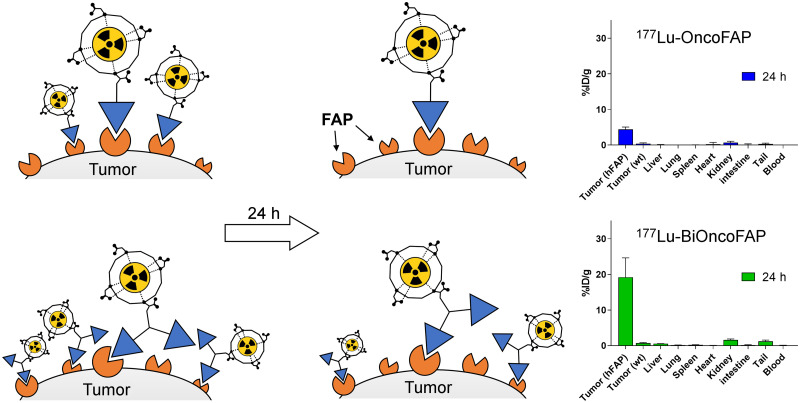
Visual Abstract
Keywords: fibroblast activation protein, theranostics, OncoFAP, targeted radiotherapy, dimeric targeting ligands
Abstract
Imaging procedures based on small-molecule radioconjugates targeting fibroblast activation protein (FAP) have recently emerged as a powerful tool for the diagnosis of a wide variety of tumors. However, the therapeutic potential of radiolabeled FAP-targeting agents is limited by their short residence time in neoplastic lesions. In this work, we present the development and in vivo characterization of BiOncoFAP, a new dimeric FAP-binding motif with an extended tumor residence time and favorable tumor-to-organ ratio. Methods: The binding properties of BiOncoFAP and its monovalent OncoFAP analog were assayed against recombinant human FAP. Preclinical experiments with 177Lu-OncoFAP-DOTAGA (177Lu-OncoFAP) and 177Lu-BiOncoFAP-DOTAGA (177Lu-BiOncoFAP) were performed on mice bearing FAP-positive HT-1080 tumors. Results: OncoFAP and BiOncoFAP displayed comparable subnanomolar dissociation constants toward recombinant human FAP in solution, but the bivalent BiOncoFAP bound more avidly to the target immobilized on solid supports. In a comparative biodistribution study, 177Lu-BiOncoFAP exhibited a more stable and prolonged tumor uptake than 177Lu-OncoFAP (∼20 vs. ∼4 percentage injected dose/g, respectively, at 24 h after injection). Notably, 177Lu-BiOncoFAP showed favorable tumor-to-organ ratios with low kidney uptake. Both 177Lu-OncoFAP and 177Lu-BiOncoFAP displayed potent antitumor efficacy when administered at therapeutic doses to tumor-bearing mice. Conclusion: 177Lu-BiOncoFAP is a promising candidate for radioligand therapy of cancer, with favorable in vivo tumor-to-organ ratios, a long tumor residence time, and potent anticancer efficacy.
Small-molecule radioconjugates (SMRCs) are pharmaceutical products comprising a small organic ligand that acts as a tumor-targeting agent and a radionuclide payload that can be exploited for both diagnostic and therapeutic applications (1–3). The theranostic potential of SMRCs—that is, the possibility to perform imaging and therapy with the same product—facilitates the clinical development of this new class of drugs (4–7). Patients who can predictably benefit from targeted radioligand therapy are accurately selected through dosimetry studies (8). 177Lu-DOTATATE (Lutathera; Advanced Accelerator Applications), a radioligand therapeutic targeting somatostatin receptor type 2, is the first SMRC product that gained marketing authorization for therapy of neuroendocrine tumors (9). The use of this drug has consistently shown high response rates and long median progression-free survival in a multicenter phase III clinical trial (10). More recently, a second product, named 177Lu-PSMA-617, was shown to provide therapeutic benefit to PSMA-positive metastatic castration-resistant prostate cancer patients in a large phase III clinical trial (11). Radioligand therapy with 177Lu-PSMA-617 prolonged imaging-based progression-free survival and overall survival when added to standard care (11).
In the last few years, a new category of pan-tumoral tumor-targeting SMRCs specific to fibroblast activation protein (FAP) has been successfully implemented for the diagnosis of solid tumors (12–15). FAP is a membrane-bound enzyme highly expressed on the surface of cancer-associated fibroblasts in the stroma of more than 90% of human epithelial cancers. FAP expression in healthy tissues is negligible (12,13,16,17). We have recently reported the discovery of OncoFAP, the small-molecule FAP-targeting agent with the highest affinity reported so far (18). Proof-of-concept targeting studies with 68Ga-OncoFAP-DOTAGA (68Ga-OncoFAP), a PET tracer based on OncoFAP, have confirmed excellent biodistribution in patients with different primary and metastatic solid malignancies (19).
The efficacy of radioligand therapeutics correlated strongly with their residence time in tumors (9,20–23). Although 177Lu-DOTATATE and PSMA-617 are characterized by a sustained tumor residence time in patients (i.e., ∼61 h for 177Lu-PSMA-617 and ∼88 h for 177Lu-DOTATATE) (24,25), SMRCs based on FAP-targeting agents are typically cleared from solid lesions in a few hours (26,27). In preclinical biodistribution experiments, 177Lu-OncoFAP selectively localized on neoplastic lesions (∼38 percentage injected dose [%ID]/g 1 h after systemic administration), but half the dose delivered to the tumor was lost within 8–12 h (18). A comparable tumor-targeting performance and pharmacokinetic profile have been reported for other FAP-targeting SMRCs by Loktev et al. (e.g., the tumor uptake of 177Lu-FAPI-46 decreased from 12.5 %ID/g at 1 h to 2.5 %ID/g at 24 h after administration) (28). Importantly, a rapid washout from tumors was observed not only in mice but also in patients treated with 177Lu-FAPI-46 (26,29).
In an attempt to extend the tumor residence time of FAP-targeting SMRCs and to maximize the exposure of cancer cells to biocidal radiation, we developed BiOncoFAP, a dimeric FAP-targeting OncoFAP derivative. In this work, we describe the in vitro characterization of BiOncoFAP and we report the first preclinical biodistribution and therapy studies with a radiolabeled preparation of this novel dimeric FAP-targeting compound.
MATERIALS AND METHODS
Chemistry and Radiochemistry
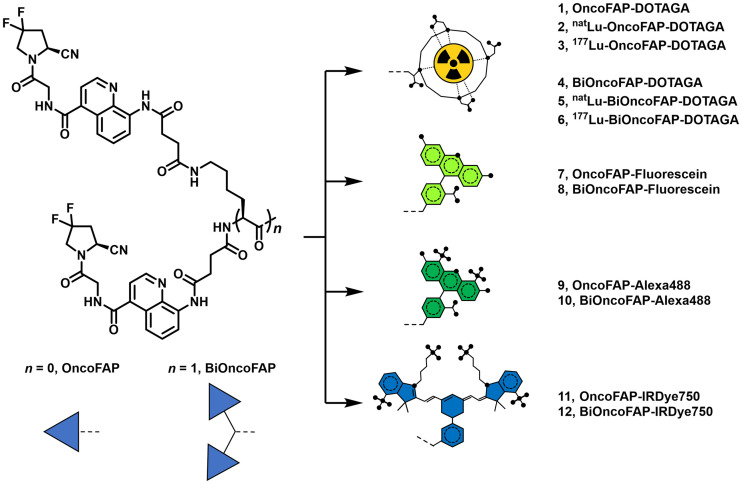
(S)-4-((4-((2-(2-cyano-4,4-difluoropyrrolidin-1-yl)-2-oxoethyl)carbamoyl)quinolin-8-yl)amino)-4-oxobutanoic acid (named OncoFAP-COOH), OncoFAP-fluorescein, OncoFAP-Alexa488, and OncoFAP-IRDye750 were synthesized as previously reported by Millul et al. (18). OncoFAP-DOTAGA (compound 1) and BiOncoFAP-DOTAGA (compound 4) were labeled with cold lutetium by incubation with [natLu]LuCl3 in acetate buffer at 90°C for 15 min to obtain natLu-OncoFAP-DOTAGA (compound 2) and natLu-BiOncoFAP-DOTAGA (compound 5), which were used as reference compounds for in vitro characterization (inhibition assay and serum stability). The structures of OncoFAP and BiOncoFAP conjugates are depicted in Figure 1. Detailed experimental chemical procedures are described in the supplemental material (available at http://jnm.snmjournals.org).
FIGURE 1.
BiOncoFAP and OncoFAP and their DOTAGA, fluorescein, Alexa488, and IRDye750 conjugates.
OncoFAP-DOTAGA (compound 1) and BiOncoFAP-DOTAGA (compound 4) were radiolabeled with 177Lu using different specific activities for the different studies (biodistribution and therapy). Before the biodistribution study, precursors (compound 1 or 4, 100 nmol) were dissolved in 100 μL of phosphate-buffered saline (PBS) and diluted with 200 μL of sodium acetate (1 M in water, pH 8). Twenty megabecquerels of 177Lu solution were added, and the mixture was heated at 90°C for 15 min, followed by dilution with 1,600 μL of PBS to achieve a final volume of 2 mL. Before the therapy studies, precursors (compound 1 or 4, 5 nmol) were dissolved in 5 μL of PBS, and then sodium acetate buffer (30 μL, 1 M in water) and 15 or 70 MBq of 177Lu solution were added. The mixture was heated at 90°C for 15 min, followed by dilution with 130 μL of PBS to afford a final volume of 200 μL. Quality control of the radiosynthesis was performed using radio-high-performance liquid chromatography. The possibility of forming a stable complex between the so-obtained 177Lu-radiolabeled derivatives and the target antigen was tested by coincubating the compounds with recombinant human FAP (hFAP) and loading the mixture onto a desalting PD-10 column run by gravity (Supplemental Fig. 1).
In Vitro Inhibition Assay on hFAP
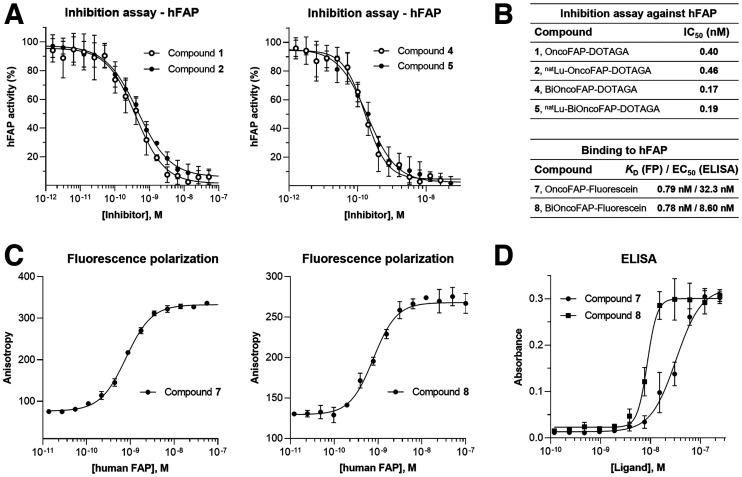
The enzymatic activity of hFAP on the Z-Gly-Pro-AMC substrate was measured at room temperature on a microtiter plate reader, monitoring the fluorescence at an excitation wavelength of 360 nm and an emission wavelength of 465 nm. The reaction mixture contained substrate (20 μM), protein (200 pM, constant), assay buffer (50 mM Tris, 100 mM NaCl, and 1 mM ethylenediaminetetraacetic acid, pH 7.4), and inhibitors (compounds 1, 2, 4, and 5) with serial dilution from 1.67 μM to 800 fM, 1:2 in a total volume of 20 μL. Experiments were performed in triplicate, and the mean fluorescence values were fitted using Prism, version 7 (GraphPad) [y = bottom + (top − bottom)/(1 + 10((LogIC50 − X)*HillSlope))]. The value is defined as the concentration of inhibitor required to reduce the enzyme activity by 50% after addition of the substrate (Fig. 2).
FIGURE 2.
(A) Enzymatic assays performed with OncoFAP-DOTAGA (compound 1), BiOncoFAP-DOTAGA (compound 2), and their corresponding cold natLu-labeled derivatives (compounds 2 and 5). (B) Binding affinity and IC50 values for OncoFAP and BiOncoFAP derivatives toward hFAP. (C) Affinity measurement of OncoFAP-fluorescein (compound 7) and BiOncoFAP-fluorescein (compound 8) to recombinant hFAP by fluorescence polarization. Both compounds showed ultrahigh affinity for FAP target. (D) ELISA experiment on OncoFAP-fluorescein (compound 7) and BiOncoFAP-fluorescein (compound 8) against hFAP. FP = fluorescence polarization; KD = affinity constant.
Affinity Measurement to hFAP by Fluorescence Polarization
Fluorescence polarization experiments were performed in 384-well plates (nonbinding, polystyrene, flat-bottom, black, high volume, 30 μL final volume). Stock solutions of proteins were serially diluted (1:2) with buffer (50 mM Tris, 100 mM NaCl, and 1 mM ethylenediaminetetraacetic acid, pH 7.4), whereas the final concentration of the binders (OncoFAP-fluorescein and BiOncoFAP-fluorescein) was kept constant at 10 nM. The fluorescence anisotropy was measured on a microtiter plate reader (Tecan Life Sciences). Experiments were performed in triplicate, and the mean anisotropy values were fitted using Prism y = bottom + (top − bottom)/(1 + 10((LogIC50 − X)*HillSlope)). The data are reported in Supplemental Figure 2.
Affinity Measurement to hFAP by Enzyme-Linked Immunosorbent Assay (ELISA)
Recombinant hFAP (1 μM, 5 mL) was biotinylated with biotin-LC-N-hydroxysuccinimide (100 equivalents) by incubation at room temperature under gentle agitation in 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; VWR) and 100 mM NaCl buffer (pH 7.4). After 2 h, biotinylated hFAP was purified via a PD-10 column and dialyzed overnight in HEPES buffer. The following day, a StreptaWell (Roche) (transparent 96-well plate) was incubated with biotinylated hFAP (100 nM, 100 μL/well) for 1 h at room temperature and washed with PBS (3 times, 200 μL/well). The protein was blocked by adding 4% milk in PBS (200 μL/well, 30 min at room temperature) and then washed with PBS (3 times, 200 μL/well). Immobilized hFAP was incubated for 30 min in the dark with serial dilutions of OncoFAP-fluorescein (compound 7) and BiOncoFAP-fluorescein (compound 8) and then washed with PBS (3 times, 200 μL/well). A solution of rabbit antifluorescein isothiocyanate antibody (1 μg/mL, product 4510-7804; Bio-Rad) in 2% milk-PBS was added to each well (100 μL/well) and incubated for an additional 30 min in the dark. The resulting complex was washed with PBS (3 times, 200 μL/well) and incubated for an additional 30 min with protein A-horseradish peroxidase (1 μg/mL in 2% milk-PBS, 100 μL/well). Each well was washed with PBS with 0.1% polysorbate (3 times, 200 μL/well) and PBS (3 times, 200 μL/well). The substrate (3,3′,5,5′-tetramethylbenzidine) was added (100 μL/well) and developed in the dark for 2 min. The reaction was stopped by adding 50 μL of 1 M sulfuric acid. The absorbance was measured at 450 nm (reference level, 620–650 nm) with a Spark multimode microplate reader (Tecan Life Sciences).
Internalization Studies by Confocal Microscopy Analysis
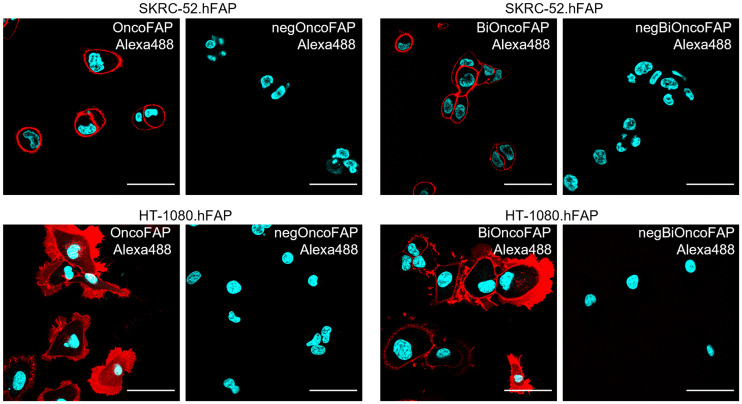
SK-RC-52.hFAP and HT-1080.hFAP cells were seeded into 4-well coverslip chamber plates (Sarstedt, Inc.) at a density of 104 cells per well in RPMI-1640 medium (Gibco) or Dulbecco modified Eagle medium (Gibco), respectively (1 mL; Invitrogen) supplemented with 10% fetal bovine serum (Gibco), Antibiotic-Antimycotic (Gibco), and 10 mM HEPES. Cells were allowed to grow overnight under standard culture conditions. The culture medium was replaced with fresh medium containing the suitable Alexa488-conjugated probes (100 nM) and Hoechst 33342 nuclear dye (Invitrogen, 1 μg/mL). Colonies were randomly selected and imaged 30 min after incubation on an SP8 confocal microscope equipped with an acoustooptical beam splitterD (Leica Microsystems) (Fig. 3).
FIGURE 3.
Confocal microscopy images after incubation of OncoFAP-Alexa488 (compound 9) and BiOncoFAP-Alexa488 (compound 10) with SK-RC-52.hFAP or HT-1080.hFAP. Red = fluorescein derivative staining; blue = Hoechst 33342 staining; scale bar = 50 μm.
Animal Studies
All animal experiments were conducted in accordance with Swiss animal welfare laws and regulations under license ZH006/2021 granted by the Veterinäramt des Kantons Zürich.
Implantation of Subcutaneous Tumors
Tumor cells were grown to 80% confluence in Dulbecco modified Eagle medium or RPMI-1640 medium with 10% fetal bovine serum and 1% Antibiotic-Antimycotic and detached with trypsin-ethylenediaminetetraacetic acid, 0.05%. Tumor cells were resuspended in Hank’s balanced salt solution medium. Aliquots of 5 × 106 cells (100 μL of suspension) were injected subcutaneously in the flank of female athymic BALB/c AnNRj-Foxn1 mice (6–8 wk old; Janvier).
Quantitative Biodistribution of 177Lu-OncoFAP and 177Lu-BiOncoFAP in Tumor-Bearing Mice
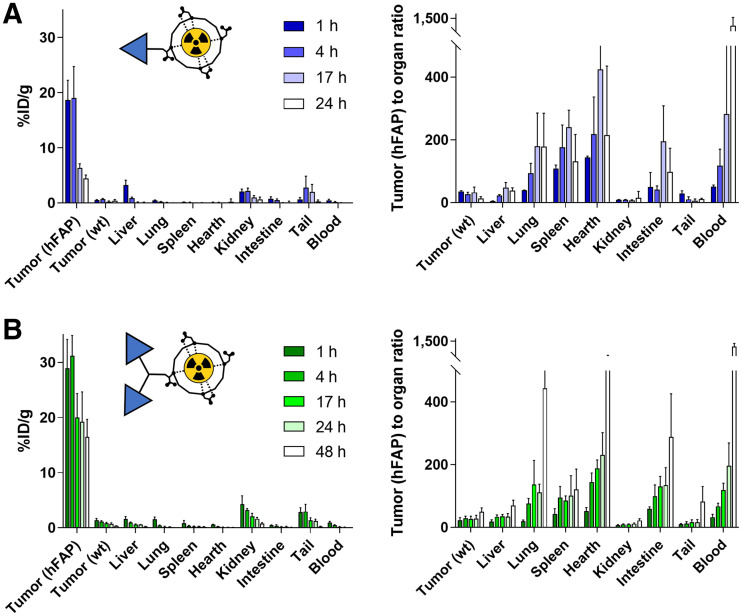
OncoFAP-DOTAGA (compound 1) and BiOncoFAP-DOTAGA (compound 4) were radiolabeled with 177Lu (as described in the supplemental material). Tumors were allowed to grow to an average volume of 500 mm3. Mice were randomized (4 or 5 per group) and injected intravenously with radiolabeled preparations of 177Lu-OncoFAP and 177Lu-BiOncoFAP (250 nmol/kg; 50 MBq/kg). The mice were euthanized by CO2 asphyxiation at different time points (1, 4, 17, and 24 h) after the intravenous injection. Tumors, organs, and blood were harvested and weighed, and radioactivity was measured with a Packard Cobra γ-counter. Values are expressed as %ID/g ± SD (Fig. 4). The %ID/g in the tumors was corrected by tumor growth rate (30).
FIGURE 4.
Quantitative in vivo biodistribution and tumor-to-organ ratio of 177Lu-OncoFAP (compound 3) (A) and 177Lu-BiOncoFAP (compound 6) (B) at different time points after intravenous administration (250 nmol/kg, 50 MBq/kg) in mice bearing HT-1080.wt and HT-1080.hFAP tumors.
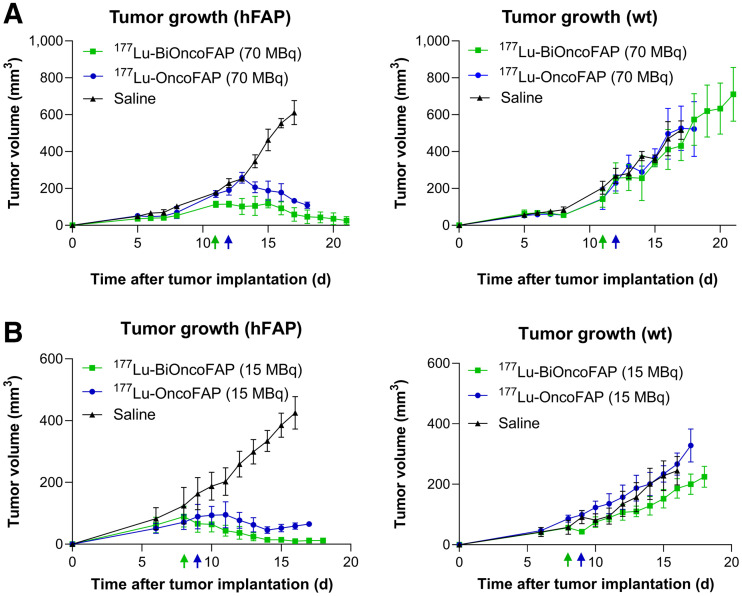
Therapy Studies with 177Lu-OncoFAP and 177Lu-BiOncoFAP in Tumor-Bearing Mice
The anticancer efficacy of 177Lu-OncoFAP and 177Lu-BiOncoFAP was assessed in athymic BALB/c AnNRj-Foxn1 mice bearing HT-1080.hFAP (right flank) and HT-1080.wt (wild type, left flank). 177Lu-OncoFAP or 177Lu-BiOncoFAP was intravenously administered at a dose of 250 nmol/kg, with 15 or 70 MBq/mouse (single administration, following the schedule indicated in Fig. 5). Therapy experiments started when the average volume of established tumors had reached 100–150 mm3. The body weight of the animals and tumor volume were measured daily and recorded. Tumor dimensions were measured with an electronic caliper, and tumor volume was calculated with the formula (long side, mm) × (short side, mm) × (short side, mm) × 0.5. The animals were euthanized when one or more termination criteria indicated by the experimental license were reached (e.g., weight loss > 15%). Prism software was used for data analysis.
FIGURE 5.
Therapeutic activity after single administration (250 nmol/kg) of 177Lu-OncoFAP (compound 3) and 177Lu-BiOncoFAP (compound 6) in BALB/c nu/nu-mice bearing HT-1080.hFAP tumor in right flank and HT-1080.wt tumor in left flank at dose of 70 MBq/mouse (A) or 15 MBq/mouse (B). Efficacy of different treatments was assessed by daily measurement of tumor volume (mm3) after administration of different compounds. Data points represent mean tumor volume ± SEM.
RESULTS
Preparation of OncoFAP and BiOncoFAP Conjugates
The dimeric ligand (BiOncoFAP-COOH, compound 13) was chemically synthesized exploiting l-lysine for the multimerization of the OncoFAP targeting moiety. The free carboxylic acid served as a functional group for the conjugation of fluorophores (BiOncoFAP-fluorescein, compound 8; BiOncoFAP-Alexa488, compound 10; and BiOncoFAP-IRDye750, compound 12) and of DOTAGA chelator (compound 4). All compounds were produced in high yields and purities (supplemental material). Monovalent OncoFAP and the corresponding conjugates (OncoFAP-fluorescein, compound 7; OncoFAP-Alexa488, compound 9; and OncoFAP-IRDye750, compound 11) were synthesized following established procedures (18). The chemical structures of OncoFAP and BiOncoFAP derivatives are illustrated in Figure 1 and in the supplemental material. Radiolabeling of OncoFAP-DOTAGA (compound 1) and BiOncoFAP-DOTAGA (compound 4) with 177Lu was achieved in high yield and purity (supplemental material). After radiolabeling, 177Lu-OncoFAP and 177Lu-BiOncoFAP retained the ability to form stable complexes with recombinant hFAP, as assessed by a PD-10 coelution experiment. Both compounds were highly hydrophilic, with experimental LogD7.4 values of –4.02 ± 0.22 (n = 5) and –3.60 ± 0.31 (n = 5), respectively (supplemental material).
In Vitro Inhibition Assay Against hFAP
We evaluated the inhibitory activity of OncoFAP-DOTAGA (compound 1), BiOncoFAP-DOTAGA (compound 4), and their natLu cold-labeled derivatives (compounds 2 and 5, respectively) against hFAP. Compounds 4 and 5 displayed enhanced inhibitory activity against the target (IC50, 168 and 192 pM, respectively), compared with their monovalent counterparts (OncoFAP-DOTAGA: IC50, 399 pM; natLu-OncoFAP-DOTAGA: IC50, 456 pM) (Figs. 2A and 2B).
Assessment of Binding Properties of BiOncoFAP to Soluble and Immobilized hFAP
To study the binding properties of OncoFAP and BiOncoFAP to soluble hFAP, we measured the affinity constant of the corresponding fluorescein conjugates (compound 8, OncoFAP-fluorescein, and compound 9, BiOncoFAP-fluorescein) in fluorescence polarization assays (Fig. 2C). Compounds 8 and 9 exhibited comparable subnanomolar affinity constants against hFAP (respectively, 795 and 781 pM). Moreover, both compounds were selective for FAP and did not bind to a set of nontarget proteins up to micromolar concentrations (Supplemental Fig. 2). Our data confirm that the dimerization does not impair the affinity and selectivity of BiOncoFAP for its target. Then, we studied the binding affinity to hFAP immobilized on a solid support of the dimeric ligand. In a comparative ELISA, BiOncoFAP-fluorescein exhibited a lower affinity constant than OncoFAP-fluorescein (8.60 vs. 32.3 nM, respectively) (Figs. 2B and 2D).
Confocal Microscopy Analysis on Tumor Cells
Binding of BiOncoFAP to FAP-positive SK-RC-52 and HT-1080 cancer cells and internalization were assessed by confocal microscopy analysis using the corresponding Alexa-488 conjugate (compound 10). OncoFAP-Alexa488 (compound 9) was used in the same experiment as a positive control, whereas untargeted analogs were included as nonbinding negative controls (chemical structures are depicted in the supplemental material). OncoFAP and BiOncoFAP displayed comparable binding features on living tumor cells. Both compounds showed lack of internalization on FAP-positive SK-RC-52 cells, whereas high membrane trafficking was observed when compounds were incubated on HT-1080.hFAP cells (Fig. 3).
Stability Studies
The stability of cold-labeled natLu-BiOncoFAP-DOTAGA was assessed in human and mouse serum after incubation at 37°C for 24, 48, 72 and 120 h. The test compound exhibited a half-life longer than 5 d in all experimental conditions. No loss of lutetium (natLu) from the DOTAGA chelator was detected (Supplemental Fig. 3).
Biodistribution of OncoFAP and BiOncoFAP in Tumor-Bearing Mice
The qualitative biodistribution of OncoFAP and BiOncoFAP was assessed in tumor-bearing mice using a near-infrared fluorophore (IRDye750) as a detection agent. Macroscopic imaging of mice implanted with SK-RC-52.hFAP (right flank) and SK-RC-52.wt (left flank) tumors revealed that both OncoFAP-IRDye750 (compound 11) and BiOncoFAP-IRDye750 (compound 12) selectively accumulated in FAP-positive tumors (Supplemental Figs. 4 and 5). Interestingly, the BiOncoFAP-IRDye750 conjugate exhibited a longer residence time at the site of disease. Encouraged by these results, we studied the quantitative biodistribution of 177Lu-BiOncoFAP in athymic BALB/c mice bearing HT-1080.hFAP (right flank) and HT-1080.wt (left flank) tumors. A direct comparison with 177Lu-OncoFAP was included in the experiment (Fig. 4). Both compounds accumulated selectively in FAP-positive tumors shortly after intravenous administration. The dimeric 177Lu-BiOncoFAP product exhibited a more stable and prolonged tumor uptake than its monovalent counterpart (∼20 vs. ∼4 %ID/g, 24 h after systemic administration). Notably, 177Lu-BiOncoFAP did not show significant uptake in healthy organs, with a favorable tumor-to-organ ratio (e.g., 22-to-1 tumor-to-kidney ratio and 70-to-1 tumor-to-liver ratio, at the 48-h time point) (Supplemental Tables 1–4).
In Vitro Cell Binding and Efflux Assays with 177Lu-OncoFAP and 177Lu-BiOncoFAP on HT-1080.hFAP Cells
Cell binding of 177Lu-OncoFAP and 177Lu-BiOncoFAP was assessed on HT-1080.hFAP cells, following literature procedures (29). Both compounds showed high binding properties toward the FAP-positive cell line. The binding was efficiently antagonized by a large excess of cold competitors (OncoFAP-DOTAGA or BiOncoFAP-DOTAGA) (Supplemental Fig. 6A). Cell efflux experiments revealed a longer half-life for 177Lu-BiOncoFAP (∼36 h) than for the monovalent counterpart (∼18 h) (Supplemental Fig. 6B).
Therapy Study
The therapeutic efficacy of 177Lu-OncoFAP and of 177Lu-BiOncoFAP was assessed in mice bearing HT-1080.hFAP tumors on the right flank and HT-1080.wt tumors on the left flank (Fig. 5; Supplemental Fig. 7). Systemic administration of both compounds at therapeutic doses (15 or 70 MBq/mouse, 250 nmol/kg) resulted in selective and potent anticancer activity against the growth of HT-1080.hFAP as compared with mice injected with saline. The most active compound in our therapy studies was 177Lu-BiOncoFAP. Tumor growth of FAP-negative lesions (HT-1080.wt) was not influenced by the treatment with 177Lu-OncoFAP or with 177Lu-BiOncoFAP. No significant change in mouse body weight was detected with either the 15- or the 70-MBq dose (Supplemental Fig. 8).
DISCUSSION
FAP-targeting radiopharmaceuticals may revolutionize the field of radioligand imaging of cancer because of their applicability to many types of malignancies and their excellent tumor selectivity, which has already been proven at the clinical level (12,13,19). Other SMRC products—177Lu-PSMA-617 and 177Lu-DOTATATE—are limited to certain specific cancer indications and may be taken up by certain normal organ structures (31,32). FAP is expressed mainly in the stroma of solid malignancies and on the tumor cell surface of mesenchymal tumors, thus adding a new element of differentiation compared with previously established targeting platforms, based on somatostatin receptor type 2 and PSMA (12,13,16,17), which are expressed on the surface of cancer cells. In this context, accurate selection of the radionuclide payload is crucial to the success of FAP-targeting radiotherapy. Although α-emitters are characterized by a short range, typically more than 100 μm (33) which may be insufficient, the use of a β-emitter radionuclide such as 177Lu (pathlength of ∼1.5 mm) (33) may enable the killing of stromal cells and surrounding tumor cells (34,35).
Sustained accumulation of SMRCs in tumors is fundamental to the effective delivery of high radiation doses over time at the site of disease and, therefore, to the success of the treatment. Among different approaches used in the past, dimerization of high-affinity ligands has been proposed as a strategy to enhance residence time in antigen-positive structures (i.e., in FAP-positive tumors) (23,36–39). Dimeric ligands present higher chances of rebinding to their target, with slower off-rates than are seen with their monovalent counterparts (40). However, an increase in the binding valency typically leads to higher uptake in healthy tissues (21,38,39). To the best of our knowledge, only 3 dimeric FAP-targeting radionuclides—DOTA/DOTAGA.(SA.FAPi)2 (41,42), DOTA-2P(FAPi)2 (21), and ND-bisFAPI (29)—have recently been described. Although preclinical biodistribution data are not available for DOTA/DOTAGA.(SA.FAPi)2, DOTA-2P(FAPi)2 was extensively characterized in an HCC-PDX-1 mouse model. Despite its slightly increased tumor uptake compared with the monovalent FAPI-46 (∼9% vs. ∼4 %ID/g 1 h after injection), the dimeric ligand presents low tumor-to-organ ratios both at 1 h and 4 h, with a particular liability for the kidney (∼1.2-to-1 and ∼1.5-to-1 tumor-to-kidney ratios, respectively) (21). Similarly, ND-bisFAPI exhibited increased tumor uptake in A549-FAP xenografts, with low tumor-to-organ ratios at all investigated time points (i.e., from 1 to 72 h after systemic administration) (29). BiOncoFAP, the novel homodimeric FAP-targeting small organic ligand described in this article, shows specific and persistent tumor uptake (∼30 %ID/g 1 h after injection and ∼16 %ID/g at 48 h after injection) in HT-1080.hFAP tumor–bearing mice. Remarkably, 177Lu-BiOncoFAP presents a clean preclinical biodistribution profile with high tumor-to-organ ratios even at early time points (e.g., ∼7-to-1 and ∼10-to-1 tumor-to-kidney ratio and ∼20-to-1 and ∼34-to-1 tumor-to-liver ratio at the 1 and 4 h time points, respectively).
The in vivo anticancer activity of 177Lu-FAPI-46 (β-emitter) and 225Ac-FAPI-46 (α-emitter) has recently been evaluated in PANC-1 tumor–bearing mice, a xenograft model of pancreatic cancer characterized by high stromal expression of FAP (35). Both products showed only limited tumor growth suppression even at the highest dose (i.e., 30 kBq/mouse for 225Ac-FAPI-46 and 30 MBq/mouse for 177Lu-FAPI-46).
Collectively, our biodistribution and therapy results show that both 177Lu-OncoFAP and 177Lu-BiOncoFAP are able to efficiently localize at the tumor site and produce a potent anticancer effect in mice bearing subcutaneous FAP-positive tumors, after a single administration at a dose of 70 MBq (∼2 mCi)/mouse or 15 MBq (∼0.4 mCi)/mouse. Compared with the monomeric 177Lu-OncoFAP, our new bivalent 177Lu-BiOncoFAP displayed an enhanced in vivo antitumor activity. As expected, lack of tumor suppression was observed for the FAP-negative tumors (HT-1080.wt), which were used as an internal control to appreciate the specificity of OncoFAP-based theranostic products toward FAP-positive solid lesions. In this article, we have presented the favorable biodistribution profile and therapeutic efficacy of 177Lu-OncoFAP and 177Lu-BiOncoFAP obtained in xenograft models with stable, homogeneous expression of hFAP on the surface of tumor cells. Further investigations in tumor models with a stromal pattern of FAP expression (e.g., patient-derived xenografts) will be of pivotal importance to predict the therapeutic performance of OncoFAP and BiOncoFAP-based therapeutics in the view of future clinical studies.
Considering the exquisite selectivity for cancer lesions and pan-tumoral properties of FAP-targeting radioligand therapeutics, this new class of radiopharmaceutical products may represent a breakthrough in cancer therapy (12). Interim reports on the efficacy of the FAP-targeting peptides and small organic ligands developed so far have shown this therapeutic strategy to have limitations (26,43). Escalation of the dose of radiolabeled FAP-targeting peptides is limited by their intrinsically high kidney uptake at late time points (43–45). Therapy with small organic ligands based on FAPI-46 may be limited by their short residence time in the tumor (26,46). We have developed 177Lu-BiOncoFAP, a new radioligand therapeutic product with prolonged in vivo tumor uptake and highly favorable tumor-to-kidney ratios. Future clinical studies on a basket of indications will provide clarity on the therapeutic efficacy of this novel FAP-targeting product.
CONCLUSION
177Lu-BiOncoFAP is a promising FAP-targeting SMRC product for tumor therapy. This novel bivalent FAP-targeting compound binds its target with high affinity and shows a long residence time in tumor lesions, with favorable tumor-to-organ ratios. Once administered at therapeutic doses, 177Lu-BiOncoFAP potently inhibits growth of FAP-positive tumors in mice. Our data support clinical development of 177Lu-BiOncoFAP in the frame of targeted radioligand therapy.
DISCLOSURE
Dario Neri is a cofounder and shareholder of Philogen (http://www.philogen.com/en/), a Swiss–Italian Biotech company that operates in the field of ligand-based pharmacodelivery. Andrea Galbiati, Aureliano Zana, Matilde Bocci, Jacopo Millul, Abdullah Elsayed, Jacqueline Mock, and Samuele Cazzamalli are employees of Philochem AG, the daughter company of Philogen that owns and has patented OncoFAP (PCT/EP2021/053494) and BiOncoFAP (PCT/EP2022/053404). No other potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
We thank Ettore Gilardoni for performing an exact mass analysis of compounds presented in this article, and Frederik Peissert and Luca Prati for their support with small-molecule ELISA experiments.
KEY POINTS
QUESTION: Does ligand dimerization enhance the tumor retention time and therapeutic potential of FAP-targeting radioconjugates?
PERTINENT FINDINGS: Compared with its OncoFAP monovalent counterpart, dimeric 177Lu-BiOncoFAP shows higher and longer tumor uptake in tumor-bearing mice. 177Lu-BiOncoFAP displays a potent in vivo anticancer effect in preclinical murine models.
IMPLICATIONS FOR PATIENT CARE: The prolonged tumor uptake of 177Lu-BiOncoFAP supports clinical development for the targeted radioligand therapy of multiple FAP-positive cancer lesions.
REFERENCES
- 1. Dal Corso A. Targeted small-molecule conjugates: the future is now. ChemBioChem. 2020;21:3321–3322. [DOI] [PubMed] [Google Scholar]
- 2. Sun X, Li Y, Liu T, Li Z, Zhang X, Chen X. Peptide-based imaging agents for cancer detection. Adv Drug Deliv Rev. 2017;110–111:38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siva S, Udovicich C, Tran B, Zargar H, Murphy DG, Hofman MS. Expanding the role of small-molecule PSMA ligands beyond PET staging of prostate cancer. Nat Rev Urol. 2020;17:107–118. [DOI] [PubMed] [Google Scholar]
- 4. Ballinger JR. Theranostic radiopharmaceuticals: established agents in current use. Br J Radiol. 2018;91:20170969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Turner JH. An introduction to the clinical practice of theranostics in oncology. Br J Radiol. 2018;91:20180440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lenzo NP, Meyrick D, Turner JH. Review of gallium-68 PSMA PET/CT imaging in the management of prostate cancer. Diagnostics (Basel). 2018;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Turner JH. Recent advances in theranostics and challenges for the future. Br J Radiol. 2018;91:20170893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Herrero Álvarez N, Bauer D, Hernández-Gil J, Lewis JS. Recent advances in radiometals for combined imaging and therapy in cancer. ChemMedChem. 2021;16:2909–2941. [DOI] [PubMed] [Google Scholar]
- 9. Hennrich U, Kopka K. Lutathera®: the first FDA- and EMA-approved radiopharmaceutical for peptide receptor radionuclide therapy. Pharmaceuticals (Basel). 2019;12:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 trial of 177Lu-DOTATATE for midgut neuroendocrine tumours. N Engl J Med. 2017;376:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sartor O, de Bono J, Chi KN, et al. Lutetium-177–PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385:1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Calais J. FAP: the next billion dollar nuclear theranostics target? J Nucl Med. 2020;61:163–165. [DOI] [PubMed] [Google Scholar]
- 13. Kratochwil C, Flechsig P, Lindner T, et al. 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med. 2019;60:801–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Backhaus P, Burg M, Roll W, et al. A new horizon for breast cancer staging: first evidence from simultaneous PET-MRI targeting the fibroblast activating protein (FAP) [abstract]. Nuklearmedizin. 2021;60:L10. [Google Scholar]
- 15. Backhaus P, Burg MC, Roll W, et al. Simultaneous FAPI PET/MRI targeting the fibroblast-activation protein for breast cancer. Radiology. 2022;302:39–47. [DOI] [PubMed] [Google Scholar]
- 16. Lo A, Wang LCS, Scholler J, et al. Tumour-promoting desmoplasia is disrupted by depleting FAP-expressing stromal cells. Cancer Res. 2015;75:2800–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mona CE, Benz MR, Hikmat F, et al. Correlation of 68Ga-FAPi-46 PET biodistribution with FAP expression by immunohistochemistry in patients with solid cancers: interim analysis of a prospective translational exploratory study. J Nucl Med. 2022;63:1021–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Millul J, Bassi G, Mock J, et al. An ultra-high-affinity small organic ligand of fibroblast activation protein for tumour-targeting applications. Proc Natl Acad Sci USA. 2021;118:e2101852118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Backhaus P, Gierse F, Burg MC, et al. Translational imaging of the fibroblast activation protein (FAP) using the new ligand [68Ga]Ga-OncoFAP-DOTAGA. Eur J Nucl Med Mol Imaging. 2022;49:1822–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mansi R, Fani M. Radiolabeled peptides for cancer imaging and therapy: from bench-to-bedside. Chimia (Aarau). 2021;75:500–504. [DOI] [PubMed] [Google Scholar]
- 21. Zhao L, Niu B, Fang J, et al. Synthesis, preclinical evaluation, and a pilot clinical PET imaging study of 68Ga-labeled FAPI dimer. J Nucl Med. 2022;63:862–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jones W, Griffiths K, Barata PC, Paller CJ. PSMA theranostics: review of the current status of PSMA-targeted imaging and radioligand therapy. Cancers (Basel). 2020;12:1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schäfer M, Bauder-Wüst U, Leotta K, et al. A dimerized urea-based inhibitor of the prostate-specific membrane antigen for 68Ga-PET imaging of prostate cancer. EJNMMI Res. 2012;2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gupta SK, Singla S, Thakral P, Bal CS. Dosimetric analyses of kidneys, liver, spleen, pituitary gland, and neuroendocrine tumours of patients treated with 177Lu-DOTATATE. Clin Nucl Med. 2013;38:188–194. [DOI] [PubMed] [Google Scholar]
- 25. Schuchardt C, Zhang J, Kulkarni HR, Chen X, Müller D, Baum RP. Prostate-specific membrane antigen radioligand therapy using 177Lu-PSMA I&T and 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: comparison of safety, biodistribution, and dosimetry. J Nucl Med. 2022;63:1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaghazchi F, Aghdam RA, Haghighi S, Vali R, Adinehpour Z. 177Lu-FAPI therapy in a patient with end-stage metastatic pancreatic adenocarcinoma. Clin Nucl Med. 2022;47:e243–e245. [DOI] [PubMed] [Google Scholar]
- 27. Meyer C, Dahlbom M, Lindner T, et al. Radiation dosimetry and biodistribution of 68Ga-FAPI-46 PET imaging in cancer patients. J Nucl Med. 2020;61:1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Loktev A, Lindner T, Burger EM, et al. Development of fibroblast activation protein–targeted radiotracers with improved tumour retention. J Nucl Med. 2019;60:1421–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li H, Ye S, Li L, et al. 18F- or 177Lu-labeled bivalent ligand of fibroblast activation protein with high tumor uptake and retention. Eur J Nucl Med Mol Imaging. 2022;49:2705–2715. [DOI] [PubMed] [Google Scholar]
- 30. Tarli L, Balza E, Viti F, et al. A high-affinity human antibody that targets tumoural blood vessels. Blood. 1999;94:192–198. [PubMed] [Google Scholar]
- 31. Tönnesmann R, Meyer PT, Eder M, Baranski AC. [177Lu]Lu-PSMA-617 salivary gland uptake characterized by quantitative in vitro autoradiography. Pharmaceuticals (Basel). 2019;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Geenen L, Nonnekens J, Konijnenberg M, Baatout S, De Jong M, Aerts A. Overcoming nephrotoxicity in peptide receptor radionuclide therapy using [177Lu]Lu-DOTA-TATE for the treatment of neuroendocrine tumours. Nucl Med Biol. 2021;102–103:1–11. [DOI] [PubMed] [Google Scholar]
- 33. Navalkissoor S, Grossman A. Targeted alpha particle therapy for neuroendocrine tumours: the next generation of peptide receptor radionuclide therapy. Neuroendocrinology. 2019;108:256–264. [DOI] [PubMed] [Google Scholar]
- 34. Frey K, Neri D. Antibody-based targeting of tumor vasculature and stroma. In: Tumor-Associated Fibroblasts and Their Matrix. Springer; 2011:419–450. [Google Scholar]
- 35. Liu Y, Watabe T, Kaneda-Nakashima K, et al. Fibroblast activation protein targeted therapy using [177Lu]FAPI-46 compared with [225Ac]FAPI-46 in a pancreatic cancer model. Eur J Nucl Med Mol Imaging. 2022;49:871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gaertner FC, Kessler H, Wester HJ, Schwaiger M, Beer AJ. Radiolabelled RGD peptides for imaging and therapy. Eur J Nucl Med Mol Imaging. 2012;39(suppl):S126–S138. [DOI] [PubMed] [Google Scholar]
- 37. Liu S. Radiolabeled cyclic RGD peptides as integrin αvβ3-targeted radiotracers: maximizing binding affinity via bivalency. Bioconjug Chem. 2009;20:2199–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krall N, Pretto F, Neri D. A bivalent small molecule-drug conjugate directed against carbonic anhydrase IX can elicit complete tumour regression in mice. Chem Sci. 2014;5:3640–3644. [Google Scholar]
- 39. Liu S. Radiolabeled multimeric cyclic RGD peptides as integrin αvβ3 targeted radiotracers for tumour imaging. Mol Pharm. 2006;3:472–487. [DOI] [PubMed] [Google Scholar]
- 40. Chittasupho C. Multivalent ligand: design principle for targeted therapeutic delivery approach. Ther Deliv. 2012;3:1171–1187. [DOI] [PubMed] [Google Scholar]
- 41. Ballal S, Yadav MP, Moon ES, et al. First-in-human results on the biodistribution, pharmacokinetics, and dosimetry of [177Lu]Lu-DOTA.SA.FAPi and [177Lu]Lu-DOTAGA.(SA.FAPi)2. Pharmaceuticals (Basel). 2021;14:1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Qin C, Song Y, Cai W, Lan X. Dimeric FAPI with potential for tumour theranostics. Am J Nucl Med Mol Imaging. 2021;11:537–541. [PMC free article] [PubMed] [Google Scholar]
- 43. Baum RP, Schuchardt C, Singh A, et al. Feasibility, biodistribution, and preliminary dosimetry in peptide-targeted radionuclide therapy of diverse adenocarcinomas using 177Lu-FAP-2286: first-in-humans results. J Nucl Med. 2022;63:415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhao L, Shang Q, Wu H, Lin Q. Fibroblast activation protein-based theranostics in cancer research: a state-of-the-art review. Theranostics. 2022;12:1557–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nishio M, Okamoto I, Murakami H, et al. Preclinical evaluation of FAP-2286, a peptide-targeted radionuclide therapy (PTRT) to fibroblast activation protein alpha (FAP) [abstract]. Ann Oncol. 2020;31(suppl 4):S488. [Google Scholar]
- 46. Assadi M, Rekabpour SJ, Jafari E, et al. Feasibility and therapeutic potential of 177Lu-fibroblast activation protein inhibitor-46 for patients with relapsed or refractory cancers: a preliminary study. Clin Nucl Med. 2021;46:e523–e530. [DOI] [PubMed] [Google Scholar]