Physical and Chemical Properties of Highly Weathered Soils in the Tropics with Charcoal – a Review. Biology and Fertility of Soils 2002, 35 (4), 219–230. 10.1007/s00374-002-0466-4. [DOI] [Google Scholar]
- Abit S. M.; Bolster C. H.; Cai P.; Walker S. L. Influence of Feedstock and Pyrolysis Temperature of Biochar Amendments on Transport of Escherichia Coli in Saturated and Unsaturated Soil. Environ. Sci. Technol. 2012, 46 (15), 8097–8105. 10.1021/es300797z. [DOI] [PubMed] [Google Scholar]
- Lehmann J.; Rillig M. C.; Thies J.; Masiello C. A.; Hockaday W. C.; Crowley D. Biochar Effects on Soil Biota - A Review. Soil Biol. Biochem. 2011, 43, 1812. 10.1016/j.soilbio.2011.04.022. [DOI] [Google Scholar]
- Araujo J.; Díaz-Alcántara C.-A.; Urbano B.; González-Andrés F. Inoculation with Native Bradyrhizobium Strains Formulated with Biochar as Carrier Improves the Performance of Pigeonpea (Cajanus Cajan L.). European Journal of Agronomy 2020, 113, 125985. 10.1016/j.eja.2019.125985. [DOI] [Google Scholar]
- Ajeng A. A.; Abdullah R.; Ling T. C.; Ismail S.; Lau B. F.; Ong H. C.; Chew K. W.; Show P. L.; Chang J.-S. Bioformulation of Biochar as a Potential Inoculant Carrier for Sustainable Agriculture. Environmental Technology & Innovation 2020, 20, 101168. 10.1016/j.eti.2020.101168. [DOI] [Google Scholar]
- Hale L.; Luth M.; Kenney R.; Crowley D. Evaluation of Pinewood Biochar as a Carrier of Bacterial Strain Enterobacter Cloacae UW5 for Soil Inoculation. Appl. Soil Ecol. 2014, 84, 192. 10.1016/j.apsoil.2014.08.001. [DOI] [Google Scholar]
- Chen H.; Zhang J.; Tang L.; Su M.; Tian D.; Zhang L.; Li Z.; Hu S. Enhanced Pb Immobilization via the Combination of Biochar and Phosphate Solubilizing Bacteria. Environ. Int. 2019, 127, 395–401. 10.1016/j.envint.2019.03.068. [DOI] [PubMed] [Google Scholar]
- Chuaphasuk C.; Prapagdee B. Effects of Biochar-Immobilized Bacteria on Phytoremediation of Cadmium-Polluted Soil. Environmental Science and Pollution Research 2019, 26 (23), 23679–23688. 10.1007/s11356-019-05661-6. [DOI] [PubMed] [Google Scholar]
- Li X.; Wang Y.; Luo T.; Ma Y.; Wang B.; Huang Q. Remediation Potential of Immobilized Bacterial Strain with Biochar as Carrier in Petroleum Hydrocarbon and Ni Co-Contaminated Soil. Environ. Technol. 2020, 1–14. 10.1080/09593330.2020.1815858. [DOI] [PubMed] [Google Scholar]
- Li D. C.; Jiang H. The Thermochemical Conversion of Non-Lignocellulosic Biomass to Form Biochar: A Review on Characterizations and Mechanism Elucidation. Bioresour. Technol. 2017, 246, 57–68. 10.1016/j.biortech.2017.07.029. [DOI] [PubMed] [Google Scholar]
- Tomczyk A.; Sokołowska Z.; Boguta P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Reviews in Environmental Science and Bio/Technology 2020, 19 (1), 191–215. 10.1007/s11157-020-09523-3. [DOI] [Google Scholar]
- Guo S.; Liu X.; Wang L.; Liu Q.; Xia C.; Tang J. Ball-Milled Biochar Can Act as a Preferable Biocompatibility Material to Enhance Phenanthrene Degradation by Stimulating Bacterial Metabolism. Bioresour. Technol. 2022, 350, 126901. 10.1016/j.biortech.2022.126901. [DOI] [PubMed] [Google Scholar]
- Guo S.; Liu X.; Zhao H.; Wang L.; Tang J. High Pyrolysis Temperature Biochar Reduced the Transport of Petroleum Degradation Bacteria Corynebacterium Variabile HRJ4 in Porous Media. Journal of Environmental Sciences 2021, 100, 228–239. 10.1016/j.jes.2020.07.012. [DOI] [PubMed] [Google Scholar]
- Lee Y.; Park J.; Ryu C.; Gang K. S.; Yang W.; Park Y.-K.; Jung J.; Hyun S. Comparison of Biochar Properties from Biomass Residues Produced by Slow Pyrolysis at 500°C. Bioresour. Technol. 2013, 148, 196–201. 10.1016/j.biortech.2013.08.135. [DOI] [PubMed] [Google Scholar]
- Schimmelpfennig S.; Glaser B. One Step Forward toward Characterization: Some Important Material Properties to Distinguish Biochars. Journal of Environmental Quality 2012, 41 (4), 1001–1013. 10.2134/jeq2011.0146. [DOI] [PubMed] [Google Scholar]
- Wilson B. K.; Reed D. Implications and Risks of Potential Dioxin Presence in Biochar. International Biochar Initiative 2012, 1–11. [Google Scholar]
- Odinga E. S.; Waigi M. G.; Gudda F. O.; Wang J.; Yang B.; Hu X.; Li S.; Gao Y. Occurrence, Formation, Environmental Fate and Risks of Environmentally Persistent Free Radicals in Biochars. Environ. Int. 2020, 134, 105172. 10.1016/j.envint.2019.105172. [DOI] [PubMed] [Google Scholar]
- Major J.Guidelines on Practical Aspects of Biochar Application to Field Soil in Various Soil Management Systems. A Report from International Biochar Initiative. 2010.
- Oliveira F. R.; Patel A. K.; Jaisi D. P.; Adhikari S.; Lu H.; Khanal S. K. Environmental Application of Biochar: Current Status and Perspectives. Bioresour. Technol. 2017, 246, 110–122. 10.1016/j.biortech.2017.08.122. [DOI] [PubMed] [Google Scholar]
- Abbas Z.; Ali S.; Rizwan M.; Zaheer I. E.; Malik A.; Riaz M. A.; Shahid M. R.; Rehman M. Z. ur; Al-Wabel M. I. A Critical Review of Mechanisms Involved in the Adsorption of Organic and Inorganic Contaminants through Biochar. Arabian Journal of Geosciences 2018, 11 (16), 448. 10.1007/s12517-018-3790-1. [DOI] [Google Scholar]
- Chen W.; Zhang H.; Wang C.; Yang L.; Ni J.; Wei R. Differential Roles of Ash in Sorption of Triclosan to Wood-Derived Biochars Produced at Different Temperatures. Journal of Environmental Quality 2020, 49 (2), 335–345. 10.1002/jeq2.20001. [DOI] [PubMed] [Google Scholar]
- Chen B.; Zhou D.; Zhu L. Transitional Adsorption and Partition of Nonpolar and Polar Aromatic Contaminants by Biochars of Pine Needles with Different Pyrolytic Temperatures. Environ. Sci. Technol. 2008, 42 (14), 5137–5143. 10.1021/es8002684. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Lonappan L.; Brar S. K.; Yang S. Impact of Biochar Amendment in Agricultural Soils on the Sorption, Desorption, and Degradation of Pesticides: A Review. Science of The Total Environment 2018, 645, 60–70. 10.1016/j.scitotenv.2018.07.099. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Wang H.; He L.; Lu K.; Sarmah A.; Li J.; Bolan N. S.; Pei J.; Huang H. Using Biochar for Remediation of Soils Contaminated with Heavy Metals and Organic Pollutants. Environmental Science and Pollution Research 2013, 20 (12), 8472–8483. 10.1007/s11356-013-1659-0. [DOI] [PubMed] [Google Scholar]
- Ahmad M.; Rajapaksha A. U.; Lim J. E.; Zhang M.; Bolan N.; Mohan D.; Vithanage M.; Lee S. S.; Ok Y. S. Biochar as a Sorbent for Contaminant Management in Soil and Water: A Review. Chemosphere 2014, 99, 19. 10.1016/j.chemosphere.2013.10.071. [DOI] [PubMed] [Google Scholar]
- Nguyen T. H.; Cho H.-H.; Poster D. L.; Ball W. P. Evidence for a Pore-Filling Mechanism in the Adsorption of Aromatic Hydrocarbons to a Natural Wood Char. Environ. Sci. Technol. 2007, 41 (4), 1212–1217. 10.1021/es0617845. [DOI] [PubMed] [Google Scholar]
- Kloss S.; Zehetner F.; Dellantonio A.; Hamid R.; Ottner F.; Liedtke V.; Schwanninger M.; Gerzabek M. H.; Soja G. Characterization of Slow Pyrolysis Biochars: Effects of Feedstocks and Pyrolysis Temperature on Biochar Properties. Journal of Environmental Quality 2012, 41 (4), 990–1000. 10.2134/jeq2011.0070. [DOI] [PubMed] [Google Scholar]
- Qiu Y.; Zheng Z.; Zhou Z.; Sheng G. D. Effectiveness and Mechanisms of Dye Adsorption on a Straw-Based Biochar. Bioresour. Technol. 2009, 100 (21), 5348–5351. 10.1016/j.biortech.2009.05.054. [DOI] [PubMed] [Google Scholar]
- Xu R.-k.; Xiao S.-c.; Yuan J.-h.; Zhao A.-z. Adsorption of Methyl Violet from Aqueous Solutions by the Biochars Derived from Crop Residues. Bioresour. Technol. 2011, 102, 10293–10298. 10.1016/j.biortech.2011.08.089. [DOI] [PubMed] [Google Scholar]
- Chen X.; Xia X.; Wang X.; Qiao J.; Chen H. A Comparative Study on Sorption of Perfluorooctane Sulfonate (PFOS) by Chars, Ash and Carbon Nanotubes. Chemosphere 2011, 83 (10), 1313–1319. 10.1016/j.chemosphere.2011.04.018. [DOI] [PubMed] [Google Scholar]
- Pignatello J. J.; Mitch W. A.; Xu W. Activity and Reactivity of Pyrogenic Carbonaceous Matter toward Organic Compounds. Environ. Sci. Technol. 2017, 51 (16), 8893–8908. 10.1021/acs.est.7b01088. [DOI] [PubMed] [Google Scholar]
- Luo Z.; Yao B.; Yang X.; Wang L.; Xu Z.; Yan X.; Tian L.; Zhou H.; Zhou Y. Novel Insights into the Adsorption of Organic Contaminants by Biochar: A Review. Chemosphere 2022, 287, 132113. 10.1016/j.chemosphere.2021.132113. [DOI] [PubMed] [Google Scholar]
- You X.; Jiang H.; Zhao M.; Suo F.; Zhang C.; Zheng H.; Sun K.; Zhang G.; Li F.; Li Y. Biochar Reduced Chinese Chive (Allium Tuberosum) Uptake and Dissipation of Thiamethoxam in an Agricultural Soil. Journal of Hazardous Materials 2020, 390, 121749. 10.1016/j.jhazmat.2019.121749. [DOI] [PubMed] [Google Scholar]
- Rajapaksha A. U.; Chen S. S.; Tsang D. C. W.; Zhang M.; Vithanage M.; Mandal S.; Gao B.; Bolan N. S.; Ok Y. S. Engineered/Designer Biochar for Contaminant Removal/Immobilization from Soil and Water: Potential and Implication of Biochar Modification. Chemosphere 2016, 148, 276–291. 10.1016/j.chemosphere.2016.01.043. [DOI] [PubMed] [Google Scholar]
- Wang S.; Zhao M.; Zhou M.; Li Y. C.; Wang J.; Gao B.; Sato S.; Feng K.; Yin W.; Igalavithana A. D.; Oleszczuk P.; Wang X.; Ok Y. S. Biochar-Supported NZVI (NZVI/BC) for Contaminant Removal from Soil and Water: A Critical Review. Journal of Hazardous Materials 2019, 373, 820–834. 10.1016/j.jhazmat.2019.03.080. [DOI] [PubMed] [Google Scholar]
- Zhang P.; Duan W.; Peng H.; Pan B.; Xing B. Functional Biochar and Its Balanced Design. ACS Environmental Au 2022, 2 (2), 115–127. 10.1021/acsenvironau.1c00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.; Pan B.; Li H.; Liao S.; Zhang D.; Wu M.; Xing B. Degradation of P-Nitrophenol on Biochars: Role of Persistent Free Radicals. Environ. Sci. Technol. 2016, 50 (2), 694–700. 10.1021/acs.est.5b04042. [DOI] [PubMed] [Google Scholar]
- Ruan X.; Sun Y.; Du W.; Tang Y.; Liu Q.; Zhang Z.; Doherty W.; Frost R. L.; Qian G.; Tsang D. C. W. Formation, Characteristics, and Applications of Environmentally Persistent Free Radicals in Biochars: A Review. Bioresour. Technol. 2019, 281, 457–468. 10.1016/j.biortech.2019.02.105. [DOI] [PubMed] [Google Scholar]
- Fang G.; Gao J.; Liu C.; Dionysiou D. D.; Wang Y.; Zhou D. Key Role of Persistent Free Radicals in Hydrogen Peroxide Activation by Biochar: Implications to Organic Contaminant Degradation. Environ. Sci. Technol. 2014, 48 (3), 1902–1910. 10.1021/es4048126. [DOI] [PubMed] [Google Scholar]
- Fang G.; Liu C.; Gao J.; Dionysiou D. D.; Zhou D. Manipulation of Persistent Free Radicals in Biochar To Activate Persulfate for Contaminant Degradation. Environ. Sci. Technol. 2015, 49 (9), 5645–5653. 10.1021/es5061512. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Zhang M.; Li Y.; Li J.; Jing Y.; Xiang Y.; Yao B.; Deng Q. Linkage of Crop Productivity to Soil Nitrogen Dynamics under Biochar Addition: A Meta-Analysis across Field Studies. Agronomy 2022, 10.3390/agronomy12020247. [DOI] [Google Scholar]
- Harindintwali J. D.; Zhou J.; Muhoza B.; Wang F.; Herzberger A.; Yu X. Integrated Eco-Strategies towards Sustainable Carbon and Nitrogen Cycling in Agriculture. Journal of Environmental Management 2021, 293, 112856. 10.1016/j.jenvman.2021.112856. [DOI] [PubMed] [Google Scholar]
- Zheng J.; Han J.; Liu Z.; Xia W.; Zhang X.; Li L.; Liu X.; Bian R.; Cheng K.; Zheng J.; Pan G. Biochar Compound Fertilizer Increases Nitrogen Productivity and Economic Benefits but Decreases Carbon Emission of Maize Production. Agriculture, Ecosystems & Environment 2017, 241, 70–78. 10.1016/j.agee.2017.02.034. [DOI] [Google Scholar]
- Puga A. P.; Grutzmacher P.; Cerri C. E. P.; Ribeirinho V. S.; Andrade C. A. de. Biochar-Based Nitrogen Fertilizers: Greenhouse Gas Emissions, Use Efficiency, and Maize Yield in Tropical Soils. Science of The Total Environment 2020, 704, 135375. 10.1016/j.scitotenv.2019.135375. [DOI] [PubMed] [Google Scholar]
- Xiu L.; Zhang W.; Wu D.; Sun Y.; Zhang H.; Gu W.; Wang Y.; Meng J.; Chen W. Biochar Can Improve Biological Nitrogen Fixation by Altering the Root Growth Strategy of Soybean in Albic Soil. Science of The Total Environment 2021, 773, 144564. 10.1016/j.scitotenv.2020.144564. [DOI] [PubMed] [Google Scholar]
- Park J. H.; Bolan N.; Megharaj M.; Naidu R. Isolation of Phosphate Solubilizing Bacteria and Their Potential for Lead Immobilization in Soil. Journal of Hazardous Materials 2011, 185 (2), 829–836. 10.1016/j.jhazmat.2010.09.095. [DOI] [PubMed] [Google Scholar]
- Prabhu N.; Borkar S.; Garg S. In Chapter 11 - Phosphate Solubilization by Microorganisms: Overview, Mechanisms, Applications and Advances; Academic Press, 2019; pp 161–176. 10.1016/B978-0-12-817497-5.00011-2 [DOI] [Google Scholar]
- Liu Z.; Li Y. C.; Zhang S.; Fu Y.; Fan X.; Patel J. S.; Zhang M. Characterization of Phosphate-Solubilizing Bacteria Isolated from Calcareous Soils. Applied Soil Ecology 2015, 96, 217–224. 10.1016/j.apsoil.2015.08.003. [DOI] [Google Scholar]
- Tabassum B.; Khan A.; Tariq M.; Ramzan M.; Iqbal Khan M. S.; Shahid N.; Aaliya K. Bottlenecks in Commercialisation and Future Prospects of PGPR. Applied Soil Ecology 2017, 121, 102–117. 10.1016/j.apsoil.2017.09.030. [DOI] [Google Scholar]
- Kurth F.; Zeitler K.; Feldhahn L.; Neu T. R.; Weber T.; Krištůfek V.; Wubet T.; Herrmann S.; Buscot F.; Tarkka M. T. Detection and Quantification of a Mycorrhization Helper Bacterium and a Mycorrhizal Fungus in Plant-Soil Microcosms at Different Levels of Complexity. BMC Microbiol. 2013, 13, 205. 10.1186/1471-2180-13-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. E.; Smith F. A.; Jakobsen I. Mycorrhizal Fungi Can Dominate Phosphate Supply to Plants Irrespective of Growth Responses. Plant Physiol. 2003, 133 (1), 16–20. 10.1104/pp.103.024380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser B.; Lehr V.-I. Biochar Effects on Phosphorus Availability in Agricultural Soils: A Meta-Analysis. Sci. Rep. 2019, 9 (1), 9338. 10.1038/s41598-019-45693-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L.; Li H.; Tan F.; Zhu N.; He M.; Hu G. Biochar: A Potential Route for Recycling of Phosphorus in Agricultural Residues. GCB Bioenergy 2016, 8 (5), 852–858. 10.1111/gcbb.12365. [DOI] [Google Scholar]
- Zhao L.; Cao X.; Zheng W.; Scott J. W.; Sharma B. K.; Chen X. Copyrolysis of Biomass with Phosphate Fertilizers To Improve Biochar Carbon Retention, Slow Nutrient Release, and Stabilize Heavy Metals in Soil. ACS Sustainable Chem. Eng. 2016, 4 (3), 1630–1636. 10.1021/acssuschemeng.5b01570. [DOI] [Google Scholar]
- Zheng B.-X.; Ding K.; Yang X.-R.; Wadaan M. A. M.; Hozzein W. N.; Peñuelas J.; Zhu Y.-G. Straw Biochar Increases the Abundance of Inorganic Phosphate Solubilizing Bacterial Community for Better Rape (Brassica Napus) Growth and Phosphate Uptake. Science of The Total Environment 2019, 647, 1113–1120. 10.1016/j.scitotenv.2018.07.454. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Lin Y.; Chiu P. C.; Imhoff P. T.; Guo M. Phosphorus Release Behaviors of Poultry Litter Biochar as a Soil Amendment. Science of The Total Environment 2015, 512–513, 454–463. 10.1016/j.scitotenv.2015.01.093. [DOI] [PubMed] [Google Scholar]
- Ayangbenro A. S.; Babalola O. O. Reclamation of Arid and Semi-Arid Soils: The Role of Plant Growth-Promoting Archaea and Bacteria. Curr. Plant Biol. 2020, 100173. 10.1016/j.cpb.2020.100173. [DOI] [Google Scholar]
- Sattar A.; Naveed M.; Ali M.; Zahir Z. A.; Nadeem S. M.; Yaseen M.; Meena V. S.; Farooq M.; Singh R.; Rahman M.; Meena H. N. Perspectives of Potassium Solubilizing Microbes in Sustainable Food Production System: A Review. Applied Soil Ecology 2019, 133, 146–159. 10.1016/j.apsoil.2018.09.012. [DOI] [Google Scholar]
- Harindintwali J. D.; Zhou J.; Yang W.; Gu Q.; Yu X. Biochar-Bacteria-Plant Partnerships: Eco-Solutions for Tackling Heavy Metal Pollution. Ecotoxicology and Environmental Safety 2020, 204, 111020. 10.1016/j.ecoenv.2020.111020. [DOI] [PubMed] [Google Scholar]
- Wang L.; Xue C.; Nie X.; Liu Y.; Chen F. Effects of Biochar Application on Soil Potassium Dynamics and Crop Uptake. Journal of Plant Nutrition and Soil Science 2018, 181 (5), 635–643. 10.1002/jpln.201700528. [DOI] [Google Scholar]
- Liu S.; Tang W.; Yang F.; Meng J.; Chen W.; Li X. Influence of Biochar Application on Potassium-Solubilizing Bacillus Mucilaginosus as Potential Biofertilizer. Preparative Biochemistry and Biotechnology 2017, 10.1080/10826068.2016.1155062. [DOI] [PubMed] [Google Scholar]
- Pourbabaee A. A.; Koohbori Dinekaboodi S.; Seyed Hosseini H. M.; Alikhani H. A.; Emami S. Potential Application of Selected Sulfur-Oxidizing Bacteria and Different Sources of Sulfur in Plant Growth Promotion under Different Moisture Conditions. Commun. Soil Sci. Plant Anal. 2020, 51 (6), 735–745. 10.1080/00103624.2020.1729377. [DOI] [Google Scholar]
- Anandham R.; Gandhi P. I.; SenthilKumar M.; Sridar R.; Nalayini P.; Sa T.-M.. Sulfur-Oxidizing Bacteria: A Novel Bioinoculant for Sulfur Nutrition and Crop Production. In Bacteria in Agrobiology: Plant Nutrient Management; 2011. 10.1007/978-3-642-21061-7_5 [DOI] [Google Scholar]
- Cheah S.; Malone S. C.; Feik C. J. Speciation of Sulfur in Biochar Produced from Pyrolysis and Gasification of Oak and Corn Stover. Environ. Sci. Technol. 2014, 48, 8474. 10.1021/es500073r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J.; Özkaya Ö.; Kümmerli R. Bacterial Siderophores in Community and Host Interactions. Nature Reviews Microbiology 2020, 18 (3), 152–163. 10.1038/s41579-019-0284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira M. J.; Silva H.; Cunha A. Siderophore-Producing Rhizobacteria as a Promising Tool for Empowering Plants to Cope with Iron Limitation in Saline Soils: A Review. Pedosphere 2019, 29 (4), 409–420. 10.1016/S1002-0160(19)60810-6. [DOI] [Google Scholar]
- Radić S.; Babić M.; Škobić D.; Roje V.; Pevalek-Kozlina B. Ecotoxicological Effects of Aluminum and Zinc on Growth and Antioxidants in Lemna Minor L. Ecotoxicology and Environmental Safety 2010, 73 (3), 336–342. 10.1016/j.ecoenv.2009.10.014. [DOI] [PubMed] [Google Scholar]
- da Silva Lobato A. K.; Alvino Lima E. J.; Guedes Lobato E. M. S.; Maciel G. M.; Marques D. J.. Tolerance of Plants to Toxicity Induced by Micronutrients. In Abiotic and Biotic Stress in Plants - Recent Advances and Future Perspectives; 2016. 10.5772/62046 [DOI] [Google Scholar]
- Kamran M.; Malik Z.; Parveen A.; Zong Y.; Abbasi G. H.; Rafiq M. T.; Shaaban M.; Mustafa A.; Bashir S.; Rafay M.; Mehmood S.; Ali M. Biochar Alleviates Cd Phytotoxicity by Minimizing Bioavailability and Oxidative Stress in Pak Choi (Brassica chinensis L.) Cultivated in Cd-Polluted Soil. J. Environ. Manage. 2019, 250, 109500. 10.1016/j.jenvman.2019.109500. [DOI] [PubMed] [Google Scholar]
- Chen X.; Chen G.; Chen L.; Chen Y.; Lehmann J.; McBride M. B.; Hay A. G. Adsorption of Copper and Zinc by Biochars Produced from Pyrolysis of Hardwood and Corn Straw in Aqueous Solution. Bioresour. Technol. 2011, 102 (19), 8877–8884. 10.1016/j.biortech.2011.06.078. [DOI] [PubMed] [Google Scholar]
- Ferreira C. M. H.; Soares H. M. V. M.; Soares E. V. Promising Bacterial Genera for Agricultural Practices: An Insight on Plant Growth-Promoting Properties and Microbial Safety Aspects. Sci. Total Environ. 2019, 682, 779. 10.1016/j.scitotenv.2019.04.225. [DOI] [PubMed] [Google Scholar]
- Li W.; Wang D.; Hu F.; Li H.; Ma L.; Xu L. Exogenous IAA Treatment Enhances Phytoremediation of Soil Contaminated with Phenanthrene by Promoting Soil Enzyme Activity and Increasing Microbial Biomass. Environ. Sci. Pollut. Res. 2016, 10.1007/s11356-016-6170-y. [DOI] [PubMed] [Google Scholar]
- Hansen H.; Grossmann K. Auxin-Induced Ethylene Triggers Abscisic Acid Biosynthesis and Growth Inhibition. Plant physiology 2000, 124 (3), 1437–1448. 10.1104/pp.124.3.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostin A.; Kowalyczk M.; Bhalerao R. P.; Sandberg G. Metabolism of Indole-3-Acetic Acid in Arabidopsis. Plant physiology 1998, 118 (1), 285–296. 10.1104/pp.118.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadeniz A.; Topcuoğlu Ş. F.; İnan S. Auxin, Gibberellin, Cytokinin and Abscisic Acid Production in Some Bacteria. World J. Microbiol. Biotechnol. 2006, 22 (10), 1061–1064. 10.1007/s11274-005-4561-1. [DOI] [Google Scholar]
- Kudoyarova G. R.; Arkhipova T. N.; Melent’ev A. I. In Role of Bacterial Phytohormones in Plant Growth Regulation and Their Development BT - Bacterial Metabolites in Sustainable Agroecosystem; Maheshwari D. K., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp 69–86. 10.1007/978-3-319-24654-3_4 [DOI] [Google Scholar]
- French E.; Iyer-Pascuzzi A. S. A Role for the Gibberellin Pathway in Biochar-Mediated Growth Promotion. Sci. Rep. 2018, 8 (1), 5389. 10.1038/s41598-018-23677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viger M.; Hancock R. D.; Miglietta F.; Taylor G. More Plant Growth but Less Plant Defence? First Global Gene Expression Data for Plants Grown in Soil Amended with Biochar. GCB Bioenergy 2015, 7 (4), 658–672. 10.1111/gcbb.12182. [DOI] [Google Scholar]
- Hao D.; Sun X.; Ma B.; Zhang J.-S.; Guo H. In 6 - Ethylene; Li J., Li C., Smith S. M., et al., Eds.; Academic Press, 2017; pp 203–241. 10.1016/B978-0-12-811562-6.00006-2 [DOI] [Google Scholar]
- Weisman D.; Alkio M.; Colón-Carmona A. Transcriptional Responses to Polycyclic Aromatic Hydrocarbon-Induced Stress in Arabidopsis Thaliana Reveal the Involvement of Hormone and Defense Signaling Pathways. BMC plant biology 2010, 10, 59. 10.1186/1471-2229-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Váňová L.; Kummerová M.; Votrubová O. Fluoranthene-Induced Production of Ethylene and Formation of Lysigenous Intercellular Spaces in Pea Plants Cultivated in Vitro. Acta Physiologiae Plantarum 2011, 33 (3), 1037–1042. 10.1007/s11738-010-0618-3. [DOI] [Google Scholar]
- Iqbal N.; Khan N. A.; Ferrante A.; Trivellini A.; Francini A.; Khan M. I. R. Ethylene Role in Plant Growth, Development and Senescence: Interaction with Other Phytohormones. Front. Plant Sci. 2017, 8, 475. 10.3389/fpls.2017.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M.; Van den Broeck L.; Inzé D. The Pivotal Role of Ethylene in Plant Growth. Trends in Plant Science 2018, 23 (4), 311–323. 10.1016/j.tplants.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick B. R. Bacteria with ACC Deaminase Can Promote Plant Growth and Help to Feed the World. Microbiol. Res. 2014, 169, 30. 10.1016/j.micres.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Arshad M.; Saleem M.; Hussain S. Perspectives of Bacterial ACC Deaminase in Phytoremediation. Trends Biotechnol. 2007, 25 (8), 356–362. 10.1016/j.tibtech.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Grichko V. P.; Filby B.; Glick B. R. Increased Ability of Transgenic Plants Expressing the Bacterial Enzyme ACC Deaminase to Accumulate Cd, Co, Cu, Ni, Pb, and Zn. J. Biotechnol. 2000, 81 (1), 45–53. 10.1016/S0168-1656(00)00270-4. [DOI] [PubMed] [Google Scholar]
- Compant S.; Duffy B.; Nowak J.; Clément C.; Barka E. A. Use of Plant Growth-Promoting Bacteria for Biocontrol of Plant Diseases: Principles, Mechanisms of Action, and Future Prospects. Appl. Environ. Microbiol. 2005, 71 (9), 4951–4959. 10.1128/AEM.71.9.4951-4959.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin S. A.; Green D. H.; Hart M. C.; Küpper F. C.; Sunda W. G.; Carrano C. J. Photolysis of Iron–Siderophore Chelates Promotes Bacterial–Algal Mutualism. Proc. Natl. Acad. Sci. U. S. A. 2009, 106 (40), 17071–17076. 10.1073/pnas.0905512106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak S.; Cordero O. X. Rhizobiome Shields Plants from Infection. Nature Microbiology 2020, 5 (8), 978–979. 10.1038/s41564-020-0766-1. [DOI] [PubMed] [Google Scholar]
- Gu S.; Wei Z.; Shao Z.; Friman V.-P.; Cao K.; Yang T.; Kramer J.; Wang X.; Li M.; Mei X.; Xu Y.; Shen Q.; Kümmerli R.; Jousset A. Competition for Iron Drives Phytopathogen Control by Natural Rhizosphere Microbiomes. Nature Microbiology 2020, 5 (8), 1002–1010. 10.1038/s41564-020-0719-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flury P.; Vesga P.; Péchy-Tarr M.; Aellen N.; Dennert F.; Hofer N.; Kupferschmied K. P.; Kupferschmied P.; Metla Z.; Ma Z.; Siegfried S.; de Weert S.; Bloemberg G.; Höfte M.; Keel C. J.; Maurhofer M. Antimicrobial and Insecticidal: Cyclic Lipopeptides and Hydrogen Cyanide Produced by Plant-Beneficial Pseudomonas Strains CHA0, CMR12a, and PCL1391 Contribute to Insect Killing. Frontiers in microbiology 2017, 8, 100. 10.3389/fmicb.2017.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H.-S.; Kim Y.-S.; Kim S.-D. Pseudomonas Stutzeri YPL-1 Genetic Transformation and Antifungal Mechanism against Fusarium Solani, an Agent of Plant Root Rot. Appl. Environ. Microbiol. 1991, 57 (2), 510–516. 10.1128/aem.57.2.510-516.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamensky M.; Ovadis M.; Chet I.; Chernin L. Soil-Borne Strain IC14 of Serratia Plymuthica with Multiple Mechanisms of Antifungal Activity Provides Biocontrol of Botrytis Cinerea and Sclerotinia Sclerotiorum Diseases. Soil Biology and Biochemistry 2003, 35 (2), 323–331. 10.1016/S0038-0717(02)00283-3. [DOI] [Google Scholar]
- Fridlender M.; Inbar J.; Chet I. Biological Control of Soilborne Plant Pathogens by a β-1,3 Glucanase-Producing Pseudomonas Cepacia. Soil Biology and Biochemistry 1993, 25 (9), 1211–1221. 10.1016/0038-0717(93)90217-Y. [DOI] [Google Scholar]
- Zhang L.; Birch R. G. Biocontrol of Sugar Cane Leaf Scald Disease by an Isolate of Pantoea Dispersa Which Detoxifies Albicidin Phytotoxins. Letters in Applied Microbiology 1996, 22 (2), 132–136. 10.1111/j.1472-765X.1996.tb01126.x. [DOI] [Google Scholar]
- Pieterse C. M. J.; Zamioudis C.; Berendsen R. L.; Weller D. M.; Van Wees S. C. M.; Bakker P. A. H. M. Induced Systemic Resistance by Beneficial Microbes. Annual Review of Phytopathology 2014, 52 (1), 347–375. 10.1146/annurev-phyto-082712-102340. [DOI] [PubMed] [Google Scholar]
- Fincheira P.; Quiroz A. Microbial Volatiles as Plant Growth Inducers. Microbiological Research 2018, 208, 63–75. 10.1016/j.micres.2018.01.002. [DOI] [PubMed] [Google Scholar]
- Ryu C.-M.; Farag M. A.; Hu C.-H.; Reddy M. S.; Kloepper J. W.; Paré P. W. Bacterial Volatiles Induce Systemic Resistance in Arabidopsis. Plant Physiology 2004, 134 (3), 1017–1026. 10.1104/pp.103.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desaki Y.; Miya A.; Venkatesh B.; Tsuyumu S.; Yamane H.; Kaku H.; Minami E.; Shibuya N. Bacterial Lipopolysaccharides Induce Defense Responses Associated with Programmed Cell Death in Rice Cells. Plant and Cell Physiology 2006, 47 (11), 1530–1540. 10.1093/pcp/pcl019. [DOI] [PubMed] [Google Scholar]
- Aznar A.; Dellagi A. New Insights into the Role of Siderophores as Triggers of Plant Immunity. Journal of Experimental Botany 2015, 66 (11), 3001–3010. 10.1093/jxb/erv155. [DOI] [PubMed] [Google Scholar]
- Ongena M.; Jourdan E.; Adam A.; Paquot M.; Brans A.; Joris B.; Arpigny J.-L.; Thonart P. Surfactin and Fengycin Lipopeptides of Bacillus Subtilis as Elicitors of Induced Systemic Resistance in Plants. Environmental Microbiology 2007, 9 (4), 1084–1090. 10.1111/j.1462-2920.2006.01202.x. [DOI] [PubMed] [Google Scholar]
- Weller D. M.; Mavrodi D. V.; van Pelt J. A.; Pieterse C. M. J.; van Loon L. C.; Bakker P. A. H. M. Induced Systemic Resistance in Arabidopsis Thaliana Against Pseudomonas Syringae Pv. Tomato by 2,4-Diacetylphloroglucinol-Producing Pseudomonas Fluorescens. Phytopathology 2012, 102 (4), 403–412. 10.1094/PHYTO-08-11-0222. [DOI] [PubMed] [Google Scholar]
- Meziane H.; Van Der Sluis I.; Van Loon L. C.; Höfte M.; Bakker P. A. H. M. Determinants of Pseudomonas Putida WCS358 Involved in Inducing Systemic Resistance in Plants. Molecular Plant Pathology 2005, 6 (2), 177–185. 10.1111/j.1364-3703.2005.00276.x. [DOI] [PubMed] [Google Scholar]
- Yang J.; Kloepper J. W.; Ryu C.-M. Rhizosphere Bacteria Help Plants Tolerate Abiotic Stress. Trends in Plant Science 2009, 14 (1), 1–4. 10.1016/j.tplants.2008.10.004. [DOI] [PubMed] [Google Scholar]
- van Loon L. C.; Bakker P. A. H. M.; Pieterse C. M. J. SYSTEMIC RESISTANCE INDUCED BY RHIZOSPHERE BACTERIA. Annual Review of Phytopathology 1998, 36 (1), 453–483. 10.1146/annurev.phyto.36.1.453. [DOI] [PubMed] [Google Scholar]
- Bakker P. A. H. M.; Doornbos R. F.; Zamioudis C.; Berendsen R. L.; Pieterse C. M. J. Induced Systemic Resistance and the Rhizosphere Microbiome. plant pathology journal 2013, 29 (2), 136–143. 10.5423/PPJ.SI.07.2012.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal A. K.; Alkan N.; Elad Y.; Sela N.; Philosoph A. M.; Graber E. R.; Frenkel O. Molecular Insights into Biochar-Mediated Plant Growth Promotion and Systemic Resistance in Tomato against Fusarium Crown and Root Rot Disease. Sci. Rep. 2020, 10 (1), 13934. 10.1038/s41598-020-70882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egamberdieva D.; Shurigin V.; Alaylar B.; Ma H.; Müller M. E. H.; Wirth S.; Reckling M.; Bellingrath-Kimura S. D. The Effect of Biochars and Endophytic Bacteria on Growth and Root Rot Disease Incidence of Fusarium Infested Narrow-Leafed Lupin (Lupinus Angustifolius L.). Microorganisms 2020, 8, 496. 10.3390/microorganisms8040496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogovska N.; Laird D.; Leandro L.; Aller D. Biochar Effect on Severity of Soybean Root Disease Caused by Fusarium Virguliforme. Plant and Soil 2017, 413 (1), 111–126. 10.1007/s11104-016-3086-8. [DOI] [Google Scholar]
- Frenkel O.; Jaiswal A. K.; Elad Y.; Lew B.; Kammann C.; Graber E. R. The Effect of Biochar on Plant Diseases: What Should We Learn While Designing Biochar Substrates?. Journal of Environmental Engineering and Landscape Management 2017, 10.3846/16486897.2017.1307202. [DOI] [Google Scholar]
- Graber E. R.; Frenkel O.; Jaiswal A. K.; Elad Y. How May Biochar Influence Severity of Diseases Caused by Soilborne Pathogens?. Carbon Manage. 2014, 5, 169. 10.1080/17583004.2014.913360. [DOI] [Google Scholar]
- Yu X. Z.; Wu S. C.; Wu F. Y.; Wong M. H. Enhanced Dissipation of PAHs from Soil Using Mycorrhizal Ryegrass and PAH-Degrading Bacteria. Journal of Hazardous Materials 2011, 186 (2), 1206–1217. 10.1016/j.jhazmat.2010.11.116. [DOI] [PubMed] [Google Scholar]
- Leigh M. B.; Prouzová P.; Macková M.; Macek T.; Nagle D. P.; Fletcher J. S. Polychlorinated Biphenyl (PCB)-Degrading Bacteria Associated with Trees in a PCB-Contaminated Site. Applied and environmental microbiology 2006, 72 (4), 2331–2342. 10.1128/AEM.72.4.2331-2342.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreolli M.; Lampis S.; Poli M.; Gullner G.; Biró B.; Vallini G. Endophytic Burkholderia Fungorum DBT1 Can Improve Phytoremediation Efficiency of Polycyclic Aromatic Hydrocarbons. Chemosphere 2013, 92 (6), 688–694. 10.1016/j.chemosphere.2013.04.033. [DOI] [PubMed] [Google Scholar]
- Joutey N. T. In Biodegradation: Involved Microorganisms and Genetically Engineered Microorganisms; Bahafid W., Ed.; IntechOpen: Rijeka, Croatia, 2013; Chapter 11. 10.5772/56194 [DOI] [Google Scholar]
- Ma J.; Xu L.; Jia L. Degradation of Polycyclic Aromatic Hydrocarbons by Pseudomonas Sp. JM2 Isolated from Active Sewage Sludge of Chemical Plant. Journal of Environmental Sciences 2012, 24 (12), 2141–2148. 10.1016/S1001-0742(11)61064-4. [DOI] [PubMed] [Google Scholar]
- Singer A. C.; Crowley D. E.; Thompson I. P. Secondary Plant Metabolites in Phytoremediation and Biotransformation. Trends Biotechnol. 2003, 21 (3), 123–130. 10.1016/S0167-7799(02)00041-0. [DOI] [PubMed] [Google Scholar]
- Miya R. K.; Firestone M. K. Enhanced Phenanthrene Biodegradation in Soil by Slender Oat Root Exudates and Root Debris. Journal of Environmental Quality 2001, 30 (6), 1911–1918. 10.2134/jeq2001.1911. [DOI] [PubMed] [Google Scholar]
- Musilova L.; Ridl J.; Polivkova M.; Macek T.; Uhlik O. Effects of Secondary Plant Metabolites on Microbial Populations: Changes in Community Structure and Metabolic Activity in Contaminated Environments. International journal of molecular sciences 2016, 17 (8), 1205. 10.3390/ijms17081205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.-H.; Aitken M. D. Salicylate Stimulates the Degradation of High-Molecular Weight Polycyclic Aromatic Hydrocarbons by Pseudomonas Saccharophila P15. Environ. Sci. Technol. 1999, 33 (3), 435–439. 10.1021/es9805730. [DOI] [Google Scholar]
- Slater H.; Gouin T.; Leigh M. B. Assessing the Potential for Rhizoremediation of PCB Contaminated Soils in Northern Regions Using Native Tree Species. Chemosphere 2011, 84 (2), 199–206. 10.1016/j.chemosphere.2011.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hamme J. D.Bioavailability and Biodegradation of Organic Pollutants - A Microbial Perspective. Biodegradation and Bioremediation; 2004; Vol. 2. 10.1007/978-3-662-06066-7_3 [DOI] [Google Scholar]
- Reid B. J.; Jones K. C.; Semple K. T. Bioavailability of Persistent Organic Pollutants in Soils and Sediments—a Perspective on Mechanisms, Consequences and Assessment. Environ. Pollut. 2000, 108 (1), 103–112. 10.1016/S0269-7491(99)00206-7. [DOI] [PubMed] [Google Scholar]
- Alexander M. Aging, Bioavailability, and Overestimation of Risk from Environmental Pollutants. Environ. Sci. Technol. 2000, 34 (20), 4259–4265. 10.1021/es001069. [DOI] [Google Scholar]
- Elumalai P.; Parthipan P.; Huang M.; Muthukumar B.; Cheng L.; Govarthanan M.; Rajasekar A. Enhanced Biodegradation of Hydrophobic Organic Pollutants by the Bacterial Consortium: Impact of Enzymes and Biosurfactants. Environ. Pollut. 2021, 289, 117956. 10.1016/j.envpol.2021.117956. [DOI] [PubMed] [Google Scholar]
- Marchant R.; Banat I. M. Microbial Biosurfactants: Challenges and Opportunities for Future Exploitation. Trends Biotechnol. 2012, 30 (11), 558–565. 10.1016/j.tibtech.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Mondal M.; Halder G.; Oinam G.; Indrama T.; Tiwari O. N. In Chapter 17 - Bioremediation of Organic and Inorganic Pollutants Using Microalgae; Gupta V. K., Pandey A. B., et al., Eds.; Elsevier: Amsterdam, 2019; pp 223–235. 10.1016/B978-0-444-63504-4.00017-7 [DOI] [Google Scholar]
- Wood T. L.; Gong T.; Zhu L.; Miller J.; Miller D. S.; Yin B.; Wood T. K. Rhamnolipids from Pseudomonas Aeruginosa Disperse the Biofilms of Sulfate-Reducing Bacteria. npj Biofilms and Microbiomes 2018, 4 (1), 22. 10.1038/s41522-018-0066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmyttere H.; Deweer C.; Muchembled J.; Sahmer K.; Jacquin J.; Coutte F.; Jacques P. Antifungal Activities of Bacillus Subtilis Lipopeptides to Two Venturia Inaequalis Strains Possessing Different Tebuconazole Sensitivity. Front. Microbiol. 2019, 2327. 10.3389/fmicb.2019.02327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. G.; Zajic J. E.; Gerson D. F. Production of Surface-Active Lipids by Corynebacterium Lepus. Applied and environmental microbiology 1979, 37 (1), 4–10. 10.1128/aem.37.1.4-10.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahhan R.; R S. T.; A B. A.; M M. R. Rhamnolipid-Induced Removal of Lipopolysaccharide from Pseudomonas Aeruginosa: Effect on Cell Surface Properties and Interaction with Hydrophobic Substrates. Appl. Environ. Microbiol. 2000, 66 (8), 3262–3268. 10.1128/AEM.66.8.3262-3268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamp S. J.; Tolker-Nielsen T. Multiple Roles of Biosurfactants in Structural Biofilm Development by Pseudomonas Aeruginosa. Journal of bacteriology 2007, 189 (6), 2531–2539. 10.1128/JB.01515-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S. K.; Mangwani N.; Rao T. S.; Das S. 8 - Biofilm-Mediated Bioremediation of Polycyclic Aromatic Hydrocarbons. Microb. Biodegrad. Biorem. 2014, 203–232. 10.1016/B978-0-12-800021-2.00008-X. [DOI] [Google Scholar]
- Leng L.; Yuan X.; Zeng G.; Shao J.; Chen X.; Wu Z.; Wang H.; Peng X. Surface Characterization of Rice Husk Bio-Char Produced by Liquefaction and Application for Cationic Dye (Malachite Green) Adsorption. Fuel 2015, 155, 77. 10.1016/j.fuel.2015.04.019. [DOI] [Google Scholar]
- Sun K.; Keiluweit M.; Kleber M.; Pan Z.; Xing B. Sorption of Fluorinated Herbicides to Plant Biomass-Derived Biochars as a Function of Molecular Structure. Bioresour. Technol. 2011, 102, 9897. 10.1016/j.biortech.2011.08.036. [DOI] [PubMed] [Google Scholar]
- Zhang G.; Zhang Q.; Sun K.; Liu X.; Zheng W.; Zhao Y. Sorption of Simazine to Corn Straw Biochars Prepared at Different Pyrolytic Temperatures. Environ. Pollut. 2011, 159, 2594. 10.1016/j.envpol.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Sun K.; Ro K.; Guo M.; Novak J.; Mashayekhi H.; Xing B. Sorption of Bisphenol A, 17α-Ethinyl Estradiol and Phenanthrene on Thermally and Hydrothermally Produced Biochars. Bioresour. Technol. 2011, 102, 5757. 10.1016/j.biortech.2011.03.038. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Sheng G. Enhanced Pesticide Sorption by Soils Containing Particulate Matter from Crop Residue Burns. Environ. Sci. Technol. 2003, 37, 3635. 10.1021/es034006a. [DOI] [PubMed] [Google Scholar]
- Tian S.-Q.; Wang L.; Liu Y.-L.; Ma J. Degradation of Organic Pollutants by Ferrate/Biochar: Enhanced Formation of Strong Intermediate Oxidative Iron Species. Water Res. 2020, 183, 116054. 10.1016/j.watres.2020.116054. [DOI] [PubMed] [Google Scholar]
- Leng L.; Xu S.; Liu R.; Yu T.; Zhuo X.; Leng S.; Xiong Q.; Huang H. Nitrogen Containing Functional Groups of Biochar: An Overview. Bioresour. Technol. 2020, 298, 122286. 10.1016/j.biortech.2019.122286. [DOI] [PubMed] [Google Scholar]
- Yuan Y.; Bolan N.; Prévoteau A.; Vithanage M.; Biswas J. K.; Ok Y. S.; Wang H. Applications of Biochar in Redox-Mediated Reactions. Bioresour. Technol. 2017, 246, 271–281. 10.1016/j.biortech.2017.06.154. [DOI] [PubMed] [Google Scholar]
- Kizito S.; Lv T.; Wu S.; Ajmal Z.; Luo H.; Dong R. Treatment of Anaerobic Digested Effluent in Biochar-Packed Vertical Flow Constructed Wetland Columns: Role of Media and Tidal Operation. Science of The Total Environment 2017, 592, 197–205. 10.1016/j.scitotenv.2017.03.125. [DOI] [PubMed] [Google Scholar]
- Tsang D. C. W.; Yu I. K. M.; Xiong X. In Chapter 18 - Novel Application of Biochar in Stormwater Harvesting; Ok Y. S., Tsang D. C. W., Bolan N., Novak J. M., et al., Eds.; Elsevier, 2019; pp 319–347. 10.1016/B978-0-12-811729-3.00018-2 [DOI] [Google Scholar]
- Ma L.; Hu T.; Liu Y.; Liu J.; Wang Y.; Wang P.; Zhou J.; Chen M.; Yang B.; Li L. Combination of Biochar and Immobilized Bacteria Accelerates Polyacrylamide Biodegradation in Soil by Both Bio-Augmentation and Bio-Stimulation Strategies. J. Hazard. Mater. 2020, 124086. 10.1016/j.jhazmat.2020.124086. [DOI] [PubMed] [Google Scholar]
- Dai Y.; Zhang N.; Xing C.; Cui Q.; Sun Q. The Adsorption, Regeneration and Engineering Applications of Biochar for Removal Organic Pollutants: A Review. Chemosphere 2019, 223, 12–27. 10.1016/j.chemosphere.2019.01.161. [DOI] [PubMed] [Google Scholar]
- Barac T.; Taghavi S.; Borremans B.; Provoost A.; Oeyen L.; Colpaert J. V.; Vangronsveld J.; van der Lelie D. Engineered Endophytic Bacteria Improve Phytoremediation of Water-Soluble, Volatile, Organic Pollutants. Nat. Biotechnol. 2004, 22 (5), 583–588. 10.1038/nbt960. [DOI] [PubMed] [Google Scholar]
- Song Y.; Li Y.; Zhang W.; Wang F.; Bian Y.; Boughner L. A.; Jiang X. Novel Biochar-Plant Tandem Approach for Remediating Hexachlorobenzene Contaminated Soils: Proof-of-Concept and New Insight into the Rhizosphere. J. Agric. Food Chem. 2016, 64 (27), 5464–5471. 10.1021/acs.jafc.6b01035. [DOI] [PubMed] [Google Scholar]
- Turkovskaya O.; Muratova A. Plant–Bacterial Degradation of Polyaromatic Hydrocarbons in the Rhizosphere. Trends Biotechnol. 2019, 37 (9), 926–930. 10.1016/j.tibtech.2019.04.010. [DOI] [PubMed] [Google Scholar]
- Hussain F.; Hussain I.; Khan A. H. A.; Muhammad Y. S.; Iqbal M.; Soja G.; Reichenauer T. G.; Zeshan; Yousaf S. Combined Application of Biochar, Compost, and Bacterial Consortia with Italian Ryegrass Enhanced Phytoremediation of Petroleum Hydrocarbon Contaminated Soil. Environ. Exp. Bot. 2018, 153, 80. 10.1016/j.envexpbot.2018.05.012. [DOI] [Google Scholar]
- Sarma H.; Sonowal S.; Prasad M. N. V. Plant-Microbiome Assisted and Biochar-Amended Remediation of Heavy Metals and Polyaromatic Compounds - a Microcosmic Study. Ecotoxicol. Environ. Saf. 2019, 176, 288. 10.1016/j.ecoenv.2019.03.081. [DOI] [PubMed] [Google Scholar]
- Take Action for the Sustainable Development Goals - United Nations Sustainable Development; United Nations, 2022. [Google Scholar]
- Lal R.; Bouma J.; Brevik E.; Dawson L.; Field D. J.; Glaser B.; Hatano R.; Hartemink A. E.; Kosaki T.; Lascelles B.; Monger C.; Muggler C.; Ndzana G. M.; Norra S.; Pan X.; Paradelo R.; Reyes-Sánchez L. B.; Sandén T.; Singh B. R.; Spiegel H.; Yanai J.; Zhang J. Soils and Sustainable Development Goals of the United Nations: An International Union of Soil Sciences Perspective. Geoderma Regional 2021, 25, e00398. 10.1016/j.geodrs.2021.e00398. [DOI] [Google Scholar]
- Wan X.; Lei M.; Chen T. Cost–Benefit Calculation of Phytoremediation Technology for Heavy-Metal-Contaminated Soil. Science of The Total Environment 2016, 563–564, 796–802. 10.1016/j.scitotenv.2015.12.080. [DOI] [PubMed] [Google Scholar]
- U.S. Federal Remediation Technologies Roundtable (US FRTR) . Remediation Technologies Screening Matrix and Reference Guide, ver. 4.0; 2020.
- Cunningham S. D.; Berti W. R.; Huang J. W. Phytoremediation of Contaminated Soils. Trends Biotechnol. 1995, 13 (9), 393–397. 10.1016/S0167-7799(00)88987-8. [DOI] [Google Scholar]
- Watanabe M. E. Phytoremediation on the Brink of Commericialization. Environ. Sci. Technol. 1997, 31 (4), 182A–186A. 10.1021/es972219s. [DOI] [PubMed] [Google Scholar]
- Vangronsveld J.; Herzig R.; Weyens N.; Boulet J.; Adriaensen K.; Ruttens A.; Thewys T.; Vassilev A.; Meers E.; Nehnevajova E.; van der Lelie D.; Mench M. Phytoremediation of Contaminated Soils and Groundwater: Lessons from the Field. Environmental Science and Pollution Research 2009, 16 (7), 765–794. 10.1007/s11356-009-0213-6. [DOI] [PubMed] [Google Scholar]
- Mitton F. M.; Gonzalez M.; Monserrat J. M.; Miglioranza K. S. B. Potential Use of Edible Crops in the Phytoremediation of Endosulfan Residues in Soil. Chemosphere 2016, 148, 300–306. 10.1016/j.chemosphere.2016.01.028. [DOI] [PubMed] [Google Scholar]
- Sharma P.; Jha A. B.; Bauddh K.; Korstad J.; Dubey R. S.. Chapter 3 - Efficient Utilization of Plant Biomass after Harvesting the Phytoremediator Plants. In Phytorestoration of Abandoned Mining and Oil Drilling Sites; Bauddh K., Korstad J., Sharma P. B., et al., Eds.; Elsevier, 2021; pp 57–84. 10.1016/B978-0-12-821200-4.00003-0 [DOI] [Google Scholar]
- Tripathi S.; Singh V. K.; Srivastava P.; Singh R.; Devi R. S.; Kumar A.; Bhadouria R.. Chapter 4 - Phytoremediation of Organic Pollutants: Current Status and Future Directions. In Abatement of Environmental Pollutants; Singh P., Kumar A., Borthakur A. B., et al., Eds.; Elsevier, 2020; pp 81–105. 10.1016/B978-0-12-818095-2.00004-7 [DOI] [Google Scholar]
- Line M. A.; Garland C. D.; Crowley M. Evaluation of Landfarm Remediation of Hydrocarbon-Contaminated Soil at the Inveresk Railyard, Launceston, Australia. Waste Management 1996, 16 (7), 567–570. 10.1016/S0956-053X(96)00077-3. [DOI] [Google Scholar]
- U.S. Federal Remediation Technologies Roundtable (US FRTR) . Enhanced Bioremediation; 2020.
- Kochanek J.; Soo R. M.; Martinez C.; Dakuidreketi A.; Mudge A. M. Biochar for Intensification of Plant-Related Industries to Meet Productivity, Sustainability and Economic Goals: A Review. Resources, Conservation and Recycling 2022, 179, 106109. 10.1016/j.resconrec.2021.106109. [DOI] [Google Scholar]
- Xiao L.; Feng L.; Yuan G.; Wei J. Low-Cost Field Production of Biochars and Their Properties. Environmental Geochemistry and Health 2020, 42 (6), 1569–1578. 10.1007/s10653-019-00458-5. [DOI] [PubMed] [Google Scholar]
- Maroušek J.; Vochozka M.; Plachý J.; Žák J. Glory and Misery of Biochar. Clean Technologies and Environmental Policy 2017, 19 (2), 311–317. 10.1007/s10098-016-1284-y. [DOI] [Google Scholar]
- Galinato S. P.; Yoder J. K.; Granatstein D. The Economic Value of Biochar in Crop Production and Carbon Sequestration. Energy Policy 2011, 39 (10), 6344–6350. 10.1016/j.enpol.2011.07.035. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.