To the Editor: Bivalent messenger RNA (mRNA) vaccines containing the ancestral severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and B.1.1.529 (omicron) variant spike sequences were recently made available to address the waves of infection and coronavirus disease 2019 (Covid-19) caused by omicron variants. The omicron BA.1–containing bivalent vaccine mRNA-1273.214, currently authorized for use in multiple countries, elicits strong neutralizing antibody responses against omicron BA.1 and the epidemiologically dominant BA.4 and BA.5 subvariants.1 The omicron BA.2.75 subvariant, which has steadily increased in prevalence in at least 36 countries, contains potential antibody-escape spike mutations.2 We aimed to characterize the neutralization of BA.2.75 after mRNA-1273.214 boosting and to further elucidate the cross-neutralization potential of this bivalent vaccine against multiple omicron variants.1
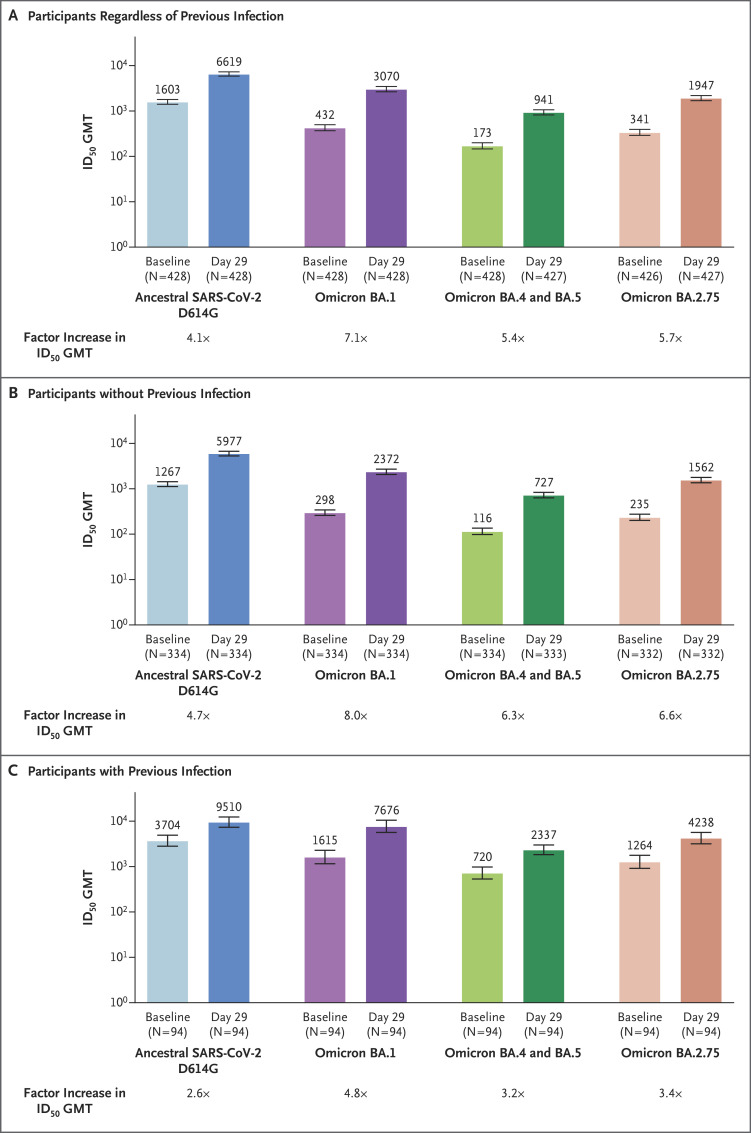
In this phase 2–3 study, geometric mean titers (GMTs) of neutralizing antibodies at a 50% inhibitory dilution were assessed in serum samples collected at day 29 after the administration of 50 μg of mRNA-1273.214 as a second booster dose in adults who had previously received both the mRNA-1273 primary series and a first booster dose of 50 μg of mRNA-1273 at least 3 months earlier and had no evidence of SARS-CoV-2 infection within 3 months before study enrollment. The neutralization assay used lentivirus-based pseudoviruses and was performed in 293T cells that were stably transduced to overexpress angiotensin-converting enzyme 2 (see the Supplementary Methods section in the Supplementary Appendix, available with the full text of this letter at NEJM.org).3 In all 428 participants in the per-protocol immunogenicity population, mRNA-1273.214 elicited a potent neutralizing antibody response against the BA.2.75 subvariant, regardless of previous SARS-CoV-2 infection (Figure 1), with a GMT of 1947 (95% confidence interval [CI], 1711 to 2215). This response was 2.1 (95% CI, 1.9 to 2.2) times as high as those against BA.4 and BA.5 subvariants (GMT, 941; 95% CI, 826 to 1071) and 3.4 (95% CI, 3.1 to 3.7) and 1.6 (95% CI, 1.5 to 1.7) times as low as those against the ancestral SARS-CoV-2 D614G strain (GMT, 6619; 95% CI, 5942 to 7374) and the BA.1 subvariant (GMT, 3070; 95% CI, 2685 to 3511), respectively (Tables S1 and S2 in the Supplementary Appendix). The GMTs in the participants without previous infection were generally similar to those in all participants regardless of previous infection, and the GMTs in the participants with previous infection were higher than those in all participants regardless of previous infection.
Figure 1. Neutralization of the Ancestral SARS-CoV-2 D614G Strain and Omicron Subvariants after Receipt of mRNA-1273.214 as a Second Booster Dose.
Pseudovirus neutralizing antibody titers against the ancestral severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) D614G strain and the omicron subvariants BA.1, BA.4 and BA.5 (these subvariants have identical sequences and were therefore designated as a composite), and BA.2.75 were assessed in serum samples from adult participants in the per-protocol immunogenicity population who received a two-dose primary vaccination series of mRNA-1273 (100 μg in each dose), a first booster dose of 50 μg of mRNA-1273, and a second booster dose of 50 μg of mRNA-1273.214 (Fig. S2 in the Supplementary Appendix).1 Panel A shows the results in the participants regardless of previous SARS-CoV-2 infection; Panel B, the results in those without previous infection; and Panel C, the results in those with previous infection. The spike mutations for the ancestral SARS-CoV-2 D614G strain and the omicron subvariants are provided in Figure S3. The geometric mean titers (GMTs) of neutralizing antibodies at a 50% inhibitory dilution (ID50) were assessed at baseline (before receipt on the day of the second booster) and at day 29 after receipt of the second booster dose. The ID50 GMTs at baseline and at day 29 and the factor increases in the ID50 GMTs at day 29 relative to baseline values are provided. The 95% confidence intervals (indicated by 𝙸 bars) were calculated on the basis of the t-distribution of the log-transformed GMT values and were then back-transformed to the original scale for presentation. The lower limits of quantitation for the pseudovirus neutralization assay (PsVNA) were ID50 GMTs of 18.5 for the ancestral SARS-CoV-2 D614G strain and 19.9 for the omicron subvariant BA.1, and the upper limits of quantitation were 45,118 and 15,503, respectively. The limit of detection of the PsVNA assay for the omicron subvariants BA.4 or BA.5 and BA.2.75 was 10; values below the limit of detection were assigned a value of 5.
These data further support the cross-neutralization ability of the omicron-containing bivalent booster vaccine against emerging omicron subvariants that are not contained in the vaccine. Real-world data on the effectiveness of booster vaccines are needed to evaluate whether the potent and broad neutralizing antibody responses elicited by bivalent vaccines confer enhanced protection against Covid-19.
Supplementary Appendix
Disclosure Forms
This letter was published on November 23, 2022, at NEJM.org.
Because the trial is ongoing, access to patient-level data and supporting clinical documents by qualified external researchers may be available on request and subject to review once the trial has been completed.
Footnotes
Supported by Moderna.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Chalkias S, Harper C, Vrbicky K, et al. A bivalent omicron-containing booster vaccine against Covid-19. N Engl J Med 2022;387:1279-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen X, Chalkias S, Feng J, et al. Neutralization of SARS-CoV-2 omicron BA.2.75 after mRNA-1273 vaccination. N Engl J Med 2022;387:1234-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen X, Tang H, McDanal C, et al. SARS-CoV-2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Cell Host Microbe 2021;29(4):529-539.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.