Abstract
Many previous studies have shown that certain cardiovascular conditions (including myocarditis, arrhythmias, and cardiomyopathy) are more prevalent in individuals with celiac disease compared to individuals without the disease. Association of celiac disease with dilated cardiomyopathy (DCMP) is a rare occurrence and a few cases have been reported and even fewer in children. Here, we report an interesting case of a 10-year-old male child who presented to the pediatric emergency in a life-threatening condition with congestive cardiac failure manifested by dyspnea, hepatomegaly, pedal edema, and raised JVP with underlying severe anemia. The diagnosis of DCMP associated with celiac disease was made. The child was advised for strict gluten-free diet and hematinics, and ivabradine was started for managing DCMP. Early diagnosis with screening tests may prevent serious complications and also are essential to prevent progression of the disease.
Keywords: Association, celiac, child, dilated cardiomyopathy, disease, gluten free, heart failure
Introduction
Celiac disease (CD) is caused by inflammation of the small intestinal mucosa due to ingestion of gluten-containing diet in genetically susceptible individuals, and has an estimated prevalence of about 1% in the general population.[1] It is characterized by chronic malabsorption associated with ingestion of grains containing gluten, such as wheat, barley, and rye. It is associated with various autoimmune disorders including autoimmune thyroid disease, type I diabetes mellitus, dermatitis herpetiformis, selective Ig A deficiency, sclerosing cholangitis, epilepsy, and others.[2,3] Many previous studies have shown that certain cardiovascular conditions (including myocarditis, arrhythmias, and cardiomyopathy) are more prevalent in individuals with CD compared to individuals without the disease.[4] Association of CD with dilated cardiomyopathy (DCMP) is a rare occurrence, and a few cases have been reported and even fewer in children.[5] Here, we report an interesting case of a 10-year-old boy who had two previous episodes of congestive heart failure with underlying anemia over a span of 2 months. He was later diagnosed to have CD along with DCMP.
Case Report
Here, we report an interesting case of a 10-year-old male child who presented to the pediatric emergency in a life-threatening condition with congestive cardiac failure manifested by dyspnea, hepatomegaly, pedal edema, and raised Jugular Venous Pressure (JVP) with underlying severe anemia (hemoglobin [Hb] = 2.8 gm%). The child was transfused with 2 units of packed red blood cells (PRBC). He was also given 100% supplemental oxygen inhalation and intravenous fluids. After stabilization, the child was shifted to pediatric ward. The child had a similar history 2 months before this hospitalization. He was then treated in a private hospital, where 2 units of PRBC were transfused and he was discharged thereafter on medical management, which he did not take. There was no other significant past history. There was no significant family history of any chronic illness, like history of blood transfusions, heart disease, or early sudden death in family.
In the ward, at day 2 of admission, except pallor, no other findings were observed on general and systemic examination. Anthropometry of the child was as per the World Health Organization (WHO) standards. Workup of the child was done to ascertain the cause of anemia and congestive cardiac failure. Laboratory findings revealed microcytic hypochromic anemia (Hb = 2.8 gm/dL), low serum ferritin, and low transferrin saturation, suggestive of iron deficiency anemia. Bone marrow aspiration was done and it confirmed similar findings. Liver function tests showed hypoalbuminemia (2.4 gm%), raised transaminases (alanine transaminase [ALT] 500 U/L, AST 200 U/L), and raised alkaline phosphatase (1123 KAU) levels. Serum cholesterol, triglyceride levels, blood urea nitrogen, and serum creatinine levels were within normal limits. Thyroid function tests were done to ascertain the cause of Congestive heart failure (CHF) and were found to be normal. Creatine phosphokinase (CPK) levels (1500 U/L) were significantly raised. Chest radiography revealed cardiomegaly [Figure 1]. Electrocardiogram (ECG) showed left bundle branch block. Echocardiography showed global hypokinesia with a left ventricular dilatation (End diastolic diameter (EDD) 41 mm) and ejection fraction of 25%–30%. The pulmonary arterial pressures were also elevated. To find the cause of severe anemia, tissue transglutaminase antibody levels was measured, which were found to be raised 220 U/ml (reference range, <20 U/mL), and anti-endomysial antibody titer was also positive. Endoscopic biopsy of the second part of the duodenum was done, which showed villous atrophy, confirming CD with stage Marsh 3b [Figure 2]. The diagnosis of DCMP associated with CD was made. The child was advised strict gluten-free diet and hematinics, and ivabradine (hyperpolarization-activated cyclic nucleotide-gated channel blocker) was started for managing DCMP after consultation with a cardiologist. The child was discharged in a stable condition and is currently on regular follow-up and is doing well. IgA transglutaminase antibodies levels are being tested for assessing compliance to follow-up and they are showing decreasing trend. Echocardiography is also planned on follow-up after 6 months. A written informed consent was obtained from the parents. Institutional ethics committee reviewed and cleared the case report for publication.
Figure 1.
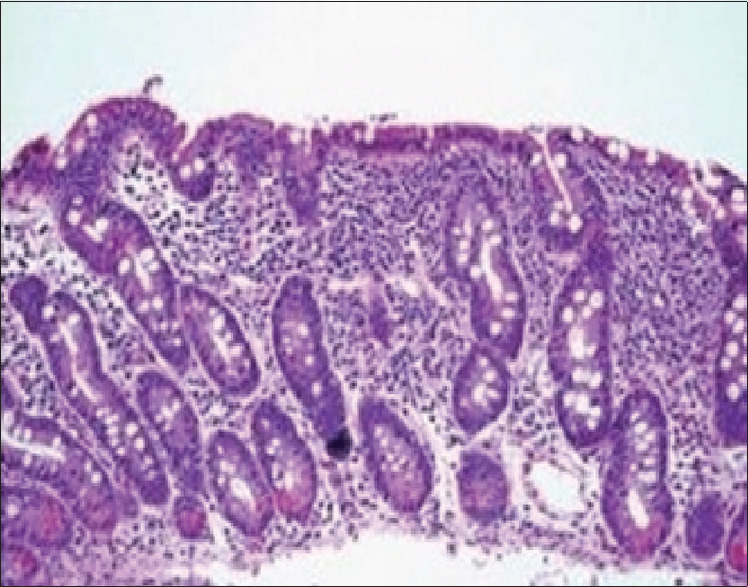
Duodenal biopsy showing Marsh 3B stage
Figure 2.
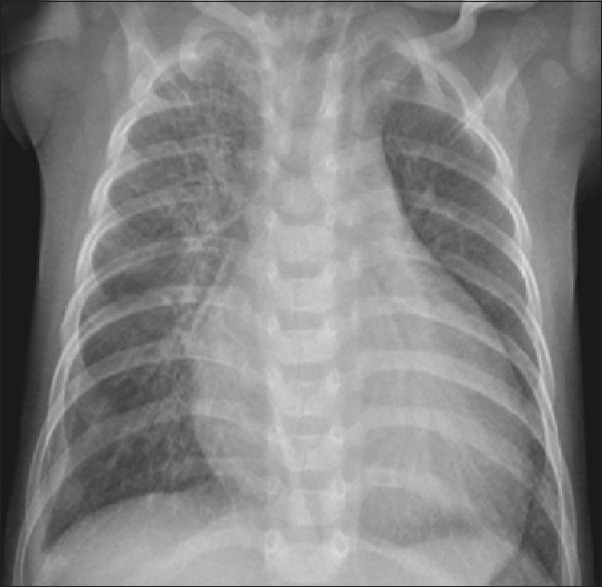
Chest X-ray showing cardiomegaly
Discussion
CD is a multisystem disorder of autoimmune etiology, associated with intolerance to gluten. It is a disease of the small intestine that develops in genetically susceptible individuals. Gluten ingestion causes inflammation of the intestinal mucosa leading to hyperplasia of the crypts and villous atrophy. Patients classically present with failure to thrive, chronic diarrhea, poor appetite, abdominal distension, and pain.[6] Other clinical findings include iron deficiency anemia, osteomalacia, coagulopathy, and peripheral neuropathy. It has approximately 10% prevalence among first-degree relatives. Other autoimmune disorders, such as diabetes mellitus and thyroid disorders, are commonly associated with CD. Many studies conducted on adults previously have shown association of CD and DCMP.[7,8,9] It has been reported that there is an increased incidence of CD in patients with DCMP, but secondary cardiomyopathy has also been reported in known CD patients.[10,11] DCMP is defined as the presence of left ventricular dysfunction with chamber dilatation. Where the etiology is unknown, an autoimmune pathogenesis has been suggested as in cases of CD.[12] It may be related to the phenomenon of antigenic mimicry, where the autoimmune response is directed at the myocardium and small bowel. It has been shown that patients of CD with good adherence to gluten-free diet demonstrate significant recovery of ventricular volumes and function.[13] Malabsorption resulting in micronutrient deficiencies, for example, of thiamine, calcium, selenium, and carnitine, which are essential components of myocardial contractility and associated gut edema due to cardiac failure, contributes to it.[14] Other hypotheses which have been suggested include nutritional deficiencies. Chronic anemia leads to a hyperdynamic state and can progress to cardiac failure.
In pediatric population, data regarding association of CD and myocarditis or cardiomyopathy is very limited. Dogan et al.[15] reported an 8-year-old girl who was a known case of CD, presenting with stroke and DCMP. Frustaci et al.[10] reported few cases of lymphocytic myocarditis and associated CD. A nationwide study done in the USA in 2012 found a moderately increased risk of idiopathic DCMP in patients with biopsy-verified CD.[16]
CD goes undiagnosed in the community as classical symptoms may be lacking and anemia, in general, is very common and also a part of CD presentation itself. Hence, in community settings, the family may keep going for treatment with supplements or transfusion without getting to the root of the problem, ultimately leading to complications in children. As it is an easily treatable disease, family physicians and primary care physicians should know the uncommon presentations of CD also for timely diagnosis and management.
Conclusion
DCMP associated with CD is potentially a fatal condition. It requires a multidisciplinary approach for appropriate management. Early diagnosis with screening tests may prevent serious complications and also are essential to prevent progression of the disease. It has been proven that strict adherence to gluten-free diet is effective in reversing the cardiac changes and improving the quality of life of the patient. Children who present with DCMP or myocarditis with no known etiologies should be thoroughly investigated.
Key points
DCMP associated with CD is a potentially fatal condition. It requires a multidisciplinary approach for appropriate management.
Early diagnosis with screening tests may prevent serious complications and also are essential to prevent progression of the disease.
Children who present with DCMP or myocarditis with no known etiologies should be thoroughly investigated.
As it is an easily treatable disease, family physicians and primary care physicians should know the uncommon presentations of CD also for timely diagnosis and management.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Maki M, Mustalahti K, Kokkonen J, Kulmala P, Haapalahti M, Karttunen T, et al. Prevalence of celiac disease among children in Finland. N Engl J Med. 2003;348:2517–24. doi: 10.1056/NEJMoa021687. [DOI] [PubMed] [Google Scholar]
- 2.Counsell CE, Taha A, Ruddell WSJ. Coeliac disease and autoimmune thyroid disease. Gut. 1994;35:844–6. doi: 10.1136/gut.35.6.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meini A, Pillan NM, Villanacci V, Monafo V, Ugazio AG, Plebani A. Prevalence and diagnosis of celiac disease in IgA-deficient children. Ann Allergy Asthma Immunol. 1996;77:333–6. doi: 10.1016/S1081-1206(10)63329-7. [DOI] [PubMed] [Google Scholar]
- 4.Bayar N, Çagırcı G, Ureyen ÇM, Kuş G, Küçükseymen S, Arslan S. The relationship between spontaneous multi-vessel coronary artery dissection and celiac disease. Korean Circ J. 2015;45:242–4. doi: 10.4070/kcj.2015.45.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curione M, Barbato M, DeBiase L, Viola F, LoRusso L, Cardi E. Prevalence of coeliac disease in idiopathic dilated cardiomyopathy. Lancet. 1999;354:222–3. doi: 10.1016/s0140-6736(99)01501-9. [DOI] [PubMed] [Google Scholar]
- 6.Farrell RJ, Kelly CP. Celiac sprue. N Engl J Med. 2002;346:180–8. doi: 10.1056/NEJMra010852. [DOI] [PubMed] [Google Scholar]
- 7.Chuaqui B, Garrido J, Casanegra P. Actin-deficient cardiomyopathy coexisting with celiac disease:A chance association? Pathol Res Pract. 1986;181:604–14. doi: 10.1016/S0344-0338(86)80155-8. [DOI] [PubMed] [Google Scholar]
- 8.Makhdoom Z, Randall N. Dilated cardiomyopathy due to anticardiolipin syndrome in association with celiac sprue. J Clin Gastroenterol. 2000;31:91–2. doi: 10.1097/00004836-200007000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Curione M, Barbato M, Viola F, Francia P, De Biase L, Cucchiara S. Idiopathic dilated cardiomyopathy associated with celiac disease:The effect of a gluten-free diet on cardiac performance. Dig Liver Dis. 2002;34:866–9. doi: 10.1016/s1590-8658(02)80258-4. [DOI] [PubMed] [Google Scholar]
- 10.Frustaci A, Cuoco L, Chimenti C, Pieroni M, Fioravanti G, Gentiloni N, et al. Celiac disease associated with autoimmune myocarditis. Circulation. 2002;105:2611–8. doi: 10.1161/01.cir.0000017880.86166.87. [DOI] [PubMed] [Google Scholar]
- 11.Patel P, Smith F, Kilcullen N, Artis N. Dilated cardiomyopathy as the first presentation of coeliac disease:Association or causation? Clin Med. 2018;18:177–9. doi: 10.7861/clinmedicine.18-2-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chimenti C, Pieroni M, Maseri A, Frustaci A. Dilated cardiomyopathy and celiac disease. Ital Heart J. 2002;384:5. [PubMed] [Google Scholar]
- 13.Milisavljević N, Cvetković M, Nikolić G, Filipović B, Milinić N. Dilated cardiomiopathy associated with celiac disease:Case report and literature review. Srp Arh Celok Lek. 2012;140:641–3. [PubMed] [Google Scholar]
- 14.Witte KK, Clark AL, Cleland JG. Chronic heart failure and micronutrients. J Am Coll Cardiol. 2001;37:1765–74. doi: 10.1016/s0735-1097(01)01227-x. [DOI] [PubMed] [Google Scholar]
- 15.Dogan M, Peker E, Cagan E, Akbayram S, Acikgoz M, Caksen H, et al. Stroke and dilated cardiomyopathy associated with celiac disease. World J Gastroenterol. 2010;16:2302–4. doi: 10.3748/wjg.v16.i18.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emilsson L, Andersson B, Elfström P, Green PH, Ludvigsson JF. Risk of idiopathic dilated cardiomyopathy in 29 000 patients with celiac disease. J Am Heart Assoc. 2012;1:e001594. doi: 10.1161/JAHA.112.001594. [DOI] [PMC free article] [PubMed] [Google Scholar]


