Abstract
OBJECTIVES
This study aimed to evaluate hemodynamic correlates of inducible blood pressure (BP) pulsatility with exercise in heart failure with preserved ejection fraction (HFpEF), to identify relationships to outcomes, and to compare this with heart failure with reduced ejection fraction (HFrEF).
BACKGROUND
In HFpEF, determinants and consequences of exercise BP pulsatility are not well understood.
METHODS
We measured exercise BP in 146 patients with HFpEF who underwent invasive cardiopulmonary exercise testing. Pulsatile BP was evaluated as proportionate pulse pressure (PrPP), the ratio of pulse pressure to systolic pressure. We measured pulmonary arterial catheter pressures, Fick cardiac output, respiratory gas exchange, and arterial stiffness. We correlated BP changes to central hemodynamics and cardiovascular outcome (nonelective cardiovascular hospitalization) and compared findings with 57 patients with HFrEF from the same referral population.
RESULTS
In HFpEF, only age (standardized beta = 0.593; P < 0.001), exercise stroke volume (standardized beta = 0.349; P < 0.001), and baseline arterial stiffness (standardized beta = 0.182; P = 0.02) were significant predictors of peak exercise PrPP in multivariable analysis (R = 0.661). In HFpEF, lower PrPP was associated with lower risk of cardiovascular events, despite adjustment for confounders (HR:0.53 for PrPP below median; 95% CI: 0.28-0.98; P = 0.043). In HFrEF, lower exercise PrPP was not associated with arterial stiffness but was associated with lower peak exercise stroke volume (P = 0.013) and higher risk of adverse cardiovascular outcomes (P = 0.004).
CONCLUSIONS
In HFpEF, greater inducible BP pulsatility measured using exercise PrPP reflects greater arterial stiffness and higher risk of adverse cardiovascular outcomes, in contrast to HFrEF where inducible exercise BP pulsatility relates to stroke volume reserve and favorable outcome.
Keywords: arterial stiffness, blood pressure, exercise, heart failure with preserved ejection fraction, hemodynamics
Heart failure with preserved ejection fraction (HFpEF) is a major cause of morbidity and mortality, yet diagnosis is challenging, and therapies are limited. These difficulties are in part due to a limited understanding of disease mechanisms. Exercise testing can unmask physiologic abnormalities not present during rest, offering insight into the pathophysiology of HFpEF.1,2 To date, exercise response patterns in HFpEF have largely focused on pulmonary capillary wedge pressure (PCWP). Blood pressure (BP) is a manifestation of ventricular-vascular interaction in HFpEF3,4 but little is known about the relationship between pulsatile BP responses to exercise and relationships to ventricular-vascular function.
Pulsatile BP, defined as the proportionate pulse pressure (PrPP), is the ratio of pulse pressure (pulse pressure = systolic BP [SBP] − diastolic BP [DBP], ie, PP = SBP − DBP) to SBP. Higher resting PrPP has been shown to be a favorable feature in heart failure with reduced ejection fraction (HFrEF) due to an association with preserved left ventricular contractility.5,6 Data suggest that BP pulsatility in HFpEF, however, may be unfavorable and due to increased arterial stiffness and wave reflection.7-10 HFpEF is often incompletely characterized by resting measures alone and repeated measurements during exercise can help to clearly delineate underlying pathology.11 No study has evaluated the mechanism and consequence of inducible BP pulsatility in HFpEF during exercise. We hypothesized that greater inducible BP pulsatility in HFpEF would be associated with worse underlying arterial stiffness and worse cardiovascular outcomes in contrast to patients with HFrEF, where inducible BP pulsatility would indicate contractile reserve and favorable outcome.
To test this hypothesis, we evaluated exercise BP responses in HFpEF in a prospective cohort undergoing cardiopulmonary exercise testing (CPET) with invasive hemodynamic profiling and characterization of arterial stiffness. We aimed to identify hemodynamic correlates and outcome prediction value of pulsatile exercise BP response in HFpEF and to compare them with HFrEF.
MATERIALS AND METHODS
SUBJECTS, EXERCISE PROTOCOL, AND HEMODYNAMICS.
We performed a retrospective analysis of exercise BP responses within a cohort of patients with HFpEF who underwent invasive CPET between 2006 and 2018. We evaluated 146 patients who met a strict published definition of HFpEF, which included clinical diagnosis of heart failure (HF) with New York Heart Association functional class II-IV symptoms, recent hospitalization, acute or chronic therapy for HF, or hemodynamic evidence of HF at right heart catheter, normal ejection fraction >50% on echocardiography or ventriculography, and biomarker evidence of HF using N-terminal pro–B-type natriuretic peptide.12 Although patients comprised heterogenous timeframes for when the initial diagnosis of HF was made, importantly all patients met the physiologic diagnostic criteria for HF at the time of study based on same-day measurements on the day of CPET. Patients were studied during periods of clinical stability without active medication titration and were instructed to take all of their usual cardiovascular medications on the day of testing. The time duration between medication consumption and testing was not prespecified. Supplemental Table 1 outlines the HFpEF definition used for this study in detail. Patients underwent invasive CPET using our laboratory’s protocol as previously described.2 Upright maximum incremental ramp exercise testing was performed using a cycle ergometer. After a resting period ≥3 min, there was a period of unloaded exercise for 3 min and then a continuous ramp (5-30 W/min, based on estimated exercise capacity) designed to achieve 8-12 min of total exercise at a cadence of 60 revolutions/min. Hemodynamic parameters were measured at baseline and at 1-min intervals. BP was measured using a radial artery catheter. PCWP was measured using inflation of a Swan-Ganz catheter placed in the pulmonary artery. Stroke volume was measured using the Fick equation applied to blood gas sampling from the radial arterial catheter and right atrium. Hemodynamic measurements were recorded using a digital archiving system (Witt Biomedical). PrPP was defined as the ratio of pulse pressure to systolic pressure5 as measured using the radial arterial pressure transducer. Arterial stiffness was assessed by quantifying wave reflection using the augmented pressure (AP), which was determined from central pressure waveform analysis using a generalized transfer function applied to radial arterial waveforms (SphygmoCor, AtCor Medical).8,13 Respiratory gas exchange was assessed using breath-by-breath analysis (Medgraphics). All data were collected prospectively as part of our institutional registry and this study was performed as a retrospective analysis of these prospectively collected data.
We compared BP response patterns in our HFpEF cohort with 57 patients with HFrEF (defined as left ventricular ejection fraction <40% based on transthoracic echocardiography or radionuclide imaging) undergoing invasive CPET (again between 2006 and 2018) with the protocol described. Arterial stiffness measures were available in 110/146 (75%) patients with HFpEF and 23/57 (40%) patients with HFrEF.
All subjects gave written informed consent and all studies were approved by the Mass General Brigham (formerly Partners Healthcare) Institutional Review Board.
STATISTICAL ANALYSIS.
Associations between BP parameters and invasive hemodynamic correlates were evaluated using multivariable linear regression models. Age, sex, heart rate change, body mass index (BMI), maximal external workload achieved (W), and baseline SBP or DBP were included as covariates for models evaluating change in systolic and diastolic BP. A stepwise selection model including arterial stiffness (AP), peak exercise stroke volume, sex, BMI, maximum external workload, and heart rate change was used to identify key determinants of PrPP.
Two-group comparisons between HFpEF and HFrEF were performed using the: 1) Student t test (with 2-tailed significance assuming unequal variance) for normally distributed data (defined as skewness statistic between −0.5 and 0.5); 2) Mann-Whitney U test for non-normal data; and 3) Fisher exact test for proportions.
We binarized the PrPP response to exercise by splitting peak exercise PrPP at the median. We evaluated the effect of peak exercise PrPP above vs below the median on time to cardiovascular events using Kaplan-Meier analysis with log-rank test and Cox proportional hazards models adjusted for age, sex, BMI, and maximal workload achieved. Maximal workload was assessed using watts. While peak oxygen consumption (VO2) could have been used as the workload marker, we aimed to make our findings as generalizable as possible. We recognize that many exercise-based evaluations are conducted without measurement of gas exchange variables, but workload can be readily derived from a cycle ergometer or treadmill, hence our decision to use watts as the marker of workload. A cardiovascular event was defined as a nonelective cardiovascular hospital admission (for reasons including arrhythmia, acute coronary syndrome, coronary revascularization, or HF) up to October 2018. Outcomes were available in all patients. Outcome assessment in the HFpEF group was across the entirety of follow-up but displayed graphically to 5 years. Outcome assessment in the HFrEF group was evaluated as freedom from events at 1 year due to the overall high cumulative event rate in this selected population (HFrEF patients undergoing invasive CPET) and also included all-cause mortality, ventricular assist device implantation, or cardiac transplantation at 1 year in the composite outcome.
All statistical analyses were performed using SPSS Version 25.0 (IBM Corporation). Statistical significance was taken as P < 0.05.
INVESTIGATOR DIVERSITY STATEMENT.
There was diversity in our investigator group, with regard to age, career stage, sex (9 male and 8 female authors), and race.
RESULTS
BASELINE CHARACTERISTICS.
Baseline characteristics of both the HFpEF and HFrEF cohort are shown in Table 1. Patients with HFpEF had a mean age of 62.7 ± 12.5 years and 49% of patients were female. The HFpEF cohort was on average obese and hypertensive at rest (BMI: 32.9 ± 7.5 kg/m2; SBP: 148 ± 23 mm Hg). Patients with HFrEF had a mean age of 60.3 ± 12.8 years and 23% of patients were female.
TABLE 1.
Baseline Characteristics
| HFpEF (n = 146) |
HFrEF (n = 57) |
P Value | |
|---|---|---|---|
| Age, y | 63 ± 13 | 60 ± 13 | 0.23 |
| Female | 72/146 (49.3) | 13/57 (22.8) | 0.001 |
| Weight, kg | 93.7 ± 21.5 | 83.0 ± 17.6 | <0.001 |
| Height, m | 1.69 ± 0.10 | 1.73 ± 0.11 | 0.006 |
| BMI, kg/m2 | 32.9 ± 7.5 | 27.4 ± 4.6 | <0.001 |
| Hemoglobin, mg/dL | 13.1 ± 1.8 | 12.7 ± 1.9 | 0.22 |
| Diabetes mellitus | 36/145 (24.8) | 17/56 (30.4) | 0.42 |
| Myocardial infarction | 10/145 (6.9) | 12/57 (28.2) | 0.006 |
| Hypertension | 100/146 (68.4) | 30/56 (53.6) | 0.05 |
| Peripheral arterial disease | 6/145 (4.1) | 12/57 (21.1) | <0.001 |
| Smoking | 78/137 (56.9) | 34/54 (63.0) | 0.44 |
| Aspirin | 66/146 (45.2) | 38/57 (66.7) | 0.008 |
| Beta-blocker | 75/146 (51.4) | 42/57 (73.7) | 0.004 |
| Calcium-channel blocker | 26/146 (17.8) | 6/57 (10.5) | 0.28 |
| ACE inhibitor | 36/146 (24.7) | 24/57 (42.1) | 0.017 |
| Angiotensin receptor blocker | 23/146 (15.8) | 12/57 (21.1) | 0.41 |
| Aldosterone inhibitor | 4/145 (2.8) | 27/57 (47.4) | <0.001 |
| Neprolysin inhibitor | 0/145 (0.0) | 3/57 (5.0) | 0.005 |
| Nitroglycerin | 15/146 (10.3) | 7/57 (12.3) | 0.80 |
| Diuretic | 68/146 (46.6) | 45/57 (78.9) | <0.001 |
| Statin | 81/146 (55.5) | 31/57 (54.4) | 1.00 |
| VO2 rest, mL/min | 297.7 ± 66.1 | 279.0 ± 61.1 | 0.06 |
| Indexed VO2 rest, mL/kg/min | 3.2 ± 0.6 | 3.4 ± 0.8 | 0.06 |
| CavO2 rest, mL/dL | 6.3 ± 1.1 | 7.3 ± 1.5 | <0.001 |
| Heart rate rest, beats/min | 75 ± 14 | 74 ± 14 | 0.73 |
| SBP rest, mm Hg | 148 ± 23 | 122 ± 22 | <0.001 |
| DBP rest, mm Hg | 76 ± 13 | 68 ± 11 | <0.001 |
| PP rest, mm Hg | 72 ± 19 | 54 ± 18 | <0.001 |
| PrPP rest, mm Hg | 0.48 ± 0.08 | 0.43 ± 0.09 | <0.001 |
| Aortic SBP rest, mm Hg | 139 ± 20 | 117 ± 21 | <0.001 |
| Aortic DBP rest, mm Hg | 82 ± 13 | 73 ± 11 | 0.002 |
| Aortic PP rest, mm Hg | 57 ± 17 | 44 ± 15 | 0.002 |
| Stroke volume rest, mL | 67.8 ± 20.6 | 54.9 ± 16.8 | <0.001 |
| Cardiac output rest, L/min | 4.9 ± 1.3 | 4.0 ± 1.0 | <0.001 |
| Upright right atrial pressure rest, mm Hg | 3 ± 3 | 3 ± 4 | 0.37 |
| Upright mean PAP rest, mm Hg | 19 ± 5 | 23 ± 8 | 0.003 |
| Upright mean PCWP rest, mm Hg | 8 ± 4 | 12 ± 8 | 0.002 |
| Supine right atrial pressure rest, mm Hg | 9 ± 4 | 8 ± 5 | 0.25 |
| Supine mean PAP, mm Hg | 25 ± 6 | 29 ± 11 | 0.028 |
| Supine mean PCWP rest, mm Hg | 16 ± 6 | 18 ± 9 | 0.22 |
| Aortic augmented pressure rest, mm Hg | 15 ± 11 | 11 ± 10 | 0.033 |
| Aortic augmentation index, % | 24.3 ±12.2 | 22.2 ± 15.3 | 0.54 |
Values are mean ± SD or n/N (%).
ACE = angiotensin converting enzyme; BMI = body mass index; CavO2 = peripheral oxygen extraction; DBP = diastolic blood pressure; HFpEF = heart failure with preserved ejection fraction; HRrEF = heart failure with reduced ejection fraction; PAP = pulmonary arterial pressure; PCWP = pulmonary capillary wedge pressure; PP = pulse pressure; PrPP = proportionate pulse pressure; SBP = systolic blood pressure; VO2 = oxygen consumption.
REST AND EXERCISE HEMODYNAMICS AND CARDIOPULMONARY METRICS.
Resting hemodynamic measurements in the HFpEF and HFrEF cohorts are outlined in Table 1. Peak exercise hemodynamic and gas exchange parameters are shown in Table 2. Compared with patients with HFrEF, patients with HFpEF had a higher peak exercise VO2 and greater augmentation in heart rate and BP with exercise. Inducible BP pulsatility (PrPP at peak exercise) was also greater in the HFpEF cohort, as was exertional PCWP change, and peak stroke volume, cardiac output, and external workload achieved. Tissue extraction of oxygen (CavO2) at peak exercise was lower in HFpEF.
TABLE 2.
Peak Exercise Values for Cardiopulmonary Physiological Parameters
| HFpEF | HFrEF | P Value | |
|---|---|---|---|
| VO2 peak exercise, mL | 1,287.3 ± 430.1 | 973.6 ± 389.7 | <0.001 |
| ΔVO2, mL | 992.4 ± 393.6 | 694.7 ± 374.1 | <0.001 |
| Indexed VO2 max, mL/kg | 13.7 ± 3.1 | 11.7 ± 3.6 | <0.001 |
| CavO2 peak exercise | 11.7 ± 2.1 | 12.7 ± 2.4 | 0.007 |
| ΔCavO2 | 5.4 ± 2.1 | 5.4 ± 2.0 | 0.73 |
| HR peak exercise, beats/min | 126.8 ± 24.7 | 111.2 ± 22.3 | <0.001 |
| Δ, beats/min | 51.8 ± 21.2 | 36.9 ± 21.3 | <0.001 |
| SBP peak exercise, mm Hg | 185.4 ± 34.2 | 141.6 ± 32.6 | <0.001 |
| ΔSBP, mm Hg | 37.6 ± 26.9 | 20.0 ± 26.1 | <0.001 |
| DBP peak exercise, mm Hg | 87.1 ± 18.9 | 71.7 ± 15.3 | <0.001 |
| ΔDBP, mm Hg | 11.5 ± 14.2 | 3.8 ± 12.3 | <0.001 |
| PP peak exercise, mm Hg | 98.3 ± 24.4 | 70.0 ± 23.4 | <0.001 |
| ΔPP, mm Hg | 26.1 ± 18.6 | 16.2 ± 17.8 | 0.001 |
| PrPP peak exercise, mm Hg | 0.53 ± 0.07 | 0.48 ± 0.08 | <0.001 |
| ΔPrPP, mm Hg | 0.04 ± 0.06 | 0.05 ± 0.06 | 0.91 |
| PCWP peak exercise, mm Hg | 26.5 ± 7.9 | 25.8 ± 8.8 | 0.50 |
| ΔPCWP, mm Hg | 18.4 ± 7.3 | 13.6 ± 8.1 | <0.001 |
| SV peak exercise, mL | 89.2 ± 25.7 | 70.6 ± 22.6 | <0.001 |
| ΔSV, mL | 21.3 ± 20.3 | 15.7 ± 20.7 | 0.08 |
| CO peak exercise, L/min | 11.1 ± 3.5 | 7.7 ± 2.8 | <0.001 |
| ΔCO, L/min | 6.2 ± 2.7 | 3.7 ± 2.6 | <0.001 |
| Maximum external workload, watts | 89.9 ± 34.3 | 71.4 ± 35.6 | 0.001 |
Values are mean ± SD.
CO = cardiac output; HR = heart rate; SV = stroke volume; D Δ change in (from baseline to peak); other abbreviations as in Table 1.
ASSOCIATION OF EXERCISE BP CHANGES WITH PCWP AND STROKE VOLUME.
Among individuals with HFpEF, the changes in SBP and DBP from baseline to peak exercise were significantly positively correlated with exertional change in PCWP and peak exercise PCWP after multivariable adjustment for age, sex, heart rate change, BMI, maximal external workload achieved, and baseline SBP or DBP (Table 3). We found that during exercise, an approximate 5-mm Hg increase in diastolic BP was associated with a 1-mm Hg increase in peak PCWP. Specifically, in unadjusted analyses, an increase in DBP of 4.8 mm Hg was associated with a 1-mm Hg increase in peak PCWP. After correction for confounders, an increase in DBP of 5.6 mm Hg was associated with an increase in peak PCWP by 1 mm Hg.
TABLE 3.
Associations Between SBP and DBP Change and Exertional PCWP Response in HFpEF Using Multivariable Linear Regression
PrPP at peak exercise was significantly predicted by peak exercise stroke volume and resting AP in HFpEF after multivariable adjustment (Tables 3 and 4).
TABLE 4.
Determinants of PrPP at Peak Exercise in HFpEF
| Beta | 95% CI | Standardized Beta |
P Value | |
|---|---|---|---|---|
| PrPPa Model R2 = 0.437 | ||||
| SV at peak exercise | 0.001 | 0.001-0.001 | 0.349 | <0.001 |
| Baseline AP | 0.001 | 0.000-0.002 | 0.182 | 0.020 |
| Age | 0.003 | 0.002-0.004 | 0.593 | <0.001 |
Stepwise multivariable linear regression, variables removed due to nonsignificance: female sex, BMI, maximum external workload (W), and HR change. Note that absolute values for beta are low due to the order of magnitude at which PrPP is distributed; standardized beta values are more informative.
In the HFrEF cohort, after multivariable adjustment for age (overall model R = 0.586 and significance = 0.043), peak exercise stroke volume was a significant predictor of PrPP (multivariable adjusted standardized beta = 0.577; P = 0.013) but baseline arterial stiffness (resting AP), which was 27% lower than in HFpEF, was not (standardized beta = 0.137; P = 0.54).
ASSOCIATION OF INDUCIBLE BP PULSATILITY WITH CARDIOVASCULAR OUTCOMES.
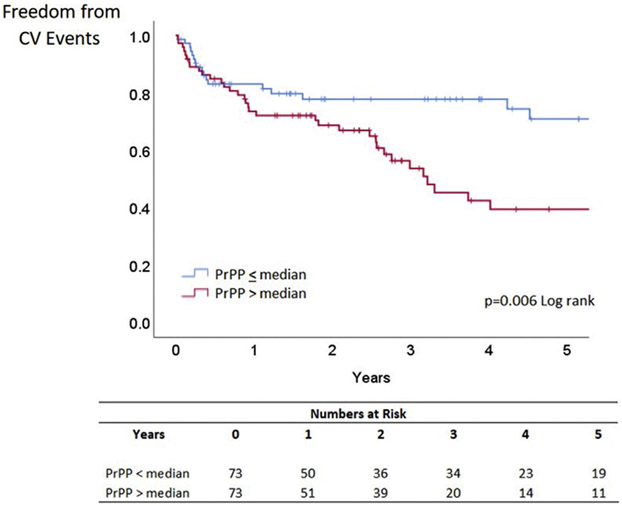
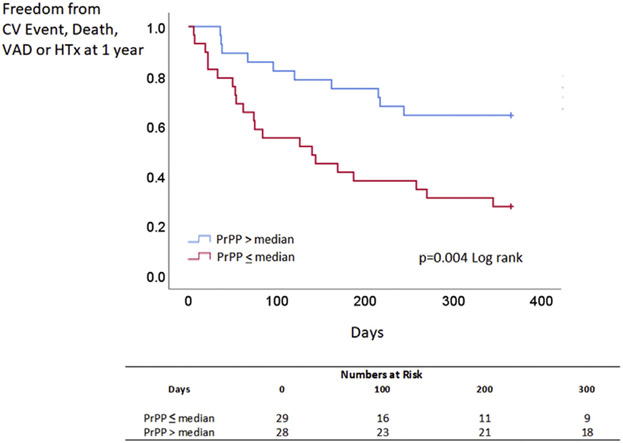
Over a median follow-up of 4.8 years, a total of 53 patients with HFpEF experienced cardiovascular events. Elevated peak exercise PrPP (above the median) was associated with a greater risk of cardiovascular events in HFpEF (Figure 1) (P = 0.006 log-rank). After multivariable adjustment for age, sex, diabetes mellitus, and myocardial infarction, lower PrPP was associated with less cardiovascular events in HFpEF (HR for cardiovascular events when PrPP is below median 0.53 [95% CI: 0.28-0.98]; P = 0.043) (Table 5). This result persisted when PrPP was analyzed as a continuous variable, rather than dichotomized at the median (P = 0.028). By contrast in the HFrEF cohort, where 31 patients experienced a cardiovascular event, death, ventricular assist device placement, or heart transplantation in the first year of follow-up, elevated PrPP (above median) was associated with better outcomes (P = 0.004) (Figure 2). This result persisted with PrPP as a continuous variable in a Cox proportionate hazards model (P = 0.013).
FIGURE 1. Blood Pressure Pulsatility and Outcomes in Heart Failure With Preserved Ejection Fraction.
Freedom from CV events based on PrPP at peak exercise (above or below median) in heart failure with preserved ejection fraction. CV = cardiovascular; PrPP = proportionate pulse pressure.
TABLE 5.
Cox Proportionate Hazards Models Evaluating Predictive Value of PrPP for Cardiovascular Events in HFpEF
| Hazard Ratio (95% CI) for Cardiovascular Events |
P Value | |
|---|---|---|
| PrPP below median | 0.525 (0.281-0.981) | 0.043 |
| Age | 0.997 (0.966-1.029) | 0.84 |
| Female | 2.228 (1.143-4.342) | 0.019 |
| BMI | 0.992 (0.950-1.034) | 0.70 |
| Maximum workload | 0.992 (0.981-1.004) | 0.20 |
Abbreviations as in Table 1.
FIGURE 2. Blood Pressure Pulsatility and Outcomes in Heart Failure With Reduced Ejection Fraction.
Freedom from CV events, death, VAD, and HTx at 1 year, based on PrPP at peak exercise in heart failure with reduced ejection fraction (above and below median). HTx = heart transplantation; VAD = ventricular assist device; other abbreviations as in Figure 1.
DISCUSSION
BP IS A WINDOW INTO VENTRICULAR-VASCULAR INTERACTION IN HFpEF.
BP responses during exercise provide a window into cardiovascular function and interaction in the setting of HF. In patients with HFpEF undergoing comprehensive hemodynamic profiling with invasive CPET, we found that exaggerated exercise changes in SBP and DBP were associated with impaired cardiac performance as measured using a steep increment in left heart filling pressure, whereas pulsatility of exercise BP was associated with both arterial stiffness and stroke volume augmentation. High pulsatility in exercise BP was associated with worse cardiovascular outcome in HFpEF, highlighting the importance of arterial stiffness in HFpEF. In contrast, in patients with HFrEF, in whom exercise stroke volume was more impaired and arterial stiffness was lower, a higher exercise BP pulsatility conferred better 1-year outcomes.
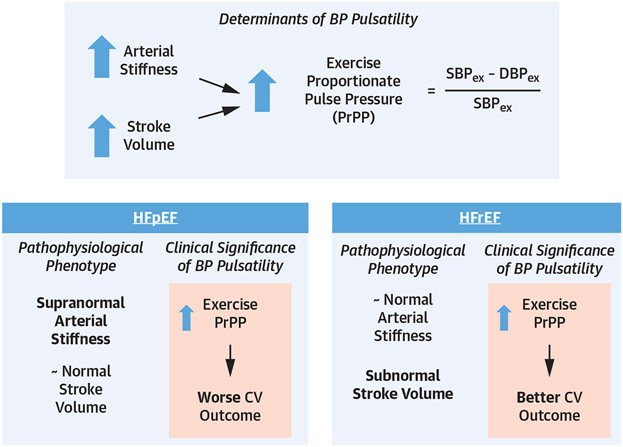
Pulsatility of exercise BP in HFpEF, as measured using PrPP, appears to be a summation of conflicting processes (Central Illustration). On the one hand, exercise PrPP is a result of stroke volume reserve capacity—the ability to augment stroke volume during exercise. On the other hand, PrPP also indicates underlying arterial stiffness and early wave reflection. To the latter point, arterial stiffness and associated wave reflection are known to increase pulsatile BP and contribute to diastolic dysfunction due to increased left ventricular afterload and impaired myocardial energetics.14,15 The fact that a particularly pulsatile BP response relative to SBP increase (PrPP) in HFpEF is associated with worse cardiovascular outcome suggests that the arterial stiffness associations with PrPP outweigh the favorable (stroke volume augmentation) aspect of elevated PrPP in driving clinical outcomes in HFpEF. Notably, others have described relative preservation of stroke volume response during exercise in HFpEF, as well as the lack of clinical consequence of poor stroke volume augmentation in HFpEF.16,17
CENTRAL ILLUSTRATION. BP Responses to Exercise in Heart Failure.
PrPP at peak exercise in HFpEF and HFrEF. In HFpEF, higher PrPP indicates not only increased stroke volume reserve, but also underlying arterial stiffness, which predisposes to worse CV outcome. In HFpEF, increased stroke volume reserve drives PrPP at peak exercise, indicating contractile reserve, leading to better CV outcome. BP = blood pressure; CV = cardiovascular; DBPex = diastolic blood pressure at peak exercise; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; PrPP = proportionate pulse pressure; SBPex = systolic blood pressure at peak exercise.
Although the role of arterial stiffness and pulsatile BP in HFpEF is established,7-10 these insights have been gained from studies evaluating resting BP measures. Our study is the first to evaluate the exercise-induced BP pulsatility to reveal underlying pathophysiological phenotype and gain insight into risks of adverse outcomes in HFpEF.
Other studies have evaluated the prognostic value of pulse pressure in HFpEF, but these have primarily focused on resting measures taken in hospitalized patients before discharge and results have been mixed. Teng et al18 showed that in HFpEF there was a trend toward worse outcome with resting higher pulse pressure but also worse outcome with low pulse pressure <50 mm Hg. Laskey et al19 also showed higher risk of adverse outcome with higher resting pulse pressure at discharge, but again a protective effect of higher pulse pressure up to 50 mm Hg. Tokitsu et al20 also showed a U-shaped curve for adverse outcome with BP pulsatility in HFpEF, but the degree of left ventricular systolic dysfunction was unknown. These data suggest that the relationship between resting pulse pressure and outcome in HFpEF is not straightforward. Through thorough exercise hemodynamic phenotyping, we have shown that inducible BP pulsatility can reveal underlying cardiovascular pathophysiology that in turn is related to adverse outcome. We note that exercise testing is simple, safe, and often undertaken in patients with HFpEF and HFrEF and suggest that this approach can help clinicians better assess patients with HF.
The role of exercise-based measurements to characterize HFpEF is increasingly recognized, with exercise assessment endorsed as part of the recently developed European Society of Cardiology HFpEF Diagnostic Algorithm.21 However, this algorithm does not include any information about BP patterns during exercise and previous societal scientific statements on exercise testing standardization only mention an unchanged SBP or a decrease in SBP with exercise being associated with adverse outcome. In the present study, we have demonstrated that exercise assessment of BP in HFpEF can reveal changes that associate with adverse hemodynamic response (elevated exercise PCWP) and adverse outcomes. Although not a substitute for detailed hemodynamic phenotyping with invasive CPET, exercise BP assessment could provide a more easily accessible insight into disease phenotype and risk of adverse outcome in HFpEF.22,23 Our findings for SBP and DBP are consistent with previous studies performed without concomitant invasive hemodynamic measurements showing that in middle-aged adults without HF, increased exercise DBP and SBP predict adverse cardiovascular outcomes.22
Although not directly assessed in this study, our findings also provide some insight into potential therapeutic avenues. The importance of wave reflection in creating an adverse hemodynamic state is well established.24-26 Targeted intervention has been shown to be effective in reducing wave reflection and might reduce the adverse ventricular-vascular interaction in patients with particularly pulsatile exertional BP responses in HFpEF.27 With emerging therapies in HF28,29 that have shown subgroup benefits in older persons and females, further investigation is required to evaluate the role assessment of BP pulsatility might play in better targeting therapies in HFpEF.
To more widely use our approach, we must gain clarity on the comparative information yielded from noninvasive BP monitoring during exercise. The RELAX (PhosphodiesteRasE-5 Inhibition to Improve CLinical Status And EXercise Capacity in Diastolic Heart Failure)-HFpEF trial used the same entry criteria to identify patients with HFpEF that were used in our study, yet relied on noninvasive BP measurement during exercise.30 In the RELAX-HFpEF cohort excursions in SBP were very similar (our study +37 mm Hg vs RELAX +34 mm Hg) and rest and exercise PrPP were within 0.03 of one another (rest: our study 0.48, RELAX 0.46; peak exercise: our study 0.53, RELAX 0.55). Of note, when considering applying these findings more widely, we highlight the fact that our study used upright exercise, which is known to more effectively gauge exercise tolerance than supine exercise.31
STUDY LIMITATIONS.
Our findings should be interpreted in context of our study’s limitations. Our cohort was composed of patients referred for invasive exercise hemodynamic testing, introducing selection bias. This allowed us to use a strict definition for HFpEF, which is a strength, but our findings should nevertheless be validated in broader populations. This selection bias also led to a younger cohort of HFpEF and HFrEF in our cohort than seen in the general community. Our use of a radial artery catheter to measure peripheral BP is a strength in being able to accurately discern BP continuously and BP changes during exercise were similar to those in other HFpEF studies,12 but our results should be confirmed using more widely used noninvasive cuff measurements during exercise.
We measured AP as a marker of wave reflection and interpreted this as a marker of arterial stiffness, rather than measuring arterial stiffness directly (with pulse wave velocity). Correlations between arterial stiffness and wave reflection are well established, however, wave reflection is not solely dependent on arterial stiffness.12,26 Our HFrEF cohort was small because invasive exercise CPET is infrequent in this group, and arterial stiffness data was also not available in all patients. The late separation in the Kaplan-Meier curves for the HFpEF cohort and P value of 0.043 indicates a low fragility index but importantly the directionality of outcomes data clearly differs from that in HFrEF. Finally, our cohort had little racial and ethnic diversity highlighting the need for larger studies in diverse cohorts with assessment of easily measured BP response patterns to ensure consistency of findings.
CONCLUSIONS
Exercise-induced BP pulsatility in HFpEF reveals underlying arterial stiffness and is associated with adverse cardiovascular outcome. This is in contrast to HFrEF, where exercise-induced BP pulsatility indicates stroke volume reserve and favorable outcome. Exercise BP assessment can provide hemodynamic and clinical insight in HF.
Supplementary Material
NOVEL FINDINGS.
The basis and implications of inducible exercise BP pulsatility are different in HFpEF and HFrEF
In HFpEF, inducible exercise BP pulsatility reveals arterial stiffness and predicts worse outcomes.
Contrastingly, in HFrEF, inducible exercise BP pulsatility reveals stroke volume reserve and predicts better outcomes.
PERSPECTIVE.
COMPETENCY IN MEDICAL KNOWLEDGE:
Ventricular-vascular interaction in HFpEF is different to that in HFrEF and can be assessed using exercise PrPP response, a marker that has a different pathophysiological basis and set of clinical implications depending upon HF phenotype of HFpEF vs HFrEF.
TRANSLATIONAL OUTLOOK:
Future studies should further evaluate the bases for the contrasting mechanisms and consequences of ventricular-vascular interactions in HFpEF and HFrEF and also evaluate implications for diagnosis and management.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
Dr Namasivayam is supported by a Clinical and Research Fellowship from the Division of Cardiology, Massachusetts General Hospital, Harvard Medical School; and is a recipient of the St. Vincent’s Clinic Travelling Fellowship Award. Dr Lewis is supported by the American Heart Association Award 15GPSGC24800006 and by the National Heart, Lung and Blood Institute Awards R01-HL 131029 and R01-HL151841. Dr Nayor is supported by National Institutes of Health/National Heart, Lung, and Blood Institute K23-HL138260. Dr Ho is supported by National Institutes of Health/National Heart, Lung, and Blood Institute R01-HL134893 and R01-HL140224. Dr Malhotra is supported by Transformation Project Award 18TPA34230025 from the American Heart Association and grant R01-HL142809 from the National Heart, Lung, and Blood Institute. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- AP
augmented pressure
- BMI
body mass index
- BP
blood pressure
- CPET
cardiopulmonary exercise test
- DBP
diastolic blood pressure
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- PCWP
pulmonary capillary wedge pressure
- PrPP
proportionate pulse pressure
- SBP
systolic blood pressure
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
APPENDIX For a supplemental table, please see the online version of this paper.
REFERENCES
- 1.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;5:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisman AS, Shah RV, DhakaL BP, et al. Pulmonary capillary wedge pressure patterns during exercise predict exercise capacity and incident heart failure. Circ Heart Fail. 2018;11:e004750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsimploulis A, Lam PH, Arundel C, et al. Systolic blood pressure and outcomes in patients with heart failure with preserved ejection fraction. JAMA Cardiol. 2018;3:288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandesara PB, O’Neal WT, Kelli HM, Topel M, Samman-Tahhan A, Sperling LS. Diastolic blood pressure and adverse outcomes in the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) Trial. J Am Heart Assoc. 2018;7:e007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA. 1989;261:884–888. [PubMed] [Google Scholar]
- 6.Petrie CJ, Ponikowski P, Metra M, et al. Proportional pulse pressure relates to cardiac index in stabilized acute heart failure patients. Clin Exp Hypertens. 2018;40:637–643. [DOI] [PubMed] [Google Scholar]
- 7.Chirinos JA, Segers P, Hughes T, Townsend R. Large-artery stiffness in health and disease. J Am Coll Cardiol. 2019;74:1237–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adji A, O’Rourke MF, Namasivayam M. Arterial stiffness, its assessment, prognostic value and implications for treatment. Am J Hypertens. 2011;24:5–17. [DOI] [PubMed] [Google Scholar]
- 9.Ohyama Y, Ambale-Venkatesh B, Noda C, et al. Association of aortic stiffness with left ventricular remodeling and reduced left ventricular function measured by magnetic resonance imaging: the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2016;9: e004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chirinos JA. Deep phenotyping of systemic arterial hemodynamics in HFpEF (Part 2): clinical and therapeutic considerations. J Cardiovasc Transl Res. 2017;10:261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nayor M, Houstis NE, Namasivayam M, et al. Impaired exercise tolerance in heart failure with preserved ejection fraction: quantification of multi-organ system reserve capacity. J Am Coll Cardiol Heart Fail. 2020;8:605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Townsend RR, Wilkinson IB, Schiffrin EL, et al. Recommendations for improving and standardizing vascular research on arterial stiffness. Hypertension. 2015;66:698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Namasivayam M, McEniery CM, Wilkinson IB, et al. Different effects of vascular aging on ischemic predisposition in healthy men and women. Hypertension. 2018;72:1294–1300. [DOI] [PubMed] [Google Scholar]
- 15.Russo C, Jin Z, Palmieri V, et al. Arterial stiffness and wave reflection: sex differences and relationship with left ventricular diastolic function. Hypertension. 2012;60:362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimiaie J, Sherez J, Aviram G, et al. Determinants of effort intolerance in patients with heart failure: combined echocardiography and cardiopulmonary stress protocol. J Am Coll Cardiol HF. 2015;3:803–814. [DOI] [PubMed] [Google Scholar]
- 17.Bhella PS, Prasad A, Heinicke K, et al. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;12:1296–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teng THK, Tay WT, Dahlstrom U, Benson L, Lam CSP, Lund LH. Different relationships between pulse pressure and mortality in heart failure with reduced, mid-range and preserved ejection fraction. Int J Cardiol. 2018;254:203–209. [DOI] [PubMed] [Google Scholar]
- 19.Laskey WK, Wu J, Schulte PJ, et al. Association of arterial pulse pressure with long-term clinical outcomes in patients with heart failure. J Am Coll Cardiol HF. 2016;4:42–49. [DOI] [PubMed] [Google Scholar]
- 20.Tokitsu T, Yamamoto E, Hirata Y, et al. Clinical significance of pulse pressure in patients with preserved left ventricular ejection fraction. Eur J Heart Fail. 2016;18:1353–1361. [DOI] [PubMed] [Google Scholar]
- 21.Pieske B, Tschope C, de Boer RA, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40:3297–3317. [DOI] [PubMed] [Google Scholar]
- 22.Schultz MG, Otahal P, Cleland VJ, Blizzard L, Marwick TH, Sharman JE. Exercise-induced hypertension, cardiovascular events, and mortality in patients undergoing exercise stress testing: a systematic review and meta-analysis. Am J Hypertens. 2013;26:357–366. [DOI] [PubMed] [Google Scholar]
- 23.Ha JW, Andersen OS, Smiseth OA. Diastolic stress test: invasive and noninvasive testing. J Am Coll Cardiol Img. 2020;13:272–282. [DOI] [PubMed] [Google Scholar]
- 24.Namasivayam M, McDonnell BJ, McEniery CM, O’Rourke MF. Does wave reflection dominate age-related change in aortic blood pressure across the human life span? Hypertension. 2009;53:979–985. [DOI] [PubMed] [Google Scholar]
- 25.Namasivayam M, Adji A, O’Rourke MF. Evaluating the hemodynamic basis of age-related central blood pressure change using aortic flow triangulation. Am J Hypertens. 2016;29:178–184. [DOI] [PubMed] [Google Scholar]
- 26.Peterson VR, Woodiwiss AJ, Libhaber CD, Raymond A, Sareli P, Norton GR. Cardiac diastolic dysfunction is associated with aortic wave reflection, but not stiffness in a predominantly young-to-middle-aged community sample. Am J Hypertens. 2016;29:1148–1157. [DOI] [PubMed] [Google Scholar]
- 27.Reddy YNV, Andersen MJ, Obokata M, et al. Arterial stiffening with exercise in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2017;70:136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solomon SD, McMurray JJV, Anand IS, et al. Angiotensin-neprolysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–1620. [DOI] [PubMed] [Google Scholar]
- 29.McMurray JJV, Jackson AM, Lam CSP, et al. Effects of sacubitril-valsartan versus valsartan in women compared with men with heart failure and preserved ejection fraction. Circulation. 2020;141: 338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohammed SF, Borlaug BA, McNulty S, et al. Resting ventricular-vascular function and exercise capacity in heart failure with preserved ejection fraction: a RELAX trial ancillary study. Circ Heart Fail. 2014;7:580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kramer B, Massie B, Topic N. Hemodynamic differences between supine and upright exercise in patients with congestive heart failure. Circulation. 1982;66:820–825. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.