Abstract
Background:
More than 8% of responders who participated in the search and rescue efforts at the World Trade Center (WTC) following 9/11 developed early-onset cognitive impairment (CI). Approximately 23% were also diagnosed with chronic post-traumatic stress disorder (PTSD).
Objective:
To shed light on the pathophysiology of these WTC-related conditions, we examined diffusion connectometry to identify altered white matter tracts in WTC responders with CI and/or PTSD compared to unaffected responders.
Methods:
99 WTC responders (mean age 56 years) consisting of CI-/PTSD- (n=27), CI+/PTSD- (n=25), CI-/PTSD+ (n=24), and CI+/PTSD+ (n=23) were matched on age, sex, occupation, race, and education. Cognitive status was determined using the Montreal Cognitive Assessment and PTSD status was determined using the DSM-IV SCID. Diffusion Tensor Imaging was acquired on a 3T Siemens Biograph mMR scanner. Connectometry analysis was used to examine whole-brain tract-level differences in white matter integrity as reflected by fractional anisotropy (FA) values after adjusting for confounders.
Results:
Analyses identified that FA was negatively correlated with CI and PTSD status in the fornix, cingulum, forceps minor of the corpus callosum and the right uncinate fasciculus. Furthermore, FA was negatively correlated with PTSD status, regardless of CI status in the superior thalamic radiation and the cerebellum.
Conclusions:
This is the first connectometry study to examine altered white matter tracts in a sample of WTC responders with CI and/or PTSD. Results from this study suggest that WTC responders with early-onset CI may be experiencing an early neurodegenerative process characterized by decreased FA in white matter tracts.
Keywords: White Matter Connectometry, Cognitive Impairment, Post-traumatic Stress Disorder, Alzheimer’s disease, Diffusion Tensor Imaging, World Trade Center Responders, Midlife
1. Introduction
In the aftermath of the attacks of 9/11/2001, the men and women who responded and worked in search, rescue, and clean-up efforts at the World Trade Center (WTC) and related sites experienced a host of physical and psychological exposures, that resulted in both severe and chronic posttraumatic stress disorder (PTSD) [1, 2], and higher than expected prevalence of early-onset cognitive impairment (CI) [3], among other conditions.
CI is a critical component of the prodromal phase of Alzheimer’s disease (AD) and related dementias (ADRD) [4, 5] in the presence of other signs of neuropathology [6]. The presence of neurodegeneration, particularly gray matter atrophy, is a hallmark of the pathological cascade in AD. Conceivably, one mechanism contributing to gray matter atrophy are changes occurring in white matter tracts linking cortical networks together [7]. In ADRD, white matter alterations have been linked to neural topography, deposition of β-amyloid [8, 9] as well as tau proliferation [10]. Recently, researchers have noted that interhemispheric connectivity in the posterior cortical regions is significantly worse in individuals with early-onset (aged 45–65) as compared to late-onset (aged 65 and older) ADRD [11]. Recent work has also suggested that early-onset ADRD is sometimes characterized by a parietal-dominant pattern of neurodegeneration [12] which tends to be more severe [13], and that diffusion tractography is highly sensitive to neurodegenerative diseases, noting associations between the location of cerebral β-amyloid deposition [14], tauopathy [15], and tau spreading via tracts in the white matter [16].
The larger than expected group of World Trade Center (WTC) responders, 20 years after exposure, are experiencing early-onset neurocognitive dysfunction at midlife, thus representing an emerging and concerning clinical condition within this population [17]. Investigations of brain structure among WTC responders with severe CI found reduced cortical thickness consistent with parietal-dominant ADRD [18] and hippocampal atrophy with focal reductions in the presubiculum [19]. Recognizing that many neurodegenerative conditions include widespread changes to white matter, we previously conducted a small pilot study with WTC responders with mild cognitive impairment (MCI) [n=20] and identified changes to white matter tracts consistent with both increased and decreased white matter integrity [20].
To date, no studies have examined white matter differences in WTC responders with/without CI and also those with/without concurrent PTSD. We thus used diffusion imaging to study this issue in our responder population. Traditional diffusion analyses often use either tract-based or region-based analysis to compare diffusion data, by mapping macroscopic end-to-end connections between segmented grey matter regional targets, which require a priori tracts for analysis. However, these structural connectomic approaches rely heavily on diffusion MRI tractography to reliably quantify global end-to-end connectivity, and past studies have raised concerns that these fiber tracking algorithms exhibit limited reliability at grey-to-white matter borders [21, 22]. We therefore employed local connectometry analysis instead, to avoid this limitation by limiting tractography to consecutive fiber segments that display significant associations with the study variable, rather than mapping the entire end-to-end connectome and then analyzing tracts defined from a priori hypotheses [23, 24].
We hypothesized that local connectometry analysis would identify white matter tracts with altered diffusivity in WTC-CI+ when compared to cognitively unimpaired control (CI-) responders. A secondary objective was to examine whether WTC-CI+ with concurrent PTSD differed in the extent and/or distribution of white matter integrity compared to CI+ alone, since PTSD has been shown to be a risk factor for cognitive dysfunction both in the WTC cohort [25–30] and other populations [31–36], along with disrupted white matter integrity in individuals with PTSD [37–40], we hypothesized an augmented reduction of white matter integrity in WTC CI+PTSD versus CI alone. The study of the above two hypotheses serves to further our understanding of the neurobiological changes occurring in CI and/or PTSD at midlife in two important ways, the extent of these changes in WTC responders with CI versus those without, and whether concurrent PTSD is augmenting these changes. Furthermore, this work is important as it also informs investigations of other exposed and/or traumatized populations and how those individuals, or any future affected populations, may experience such changes.
2. Methods
2.1. Population and Study Design
In 2002, the Centers for Disease Control and Prevention (CDC) began monitoring more than 50,000 WTC responders [41] using a comprehensive monitoring protocol that has been previously described [42]. Briefly, the Stony Brook University (SBU) program monitors law enforcement and non-law enforcement (e.g., construction, utilities, and volunteer) responders who mainly reside on Long Island, NY for a variety of health conditions, including cognitive status [3]. Participants recruited from a the SBU WTC monitoring program [42] were part of an epidemiologic study of cognitive aging involving serial administration of the Montreal Cognitive Assessment (MoCA) [28]. Responders were contacted if they had previously consented to being contacted to participate in research studies and met inclusion criteria (see below).
The study used a two-by-two matched case-control design involving CI (present/absent) and PTSD (present/absent). Inclusion criteria were ages 44 to 65, and fluency in English. Cognitive status was reconfirmed at the time of scanning. CI+ was defined as scoring less than ≤20 on the MoCA within three months prior to scanning. PTSD diagnosis was determined by the DSM-IV SCID, administered by trained clinical interviewers. To minimize the impact of confounding variables, the four groups (CI+/PTSD-, CI+/PTSD+, CI-/PTSD+, CI-/PTSD-) were matched on age, sex, race/ethnicity, occupation and education (see [18] for more details).
Exclusion criteria were history of psychosis; history of diagnosed neurological conditions including diagnosed ADRD, other dementias, major stroke, multiple sclerosis, and Parkinson’s disease; any head injury during their WTC efforts or a history of military head trauma including combustive blasts; current renal or liver disease; and current use of cognitively active medications. Subjects also satisfied eligibility criteria for MRI scanning including body mass index <40, absence of claustrophobia, no known pregnancy, and no known metal implants or shrapnel that were not deemed MRI- safe. The final study sample included 99 WTC responders, of whom completed the imaging protocols described in Section 2.2.
2.2. Image acquisition
Three-dimensional T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) images were acquired within three months of CI and/or PTSD diagnosis, using a 3T Siemens Biograph mMR (TR = 1900s, TE = 2.49 ms, TI = 900 ms; Flip Angle = 9°; acquisition matrix: 256 × 256; voxel resolution: 0.89 × 0.89 × 0.89 mm) at Icahn School of Medicine, Mount Sinai, NY. Scans were acquired between 2017 and 2019. For incidental pathology screening, T2-weighted anatomical scans used a turbo spin-echo pulse sequence (34 axial slices, TR = 6170s, TE = 96 ms; Flip angle = 150°; acquisition matrix = 320 × 320; voxel size = 0.36 × 0.36 × 3 mm) were acquired and read by a board-certified radiologist to determine incidental findings. Diffusion tensor imaging were also performed with the following parameters: TE/TR = 87.6/4680 ms, b value = 1200, 64 diffusion directions, in-plane resolution = 2 mm, slice thickness = 2 mm, matrix size = 128 × 128, multiband factor = 2. After data collection, post processing incorporated standard techniques for acquisition-based artifact elimination. Specifically, subject motion and eddy corrections are were accounted for by means of eddy from the FMRIB Software Library (FSL) [43]. Total imaging time for each WTC responder participant for this study, which included T1, T2, and DTI, was no more than 18 minutes. The T1 acquisition and the diffusion acquisition were approximately 6 minutes each.
2.3. Image processing
Diffusion images were visually inspected to check for major image artifacts or significant motion during the acquisition. All images passed inspection. No radiological abnormalities were identified in the images. The diffusion data were reconstructed in the Montreal Neurological Institute (MNI) space using q-space diffeomorphic reconstruction [44] to obtain the spin distribution function (SDF; diffusion sampling length ratio = 1.25) using DSI Studio (May 4 2019 build) [45]. The FA values were used in the connectometry analysis as they can represent axonal and myelination integrity, where lower values signify increased, unrestricted permeability (isotropic) due to axonal degradation and demyelination, whereas higher values denote restricted, anisotropic diffusion that can occur in health, myelinated axons [46–48].
2.4. Connectometry
Diffusion MRI connectometry analysis [49] (DSI Studio, build 20210813) was used to identify white matter tracts with altered diffusion. A t-score threshold was assigned to select local connectomes which were significantly associated with the study variable, i.e., cognitive and/or PTSD status, while using General Linear Modelling (GLM) analysis control for age, sex, and education. Local connectomes were tracked using a deterministic fiber tracking using the quantitative anisotropy algorithm based on Euler’s method, instead of traditional voxel-based index tracking methods, as these latter approaches have been shown to be less effective at selectively removing noisy fibers as they’re equally anisotropic within individual voxels. Instead, deterministic fiber tracking using quantitative anisotropy can lead to improved removal of noisy fibers and definition of terminal locations (see Yeh et al., 2013 for details [50]). In all analyses, three T thresholds (2, 2.5, 3) were used to ensure the stability of the findings. All tracts generated from bootstrap resampling were included and a length threshold distance of 20 voxels was used to select tracts. The seeding number for each permutation was 10,000. To estimate the false discovery rate (FDR), a total of 2,000 randomized permutations were applied to group labels to obtain the null distribution for tract length. . The generated tracts were then segmented by using “recognize and cluster” functionality in DSI studio followed by manual corrections. Isolated tracts were excluded from the segmentation.
2.5. Cognitive impairment
Participants were considered CI+ if they had evidence of cognitive impairment as measured by the Montreal Cognitive Assessment (MoCA) [51], a widely used measure of cognitive functioning developed to objectively and reliably identify age-related CI. Consistent with NIA diagnostic criteria for dementia [52]. CI+ was present when individuals had difficulties with behavioral functioning related to cognition and if they had neuropsychological dysfunction consistent with possible mild dementia using a conservative cutoff (MoCA ≤ 20) [53]. CI-responders had scores in the normal range (MoCA ≥ 26).
2.6. Posttraumatic stress disorder
PTSD diagnosis was assessed using the Structured Clinical Interview for the DSM-IV (SCID-IV) [54], a semi-structured interview schedule administered by trained clinical interviewers.
2.7. Demographics
Age in years, sex (male vs. female), and race (White, Black, and Hispanic) on September 11 (Law Enforcement vs Other) were included for matching purposes.
2.8. Statistical analyses
Descriptive characteristics for the present sample were provided using mean and standard deviations, or frequencies and percentages as noted. In this study, confounding from central variables including age was completed using matching in the study design phase. One-way analysis of variance and χ2 tabulations were used to examine differences in matching and diagnostic variables across diagnostic groups. A two-tailed α = 0.05 was used to determine nominal statistical significance and results from repeated testing analyses were adjusted to avoid Type I errors using the false discovery rate (FDR = 0.05) [55].
2.9. Ethics
The Institutional Review Boards at both Stony Brook University and the Icahn School of Medicine at Mount Sinai approved study procedures; participants provided informed written consent.
3. Results
3.1. Sample Characteristics
The descriptive characteristics of the sample (n = 99) are shown in Table 1. The study sample is all WTC responders. Groups did not differ on matching criteria, such as age, sex, or race; however, CI-/PTSD- group had significantly higher education (in years) than the CI+/PTSD- and CI+/PTSD- group, which was then adjusted for in subsequent analyses.
Table 1.
Sample Characteristics.
| Characteristic | CI−/PTSD− (n = 27) | CI+/PTSD− (n = 25) | CI−/PTSD+ (n = 24) | CI+/PTSD+ (n = 23) | F/χ 2 | P |
|---|---|---|---|---|---|---|
|
| ||||||
| Age | 57.07 (4.36) | 55.60 (6.24) | 54.58 (4.69) | 56.13 (5.45) | 1.007 | 0.393 |
| Sex | 0.933 | |||||
| Male | 77.78% | 76.00% | 83.33% | 78.26% | ||
| Female | 22.22% | 24.00% | 16.67% | 21.74% | ||
| Education (years) | 16.52 (1.87) | 14.84 (1.7) | 15.75 (2.61) | 14.78 (2.28) | 3.842 | 0.012 |
| Minority status | 10.312 | 0.112 | ||||
| Black | 11.11% | 12.00% | 0.00% | 17.39% | ||
| White | 81.48% | 68.00% | 95.83% | 60.87% | ||
| Other | 7.41% | 12.00% | 4.17% | 21.74% | ||
| Hispanic | 2.927 | 0.403 | ||||
| Yes | 11.11% | 20.00% | 4.17% | 13.04% | ||
| No | 88.89% | 80.00% | 95.83% | 86.96% | ||
| WTC exposure (months) | 3.42 (3.39) | 3.63 (3.61) | 4.11 (2.98) | 3.75 (3.44) | 0.19 | 0.903 |
Note: Means (standard deviations) or percentages (%) reported. P-values examine the extent to which noted characteristics differ across groups and were derived using χ2 tests for categorical variables, and one-way ANOVA for continuous variables. Abbreviations: WTC = World Trade Center; CI = Cognitive Impairment; PTSD = Posttraumatic stress disorder.
3.2. WTC Connectometry
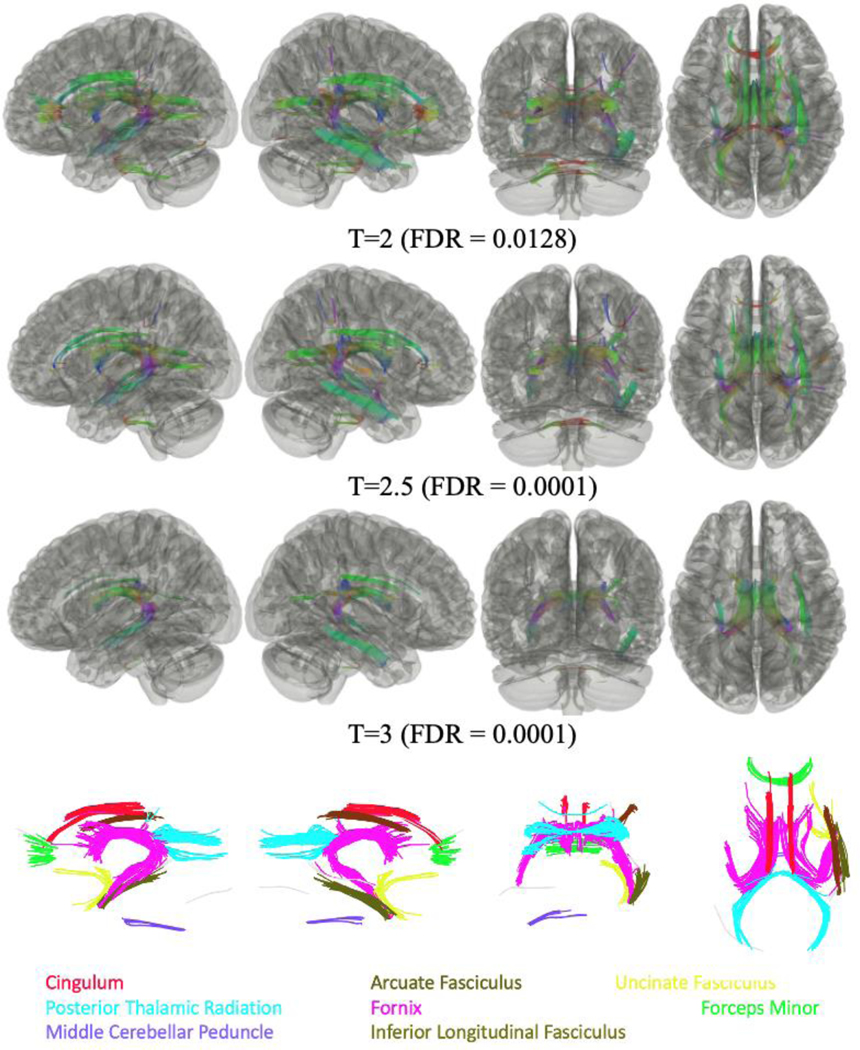
We conducted a series of four pairwise comparisons examining changes in tractography across the four groups of WTC responders in this sample using three T-thresholds (T=2, 2.5, 3) while adjusting for multiple comparisons using FDR. Non-significant findings were inferior longitudinal fasciculus omitted. As shown in Figure 2, analysis of all responders with CI+ when compared to all CI- responders, regardless of PTSD status, identified that FA was negatively associated with CI across several cortical tract bundles and subcortical tract bundles (FDR = 0.0128). No tracts were found to have significant FA values positively associated with CI status for all three T-thresholds.
Figure 2.
Connectometry comparisons between WTC responders with Cognitively Impairment (CI+) [n=48] and those who were Cognitively Unimpaired (CI-) [n=51] responders, regardless of PTSD status, using three T-thresholds (T=2, 2.5, 3), identified that FA values were lower in responders with CI+ compared to CI- responders. Significantly different tracts included the left and right fornix, the right posterior thalamic radiation, and the middle cerebellar peduncle for all three T thresholds; and the forceps minor, the left posterior thalamic radiation, the right arcuate fasciculus, the left and right cingulum, and the right uncinate fasciculus for T<=2.5.
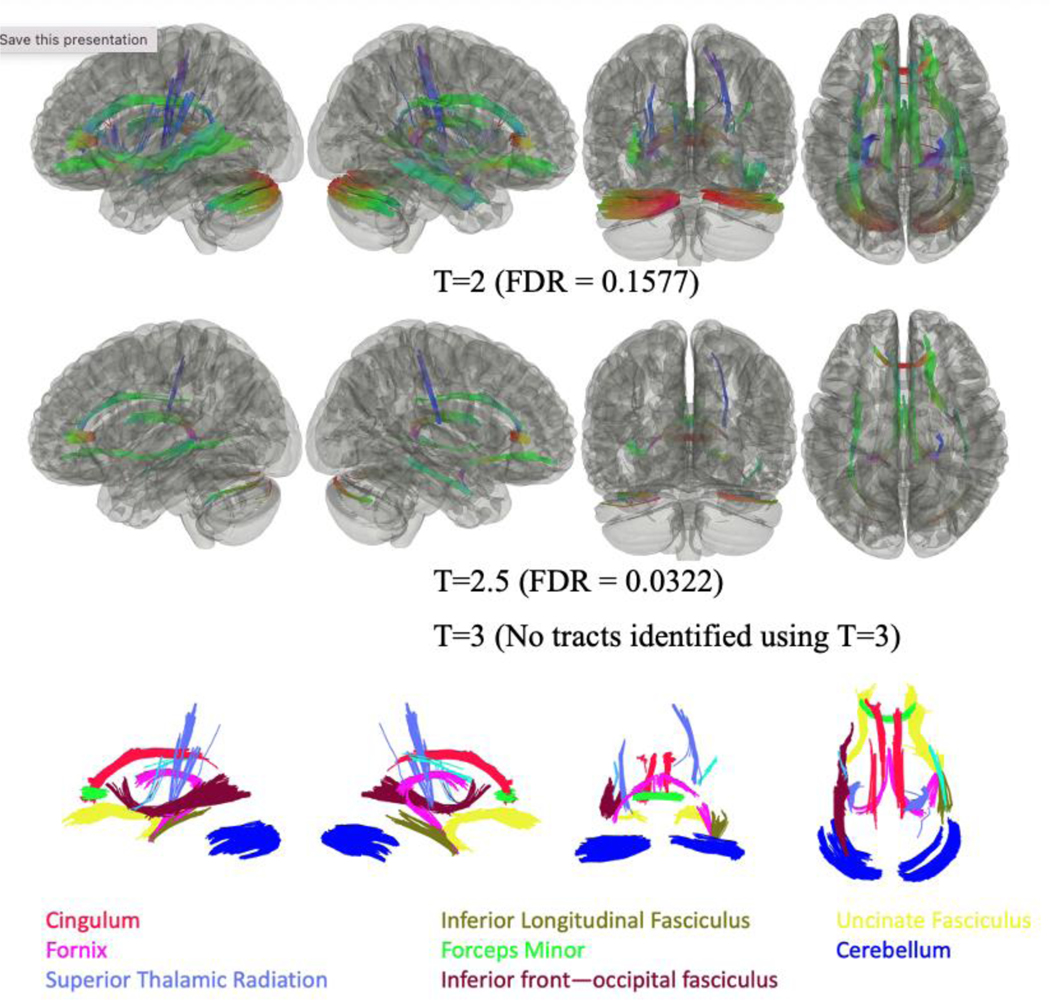
As shown in Figure 3, analysis of all responders with PTSD when compared to all responders without PTSD, regardless of cognitive status, identified that FA was negatively associated with PTSD across several cortical and subcortical tract bundles. No tracts were found to have significant FA values positively associated with PTSD status.
Figure 3.
Connectometry comparisons between WTC responders with PTSD+ (n=47) and PTSD- (n=52), regardless of cognitive status, using three T-thresholds (T=2, 2.5, 3) identified lower FA values in responders with PTSD when compared to responders without PTSD.
Significantly different tracts included the left and right fornix, the right uncinate fasciculus, the forceps minor, the left and right cingulum, the left and right cerebellum, the right superior thalamic radiation, and the right inferior longitudinal fasciculus for T<=2.5; and the left superior thalamic radiation, the left inferior fronto-occipital fasciculus, and the left uncinate fasciculus, for T=2.
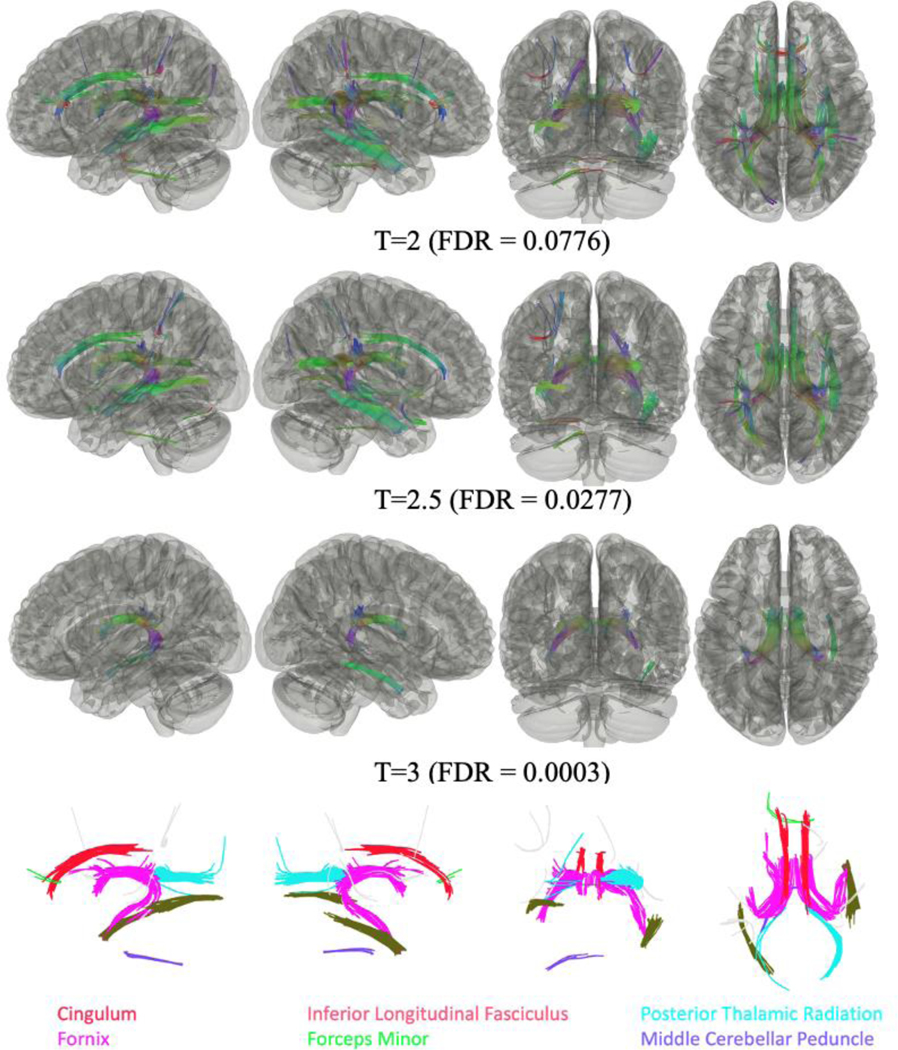
As shown in Figure 4, connectometry comparisons between CI+/PTSD- (n=25) compared to CI-/PTSD- responders (n=27) identified that FA was negatively associated in some cortical and subcortical tracts in the former group. No tracts were found to have significant FA values positively associated with CI status for all three T thresholds.
Figure 4.
Connectometry comparisons between WTC responders with Cognitive Impairment but no PTSD (CI+/PTSD-) [n=25] and no cognitive impairment or PTSD (CI-/PTSD-) [n=27] using three T-thresholds (T=2, 2.5, 3), identified that FA values were negatively associated with CI.
Significantly different tracts included the left and right fornix, the right cingulum, and the right inferior longitudinal fasciculus for all three thresholds; and the left cingulum, the left cerebellum, the left inferior longitudinal fasciculus, the right posterior thalamic radiation, and the middle cerebellar peduncle for T≤2.5; with the forceps minor, and the left posterior thalamic radiation for T=2.
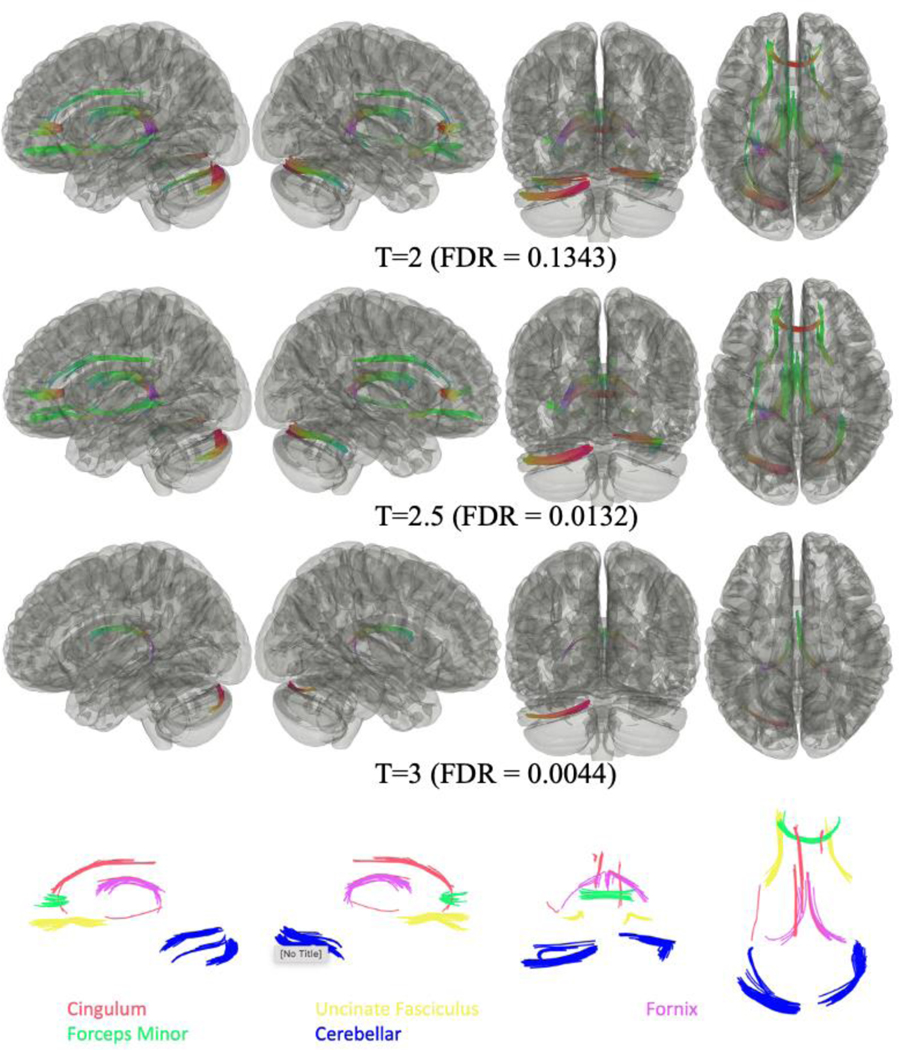
As shown in Figure 5, subgroup analysis between CI+/PTSD+ (n=23) and CI+/PTSD- (n=25) WTC responders, found negative associations of FA in some cortical and subcortical tracts in responders with CI+/PTSD+. No tracts were found to have FA values positively significantly associated with PTSD diagnosis for all three T thresholds between the two groups.
Figure 5.
Connectometry analysis of between WTC responders with Cognitive Impairment but no PTSD (CI+/PTSD-) [n=25] and those with both Cognitive Impairment and PTSD (CI+/PTSD+) [n=23], suggested that FA negatively correlated with responders with both CI+ and PTSD+ status. Significantly different tracts included the left and right fornix and the left cerebellum for all three thresholds: and the forceps minor, the right cerebellum, the left and right uncinate fasciculus and the left cingulum for T≤2.5. x
An ROI summary table of results for the four findings above identifying associations with reduced white matter FA is shown in Table 2.
Table 2:
Summary table of results showing regions of interest where white matter FA displayed significant negative associations with: cognitive impairment (CI+), regardless of posttraumatic stress disorder (PTSD) status; PTSD+, regardless of CI status; CI+/PTSD− versus CI−/PTSD−; CI+/PTSD+ versus CI+/PTSD−.
| Region of interest | CI+ (n = 48) | PTSD+ (n = 47) | CI+/PTSD− (n = 25) | CI+/PTSD+ (n = 23) |
|---|---|---|---|---|
|
|
|
|||
| L Fornix | xxx | xx | xxx | xxx |
| R Fornix | xxx | xx | xxx | xxx |
| L Posterior Thalamic Radiation | xx | x | ||
| R Posterior Thalamic Radiation | xxx | xx | ||
| L Superior Thalamic Radiation | x | |||
| R Superior Thalamic Radiation | xx | |||
| L Inferior Longitudinal Fasciculus | xx | |||
| R Inferior Longitudinal Fasciculus | xx | xxx | ||
| Forceps Minor | xx | xx | x | xx |
| R Arcuate Fasciculus | xx | |||
| Middle Cerebellar Peduncle | xxx | xx | ||
| L Cingulum | xx | xx | xx | xx |
| R Cingulum | xx | xx | xxx | |
| L Uncinate Fasciculus | x | xx | ||
| R Uncinate Fasciculus | xx | xx | xx | |
| L Cerebellum | xx | xx | xxx | |
| R Cerebellum | xx | xx | ||
| L Inferior Fronto-Occipital Fasciculus | x | |||
Note: x: T=2; xx: T=2.5; xxx: T=3; L: Left; R: Right.
4. Discussion
This is the first study to examine white matter alterations using connectometry analysis in a sample of WTC responders at midlife with and without CI as well as with and without concurrent PTSD. The goal of this study was to examine and elucidate the extent to which white matter tract integrity might be impaired in WTC responders with CI and/or PTSD. We previously reported changes in white matter diffusivity in a sample of 20 WTC responders with MCI, using diffusion spectrum imaging (DSI) measures in regions characteristic of early AD [20]. Results from the present study extend our previous finding of white matter changes in WTC responders with MCI, as we now identified bundles of decreased white matter integrity in responders with CI and PTSD, or both. Specifically, we hypothesized that local connectometry analysis would identify white matter tracts with altered diffusivity in WTC-CI+ when compared to cognitively unimpaired control (CI-) responders, and our results identified reduced white matter integrity in responders with CI+ across occipitotemporal (inferior and longitudinal fasciculus, thalamocortical (posterior and superior thalamic radiations), frontal lobe (forceps minor), and cerebellar tracts (middle cerebellar peduncle. A secondary objective was to examine whether WTC-CI+ with concurrent PTSD differed in the extent and/or distribution of white matter integrity compared to CI+ alone, and our results identified decreased connectivity in limbic regions in responders with WTC-CI+ and concurrent PTSD.
White Matter Integrity in WTC responders with cognitive impairment and PTSD.
Our most significant finding might be that white matter tracts identified here might suggest that the fornix, in the forceps minor of the corpus callosum, and the cingulum bundle may act as a convergent mechanism to link conditions affecting discussed here. The fornix, whose development peaks during late adolescence and serves to connect the hippocampus to other limbic structures, has been implicated in episodic memory recall and processing speed, where damage to this area and functions have been implicated in AD and can serve as a marker for neurodegeneration [56–61]. The forceps minor is situated in the anterior portion and serves to connect regions of the frontal cortices [62], while the inferior segment of the cingulum is an intersectional region connecting the hippocampus and parahippocampal gyrus [63], whose function serves as a compound measure of cognitive skills such as reasoning, problem solving and behavioral flexibility [64]. It has been previously demonstrated that FA in the cingulum is reduced in MCI and AD [65–69], whereby microstructural changes in this region have been shown to be predictive of decline in cognitive controls [70]. Lesions to the cingulum have also been associated with transient confusion, disorientation, disrupted verbal working memory, and memory loss [63, 71]. However, changes in this region are also implicated in PTSD (for review, see [63]). Considering that PTSD is a risk factor for CI [26, 28–30, 33–36], this result suggests that the fornix, forceps minor and cingulum bundle may serve as a shared neural correlate that is affected by either CI alone, PTSD alone, or both comorbidly. Future studies with affected WTC responders or similar populations may benefit from monitoring changes in the fornix.
Reduced FA was also identified in the right arcuate fasciculus of responders with CI, regardless of PTSD status, but not in our subgroup analyses. The arcuate fasciculus is a large axonal tract connecting the temporal and inferior parietal cortices to the inferior frontal cortex, bridging key language regions such as Broca’s and Wernicke’s areas serves as an important neural correlate implicated in language disruption and aphasia [72]. White matter disruptions and demyelination in the arcuate fasciculus have been previously demonstrated in MCI and AD [73], including conditions with are high CI risk such as schizophrenia [74] and traumatic brain injury [75]. Interestingly, we did not observe reduced FA in the right arcuate fasciculus in our subgroup analyses, such as responders with CI+/PTSD- or responders with CI+/PTSD+, suggesting a statistical power limitation that was only surpassed when we grouped them together. Therefore, future studies with sufficient sample sizes should monitor this white matter tract to establish whether PTSD contributes to our present observation, as prior studies with PTSD populations have implicated white matter abnormalities within the arcuate fasciculus [76–78].
Similarly, we identified reduced FA in the uncinate fasciculus in responders with both CI+ and PTSD+. The uncinate fasciculus is a tract that connects the limbic and temporal lobes to the inferior frontal lobe and has been implicated in several psychiatric disorders and frontotemporal dementias, where it is in involved in higher memory associations, such as assigning a name and voice with a face in addition to being involved in reward behaviors (for review, see [79]).
Reduced FA was identified in the left and right inferior longitudinal fasciculus in both responders with PTSD alone but also with CI+/PTSD-. The inferior longitudinal fasciculus is a white matter tract that connects the occipital to the temporal lobes, whereby atrophy has been associated with visuospatial, semantic, object recognition, face processing and language dysfunction in dementia populations [80–84] and has been reported to be disrupted in AD, semantic dementia, and Lewy-body dementia [85–88]. Considering the extensive cortical connective bundles that this tract serves in the brain, this result can be considered as a widespread disruption in white matter connectivity presenting in responders that is shared with both those who present with CI alone and PTSD alone. Interestngly, our responder group with both CI+/PTSD+ did not show that FA was significantly associated with the comorbid condition, suggesting that that smaller sample size of this group may have not been sufficiently large to power this result. Nevertheless, this observation was absent in responders with CI+, regardless of PTSD status, which confounds the above interpration and requires future interrogation to clarify whether reduced FA is associated by CI, PTSD, or both.
Lastly, we identified reduced FA in the middle cerebellar peduncle, which is a tract that connects the pons to the cerebellum, conveying information to the cerebrum. This tract has been reported to be implicated demyelinating diseases such as multiple sclerosis, vascular and toxic encephalopathies, and other neurodegenerative disorders [89]. In addition, we also identified reduced FA in the left and right cerebellum of responders with PTSD+, regardless of CI status, and also in our subgroup analyses of both CI+/PTSD- and CI+/PTSD+. However, and rather perplexingly, we did not observe reduced white matter FA in the group of responders with CI+, regardless of PTSD status, as we have previously reported reduced cerebellar thickness in responders with CI+, regardless of PTSD status [90]. This discrepancy may be due to the pooling of responders with/without PTSD, because as previously mentioned, we observe reduced FA in the cerebellum in our subgroup analyses. Nevertheless, the cerebellum, which is a critical structure for cognition, emotion, and coordination, has long been associated with alterations in trauma-exposed populations (for review, see [91]), but has largely been absent in dementia conditions [92]. However, taken together with our prior findings, we definitively identify that both cerebellum grey and white matter are affected in WTC responders with CI with/without PTSD.
White Matter Integrity in WTC responders with PTSD, regardless of CI status.
Here, we focus our discussion on results that demonstrated reduced FA in responders with PTSD with a T value of 2.5, regardless of CI status. We identified reduced FA in the superior thalamic radiation, where thalamocortical radiations are the primary relay tracts of the brain that connect the thalamus to the cerebral cortex, relaying sensorimotor activity throughout the cortex and back. Specifically, the superior thalamic radiations bridge the ventral nuclear group of the thalamus to the precentral and postcentral gyri [93]. While the thalamic radiations serve multiple functions, reductions in white matter integrity in the superior portion have been associated with reduced global cognition and processing speed [94, 95]. This result is in line with a prior study examining cortical thickness in WTC responders with CI [18], who show reduced cortical thickness in both the precentral and postcentral gyri, suggesting a possible mechanistic explanation to how reduced white matter integrity in the connecting superior thalamic radiation may have lead to reduced thickness in these cortical regions.
Taken together, we identified a large array of ROIs that display reduced white matter integrity in responders with CI and/or PTSD in a topographical manner that overlap with a variety of dementia-like conditions rather than a single neurological condition per se. While challenging to amalgamate and generalize these results, nevertheless, identifying these tracts is an important step toward better characterizing the etiology of the emerging neurological changes in affected WTC responders at midlife. As research efforts continue to identify the etiology for this emerging WTC neurological condition in efforts to determine which dementia subgroup these responders may present as, or if it is a unique WTC condition, it is important that we continue to interrogate underlying neurophysiological correlates. Results from the present study suggest a more widespread pattern of reduced white matter integrity, which does not necessarily ascribe to a single known neurological disorder, such as AD. Nevertheless, WTC responders with CI and/or PTSD, now at midlife, continue to demonstrate cognitive impairment and PTSD are coupled with neuropathological phenomena, as shown in prior research and this study. Ongoing research with cognitively impaired and PTSD endorsing WTC responders will be paramount to uncovering and elucidating the neural correlates for these observations.
5. Limitations
This is the first DTI study of WTC responders with CI and/or PTSD nested in a prospective cohort study of individuals at midlife. While being novel in several ways, this study also has several limitations including the small sample size and the lack of an external control group. Additionally, despite over-sampling minorities and women, the study’s sample size resulted in subgroup samples that were too small to examine separately. Additionally, this study relied on DTI imaging though studies are increasingly using DSI because of its improved capabilities to track crossing fibers and map axonal trajectories via the use of probabilistic density functions instead of single tensor analysis [96, 97]. Another limitation is that data in this study were cross-sectional, hence we cannot rule out whether the observed results emerged with aging and exposure to the WTC disaster, or were present and unchanged for the two decades prior. Finally, though meeting the criteria commonly used to diagnose dementia, the lack of certainty regarding the etiology of CI in this population has caused us to be conservative with nomenclature utilized in all of our studies.
6. Conclusions
This study examined, for the first time, white matter integrity in WTC responders with CI and/or PTSD at midlife. Results are supportive of reduced white matter integrity in responders with CI+ across occipitotemporal (inferior and longitudinal fasciculus, thalamocortical (posterior and superior thalamic radiations), frontal lobe (forceps minor), and cerebellar tracts (middle cerebellar peduncle), possibly accounting for neuropathology arising in certain regions. We also identified decreased connectivity in limbic regions in responders with PTSD, suggesting that mechanisms of depotentiated synaptic plasticity may be at work, due to the debilitating nature of chronic PTSD and CI. To date, it remains unclear how WTC exposures might have resulted in changes to white matter integrity. These results support ongoing work suggesting that WTC responders with CI and/or PTSD are experiencing neurological changes, perhaps with the involvement of neuroinflammation as the etiological substrate. This warrants future investigations of neuroinflammation, such as free water DTI techniques, as WTC responders are aging and the risk of CI increases substantially. Our results herein and in future studies, might therefore serve to inform policymakers and to direct clinical intervention strategies for WTC responders and for other trauma-exposed populations.
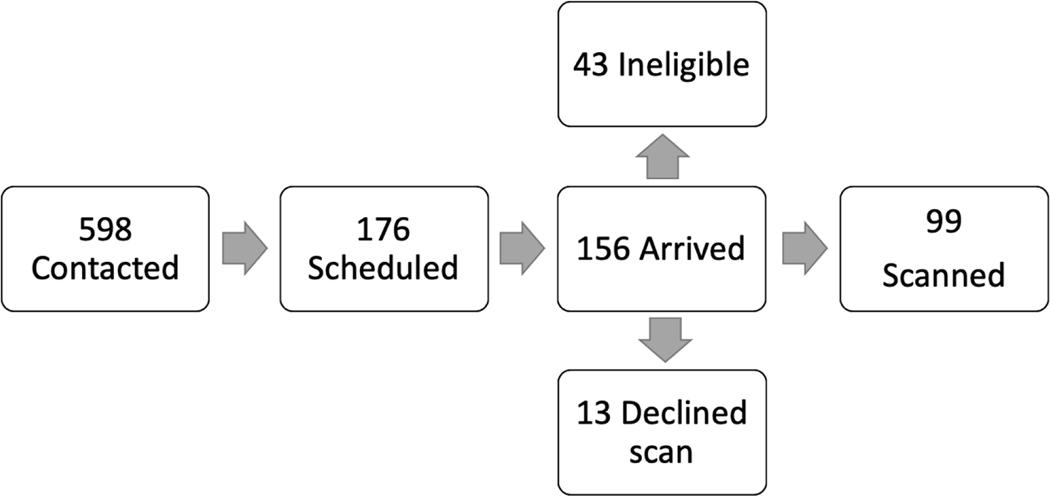
Figure 1.
Flow Chart illustrating subject enrollment into study.
7. Acknowledgements:
This work was supported by the National Institutes of Health (NIH/NIA R01 AG049953) and the Centers for Disease Control and Prevention (U01 OH011314; CDC/NIOSH 200-2011-39361).
Footnotes
The authors have no conflict of interest to report.
8. References
- [1].Galea S, Ahern J, Resnick H, Kilpatrick D, Bucuvalas M, Gold J, Vlahov D (2002) Psychological sequelae of the September 11 terrorist attacks in New York City. N Engl J Med 346, 982–987. [DOI] [PubMed] [Google Scholar]
- [2].Bromet EJ, Hobbs MJ, Clouston SA, Gonzalez A, Kotov R, Luft BJ (2016) DSM-IV post-traumatic stress disorder among World Trade Center responders 11–13 years after the disaster of 11 September 2001 (9/11). Psychol Med 46, 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Clouston SAP, Diminich ED, Kotov R, Pietrzak RH, Richards M, Spiro A 3rd, Deri Y, Carr M, Yang X, Gandy S, Sano M, Bromet EJ, Luft BJ(2019) Incidence of mild cognitive impairment in World Trade Center responders: Long-term consequences of re-experiencing the events on 9/11/2001. Alzheimers Dement (Amst) 11, 628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Backman L, Small BJ, Fratiglioni L (2001) Stability of the preclinical episodic memory deficit in Alzheimer’s disease. Brain 124, 96–102. [DOI] [PubMed] [Google Scholar]
- [5].Rodrigue KM, Kennedy KM, Devous MD Sr., Rieck JR, Hebrank AC, Diaz-Arrastia R, Mathews D, Park DC(2012) beta-Amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology 78, 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe, Scheltens P, Siemers E, Snyder HM, Sperling R, Contributors (2018) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14, 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Menon V (2011) Large-scale brain networks and psychopathology: a unifying triple network model. Trends in cognitive sciences 15, 483–506. [DOI] [PubMed] [Google Scholar]
- [8].Pandya S, Kuceyeski A, Raj A (2017) The Brain’s Structural Connectome Mediates the Relationship between Regional Neuroimaging Biomarkers in Alzheimer’s Disease. Journal of Alzheimer’s Disease 55, 1639–1657. [DOI] [PubMed] [Google Scholar]
- [9].Prescott JW, Guidon A, Doraiswamy PM, Roy Choudhury K, Liu C, Petrella JR (2014) The Alzheimer Structural Connectome: Changes in Cortical Network Topology with Increased Amyloid Plaque Burden. Radiology 273, 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yang F, Chowdhury SR, Jacobs HI, Johnson KA, Dutta J (2019) in International Conference on Information Processing in Medical Imaging Springer, pp. 384–393. [Google Scholar]
- [11].Li K-C, Luo X, Zeng Q-Z, Xu X-J, Huang P-Y, Shen Z-J, Xu J-J, Zhou J, Zhang M-M (2018) Distinct Patterns of Interhemispheric Connectivity in Patients With Early- and Late-Onset Alzheimer’s Disease. Frontiers in Aging Neuroscience 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Noh Y, Jeon S, Lee JM, Seo SW, Kim GH, Cho H, Ye BS, Yoon CW, Kim HJ, Chin J (2014) Anatomical heterogeneity of Alzheimer disease: based on cortical thickness on MRIs. Neurology 83, 1936–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Na HK, Kang DR, Kim S, Seo SW, Heilman KM, Noh Y, Na DL (2016) Malignant progression in parietal-dominant atrophy subtype of Alzheimer’s disease occurs independent of onset age. Neurobiol Aging 47, 149–156. [DOI] [PubMed] [Google Scholar]
- [14].Prescott JW, Guidon A, Doraiswamy PM, Roy Choudhury K, Liu C, Petrella JR, Initiative AsDN (2014) The Alzheimer structural connectome: changes in cortical network topology with increased amyloid plaque burden. Radiology 273, 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Strain JF, Smith RX, Beaumont H, Roe CM, Gordon BA, Mishra S, Adeyemo B, Christensen JJ, Su Y, Morris JC (2018) Loss of white matter integrity reflects tau accumulation in Alzheimer disease defined regions. Neurology 91, e313–e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vogel JW, Iturria-Medina Y, Strandberg OT, Smith R, Levitis E, Evans AC, Hansson O (2020) Spread of pathological tau proteins through communicating neurons in human Alzheimer’s disease. Nature Communications 11, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Clouston SAP, Hall CB, Kritikos M, Bennett DA, DeKosky S, Edwards J, Finch C, Kreisl WC, Mielke M, Peskind ER, Raskind M, Richards M, Sloan RP, Spiro A, Vasdev N, Brackbill R, Farfel M, Horton M, Lowe S, Lucchini RG, Prezant D, Reibman J, Rosen R, Seil K, Zeig-Owens R, Deri Y, Diminich ED, Fausto BA, Gandy S, Sano M, Bromet EJ, Luft BJ (2021) Cognitive impairment and World Trade Centre-related exposures. Nature Reviews Neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Clouston SAP, Deri Y, Horton M, Tang C, Diminich E, DeLorenzo C, Kritikos M, Pellecchia AC, Santiago-Michels S, Carr MA, Gandy S, Sano M, Bromet EJ, Lucchini RG, Luft BJ (2020) Reduced cortical thickness in World Trade Center responders with cognitive impairment. Alzheimers Dement (Amst) 12, e12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Deri Y, Clouston S, DeLorenzo C, Gardus III JD, Horton M, Tang C, Pellecchia AC, Santiago-Michels S, Carr MA, Gandy S, Sano M, Bromet EJ, Lucchini RG, Luft BJ (2021) Selective hippocampal subfield volume reductions in World Trade Center responders with cognitive impairment. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring 13, e12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Huang C, Kritikos M, Clouston SA, Deri Y, Serrano-Sosa M, Bangiyev L, Santiago-Michels S, Gandy S, Sano M, Bromet EJ (2021) White Matter Connectivity in Incident Mild Cognitive Impairment: A Diffusion Spectrum Imaging Study of World Trade Center Responders at Midlife. Journal of Alzheimer’s Disease, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Thomas C, Ye FQ, Irfanoglu MO, Modi P, Saleem KS, Leopold DA, Pierpaoli C (2014) Anatomical accuracy of brain connections derived from diffusion MRI tractography is inherently limited. Proc Natl Acad Sci U S A 111, 16574–16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Reveley C, Seth AK, Pierpaoli C, Silva AC, Yu D, Saunders RC, Leopold DA, Ye FQ (2015) Superficial white matter fiber systems impede detection of long-range cortical connections in diffusion MR tractography. Proc Natl Acad Sci U S A 112, E2820–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yeh FC, Badre D, Verstynen T (2016) Connectometry: A statistical approach harnessing the analytical potential of the local connectome. Neuroimage 125, 162–171. [DOI] [PubMed] [Google Scholar]
- [24].Yeh FC, Tang PF, Tseng WY (2013) Diffusion MRI connectometry automatically reveals affected fiber pathways in individuals with chronic stroke. Neuroimage Clin 2, 912–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Clouston SA, Diminich ED, Kotov R, Pietrzak RH, Richards M, Spiro A III, Deri Y, Carr M, Yang X, Gandy S (2019) Incidence of mild cognitive impairment in World Trade Center responders: long-term consequences of re-experiencing the events on 9/11/2001. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring 11, 628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Clouston SAP, Guralnik JM, Kotov R, Bromet EJ, Luft BJ (2017) Functional Limitations Among Responders to the World Trade Center Attacks 14 Years After the Disaster: Implications of Chronic Posttraumatic Stress Disorder. J Trauma Stress 30, 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mukherjee S, Clouston S, Kotov R, Bromet E, Luft B (2019) Handgrip Strength of World Trade Center (WTC) Responders: The Role of Re-Experiencing Posttraumatic Stress Disorder (PTSD) Symptoms. Int J Environ Res Public Health 16, 1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Clouston SA, Kotov R, Pietrzak RH, Luft BJ, Gonzalez A, Richards M, Ruggero CJ, Spiro A 3rd, Bromet EJ (2016) Cognitive impairment among World Trade Center responders: Long-term implications of re-experiencing the 9/11 terrorist attacks. Alzheimers Dement (Amst) 4, 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Clouston S, Pietrzak RH, Kotov R, Richards M, Spiro A 3rd, Scott S, Deri Y, Mukherjee S, Stewart C, Bromet E, Luft BJ(2017) Traumatic exposures, posttraumatic stress disorder, and cognitive functioning in World Trade Center responders. Alzheimers Dement (N Y) 3, 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Clouston S, Deri Y, Diminich E, Kew RR, Kotov R, Stewart C, Yang X, Gandy S, Sano M, Bromet E, Luft B (2019) Posttraumatic stress disorder associated with total amyloid burden and amyloid-ß 42/40 ratios in plasma: Results from a pilot study of World Trade Center responders. Alzheimer’s & Dementia 11, 216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Schuitevoerder S, Rosen JW, Twamley EW, Ayers CR, Sones H, Lohr JB, Goetter EM, Fonzo GA, Holloway KJ, Thorp SR (2013) A meta-analysis of cognitive functioning in older adults with PTSD. J Anxiety Disord 27, 550–558. [DOI] [PubMed] [Google Scholar]
- [32].Greenberg MS, Tanev K, Marin MF, Pitman RK (2014) Stress, PTSD, and dementia. Alzheimers Dement 10, S155–165. [DOI] [PubMed] [Google Scholar]
- [33].Dossi G, Delvecchio G, Prunas C, Soares JC, Brambilla P (2020) Neural Bases of Cognitive Impairments in Post-Traumatic Stress Disorders: A Mini-Review of Functional Magnetic Resonance Imaging Findings. Front Psychiatry 11, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hayes JP, Vanelzakker MB, Shin LM (2012) Emotion and cognition interactions in PTSD: a review of neurocognitive and neuroimaging studies. Front Integr Neurosci 6, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sullivan DR, Marx B, Chen MS, Depue BE, Hayes SM, Hayes JP (2019) Behavioral and neural correlates of memory suppression in PTSD. J Psychiatr Res 112, 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gunak MM, Billings J, Carratu E, Marchant NL, Favarato G, Orgeta V (2020) Post-traumatic stress disorder as a risk factor for dementia: systematic review and meta-analysis. Br J Psychiatry 217, 600–608. [DOI] [PubMed] [Google Scholar]
- [37].Olson EA, Cui J, Fukunaga R, Nickerson LD, Rauch SL, Rosso IM (2017) Disruption of white matter structural integrity and connectivity in posttraumatic stress disorder: A TBSS and tractography study. Depress Anxiety 34, 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Suo X, Lei D, Li W, Chen F, Niu R, Kuang W, Huang X, Lui S, Li L, Sweeney JA, Gong Q (2019) Large-scale white matter network reorganization in posttraumatic stress disorder. Hum Brain Mapp 40, 4801–4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].McCunn P, Richardson JD, Jetly R, Dunkley B (2021) Diffusion Tensor Imaging Reveals White Matter Differences in Military Personnel Exposed to Trauma with and without Post-traumatic Stress Disorder. Psychiatry Res 298, 113797. [DOI] [PubMed] [Google Scholar]
- [40].Siehl S, Wicking M, Pohlack S, Winkelmann T, Zidda F, Steiger-White F, King J, Burgess N, Flor H, Nees F (2020) Structural white and gray matter differences in a large sample of patients with Posttraumatic Stress Disorder and a healthy and trauma-exposed control group: Diffusion tensor imaging and region-based morphometry. Neuroimage Clin 28, 102424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Centers for Disease Control and Prevention, World Trade Center Health Program At A Glance,Centers for Disease Control and Prevention, https://www.cdc.gov/wtc/ataglance.html, Accessed March 22 2017. [Google Scholar]
- [42].Dasaro CR, Holden WL, Berman KD, Crane MA, Kaplan JR, Lucchini RG, Luft BJ, Moline JM, Teitelbaum SL, Tirunagari US, Udasin IG, Weiner JH, Zigrossi PA, Todd AC (2017) Cohort Profile: World Trade Center Health Program General Responder Cohort. Int J Epidemiol 46, e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012) Fsl. Neuroimage 62, 782–790. [DOI] [PubMed] [Google Scholar]
- [44].Yeh F-C, Tseng W-YI (2011) NTU-90: a high angular resolution brain atlas constructed by q-space diffeomorphic reconstruction. Neuroimage 58, 91–99. [DOI] [PubMed] [Google Scholar]
- [45].Yeh F-C, Wedeen VJ, Tseng W-YI (2010) Generalized ${q} $-sampling imaging. IEEE transactions on medical imaging 29, 1626–1635. [DOI] [PubMed] [Google Scholar]
- [46].O’Donnell LJ, Westin CF (2011) An introduction to diffusion tensor image analysis. Neurosurg Clin N Am 22, 185–196, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Soares JM, Marques P, Alves V, Sousa N (2013) A hitchhiker’s guide to diffusion tensor imaging. Front Neurosci 7, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Alba-Ferrara LM, de Erausquin GA (2013) What does anisotropy measure? Insights from increased and decreased anisotropy in selective fiber tracts in schizophrenia. Front Integr Neurosci 7, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yeh F-C, Badre D, Verstynen T (2016) Connectometry: a statistical approach harnessing the analytical potential of the local connectome. Neuroimage 125, 162–171. [DOI] [PubMed] [Google Scholar]
- [50].Yeh F-C, Verstynen TD, Wang Y, Fernández-Miranda JC, Tseng W-YI (2013) Deterministic diffusion fiber tracking improved by quantitative anisotropy. PloS one 8, e80713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society 53, 695–699. [DOI] [PubMed] [Google Scholar]
- [52].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R (2011) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia 7, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Freitas S, Simoes MR, Alves L, Santana I (2013) Montreal cognitive assessment: validation study for mild cognitive impairment and Alzheimer disease. Alzheimer Dis Assoc Disord 27, 37–43. [DOI] [PubMed] [Google Scholar]
- [54].First MB (2015) Structured Clinical Interview for theDSM(SCID) In The Encyclopedia of Clinical Psychology, pp. 1–6. [Google Scholar]
- [55].Benjamini Y, Hochberg Y (1995) Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B-Methodological 57, 289–300. [Google Scholar]
- [56].Fletcher E, Carmichael O, Pasternak O, Maier-Hein KH, DeCarli C (2014) Early Brain Loss in Circuits Affected by Alzheimer’s Disease is Predicted by Fornix Microstructure but may be Independent of Gray Matter. Front Aging Neurosci 6, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Douet V, Chang L (2014) Fornix as an imaging marker for episodic memory deficits in healthy aging and in various neurological disorders. Front Aging Neurosci 6, 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kantarci K (2014) Fractional anisotropy of the fornix and hippocampal atrophy in Alzheimer’s disease. Front Aging Neurosci 6, 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tsivilis D, Vann SD, Denby C, Roberts N, Mayes AR, Montaldi D, Aggleton JP (2008) A disproportionate role for the fornix and mammillary bodies in recall versus recognition memory. Nat Neurosci 11, 834–842. [DOI] [PubMed] [Google Scholar]
- [60].Hopper MW, Vogel FS (1976) The limbic system in Alzheimer’s disease. A neuropathologic investigation. Am J Pathol 85, 1–20. [PMC free article] [PubMed] [Google Scholar]
- [61].Alexander RP, Concha L, Snyder TJ, Beaulieu C, Gross DW (2014) Correlations between Limbic White Matter and Cognitive Function in Temporal-Lobe Epilepsy, Preliminary Findings. Front Aging Neurosci 6, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Goldstein A, Covington BP, Mahabadi N, Mesfin FB (2022) Neuroanatomy, Corpus Callosum In StatPearls StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC., Treasure Island (FL). [PubMed] [Google Scholar]
- [63].Bubb EJ, Metzler-Baddeley C, Aggleton JP (2018) The cingulum bundle: Anatomy, function, and dysfunction. Neurosci Biobehav Rev 92, 104–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Miller EK (2000) The prefrontal cortex and cognitive control. Nat Rev Neurosci 1, 59–65. [DOI] [PubMed] [Google Scholar]
- [65].Wu Y, Sun D, Wang Y, Wang Y, Ou S (2016) Segmentation of the Cingulum Bundle in the Human Brain: A New Perspective Based on DSI Tractography and Fiber Dissection Study. Front Neuroanat 10, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wisse LE, Reijmer YD, ter Telgte A, Kuijf HJ, Leemans A, Luijten PR, Koek HL, Geerlings MI, Biessels GJ, Utrecht Vascular Cognitive Impairment Study G (2015) Hippocampal disconnection in early Alzheimer’s disease: a 7 tesla MRI study. J Alzheimers Dis 45, 1247–1256. [DOI] [PubMed] [Google Scholar]
- [67].Dalboni da Rocha JL, Bramati I, Coutinho G, Tovar Moll F, Sitaram R (2020) Fractional Anisotropy changes in Parahippocampal Cingulum due to Alzheimer’s Disease. Sci Rep 10, 2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Gozdas E, Fingerhut H, Chromik LC, O’Hara R, Reiss AL, Hosseini SMH (2020) Focal white matter disruptions along the cingulum tract explain cognitive decline in amnestic mild cognitive impairment (aMCI). Sci Rep 10, 10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Luo C, Li M, Qin R, Chen H, Yang D, Huang L, Liu R, Xu Y, Bai F, Zhao H (2020) White Matter Microstructural Damage as an Early Sign of Subjective Cognitive Decline. Frontiers in Aging Neuroscience 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Metzler-Baddeley C, Jones DK, Steventon J, Westacott L, Aggleton JP, O’Sullivan MJ (2012) Cingulum microstructure predicts cognitive control in older age and mild cognitive impairment. J Neurosci 32, 17612–17619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Dougherty DD, Weiss AP, Cosgrove GR, Alpert NM, Cassem EH, Nierenberg AA, Price BH, Mayberg HS, Fischman AJ, Rauch SL (2003) Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for treatment of major depression. J Neurosurg 99, 1010–1017. [DOI] [PubMed] [Google Scholar]
- [72].Catani M, Mesulam M (2008) The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex; a journal devoted to the study of the nervous system and behavior 44, 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zhang X, Sun Y, Li W, Liu B, Wu W, Zhao H, Liu R, Zhang Y, Yin Z, Yu T, Qing Z, Zhu B, Xu Y, Nedelska Z, Hort J, Zhang B (2019) Characterization of white matter changes along fibers by automated fiber quantification in the early stages of Alzheimer’s disease. NeuroImage: Clinical 22, 101723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Salisbury DF, Wang Y, Yeh F-C, Coffman BA (2021) White Matter Microstructural Abnormalities in the Broca’s-Wernicke’s-Putamen “Hoffman Hallucination Circuit” and Auditory Transcallosal Fibers in First-Episode Psychosis With Auditory Hallucinations. Schizophrenia bulletin 47, 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wang T, Hu Y, Wang D, Liu J, Sun J, Wei C, Dai H, Li Y (2021) Arcuate Fasciculus Subsegment Impairments Distinctly Associated with Memory and Language Deficits in Acute Mild Traumatic Brain Injury Patients. J Neurotrauma 38, 3279–3287. [DOI] [PubMed] [Google Scholar]
- [76].Choi J, Jeong B, Rohan ML, Polcari AM, Teicher MH (2009) Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biol Psychiatry 65, 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Suo X, Lei D, Li W, Sun H, Qin K, Yang J, Li L, Kemp GJ, Gong Q (2022) Psychoradiological abnormalities in treatment-naive noncomorbid patients with posttraumatic stress disorder. Depress Anxiety 39, 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].David S, Heesink L, Geuze E, Gladwin T, van Honk J, Kleber R, Leemans A (2020) Regions of white matter abnormalities in the arcuate fasciculus in veterans with anger and aggression problems. Brain Struct Funct 225, 1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR (2013) Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain 136, 1692–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mishkin M, Ungerleider LG, Macko KA (1983) Object vision and spatial vision: two cortical pathways. Trends in Neurosciences 6, 414–417. [Google Scholar]
- [81].Catani M, Jones DK, Donato R, Ffytche DH (2003) Occipito-temporal connections in the human brain. Brain 126, 2093–2107. [DOI] [PubMed] [Google Scholar]
- [82].Catani M, Thiebaut de Schotten M (2008) A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44, 1105–1132. [DOI] [PubMed] [Google Scholar]
- [83].Ortibus E, Verhoeven J, Sunaert S, Casteels I, de Cock P, Lagae L (2012) Integrity of the inferior longitudinal fasciculus and impaired object recognition in children: a diffusion tensor imaging study. Dev Med Child Neurol 54, 38–43. [DOI] [PubMed] [Google Scholar]
- [84].Taddei M, Tettamanti M, Zanoni A, Cappa S, Battaglia M (2012) Brain white matter organisation in adolescence is related to childhood cerebral responses to facial expressions and harm avoidance. Neuroimage 61, 1394–1401. [DOI] [PubMed] [Google Scholar]
- [85].Kitamura S, Kiuchi K, Taoka T, Hashimoto K, Ueda S, Yasuno F, Morikawa M, Kichikawa K, Kishimoto T (2013) Longitudinal white matter changes in Alzheimer’s disease: a tractography-based analysis study. Brain Res 1515, 12–18. [DOI] [PubMed] [Google Scholar]
- [86].Kiuchi K, Morikawa M, Taoka T, Kitamura S, Nagashima T, Makinodan M, Nakagawa K, Fukusumi M, Ikeshita K, Inoue M, Kichikawa K, Kishimoto T (2011) White matter changes in dementia with Lewy bodies and Alzheimer’s disease: a tractography-based study. J Psychiatr Res 45, 1095–1100. [DOI] [PubMed] [Google Scholar]
- [87].Zimny A, Szewczyk P, Bladowska J, Trypka E, Wojtynska R, Leszek J, Sasiadek M (2012) Quantitative evaluation of changes in the selected white matter tracts using diffusion tensor imaging in patients with Alzheimer’s disease and mild cognitive impairment. Neuroradiol J 25, 300–310. [DOI] [PubMed] [Google Scholar]
- [88].Powers JP, McMillan CT, Brun CC, Yushkevich PA, Zhang H, Gee JC, Grossman M (2013) White matter disease correlates with lexical retrieval deficits in primary progressive aphasia. Front Neurol 4, 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Morales H, Tomsick T (2015) Middle cerebellar peduncles: Magnetic resonance imaging and pathophysiologic correlate. World J Radiol 7, 438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Clouston SAP, Kritikos M, Huang C, Kuan PF, Vaska P, Pellecchia AC, Santiago-Michels S, Carr MA, Gandy S, Sano M, Bromet EJ, Lucchini RG, Luft BJ (2022) Reduced cerebellar cortical thickness in World Trade Center responders with cognitive impairment. Transl Psychiatry 12, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Blithikioti C, Nuño L, Guell X, Pascual-Diaz S, Gual A, Balcells-Olivero Μ, Miquel L (2022) The cerebellum and psychological trauma: A systematic review of neuroimaging studies. Neurobiology of Stress 17, 100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Devita M, Alberti F, Fagnani M, Masina F, Ara E, Sergi G, Mapelli D, Coin A (2021) Novel insights into the relationship between cerebellum and dementia: A narrative review as a toolkit for clinicians. Ageing Research Reviews 70, 101389. [DOI] [PubMed] [Google Scholar]
- [93].Zhang Y, Zhang J, Oishi K, Faria AV, Jiang H, Li X, Akhter K, Rosa-Neto P, Pike GB, Evans A, Toga AW, Woods R, Mazziotta JC, Miller MI, van Zijl PC, Mori S (2010) Atlas-guided tract reconstruction for automated and comprehensive examination of the white matter anatomy. Neuroimage 52, 1289–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Cremers LGM, de Groot M, Hofman A, Krestin GP, van der Lugt A, Niessen WJ, Vernooij MW, Ikram MA (2016) Altered tract-specific white matter microstructure is related to poorer cognitive performance: The Rotterdam Study. Neurobiology of Aging 39, 108–117. [DOI] [PubMed] [Google Scholar]
- [95].Vergoossen LWM, Jansen JFA, Sloten TTv, Stehouwer CDA, Schaper NC, Wesselius A, Dagnelie PC, Köhler S, Boxtel MPJv, Kroon AA, Jong JJAd, Schram MT, Backes WH(2021) Interplay of White Matter Hyperintensities, Cerebral Networks, and Cognitive Function in an Adult Population: Diffusion-Tensor Imaging in the Maastricht Study. Radiology 298, 384–392. [DOI] [PubMed] [Google Scholar]
- [96].Soares J, Marques P, Alves V, Sousa N (2013) A hitchhiker’s guide to diffusion tensor imaging. Frontiers in Neuroscience 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wedeen VJ, Hagmann P, Tseng WY, Reese TG, Weisskoff RM (2005) Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Reson Med 54, 1377–1386. [DOI] [PubMed] [Google Scholar]