Abstract
Context:
Living with diabetes can be difficult since it can affect the patient in many ways. Diabetic foot syndrome (DFS) is described as a group of symptoms where neuropathy reduced blood supply and infection leads to tissue breakdown and morbidity.
Aim:
This study aims to determine the prevalence of DFS and associated sociodemographic and treatment-related factors among adults living with type 2 diabetes mellitus in a rural community.
Setting and Design:
A cross-sectional study was conducted in an area under the rural health training centre of department of Community Medicine.
Methods and Material:
The study was conducted to determine DFS by measuring neuropathy, peripheral vascular disease using Michigan neuropathy screening instrument, and clinical examination.
Statistical Analysis Used:
The data collected was analyzed using SPSS 25.
Results:
The prevalence of DFS among those with type 2 diabetes mellitus was high (51.7%). DFS was associated with advanced age (>75 years), duration of diabetes for more than 5 years and with foot ulcer. Smoking and alcohol consumption were not associated with DFS.
Conclusion:
Half of those with diabetes had DFS. People with DFS were more likely to be older and living with diabetes for longer duration. This underscores the need for early identification of DFS by the primary care physicians. Further research on the role of health professionals at the primary care level in educating and screening DFS in people with diabetes are required.
Keywords: Diabetic foot syndrome, monofilament, peripheral neuropathy, rural areas, screening
Introduction
Evidence suggests that there are around 451 million cases in people living with diabetes mellitus in 2017 which is forecasted to reach 693 million people by 2045.[1] Diabetes and its complications are expected to result in increasing morbidity, mortality, health care utilization, and higher financial burden due to the requirement of specialized care. Diabetes-related complications, such as neuropathy, peripheral vascular disease, ulceration, and infection are the leading causes of hospitalization.[2] Diabetic foot syndrome (DFS) is a serious complication of diabetes mellitus and is described as a group of symptoms including neuropathy, reduced blood supply and infection leading to tissue breakdown, morbidity that may be followed by amputation.[3]
Diabetic neuropathy is a dreaded complication leading to disability and significant impairment of quality of life. It is not uncommon to have patients presenting with symptoms of diabetic foot even at the initial diagnosis of diabetes.[2] The delay in recognizing the symptoms of neuropathy and peripheral vascular disease lead to diabetic foot ulcers, culminating in loss of lower limb.[4] The lifetime risk for developing the diabetic foot ulceration is one in four patients with diabetes, of which a vast majority will need amputation in 4 years of the diagnosis.[2] The established risk factors for diabetic ulcers are mainly advanced age, past history of ulcers, and polyneuropathy. The lesions on the foot in people with diabetes may lead to improper cellular wound healing. Neuropathy itself can have a negative impact on wound healing.[2] Defining better preventive strategies of diabetic foot ulceration is critical to reduce the associated morbidity and mortality. Primary care physicians should be watchful as individuals attending them may not have overt symptoms but would be in different stages of development of the problem. At the primary care level, there is need for screening tests to identify at risk foot at an early stage, pressure relief, care of the wound and appropriate referral.[5] Although the burden of diabetes is known to be very high in Kerala, there is scare evidence on DFS. Therefore, this study aimed at examining DFS and the associated factors at community level.
Subjects and Methods
A cross-sectional-analytical study was conducted in an area adopted under the rural health training centre (RHTC) of Department of Community Medicine in a teaching hospital in Kerala, India to satisfy the objectives of the study. After obtaining approval from Institutional Ethics Committee, the study was carried out for 2 months (July–August 2019). Adults (>18 years) with a diagnosis of type II diabetes mellitus in an area under the RHTC formed the study sample.
The minimum sample size calculated was 99 considering the prevalence of DFS to be 51%,[6] an allowable error of 10% at a confidence level of 95%. The minimum sample size was set as 120 considering a non-response rate of 20%. Patients of either gender having a diagnosis of diabetes for at least 6 months residing in the study area who understood Malayalam or English were included in the study. All patients who are severely ill, with psychiatric illness, not able to respond, patients who had a fracture or bone surgery, patients with gangrenous foot of aetiology other than infection of foot, were excluded from the study. The study tools used were (1) a semi-structured questionnaire used to collect the sociodemographic data, diabetes related history, (2)
Michigan neuropathy screening instrument (MNSI) was used to screen for diabetic peripheral neuropathy. The two components included in the instrument are history and physical assessment. First part of the screening instrument includes 15 self-administered ‘YES’ or ‘NO’ questions on the foot sensation including pain, numbness and temperature sensitivity. A higher score >13 indicates more neuropathic symptoms. The second part of MNSI is a brief physical examination comprising inspection of the foot for deformities, dry skin, hair or nail abnormalities, callous or infection, semi-quantitative assessment of vibration sensation at the dorsum of the great toe, grading of ankle reflexes, Semmes–Weinstein monofilament testing. Patients screened positive on clinical portion of MNSI was considered neuropathic (greater than 2 points on a ten-point scale). Permission to use the instrument was obtained,[7] (3) vascular assessment of feet was done by manual assessment of foot pulses in both lower limbs for posterior tibial and dorsalis pedis artery pulses and manual measurement of ankle- brachial index (ABI). Absence of pulses and ABI less than or equal to 0.9 was considered as peripheral arterial disease (PAD).[8] The subjects found to be having foot problems were classified according to The International Working Group on Diabetic Foot (IWGDF) Risk Classification System as grade 1, 2, and 3.[9]
With RHTC as centre, one lane was selected by simple random sampling, data collection was started from the first house till the end of lane and continued with the next lane on the right till the desired sample size was obtained. Eligible participants were approached, and the objectives of the research were explained. A written informed consent was taken from all the study subjects. The above-mentioned tools were used to collect the data. The at-risk patients identified were given health education on self-care management and were referred to higher centre depending on the clinical status.
Data analysis
Basic socio-demographic characteristics and prevalence were presented as frequency and percentages. Continuous data was summarized as mean and standard deviation (SD). Chi-square test was done to find the association between categorical variables. Data obtained was analyzed using SPSS 25 (Armonk, NY, USA). A confidence interval (CI) of 95% and level of significance of 0.05 was set.
Results
Among the 120 study subjects with type 2 diabetes mellitus, 63 (52.5%) were females. More than half (57.5%) belonged to >55 years age group and were unemployed (55%). The mean age of the study group was 60.21 ± 14.23. Around 14.2% of the total study subjects were either illiterate or had only primary education. The sociodemographic variables are presented in Table 1.
Table 1.
Socio-demographic characteristics of the study sample
| Variable | Groups | Frequency (n) | Percentage (%) |
|---|---|---|---|
| Gender | Male | 57 | 47.50 |
| Female | 63 | 52.50 | |
| Age | <=55 years | 51 | 42.50 |
| 56-75 years | 47 | 39.16 | |
| >75 years | 22 | 18.33 | |
| Education | Postgraduate/Graduate | 21 | 17.50 |
| Intermediate/Diploma | 36 | 30.00 | |
| High/Middle school | 46 | 38.33 | |
| Primary/Illiterate | 17 | 14.16 | |
| Occupation | Professional/Semi-professional | 14 | 11.66 |
| Clerk/Shop | 21 | 17.50 | |
| Skilledandsemi-skilled | 6 | 5.00 | |
| Unskilled | 13 | 10.83 | |
| Unemployed | 66 | 55.00 |
Figure 1 illustrates the prevalence of DFS in the study sample. The prevalence of peripheral neuropathy was 51.7%, peripheral neuropathy with peripheral vascular disease was 33.3% and peripheral neuropathy with foot ulcer was 15%. There was significant association between DFS and age (P < 0.001), people with DFS were more likely to be older compared with those without. The proportion of those with DFS was higher among males (63.2%) than in females (42.9%). There was significant association between gender and DFS (OR = 2.29, P = 0.03). In occupational class, the proportion of DFS was higher in unemployed (57.6%) and clerical/shop owner groups (57.1%); and people with lowest education (70.2%) followed by high school (58.7%) and intermediate (44.4%) in educational class. However, the association was not found to be statistically significant [Table 2].
Figure 1.
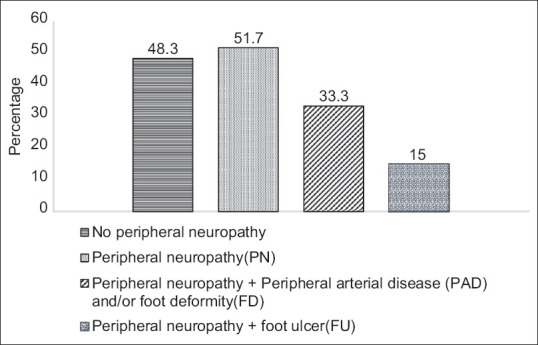
Bar diagram showing prevalence of diabetic foot syndrome in the study sample
Table 2.
Association between diabetic foot syndrome and sociodemographic characteristics in the study sample
| DFS | Crude Odds Ratio | P | Confidence interval (95%) | ||
|---|---|---|---|---|---|
|
| |||||
| Absent n (%) | Present n (%) | ||||
| Age | |||||
| <=55 (ref) | 35 (68.6) | 16 (31.4) | |||
| 56-75 | 18 (38.3) | 29 (61.7) | 3.524 | 0.003 | 1.53-8.12 |
| >75 | 4 (18.2) | 18 (81.8) | 9.844 | <0.001 | 2.87-33.83 |
| Gender | |||||
| Male | 21 (36.8) | 36 (63.2) | 2.286 | 0.027 | 1.10-4.76 |
| Female (ref) | 36 (57.1) | 27 (42.9) | |||
| Occupation | |||||
| Professional Semi professional (ref) | 10 (71.4) | 4 (28.6) | |||
| Clerk/Shop | 9 (42.9) | 12 (57.1) | 3.333 | 0.103 | 0.79-14.16 |
| Skilled and semi-skilled | 3 (50.0) | 3 (50.0) | 2.500 | 0.363 | 0.35-18.04 |
| Unskilled | 7 (53.8) | 6 (46.2) | 2.143 | 0.348 | 0.44-10.53 |
| Unemployed | 28 (42.4) | 38 (57.6) | 3.393 | 0.057 | 0.96-11.94 |
| Education | |||||
| Postgraduate Graduate (ref) | 13 (61.9) | 8 (38.1) | |||
| Intermediate Diploma | 20 (55.6) | 16 (44.4) | 1.300 | 0.640 | 0.43-3.90 |
| High school Middle school | 19 (41.3) | 27 (58.7) | 2.309 | 0.121 | 0.80-6.65 |
| Primary/illiterate | 5 (29.4) | 12 (70.2) | 3.900 | 0.051 | 0.996-15.28 |
Type of treatment was found to be significantly associated with DFS (OR = 25, P = 0.001). It was highest among people taking insulin (88.2%) followed by insulin and oral treatment (87.5%). Among participants using government health centre, 60% were having DFS when compared with 48.8% using private health services. There was no significant association between DFS and treatment centre. Among the study participants who had diabetes for more than 5 years, 62.8% had DFS compared with only 33.3% in those having diabetes for less than 5 years. Study shows significant association between development of DFS and duration of diabetes (OR = 3.38, P = 0.002) [Table 3]. The prevalence of diabetic foot ulcer in the study sample was found to be 18%. Presence of foot ulcer also showed a significant association with DFS (P = 0.001). Table 4 shows the logistic regression model with DFS as the dependent variable. A combination of oral drugs and insulin was found to be an independent risk factor associated with DFS even after adjusting for age, gender and duration of disease. (OR = 15.87, P = 0.005)
Table 3.
Association between diabetic foot syndrome and treatment details in the study sample
| Treatment details | DFS | Crude Odds Ratio | P | Confidence interval (95%) | |
|---|---|---|---|---|---|
|
| |||||
| Absent n (%) | Present n (%) | ||||
| Duration of Diabetes – | |||||
| </=5 years (ref) | 28 (66.7) | 14 (33.3) | |||
| >5 years | 29 (37.2) | 49 (62.8) | 3.379 | 0.002 | 1.54-7.44 |
| Type of treatment | |||||
| Life style modification (ref) | 10 (76.9) | 3 (23.1) | |||
| Oral | 42 (63.6) | 24 (36.4) | 1.905 | 0.362 | 0.48-7.60 |
| Insulin | 2 (11.8) | 15 (88.2) | 25.000 | 0.001 | 3.52-177.48 |
| Both oral and insulin | 3 (12.5) | 21 (87.5) | 23.333 | <0.001 | 3.98-136.80 |
| Treatment Centre | |||||
| Government | 16 (40.0) | 24 (60.0) | 1.577 | 0.246 | 0.73-3.41 |
| Private (ref) | 41 (51.3) | 39 (48.8) | |||
Table 4.
Independent factors associated with diabetic foot syndrome: Logistic regression model
| Variable | cOR | P | CI 95% | aOR | P | CI 95% |
|---|---|---|---|---|---|---|
| Age | 1.074 | <0.001 | 1.04-1.11 | 1.054 | 0.009 | 1.01-1.10 |
| Gender (ref: female gender) | 2.286 | 0.027 | 1.10-4.76 | 1.813 | 0.189 | 0.75-4.40 |
| Type of treatment (ref: life style modification) | ||||||
| Oral | 1.905 | 0.362 | 0.48-7.60 | 1.56 | 0.549 | 0.37-6.60 |
| Insulin | 25 | 0.001 | 3.5-177.4 | 12.37 | 0.019 | 1.51-101.62 |
| Insulin and oral | 23.3 | <0.001 | 3.9-136.8 | 15.87 | 0.005 | 2.30-109.50 |
| Duration of diabetes (ref:<5 years) | 3.379 | 0.002 | 1.54-7.44 | 0.561 | 0.304 | 0.19-1.69 |
cOR=crude odds ratio, aOR=adjusted odds ratio.CI at 95% = confidence interval
Discussion
The present research was a cross-sectional community-based study of 120 adults with type 2 diabetes mellitus, residing in the rural field practice areas of a teaching hospital in South Kerala. One in two patients with diabetes mellitus had DFS. DFS was assessed according to the International Working Group on the Diabetic Foot (IWGDF) where DFS is defined as “a diabetic who does not have an active foot ulcer, but has peripheral neuropathy, with or without the presence of foot deformity or peripheral artery disease, or a history of foot ulcer (s) or amputation of (a part of) the foot or leg”.[9] Evidence suggests a wide variation in the prevalence ranging from10%–60% depending upon the population studies, geographical location and the definitions used. The results in our study area show a high prevalence of DFS which was comparable to a study conducted in South India (52%)[6] whereas it was much higher than the results of a study conducted in Bangalore (12%).[10] It is logical to expect a high prevalence of DFS in the study group as Kerala has a high prevalence of diabetes in comparison to other parts of India. People with diabetes in rural and urban areas mostly consult a primary care physician and hence they can play a vital role in optimizing diabetes control and educating people with diabetes regarding foot care.[11] A quality improvement project, first of its kind in India, in primary care reported improvement in identification of at-risk foot. However, several barriers were identified such as lack of time for individual patient counselling, lack of skill building training sessions for doctors and nurses in primary care.[12]
Primary care physicians should have access to refresher trainings for managing diabetes and foot care, necessary tools for screening and assessments to identify those affected at an early stage, manage and thus prevent negative outcomes. Monofilament is a cheap, convenient tool to assess neuropathy in people with diabetes.[13] Hence it may be a feasible screening tool at the primary care level. Health professionals at different levels of care may integrate this in to their routine screening in order to detect high risk individuals. Furthermore, interventions promoting self-care and diabetes control at a community level may have an impact on the burden of DFS.
The study showed a significant association of DFS with advancing age consistent with studies in various parts of India.[14,15,16] In the study, males were more likely to have DFS compared with the results from western studies.[17] Occupation and education did not show any association with the occurrence of DFS. DFS was seen more among people taking insulin or a combination of oral medications and insulin which probably indicates the longer duration of diabetes in those patients. People with diabetes for more than five years were found to have DFS when compared to those having less duration which was consistent with another community study in India.[6] A study conducted at a tertiary care hospital in New Delhi found duration of diabetes, lower education and level of health care as the independent risk factors.[18] Poor glycemic control may be contributing to the relation between medical management and DFS. Studies have found association between glycosylated haemoglobin and DFS.[19,20] This can be a significant factor associated with DFS and may explain the independent association between medical management and DFS in the present study. However, it is a limitation of this community study that blood investigations could not be performed. There was significant association between foot ulcer and DFS in our study which has previously been demonstrated in a multicentric study in India.[14] The study gave an opportunity for diabetes self- care education and referral as necessitated.
Conclusion
More than half the adults with type 2 diabetes mellitus studied in a South Kerala rural community were found to have DFS significantly associated with age, gender, type of treatment, and duration of diabetes. The high burden of DFS has significant implications in primary care practice and research. Training and monitoring diabetes care with special emphasis to foot self-care at primary care is to be considered a public health priority. In the context of high prevalence of diabetes in a populous country like India, reducing the burden of these conditions is essential for maintaining quality of life of the affected and their families, and the national economy.
Key messages
One in two patients with diabetes mellitus had DFS.
Diabetes foot syndrome was more likely among older adults and those diagnosed with type 2 diabetes mellitus for more than five years.
The high prevalence of DFS in Kerala accentuates the need to screen for its symptoms and signs at primary care level.
Financial support and sponsorship
Short term studentship from ICMR 2019.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF diabetes atlas:Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Volmer-Thole M, Lobmann R. Neuropathy and diabetic foot syndrome. Int J Mol Sci. 2016;17:917. doi: 10.3390/ijms17060917. doi:10.3390/ijms17060917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zou S-Y, Zhao Y, Shen Y-P, Shi Y-F, Zhou H-J, Zou J-Y, et al. Identifying at-risk foot among hospitalized patients with type 2 diabetes:A cross-sectional study in one Chinese tertiary hospital. Chronic Dis Transl Med. 2015;1:210–6. doi: 10.1016/j.cdtm.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shojaiefard A, Khorgami Z, Larijani B. Independent risk factors for amputation in diabetic foot. Int J Diabetes Dev Ctries. 2008;28:32–7. doi: 10.4103/0973-3930.43096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brousseau-Foley M, Blanchette V. Multidisciplinary management of diabetic foot ulcers in primary cares in quebec:Can we do better? J Multidiscip Healthc. 2020;13:381–5. doi: 10.2147/JMDH.S251236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vibha SP, Kulkarni MM, KirthinathBallala AB, Kamath A, Maiya GA. Community based study to assess the prevalence of diabetic foot syndrome and associated risk factors among people with diabetes mellitus. BMC Endocr Disord. 2018;18:43. doi: 10.1186/s12902-018-0270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two- step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17:1281–9. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- 8.Potier L, Khalil CA, Mohammed KA, Roussel R. Use and utility of ankle-brachial index in patients with diabetes. Eur J VascEndovasc Surg. 2011;41:110–6. doi: 10.1016/j.ejvs.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Bus SA, Metten JJ, Lavery LA, Monterio-Soares M, Rasmussen A, Jubiz Y, et al. IWGDF guidance on the prevention of foot ulcers in at risk patients with diabetes mellitus. Metab Rev. 2016;32:16–24. doi: 10.1002/dmrr.2696. [DOI] [PubMed] [Google Scholar]
- 10.Nagaraj C, Ramakuri M, Konapur KS. Burden of foot problems in diabetic subjects –A community-based study among urban poor in Bangalore. Indian J Diabet Foot Complicat. 2014;6:60–6. [Google Scholar]
- 11.Elkhider ATE, Almobark AO, Badi S, Tahir H, Ramadan A, Khalil AA, et al. Risk factors associated with lower extremity amputation in Sudanese individuals with diabetes:The need for improvement in primary health care system. J Family Med Prim Care. 2021;10:985–90. doi: 10.4103/jfmpc.jfmpc_1881_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehndiratta A, Mishra SC, Bhandarkar P, Chhatbar K, Cluzeau F Team PrimaryCareDoctors. Increasing identification of foot at risk of complications in patients with diabetes:A quality improvement project in an urban primary health centre in India. BMJ Open Qual. 2020;9:e000893. doi: 10.1136/bmjoq-2019-000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayaprakash P, Bhansali S, Bhansali A, Dutta P, Anantharaman R. Magnitude of foot problems in diabetes in the developing world:A study of 1044 patients. Diabet Med. 2009;26:939–42. doi: 10.1111/j.1464-5491.2009.02781.x. [DOI] [PubMed] [Google Scholar]
- 14.Shahi SK, Kumar A, Kumar S, Singh SK. Prevalence of diabetic foot ulcer and associated risk factors in diabetic patients from North India. Age. 2012;47:55–6. [Google Scholar]
- 15.D'Souza M, Kulkarni V, Bhaskaran U, Ahmed H, Naimish H, Prakash A. Diabetic peripheral neuropathy and its determinants among patients attending a tertiary health care Centre in Mangalore, India. J Public Health Res. 2015;4:120–4. doi: 10.4081/jphr.2015.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viswanathan V, Thomas N, Tandon N, Asirvatham A, Rajasekar S, Ramachandran A, et al. Profile of diabetic foot complications and its associated complications –A multicentric study from India. J Assoc Physicians India. 2005;53:933–6. [PubMed] [Google Scholar]
- 17.Frykberg RG, Lavery LA, Pham H, Harvey C, Harkless L, Veves A. Role of neuropathy and high foot pressures in diabetic foot ulceration. Diabetes Care. 1998;21:1714–9. doi: 10.2337/diacare.21.10.1714. [DOI] [PubMed] [Google Scholar]
- 18.Kishore S, Upadhyay AD, Jyotsna VP. Categories of foot at risk in patients of diabetes at a tertiary care center:Insights into need for foot care. Indian J Endocrinol Metab. 2015;19:405–10. doi: 10.4103/2230-8210.152789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farooque U, Lohano AK, Hussain Rind S, Rind MS, Sr, Karimi S, Jaan A, et al. Correlation of Hemoglobin A1c With Wagner Classification in Patients With Diabetic Foot. Cureus. 2020;12:e9199. doi: 10.7759/cureus.9199. Doi:10.7759/cureus.9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casadei G, Filippini M, Brognara L. Glycated hemoglobin (HbA1c) as a biomarker for diabetic foot peripheral neuropathy. Diseases. 2021;9:16. doi: 10.3390/diseases9010016. doi:10.3390/diseases9010016. [DOI] [PMC free article] [PubMed] [Google Scholar]


