Abstract
Nanoparticles possess unique features that may be useful for disease diagnosis and therapy. Preclinically, many different nanodiagnostics have been explored, but only a few have made it to the market. We here provide an overview of nanoparticle-based imaging agents currently used and evaluated in the clinic and discuss preclinical progress and translational avenues for the use of nanoparticles for diagnostic and theranostic applications.
Keywords: molecular imaging, oncology, nanodiagnostics, nanomedicine, nanoparticles, theranostics
Imaging plays an important role in disease diagnosis, prediction of prognosis, and monitoring of therapeutic responses. Imaging modalities used in routine practice are ultrasound, radiography, CT, PET, SPECT, MRI, and combinations of these (i.e., SPECT/CT, PET/CT, and PET/MRI). Optical imaging and photoacoustic imaging are also gradually finding their way into the clinic, mainly in the setting of intraoperative imaging approaches. To improve the distinction between pathologic and normal tissues, contrast agents and radiolabeled probes are frequently used. In the last 2 decades, nanoparticles have received a lot of interest as imaging probes. Some nanoparticles are intrinsically magnetically or optically imageable, whereas others can be imaged only indirectly, after being labeled with radiotracers or dyes (1).
Nanoparticles tend to circulate for prolonged periods (compared with small-molecule agents) and display passive accumulation at pathologic sites such as tumors, metastases, and sites of inflammation, because of leaky vasculature and a high population of phagocytes (2). Furthermore, nanoparticles can be functionalized with targeting ligands to promote engagement with and uptake by target cells or tissues. It is because of these features that, beyond applications in imaging, nanoparticles are also extensively used for drug delivery. The therapeutic performance of drug-loaded nanomedicines relies on their ability to reach the pathologic site, which in the case of tumors usually relies on the enhanced permeability and retention (EPR) effect (3). Because EPR is a highly heterogeneous phenomenon, companion nanodiagnostics or theranostic nanoparticles are needed to stratify patients during translation, to ensure that only patients presenting good tumor accumulation are included in clinical trials (4). At the preclinical level, imaging techniques assist in better understanding nanoparticle behavior in vivo, providing fundamental insights to improve drug delivery formulations.
In this perspective, we discuss the development of nanoparticles as imaging agents, either as purely diagnostic probes for clinical disease diagnosis and staging or as imaging allies of nanoparticle therapeutics for improved formulation design, patient stratification, and nanomedicine translation.
NANOPARTICLE-BASED IMAGING
Nanoparticle-Based Diagnostics in the Clinic
Despite large numbers of preclinical studies using nanoparticles as imaging probes, only a few have moved to clinical settings (5) (Supplemental Tables 1 and 2; supplemental materials are available at http://jnm.snmjournals.org). This is because the particular pharmacokinetic properties of nanoparticles limit their use to very specific applications. For instance, nanoparticles tend to circulate for relatively long periods, have a small volume of distribution, and are taken up by phagocytes. They consequently reside and accumulate mainly in well-perfused and macrophage-rich tissues, such as liver, spleen, and lymph nodes. Hence, traditional diagnostic applications of nanoparticles include imaging of liver lesions after intravenous administration or localization of sentinel lymph nodes (SLNs) after local injection. Moreover, because of their potent contrast generation properties, nanoparticles have been used to label stem cells and track their migration to or retention in pathologic tissues. Recent clinical work has furthermore explored the use of stimulus-responsive nanoparticles, which can change their behavior or contrast generation depending on their environment. Such approaches may gradually expand nanoparticle-based imaging beyond traditional clinical applications.
99mTc-Colloids for SLN Mapping and for Inflammation and Bone Marrow Imaging
The first nanoparticles used in the clinic were 99mTc-colloids for planar scintigraphy and later SPECT imaging (6). They have been administered since the mid-1960s and are based primarily on radiolabeled sulfur colloids. 99mTc-colloids are used to identify SLNs in various tumor entities (e.g., breast cancer, melanoma, oral cavity tumors, prostate cancer, and cervical cancer) and to image lymphatic flow. Moreover, these colloids are also used for radiolabeling of leukocytes to locate sites of infection and inflammation and for imaging of bone marrow distribution. In the European Union, radiolabeled albumin nanocolloids are more commonly used than the sulfur counterparts. Although sulfur colloids have a wide range of sizes (from 10 to 1,000 nm, with filtration removing particles larger than 200 nm), albumin nanoparticles are much smaller (∼30 nm) and have a narrower size distribution (between 6 and 80 nm), which results in faster migration through the lymphatic system. Currently, these nanoparticles are still broadly used in daily clinical practice (Supplemental Table 1).
Superparamagnetic Iron Oxide Nanoparticles (SPIONs) for MRI of Liver Tumors
In 1996, ferumoxide became the first SPION-based imaging formulation approved by the U.S. Food and Drug Administration (Supplemental Table 1) (6). The large magnetic moment of SPIONs alter the transverse relaxation times (T2) of water protons, changing their signal properties in MRI. Because around 80% of intravenously injected SPIONs are cleared by Kupffer cells in the liver, and because liver tumors generally have altered vascularization and a much lower phagocytic capacity, SPIONs were initially used for carcinoma detection and dysplastic nodule evaluation. Subsequent applications outside the liver included atheroma imaging, stem cell tracking to identify postadministration location and engraftment, and dendritic cell labeling to monitor vaccine administration and lymph node trafficking. Given the more favorable pharmacokinetics and excretion profiles of gadolinium-based contrast agents (i.e., SPIONs show poor excretion and strong accumulation in the liver and spleen), as well as their positive signal generation properties, most clinical SPION applications have been discontinued, except for very specific applications such as MR angiography in patients with renal failure and use as a drug in iron-deficiency anemia (5,7). Interestingly, in recent years, the use of SPIONs has again increased in clinical settings, for new specific niche applications.
SPIONs for Imaging Tumor-Associated Macrophages (TAMs)
TAMs are involved in tumor progression and considered to be biomarkers for an unfavorable prognosis (8). Several new therapeutic agents target leukocytes, diminishing macrophage infiltration in tumors. Thus, it is important to identify tumors that present high levels of TAMs and to monitor how they respond to treatments. SPIONs (ferumoxytol) have been used to image macrophages in high-grade glioma patients (9). MRI measurements have shown a good correlation with iron-containing TAMs at tumor sites, where SPIONs were localized inside macrophages and not in tumor cells or astrocytes, as confirmed by histopathology.
SPIONs for Identifying Lymphoid Tissue
In breast cancer and melanoma, SLNs can be mapped with 99mTc-colloids and blue dyes. The use of these agents is limited by several factors, including the lack of strong optical signal in tissue (blue dye), artifacts originating from shine-through phenomena (if the SLN is too close to the primary lesion), poor spatial resolution (e.g., 10 mm for lymphoscintigraphy with 99mTc), and the need for using radioisotopes. Hence, SPIONs have been clinically explored as alternative mapping probes for SLN detection, showing diagnostic performance similar to 99mTc-based methods (10). SPIONs have also been used to identify lymph node metastases in prostate cancer patients via nanoparticle-enhanced MRI. Compared with PET/CT imaging with 68Ga-PSMA-HBED-CC, SPION-enhanced MRI was found to be superior in identifying smaller suggestive lymph nodes (11).
Fluorescent Silica Dots for Mapping SLNs
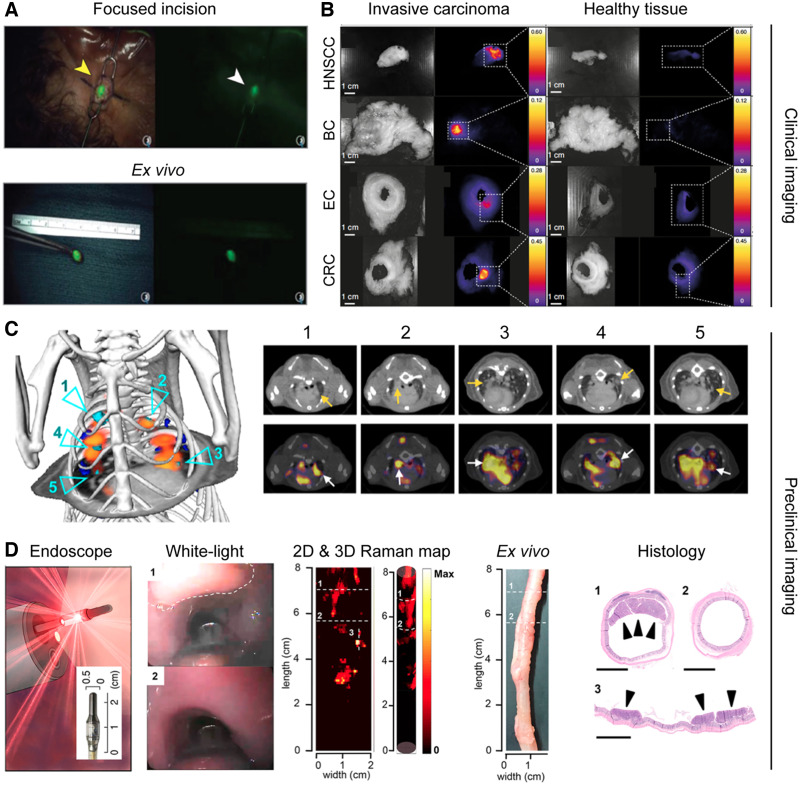
Ultrasmall integrin-targeted fluorescent core-shell silica nanoparticles (also known as Cornell dots) are being explored in the clinic to locate SLNs. Their size of less than 8 nm allows for fast renal excretion, resulting in whole-body clearance half-times of between 13 and 21 h. This rapid removal from the body favors specific molecular imaging applications and minimizes safety concerns, such as long-term accumulation in the liver. A phase I/IIa clinical study has demonstrated the feasibility and safety of Cornell dot–based SLN biopsy mapping in patients with melanoma in the head and neck area (Fig. 1A) (12). Moreover, Cornell dots radiolabeled with 124I, 89Zr, or 64Cu have also been used as hybrid probes for PET and fluorescence-based imaging for staging tumors in clinical settings (13).
FIGURE 1.
Nanoparticle-based imaging for diagnosis. (A) Real-time transcutaneous imaging of SLNs using fluorescent silica dots. (Adapted with permission of (12).) (B) Fluorescence images of different surgically removed tumor and healthy-tissue specimens after ONM-100 administration. (Adapted with permission of (14).) (C) Use of 64Cu-macrin to image tumor-associated macrophages in orthotopic mouse model of lung adenocarcinoma via PET/CT. Cyan arrows highlight tumors with high 64Cu-macrin uptake. Arrows are used to further highlight those regions in corresponding transverse sections. (Adapted with permission of (19).) (D) Endoscopy imaging premalignant colorectal lesions using nanoparticle-based SERS. Lesion is highlighted by the white dashed line in white-light image. Raman signals correlated with presence of lesions (regions 1 and 3), whereas no raman signals were detected in lesion-free region (region 2). (Adapted with permission of (22).) BC = breast cancer; EC = esophageal cancer; CRC = colorectal cancer; HNSCC = head and neck squamous cell carcinoma.
pH-Sensitive Fluorescent Polymeric Nanoparticles for Intraoperative Imaging
ONM-100 is a micellar fluorescent nanoparticle imaging agent composed of a pH-sensitive amphiphilic polymer conjugated to indocyanine green. The polymeric micelles irreversibly dissociate in the acidic extracellular tumor microenvironment, and they fluoresce as a result. In a recent clinical study, ONM-100 enabled intraoperative imaging of 4 different solid tumor types (e.g. head and neck squamous cell carcinoma, esophageal cancer, breast cancer, and colorectal cancer) both in vivo and ex vivo in 30 patients (Fig. 1B) (14). ONM-100 furthermore promoted the detection of tumor-positive resection margins in 9 (of 9) patients. Moreover, nanoparticle fluorescence was observed in 4 occult lesions missed by standard-of-care surgery or pathologic analysis.
Nanoparticle-Based Surface-Enhanced Raman Spectroscopy (SERS) for Identification of Surgical Tumor Margin Surfaces
Lumpectomy (also known as partial mastectomy) is a standard intervention for breast cancer. Unfortunately, additional surgery is required in up to 50% of patients if pathologic analysis reveals the presence of carcinoma in the resection margins. Intraoperative identification of residual carcinoma at the surgical margin surface holds promise to reduce the number of reexcision surgeries. Recently, a raman-encoded molecular imaging technique based on gold nanoparticles topically applied to the excised tissue has been developed (15). This SERS technique allows visualization of the expression of multiple cell surface biomarkers at surgical margins. In a proof-of-concept study, 57 freshly removed specimens were imaged to characterize the expression of 4 biomarkers (i.e., human epidermal growth factor receptor-2 [HER2], estrogen receptor, epidermal growth factor receptor, and CD44), and the detection of breast carcinoma was achieved with a sensitivity and specificity of 89% and 92%, respectively.
Promising Nanoparticle-Based Imaging Approaches at the Preclinical Stage
While the amount of preclinical research focusing on developing novel nanoparticles for imaging applications is vast, only very few nanodiagnostics are heading toward clinical use. This discordance is because research is driven mostly by materials science, in which developing new multifunctional nanomaterials with exotic properties prevails over trying to overcome key current pitfalls of nanoparticle imaging agents. Nonetheless, it is worth highlighting several preclinical initiatives that are trying to move toward translation. For example, some efforts have focused on already-approved SPIONs, either minimizing some of the features that resulted in their discontinuation as MRI contrast agents or exploiting them as probes for new imaging modalities previously not used for diagnostics. Beyond SPIONs, nanoparticles emitting in the second near-infrared window (NIR-II) have recently expanded the applicability of fluorescence imaging, as their deeper tissue penetration and higher spatial resolution potentially allow for more precise functional and molecular imaging.
SPIONs for Longitudinal Relaxation Time (T1)–Based MR Angiography
Despite initial approval in the United States and European Union, most SPIONs were discontinued as T2 contrast agents because of poorer pharmacokinetic properties and performance than for the much smaller gadolinium-based contrast agents. T2 MRI contrast agents have inherent limitations, including dark signal (negative contrast) and the blooming effect. Hence, T1 contrast agents tend to be preferred by clinicians. Expanding on a pioneer work in which small SPIONs were used as T1 blood-pool contrast agents, a study used extremely small SPIONs as contrast agents for high-resolution T1 MR angiography in beagle dogs and macaques (16). As a proof of concept, cerebral ischemia was imaged and identified in these large animals. Regarding a potential clinical future, SPIONs showing T1 contrast may benefit current niche applications in which conventional SPIONs are used as T2 contrast agents (e.g., SLN imaging and cell tracking), as long as SPION clustering, which can result in signal quenching, is minimized. Compared with current small-molecule gadolinium chelates, SPIONs have diagnostically less optimal pharmacokinetics (i.e., slower tissue accumulation, slower compartment exchange, and slower excretion) and are therefore unlikely to replace them as general MRI probes.
SPIONs for Magnetic Particle Imaging (MPI) of Perfusion
Invented in 2001 and commercialized in 2013, MPI has emerged as a promising imaging technique. MPI provides 3-dimensional images of SPION distribution and has distinct advantages over conventional MRI, such as quantitative imaging of nanoparticles as positive contrasts, shorter acquisition times, higher temporal resolution, and absence of signal from tissue. Hence, MPI has been used for real-time functional imaging, such as detecting perfusion deficits in ischemic brains in mice (17). Regarding its clinical future, MPI faces 2 main challenges. First, most current MPI systems are designed for animal imaging, and efforts to upscale MPI scanners to the appropriate size (while providing sufficient imaging capabilities) are still ongoing and continue to be a challenge. Second, whereas MPI may outperform SPION-based MRI, MPI is still limited by the distinct pharmacokinetic features of SPIONs (i.e., slower accumulation in tissue of interest, slower compartment exchange, and slower excretion than for small molecules). Moreover, many MPI studies have focused on imaging the vascular system. Reliable (and cheaper) techniques for imaging perfusion already exist, including CT and ultrasound, questioning the need for a more expensive imaging technique. Taking everything together, the future of MPI as a general diagnostic tool is disputable, and MPI may be limited to specific applications such as hot-spot imaging of labeled stem cells (as long as the cell properties are not disrupted).
SPIONs for Monitoring Immunotherapy
Chimeric antigen receptor T-cell therapy is approved by the Food and Drug Administration for the treatment of chemotherapy-resistant leukemia. However, in patients with solid tumors, chimeric antigen receptor T-cell therapy has shown mixed results. As with nanomedicines, one challenge facing this therapy is monitoring infiltration and accumulation of the therapeutic entities, that is, the ex vivo engineered T cells, into the tumor region on intravenous administration, as the therapeutic response strongly depends on this accumulation. Hence, there may be a need to track the location of T cells noninvasively. Following the steps of a pioneer study that monitored dendritic cell therapy with SPIONs in melanoma patients, a recent study demonstrated that SPIONs could also be used to label chimeric antigen receptor T cells, and their accumulation and distribution could be determined in osteosarcoma-bearing mice by MRI and MPI (18). From the different SPION applications described in this article, tracking of circulating cells is one with decent clinical potential, as nanoparticles are more suited than small molecule–based contrast agents for labeling and tracking stem and immune cells.
64Cu-Labeled Macrin Nanoparticles for PET Imaging of Macrophages
TAM density correlates with cancer progression and drug response (8), especially during nano- and immunotherapy. However, imaging the dynamic spatiotemporal distribution of TAMs is challenging, particularly noninvasively. In this context, 64Cu-labeled macrin nanoparticles were developed to image TAMs and their response to therapy via PET. Macrin nanoparticles comprise a 20-nm polyglucose core, which prevents renal clearance and promotes macrophage uptake (>90% of the administered dose). In a proof-of-concept study, macrin nanoparticles were used to characterize macrophage responses to chemotherapy and radiotherapy (Fig. 1C) (19). Furthermore, TAM-rich tumors identified by macrin nanoparticle–based imaging showed over a 700% higher nanomedicine accumulation than in TAM-deficient tumors. This observation corroborates that TAM imaging is useful for patient stratification in cancer nanomedicine. Beyond cancer, 64Cu-macrin nanoparticles have also been used to monitor macrophages during infections in mice, rabbits, and pigs (20). Regarding their clinical translation, a phase I clinical trial is currently recruiting participants to further study 64Cu-macrin nanoparticles. The aim of this study is to evaluate the pharmacokinetics, whole-body distribution, and safety in healthy individuals, as well as nanoparticle accumulation in disease sites in patients with cancer, sarcoidosis, and myocardial infarction.
Nanoparticles with NIR-II Emission for Imaging Immune Responses
The NIR-II ranges from 1,000 to 1,700 nm. It has become an attractive optical region for biologic imaging, as tissues show lower autofluorescence, absorption, and scattering, resulting in higher spatial resolution and deeper tissue penetration. Taking advantage of these characteristics, molecular imaging based on NIR-II has been explored to study immunotherapy responses (21). Down-converting nanoparticles functionalized with polymers and antibodies allowed the imaging of programmed death ligand 1 and CD8 in mice with colon cancer, with an impressive tumor–to–normal-tissue signal ratio of 40. Molecular imaging revealed the presence of cytotoxic T cells in tumors in response to immunotherapy. Regarding translation, NIR-II fluorescence imaging holds promise for several niche applications. However, most work done thus far has relied on quantum dots, carbon nanotubes, and lanthanide-based down-converting nanoparticles, which are unlikely to be translated because they are incompletely excreted from the body and possess intrinsic tolerability issues. Conversely, although organic dyes and polymeric nanoparticles loaded with NIR-II fluorophores have somewhat lower quantum yields, they typically display better excretion profiles and currently are the NIR-II imaging probes with the brightest clinical future.
Nanoparticle-Based SERS for Intraoperative Imaging
Intraoperative imaging of precursor lesions in live animals has been performed by contrast-enhanced raman endoscopy (Fig. 1D) (22). The nanoparticles used as SERS probes were similar to the ones used in the clinic for the identification of surgical margin tumor surfaces via SERS. With this technique, highly sensitive detection of precursor lesions of gastrointestinal tract cancer in clinically relevant transgenic animal models was achieved. Furthermore, real-time raman endoscopy systems have already been used in the clinic in humans, although not for SERS-based imaging. In this regard, although SERS is one of the most sensitive techniques for detection and analysis, it requires the use of gold (or silver) nanoparticles. At the moment, the future of gold nanoparticles in the clinic is unclear (several clinical trials are ongoing), as a fraction of the injected nanoparticles tends to remain in the liver and spleen of patients for prolonged periods. Therefore, the clinical future of gold nanoparticle–enhanced SERS remains uncertain.
IMAGING OF NANOPARTICLES
Patient Stratification in Cancer Nanomedicine
While nanomedicines usually display strong antitumor effects in preclinical studies, their benefits in clinical settings tend to be modest, primarily reducing the side effects of drugs (4). To facilitate clinical translation, oncology practice routinely uses different strategies for patient stratification, including biopsy-based companion diagnostics (e.g., in vitro testing assays) and imaging-based companion diagnostics (e.g., nontherapeutic imageable nanoparticles). For example, in the clinical trials resulting in the approval of trastuzumab and pertuzumab, only patients with high expression levels of human epidermal growth factor receptor 2 (HER2, the antigen for both antibodies) were included, as they were most likely to benefit from treatments. In the case of nanomedicines in the clinic, such strategies are not routinely used to identify patients who should be included in trials and treated with the formulations in question. This is stunning, since nanomedicine performance is known to be strongly affected by the extent of tumor accumulation (i.e., EPR effect), which is highly variable both intra- and interindividually. This lack of stratification may explain multiple recent failures of cancer nanomedicines in clinical trials (23). Taking these notions into account, several recent studies have now gradually started to explore the use of companion nanodiagnostics and nanotheranostics to visualize and quantify tumor accumulation of nanomedicines in patients (Supplemental Tables 1 and 2).
SPIONs as Companion Nanodiagnostics
Ferumoxytol is Food and Drug Administration–approved for the treatment of anemia in patients with kidney disease and can be used to characterize nanoparticle tumor accumulation and EPR heterogeneity via MRI (24). Ferumoxytol accumulation was studied in patients with different types of malignancy. As anticipated, higher levels of ferumoxytol accumulation in tumors correlated with a greater reduction in lesion size on treatment with liposomal irinotecan (which is approved for pancreatic cancer therapy). This pragmatic way of visualizing and quantifying nanoparticle accumulation in tumors via SPION application holds significant clinical potential for use as a companion diagnostic in cancer nanomedicine.
Radiolabeled Nanoparticles as Nanotheranostic Agents
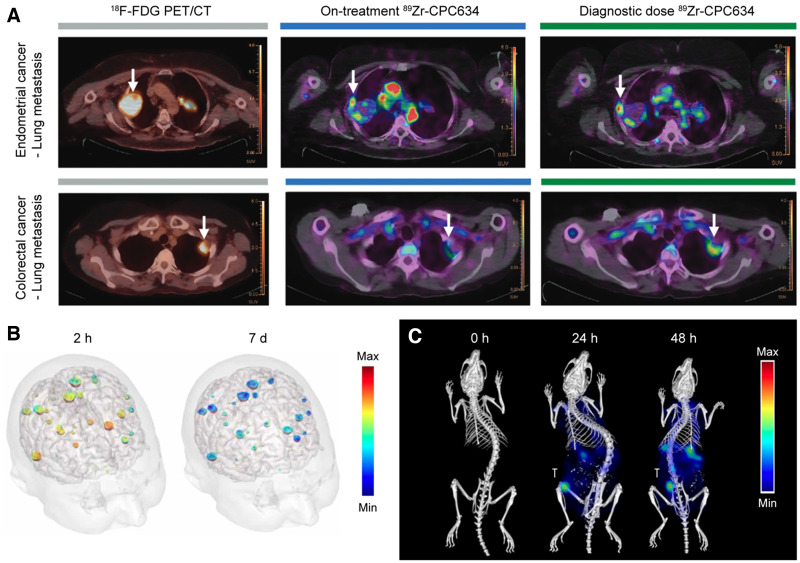
An alternative to using a companion nanodiagnostic is coloading nanomedicines with both drugs and imaging agents. For example, a clinical study showed that PET/CT can assess the tumor accumulation of 64Cu-labeled HER2-targeted liposomal doxorubicin in metastatic breast cancer patients (25). A retrospective exploratory analysis of patient outcome confirmed that the tumor deposition of 64Cu-labeled liposome correlated favorably with therapy outcome. This approach has recently been expanded to other nanoparticle platforms. For instance, the tumor accumulation of docetaxel-loaded polymeric micelles has been studied using PET/CT in 7 patients with solid tumors via radiolabeling of the theranostic nanomedicine formulations with 89Zr (Fig. 2A) (26). Looking into the future, there are several theranostic radioisotopes, such as 177Lu and 131I (β− and γ emitters), that provide both therapeutic and imaging capabilities. These isotopes have also already been loaded into nanoformulations; however, they have thus far been tested only in preclinical settings (27).
FIGURE 2.
Imaging of companion diagnostic nanomedicines and nanotheranostics. (A) PET/CT imaging of accumulation of 89Zr-labeled docetaxel-loaded polymeric micelles (89Zr-CPC634) in lung metastases at 96 h after intravenous injection. Arrows indicate tumors. (Adapted with permission of (26).) (B) Color-coded signal-enhanced MRI of upper half of the head of patient with brain metastases resulting from non–small cell lung cancer after intravenous injection of AGuIX. (Adapted with permission of (28).) (C) Biodistribution images of fluorophore-labeled polymeric micelles obtained using hybrid micro-CT fluorescence tomography. (Adapted with permission of (32).) T = orthotopically induced triple-negative breast cancer tumor.
Gadolinium Nanoparticles as Nanotheranostic Agents
AGuIX (NH TherAguix SA) nanoparticles are ultrasmall (5 nm) polysiloxane-based nanoformulations that contain approximately a dozen chelated gadolinium ions per particle and are being evaluated in the clinic, particularly for whole-brain radiotherapy enhancement (Supplemental Table 1). AGuIX nanoparticles rely on the potent radiosensitizing properties of gadolinium, which (like other elements with a high atomic number) has a high photoelectric absorption coefficient, delivering a high dose to the surrounding tissue when exposed to ionizing radiation. Thus far, the results of only a phase I completed clinical trial have been published (28), in which single intravenous administrations of AGuIX nanoparticles (doses of 15–100 mg/kg of body weight) were studied in 15 patients with 4 types of brain metastases (melanoma, lung, colon, and breast). AGuIX nanoparticles prominently accumulated in and increased image contrast in all types of brain metastases for up to a week after administration (Fig. 2B). At the moment, 7 open clinical trials (phase I/II or II) are ongoing, in which the benefits of AGuIX as a radiosensitizer against several cancers (therapeutic performance), as well as its ability to guide radiotherapy (theranostic performance), are being explored. The results of these studies will define the clinical future of AGuIX nanoparticles.
Imaging of Therapeutic Nanoparticles
In addition to nanoparticles specifically developed as imaging or theranostic probes, several nanoparticles that are approved for clinical use as therapeutics also possess intrinsic imaging properties (Supplemental Table 1). For example, NanoTherm (MagForce AG) is a SPION-based nanoformulation used for the treatment of localized cancers with magnetic hyperthermia. These nanoparticles were approved in Europe for the treatment of glioblastoma in 2011 and recently received the green light from the Food and Drug Administration to move to a stage IIb trial for the focal ablation of prostate cancer. SPIONs can be imaged via MRI and MPI, allowing the study of NanoTherm tumor accumulation, retention, or distribution if necessary. Another type of imageable therapeutic nanoparticle is the hafnium oxide nanoparticle (NBTXR3; Nanobiotix), which in 2019 was approved by the European Medicines Agency as an intratumorally injected radiosensitizer for the treatment of soft-tissue sarcoma. NBTXR3 is also in clinical trials for the treatment of other types of cancer (Supplemental Table 2). Hafnium is a high-atomic-number element and (like gadolinium) has a strong photoelectric absorption coefficient, which causes hafnium oxide nanoparticles to display both strong radiosensitizing properties and strong CT contrast. Similarly, gold nanoparticles, which are currently in clinical trials for nucleic acid delivery to glioblastomas and for photothermal ablation therapy of solid tumors (29), also show strong contrast in CT, as well as in photoacoustic imaging. Thus, whereas these nanoformulations were not initially designed as imaging probes, their intrinsic imaging capabilities may assist in promoting their clinical expansion or translation, via providing noninvasive and quantitative information on tumor accumulation and distribution and, thereby, via promoting potential patient stratification.
Preclinical Imaging of Nanoparticles for Improved In Vivo Performance
When not used as tools for clinical diagnosis and decision making, nanoparticle imaging can be performed to better understand and refine nanoparticle behavior and performance in vivo. Efforts in this regard include characterizing nanoparticle pharmacokinetics and biodistribution, performing mechanistic studies on the principles of nanoparticle tumor accumulation, and monitoring local drug release from nanoparticles on external stimuli. In the last couple of years, new studies relying heavily on multiimaging setups have challenged some of the long-standing paradigms in nanomedicine and drug delivery.
Imaging Nanoparticles in Circulation and During Extravasation
The accumulation of nanomedicines in tumors is widely believed to be caused by passive diffusion of nanoparticles through the gaps between endothelial cells in tumor blood vessels. This notion, which is an essential component of the EPR effect, has been one of the driving forces for the development of nanocarriers that can maximize convection or diffusion through interendothelial gaps. Recently, new research has questioned the prominence of this extravasation mechanism by identifying new transport processes, such as phagocyte hijacking in the bloodstream (30). By combining transmission electron microscopy, 3-dimensional microscopy, and dynamic intravital microscopy, it has been reported that although gaps between endothelial cells occur, they are not frequent and their role in nanoparticle tumor accumulation may thus be overestimated. Similar multiimaging efforts have set out to measure nanoparticle uptake rates by Kupffer cells in vivo, identifying a concentration threshold above which Kupffer cells get overwhelmed and liver clearance decreases, prolonging nanoparticle circulation and enhancing the therapeutic effect of nanotherapies (31).
Multiscale Imaging of the Biodistribution of Nanomedicines
Clinical-stage polymeric micelles were fluorophore-labeled to investigate their biodistribution and target site accumulation (32). The micelles were imaged at the whole-body, tissue, and cellular level by multimodal and multiscale optical imaging approaches (Fig. 2C), including 3-dimensional micro-CT fluorescence tomography and 2-dimensional fluorescence reflectance imaging, among others. The polymeric micelles achieved a high tumor accumulation, with values twice as high as those observed in liver and spleen. Moreover, from the observation that 66% of intratumoral polymeric micelles were extracellularly located, the authors concluded that the anticancer efficacy of polymeric micelles is likely caused by release of the drugs in the tumor microenvironment, providing key information for the design of nanoformulations. Regarding the remaining 33% of intratumoral polymeric micelles, they predominantly accumulated in phagocytes, which may provide new opportunities for nanoimmunotherapy.
Monitoring Drug Release from Nanomedicines
Nanomedicines need to release their payload at the tumor site to achieve proper therapeutic outcomes. To study drug release in vivo, different imaging strategies have been explored. One possible approach relies on the inherent fluorescence emission of chemotherapeutic drugs, such as topotecan (topoisomerase I inhibitor), which is an anticancer agent and shows strong pH-dependent fluorescence (33). Alternatively, triggerable nanomedicine formulations can be coloaded with drugs and imaging agents that are released at the same time as the drugs. In such setups, gadolinium chelates have been used on multiple occasions to monitor drug release from (thermo-, sono-, and pH-) responsive liposomes via MRI (34), confirming that therapeutic cargo is released at the tumor site. These efforts are valuable to optimize formulation design and confirm preclinical performance and clinical potential, but because of their unpragmatic nature and insufficient cost efficiency and time efficiency, it is unlikely that they will be widely implemented in the clinic.
SUMMARY AND OUTLOOK
After several decades of preclinical and clinical progress, successes, and setbacks, nanoparticle-based imaging agents are slowly but steadily making a mark in disease diagnosis and clinical decision making (Supplemental Tables 1 and 2). Initial paradigms based on smart, multimodal, or multifunctional nanomaterials that are universally useful for the detection of all sorts of diseases have shifted to more pragmatic and realistic approaches in which nanoparticles are used for very specific diagnostic applications. On the one hand, these applications are strongly dictated by the pharmacokinetic properties of the nanoparticles, as well as by their propensity to accumulate in specific tissues and cells. On the other hand, the applicability of nanoparticle-based imaging agents strongly depends on the availability of alternative diagnostic probes and protocols, or more explicitly, the lack thereof.
Theranostic nanoparticles, which combine diagnostic and therapeutic features in a single formulation, can provide information about their biodistribution and about target site accumulation, distribution, and retention. This is a potential avenue toward patient stratification, which is performed routinely in the development of oncologic treatments but hardly ever in nanomedicine. Advances in this direction will increasingly profit from combination with machine-learning techniques, which can contribute to many aspects of basic, translational, and clinical nanoparticle research, such as via formulation optimization or via pathologic and radiomic feature identification related to nanomedicine target site accumulation and efficacy. Novel nanoparticle formats are furthermore developed to align with advances in the engineering of novel imaging instrumentation, including ones implemented in surgical theaters, giving rise to new diagnostic and theranostic methods. Finally, nanoparticles are also extensively explored for ex vivo sensing applications, such as in point-of-care devices and in coronavirus self-tests.
Altogether, it can be concluded that nanoparticles are increasingly impacting clinical imaging and diagnostic decision making and that there is promising preclinical progress toward the development of novel nanoparticle-based imaging protocols.
DISCLOSURE
This work is funded under the Excellence Strategy of the Federal Government and the Länder, by the European Research Council (ERC COG Meta-Targeting; 864121), by the German Federal Ministry of Research and Education (BMBF; 16GW0319K), and by the German Research Foundation (DFG; GRK2375 [331065168], LA2937/4-1, and SFB1066). No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1. Kim J, Lee N, Hyeon T. Recent development of nanoparticles for molecular imaging. Philos Trans A Math Phys Eng Sci. 2017;375:20170022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rizzo LY, Theek B, Storm G, Kiessling F, Lammers T. Recent progress in nanomedicine: therapeutic, diagnostic and theranostic applications. Curr Opin Biotechnol. 2013;24:1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Golombek SK, May J-N, Theek B, et al. Tumor targeting via EPR: strategies to enhance patient responses. Adv Drug Deliv Rev. 2018;130:17–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van der Meel R, Sulheim E, Shi Y, Kiessling F, Mulder WJM, Lammers T. Smart cancer nanomedicine. Nat Nanotechnol. 2019;14:1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anselmo AC, Mitragotri S. Nanoparticles in the clinic: an update post COVID-19 vaccines. Bioeng Transl Med. 2021;6:e10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thakor AS, Jokerst JV, Ghanouni P, Campbell JL, Mittra E, Gambhir SS. Clinically approved nanoparticle imaging agents. J Nucl Med. 2016;57:1833–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kallianos K, Henry TS, Yeghiazarians Y, et al. Ferumoxytol MRA for transcatheter aortic valve replacement planning with renal insufficiency. Int J Cardiol. 2017;231:255–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pathria P, Louis TL, Varner JA. Targeting tumor-associated macrophages in cancer. Trends Immunol. 2019;40:310–327. [DOI] [PubMed] [Google Scholar]
- 9. Iv M, Samghabadi P, Holdsworth S, et al. Quantification of macrophages in high-grade gliomas by using ferumoxytol-enhanced MRI: a pilot study. Radiology. 2019;290:198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taruno K, Kurita T, Kuwahata A, et al. Multicenter clinical trial on sentinel lymph node biopsy using superparamagnetic iron oxide nanoparticles and a novel handheld magnetic probe. J Surg Oncol. 2019;120:1391–1396. [DOI] [PubMed] [Google Scholar]
- 11. Schilham MGM, Zamecnik P, Privé BM, et al. Head-to-head comparison of 68Ga-prostate-specific membrane antigen PET/CT and ferumoxtran-10–enhanced MRI for the diagnosis of lymph node metastases in prostate cancer patients. J Nucl Med. 2021;62:1258–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zanoni DK, Stambuk HE, Madajewski B, et al. Use of ultrasmall core-shell fluorescent silica nanoparticles for image-guided sentinel lymph node biopsy in head and neck melanoma: a nonrandomized clinical trial. JAMA Netw Open. 2021;4:e211936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Janjua TI, Cao Y, Yu C, Popat A. Clinical translation of silica nanoparticles. Nat Rev Mater. 2021;6:1072–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Voskuil FJ, Steinkamp PJ, Zhao T, et al. Exploiting metabolic acidosis in solid cancers using a tumor-agnostic pH-activatable nanoprobe for fluorescence-guided surgery. Nat Commun. 2020;11:3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang YW, Reder NP, Kang S, et al. Raman-encoded molecular imaging with topically applied SERS nanoparticles for intraoperative guidance of lumpectomy. Cancer Res. 2017;77:4506–4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu Y, Xu Y-J, Zhang G-B, et al. Iron oxide nanoclusters for T1 magnetic resonance imaging of non-human primates. Nat Biomed Eng. 2017;1:637–643. [DOI] [PubMed] [Google Scholar]
- 17. Ludewig P, Gdaniec N, Sedlacik J, et al. Magnetic particle imaging for real-time perfusion imaging in acute stroke. ACS Nano. 2017;11:10480–10488. [DOI] [PubMed] [Google Scholar]
- 18. Kiru L, Zlitni A, Tousley Aidan M, et al. In vivo imaging of nanoparticle-labeled CAR T cells. Proc Natl Acad Sci USA. 2022;119:e2102363119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim H-Y, Li R, Ng TSC, et al. Quantitative imaging of tumor-associated macrophages and their response to therapy using 64Cu-labeled macrin. ACS Nano. 2018;12:12015–12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nahrendorf M, Hoyer FF, Meerwaldt AE, et al. Imaging cardiovascular and lung macrophages with the positron emission tomography sensor 64Cu-macrin in mice, rabbits, and pigs. Circ Cardiovasc Imaging. 2020;13:e010586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhong Y, Ma Z, Wang F, et al. In vivo molecular imaging for immunotherapy using ultra-bright near-infrared-IIb rare-earth nanoparticles. Nat Biotechnol. 2019;37:1322–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harmsen S, Rogalla S, Huang R, et al. Detection of premalignant gastrointestinal lesions using surface-enhanced resonance Raman scattering–nanoparticle endoscopy. ACS Nano. 2019;13:1354–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. He H, Liu L, Morin EE, Liu M, Schwendeman A. Survey of clinical translation of cancer nanomedicines: lessons learned from successes and failures. Acc Chem Res. 2019;52:2445–2461. [DOI] [PubMed] [Google Scholar]
- 24. Ramanathan RK, Korn RL, Raghunand N, et al. Correlation between ferumoxytol uptake in tumor lesions by MRI and response to nanoliposomal irinotecan in patients with advanced solid tumors: a pilot study. Clin Cancer Res. 2017;23:3638–3648. [DOI] [PubMed] [Google Scholar]
- 25. Lee H, Shields AF, Siegel BA, et al. 64Cu-MM-302 positron emission tomography quantifies variability of enhanced permeability and retention of nanoparticles in relation to treatment response in patients with metastatic breast cancer. Clin Cancer Res. 2017;23:4190–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miedema IHC, Zwezerijnen GJC, Huisman MC, et al. PET-CT imaging of polymeric nanoparticle tumor accumulation in patients. Adv Mater. 2022;34:2201043. [DOI] [PubMed] [Google Scholar]
- 27. Pallares RM, Abergel RJ. Nanoparticles for targeted cancer radiotherapy. Nano Res. 2020;13:2887–2897. [Google Scholar]
- 28. Verry C, Dufort S, Lemasson B, et al. Targeting brain metastases with ultrasmall theranostic nanoparticles, a first-in-human trial from an MRI perspective. Sci Adv. 2020;6:eaay5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang R, Kiessling F, Lammers T, Pallares RM. Clinical translation of gold nanoparticles. Drug Deliv Transl Res. August 31, 2022. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 30. Sofias AM, Toner YC, Meerwaldt AE, et al. Tumor targeting by αvβ3-integrin-specific lipid nanoparticles occurs via phagocyte hitchhiking. ACS Nano. 2020;14:7832–7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ouyang B, Poon W, Zhang Y-N, et al. The dose threshold for nanoparticle tumour delivery. Nat Mater. 2020;19:1362–1371. [DOI] [PubMed] [Google Scholar]
- 32. Biancacci I, Sun Q, Möckel D, et al. Optical imaging of the whole-body to cellular biodistribution of clinical-stage PEG-b-pHPMA-based core-crosslinked polymeric micelles. J Control Release. 2020;328:805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Centelles MN, Wright M, So P-W, et al. Image-guided thermosensitive liposomes for focused ultrasound drug delivery: using NIRF-labelled lipids and topotecan to visualise the effects of hyperthermia in tumours. J Control Release. 2018;280:87–98. [DOI] [PubMed] [Google Scholar]
- 34. Reeβing F, Szymanski W. Following nanomedicine activation with magnetic resonance imaging: why, how, and what’s next? Curr Opin Biotechnol. 2019;58:9–18. [DOI] [PubMed] [Google Scholar]