Abstract
The COVID-19 pandemic has led to the development and rollout of several vaccines worldwide at unprecedented pace. This systematic review of published literature has been undertaken to spread awareness among general physicians and ophthalmologists about the various reported adverse effects in the eye following COVID-19 vaccination. A systematic search was performed on 25 January 2022 through PuBMed, Medline and Google scholar for publications on ocular adverse effects after COVID-19 vaccination. One brief communication, four retrospective case series, sixteen case reports, and five letters to editors were included. Ocular manifestations most commonly appear in the uvea and retina. Other manifestations are seen on the eyelid, cornea and ocular surface, and in cranial nerves innervating the eye. The incidence rate of these manifestations is quite low after COVID-19 vaccinations. Our systematic review meticulously enumerates various adverse effects of COVID -19 vaccine on the eye. Most of these adverse effects are transient and observed to resolve without any sequelae except for cases of retinal and ophthalmic vascular occlusions and corneal graft rejections. An emphasis on close follow-up and a need to delay vaccination and modified therapy to control flare up of signs and symptoms in certain sub-populations, Graves’ disease (autoimmune etiology), pre-existing uveal inflammation and corneal graft cases are warranted. We need long-term, larger, multicentric studies to substantiate our findings and establish the causal relationship with certainty. Mass vaccinations to curb this pandemic after outweighing the ocular risks associated with it is warranted.
Keywords: Cornea, coronavirus, COVID-19, eye, eyelid, neuropathy, ocular adverse effects, retinopathy, SARS-COV-2, uvea, vaccination
Introduction
The coronavirus disease, COVID-19, caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) has led to a pandemic with unprecedented effects on human lives and lifestyle. Its crippling effect on human civilization has forced the scientific community to work at breathtaking speed.
The following vaccines have been approved so far globally on an emergency basis:
BNT162b2 is a mRNA vaccine produced by PfizerBioNTech.
ChAdOx1 (AZD1222) is a non-replicating viral vector vaccine developed by AstraZeneca, Serum Institute of India.
BBIBPCorV is an inactivated virus COVID-19 vaccine developed by Sinopharm.
Few other vaccines approved are as follows:
Sputnik V is an adenoviral-based vaccine produced in Russia initially.
mRNA1273 is developed by Moderna Inc.
Ad26.COV2. S is a non-replicating viral vector vaccine developed by Janssen, Johnson & Johnson.
BBV152 (COVAXIN) is an inactivated vaccine developed by Bharat Biotech, India.
CoronaVac is an inactivated vaccine produced by Sinovac in China.
The latest vaccine added to the list, NVX-CoV2373, is a protein subunit vaccine, developed by Novavax. The efficacy of vaccines has been found to be 94%–95%.[1,2,3] As the data from all over the world is pouring in, several adverse events, including ophthalmic manifestations, of these vaccines are being reported. In this review, we analyze reported ophthalmic side effects of these vaccines.
Materials and Methods
The Preferred Reporting Items for a Systematic Review and Meta-Analyses (PRISMA) was used as a guideline.[4]
Search strategy
A systematic search was done through a computerized search on PubMed or Medline and Google Scholar on 25 January 2022 by two independent researchers (S.K and R.A). The following keywords: (“COVID-19” OR “SARS-COV-2” OR “coronavirus”) AND “vaccination” AND “ocular adverse effects” AND (“eye” OR “eyelid” OR “cornea” OR “uvea” OR “retinopathy” OR “neuropathy”) were searched. Articles were screened for relevance. Few reference articles of these were further looked for thorough information.
Inclusion criteria
Articles such as original research, case series, case reports, letters to editors on ocular adverse effects after receiving COVID-19 vaccine were included using the aforementioned search engines.
Full-text articles were selected. Articles in the English language were only selected.
Exclusion criteria
All those studies, reporting events not affecting eye, post vaccination were excluded.
Duplicate, literature reviews, systematic review or commentaries though relevant, were not included.
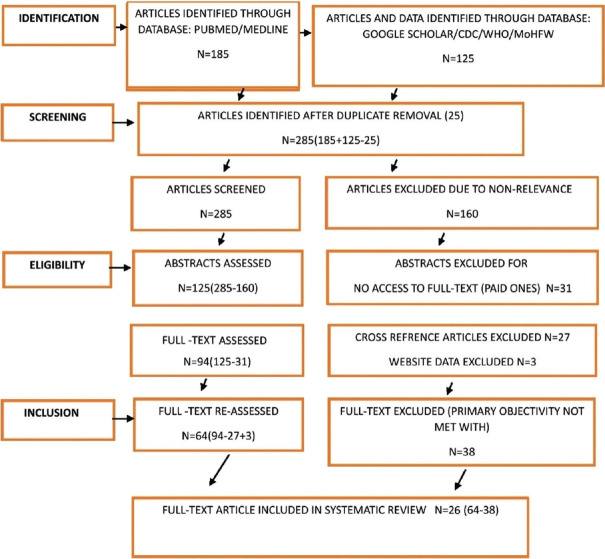
A PRISMA flowchart is depicted in Figure 1. A total of 285 articles were screened using PubMed or Medline, and Google Scholar. The Centers for Disease Control and Prevention (CDC), the World Health Organization (WHO) and the Ministry of Health and Family Welfare (MoHFW) websites were searched for different vaccines. On further assessment, 25 duplicate articles were excluded. Similar articles listed below each study were also searched to assess diverse ocular adverse events. Abstracts of 285 articles were screened. One hundred sixty articles were excluded because of non-relevance. Thirty-one abstracts were excluded as these couldn’t be accessed. Twenty-seven full-text articles from cross reference were excluded though relevant portions from these cross references were mentioned in this article. Ultimately, 64 full-text articles were evaluated. Thirty-eight full-text articles were excluded as primary objectivity was not met with. Out of these 38 full-text articles, a proposed guideline was used from one, though the study was not included in our review. Our primary objective was to include as many diverse ocular adverse events of COVID-19 vaccine and to keep similar ocular adverse events to a minimum. Finally, 26 full-text articles were included in this systematic review.
Figure 1.
PRISMA flowchart for selection of studies
Extraction of data and analysis
From each included report, the type of ocular adverse event was analyzed. An ocular adverse event was any adverse event reported in any part of the eye, ocular adnexa, orbit, nerves innervating eye and its surrounding muscles, and vision after receiving COVID-19 vaccine shot. Age, sex, type of vaccine, dose (1st or 2nd), interval between vaccine shot and appearance of ocular symptoms and outcome were mentioned in the results section of this review.
Quality rating of each study was done according to Quality Rating Scheme for Studies and Other Evidence[5] and the Centre for Evidence-based Medicine, Oxford.[6]
Statistical analysis
The statistical analysis was performed using the Chi-squared and Fisher’s exact tests. The continuous variables were expressed as mean while categorical variables were expressed as a percentage. A P value less than 0.05 was considered statistically significant.
Results
Most of the adverse effects in the eyes post COVID-19 vaccination are secondary to the immune dysregulation triggered by the antigens associated with the respective vaccines. Depending on the clinical site, the patient’s predisposition to a particular immune disorder and some yet unknown factors, these adverse reactions have varied clinical presentations in different patients.
Demographic characteristics of patients
A total of 145 patients from 26 studies [Table 1] were included in this review but individual data of 8 patients related to age and gender are missing in one study[14], and data of 4 patients are not described individually in another study. [23] The proportion of male patients was 42.85% and female was 57.14% [Figure 2]. The mean age of the patients was 41.7 years with the youngest patient being 19 years of age[26] and eldest being 83 years of age[16] A total of 44 types of ocular adverse events were reported by authors from 26 of the included studies [Figure 3]. Ocular adverse events consisted of eyelid edema and erythema, Purpuric lesions on the eyelid, reactivation of herpes simplex keratitis, herpes zoster virus corneal endotheliitis, acute graft rejection in a repeat penetrating keratoplasty (PKP), dual graft rejection in post-PK, acute endothelial rejection after Descemet’s Membrane Endothelial Keratoplasty (DMEK), reactivation of vogt-koyanagi-harada disease (VKH), anterior uveitis, posterior uveitis, scleritis, bilateral choroiditis, toxoplasma retinochoroiditis, pars planitis, retinal vasculitis, bilateral panuveitis in new-onset Behcet’s disease, multiple evanescent white dot syndromes (MEWDS), acute macular neuro-retinopathy, retinal vein occlusions, non-arteritic ischemic optic neuropathy, activations of quiescent choroidal neovascularization (CNV) secondary to myopia or uveitis, central serous chorioretinopathy, bilateral AAION, bilateral AZOOR, PAMM and AMN, disc edema and CSCR, branch retinal arterial occlusion, combined arterial and venous occlusion, venous stasis retinopathy, verve fiber layer infarction, bilateral AMN, episcleritis, forme fruste of CSR, Bell’s palsy, Abducens nerve palsy, optic neuritis, multiple cranial nerve palsies, Miller Fisher syndrome, bilateral superior ophthalmic vein thrombosis & ITP & Ischemic stroke, thyroid eye disease (TED), acute hemorrhagic conjunctivitis in a systemic lupus erythematosus case. Some of these adverse effects were reported in many patients in our study. Ocular adverse effects most commonly appear in the posterior segment of eye, that is, uvea and retinal vasculature.
Table 1.
Data of ocular adverse events after COVID-19 vaccination
| Study | Type | Age (Mean and Range) | Sex and No. (n) | Vaccine | Dose | Duration between Vaccine and Ocular symptoms (Days) | Diagnosis and Total No. of Cases (n) | Rating | Outcome and No. of Cases (n) |
|---|---|---|---|---|---|---|---|---|---|
| Austria et al.[7] | Letter to the editor | 39.3 (32-43) | F (3) | BNT162b2 | 1st/2nd | 1-2 | Eyelid edema and erythema (3) | 5 | Full Resolution (3) |
| Mazzatenta et al.[8] | Letter to the editor | 58 (44-67) | F (2) M (1) |
BNT162b2 | 1st/2nd | 10-25 | Purpuric lesions on eyelid (3) | 5 | Full Resolution (3) |
| Li et al.[9] | Case report | 55.5 (51-60) | F (1) M (1) |
Inactivated COVID 19 Vaccine (Sinovac) |
1st/2nd | 2/NA | Reactivation of herpes simplex keratitis (1) Herpes zoster virus corneal endotheliitis (1) | 5 | Resolved (1) Reduced (1) |
| Parmar et al.[10] | Case report | 35 | M | ChAdOx1 | 1st | 2 | Acute graft rejection in a repeat PKP (1) | 5 | Improved |
| Nioi et al.[11] | Case report | 44 | F | BNT162b2 | 1st | 13 | Dual graft rejection in post-PK (1) | 5 | Resolved |
| Crnej et al.[12] | Letter to the editor | 71 | M | BNT162b2 | 1st | 7 | Acute endothelial rejection after DMEK (1) | 5 | Resolved |
| Papasavvas et al.[13] | Case report | 43 | F | BNT162b2 | 2nd | 42 | Reactivation of VKH (1) | 5 | Substantial improvement |
| Testi et al.[14] | Case series | 51* (19-84) | *F (35) *M (27) | BNT162b2 ChAdOx1 m RNA-1273 BBIBP-CorV BBV152 |
1st/2nd | 1-14 | Anterior uveitis (41) Posterior uveitis (9) Scleritis (7) Others (13) [70] |
4 | Final vision not affected (65) <3 lines affected (2) >3 lines affected (3) |
| Pan et al.[15] | Case report | 50 | F | Inactivated COVID-19 Vaccine |
NA | 5 | Bilateral choroiditis (1) | 5 | Improved |
| Bolletta et al.[16] | Retrospective case series | 49.8 (18-83) | F (20) M (14) | BNT162b2 ChAdOx1 m RNA-1273 Ad26COV2.S |
1st/2nd | 1-30 | Uveitis and other complications (34) | 4 | Complete resolution (24) Significantly improved (4) Partially improved (4) Mild improvement (1) No improvement (1) |
| Maleki et al.[17] | Case report | 56 (33-79) | F (2) | BNT162b2 mRNA-1273 |
2nd | 2-10 | Bilateral AAION (1) Bilateral AZOOR (1) | 5 | Under follow-up (2) |
| Renisi et al.[18] | Case report | 23 | M | BNT162b2 | 2nd | 14 | Anterior uveitis (1) | 5 | Complete resolution |
| Bøhler et al.[19] | Brief communication | 27 | F | ChAdOx1 | 1st | 2 | AMN (1) | 5 | Not mentioned |
| Vinzamuri et al.[20] | Case report | 35 | M | ChAdOx1 | 2nd | 30 | PAMM and AMN (1) | 5 | Slight symptomatic improvement OCT-wise improved |
| Lee et al.[21] | Letter to the Editor | 41 | F | ChAdOx1 | 1st | 2 | Disc edema and CSCR (1) | 5 | Resolved |
| Girbardt et al.[22] | Retrospective case series | 46.5 (21-81) | M (4) F (2) | BNT162b2 ChAdOx1 m RNA-1273 |
1st/2nd | 2-12 | Branch retinal arterial occlusion (1) Combined arterial and venous occlusion (1) Venous stasis retinopathy (1) NAION (1) Nerve fiber layer infarction (1) Bilateral AMN (1) (6) |
4 | Under follow-up |
| Pichi et al.[23] | Retrospective case series | 41.4** (30-55) | M (3) ** | BBIBP-CorV | 1st | 1-10 | Episcleritis (1) Anterior scleritis (2) AMN (2) PAMM (1) Forme fruste of CSR (1) (7) |
4 | 1 case of AS resolved ** 1 case of AMN resolved 1 case of PAMM-result Not mentioned |
| Colella et al.[24] | Letter to the editor | 37 | M | BNT162b2 | 1st | 5 | Bell’s palsy (1) | 5 | Facial mobility partially improved |
| Pawar et al.[25] | Case report | 23 | M | ChAdOx1 | 1st | 7 | Abducens nerve palsy (1) | 5 | Mobility recovered to near normal |
| Estrada et al.[26] | Case report | 19 | F | Ad26COV2.S | 1st | 7 | Optic Neuritis (1) | 5 | Resolved |
| Tsukii et al.[27] | Case report | 55 | F | BNT162b2 | 1st | 7 | NAION (1) | 5 | Gradually Improved |
| Manea et al.[28] | Case report | 29 | M | BNT162b2 | 1st | 6 | Multiple cranial nerve palsies (1) | 5 | Improved with minimal facial palsy persisting |
| Abičić et al.[29] | Case report | 24 | F | BNT162b2 | 1st | 18 | Miller Fisher syndrome (1) | 5 | Clinically improved |
| Bayas et al.[30] | Case report | 55 | F | ChAdOx1 | 1st | 10 | Bilateral superior ophthalmic vein thrombosis (SOVT), ITP & Ischemic stroke (1) |
5 | Under Follow-up |
| Rubinstein[31] | Case report | 50 | F | BNT162b2 | 2nd | 3 | Thyroid Eye Disease (TED) (1) | 5 | Significant improvement |
| Jin et al.[32] | Case report | 28 | F | Inactive COVID-19 Vaccine | 1st | 3 | Acute hemorrhagic conjunctivitis in a systemic lupus erythematosus case (1) | 5 | Lesions faded |
M, Male ; F, Female; NA, Not available ; N, Number of cases; AAION, Arteritic anterior ischemic optic neuropathy, AMN, Acute macular neuro-retinopathy, AZOOR, Acute zonal occult outer retinopathy, CSCR, Central serous chorioretinopathy, CNV, Choroidal neovascularization, DMEK, Descemet membrane endothelial keratoplasty, ITP, Immune thrombocytopenia, NAION, Non-arteritic anterior ischemic optic neuropathy, MEWDS, Multiple evanescent white dot syndromes, PKP, Penetrating keratoplasty, PAMM, Paracentral acute middle maculopathy, SOVT, Superior ophthalmic vein thrombosis, VKH, Vogt-Koyanagi-Harada disease, TED, Thyroid eye disease. *missing data for 8 cases in age and gender **4 cases not described
Figure 2.
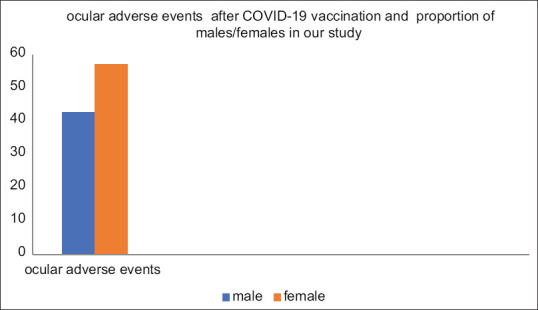
Compound bar diagram showing adverse events after covid-19 vaccination in males and females
Figure 3.
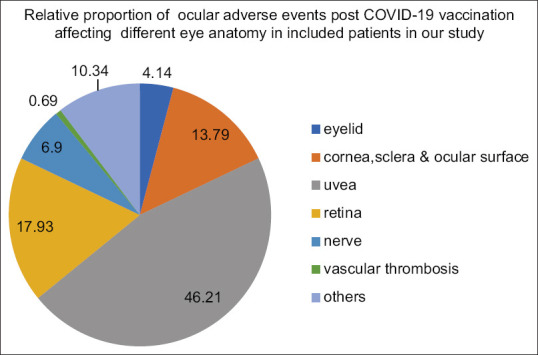
Pie chart showing adverse outcome in eye after covid-19 vaccination
Characteristics of ocular adverse events post COVID-19 vaccination, onset, duration, treatment and outcome
Ocular adverse effects were described and reported in our study based on different anatomical parts of the eye involved [Table 2].
Table 2.
Data of no. of patients affected based on eye anatomy involved post COVID-19 vaccination
| Ocular Adverse Events Based on Eye Anatomy Involved Following COVID-19 Vaccination | No of patients in each category of ocular adverse event based on eye anatomy | No. of Patients in Our Study |
|---|---|---|
| Eyelid | 6 | |
| Eyelid edema and erythema | 3 | |
| Purpuric lesions on eyelid | 3 | |
| Cornea, Ocular surface, Sclera | 20 | |
| Herpetic keratitis | 3 | |
| Reactivation of herpes simplex keratitis | 1 | |
| Herpes zoster virus corneal endotheliitis | 1 | |
| Acute graft rejection in repeat PKP | 1 | |
| Dual graft rejection in post-PK | 1 | |
| Acute endothelial rejection in DMEK | 1 | |
| Scleritis | 11 | |
| Episcleritis | 1 | |
| Uvea | 67 | |
| Anterior uveitis | 47 | |
| Pars planitis | 2 | |
| Reactivation of VKH | 3 | |
| AZOOR | 1 | |
| Bilateral choroiditis | 1 | |
| Toxoplasma retinochoroiditis | 3 | |
| Bilateral pan uveitis in Bechet’s disease | 1 | |
| Posterior uveitis | 9 | |
| Retina | 26 | |
| Retinal vasculitis | 2 | |
| Multiple evanescent white dot syndromes | 3 | |
| Acute macular neuro-retinopathy | 4 | |
| Bilateral AMN | 1 | |
| PAMM and AMN | 1 | |
| PAMM | 1 | |
| CSCR | 1 | |
| Forme fruste of CSR | 1 | |
| CSCR and disc edema | 1 | |
| Retinal vein occlusion | 5 | |
| Combined arterial and venous occlusion | 1 | |
| Venous stasis retinopathy | 1 | |
| BRAO | 1 | |
| Activation of quiescent CNV secondary to myopia or uveitis | 3 | |
| Nerve | 10 | |
| NAION | 3 | |
| AAION | 1 | |
| Nerve fiber layer infarction | 1 | |
| Bell’s palsy | 1 | |
| Abducens palsy | 1 | |
| Optic neuritis | 1 | |
| Multiple cranial nerve palsies | 1 | |
| Miller Fisher syndrome | 1 | |
| Vascular thrombosis | 1 | |
| Bilateral superior ophthalmic vein thrombosis, ITP and ischemic stroke | 1 | |
| Others | 15 | |
| TED | 1 | |
| Acute hemorrhagic conjunctivitis in a systemic lupus erythematosus case | 1 | |
| Others | 13 |
Eyelid edema and erythema
Three patients presented with unilateral lid edema and erythema, more in upper than lower, on day 1 or 2 following first or second dose of Pfizer COVID-19 mRNA vaccine.[7] They had no other associated ocular, adnexal, or systemic findings. These symptoms resolved fully in 1–2 days without any sequelae with observation, antihistamines, and oral steroids, respectively.
Eyelid purpuric lesions
Three cases of localized purpuric and ecchymotic eyelid reaction after administration of BNT162b2 mRNA COVID-19 vaccine was reported.[8] The lesions appeared after a median of 14 days post injection. There were no symptoms. Spontaneous clearing occurred after 10–15 days.
Herpes simplex keratitis or Herpes zoster corneal endotheliitis
A 60- year-old woman complained of photophobia, deteriorating vision and redness in her right eye after her first shot of inactivated COVID-19 vaccines (Sinovac). Her symptoms started two days after the dose. She had a history of corneal scarring for which she underwent penetrating keratoplasty (PKP) one year ago. Herpes simplex keratitis was the underlying cause of scarring. She was on topical steroids, tacrolimus and tear substitutes but no anti-viral therapy. There was no recurrence after surgery till she received the vaccine. A diagnosis of herpes simplex epithelial keratitis was made. She was put on anti-viral. She responded to it and received second dose of vaccine uneventfully.[9]
Another patient, a 51-year-old man complained of blurred vision and redness in his left eye. He had received his second dose of inactivated COVID-19 vaccine a few days ago. He had no history of ocular surgery. On examination, corneal edema, Descemet fold, anterior chamber reaction and keratic precipitates were noted. A diagnosis of herpes zoster corneal endotheliitis was made after polymerase chain reaction test. He was put on topical steroids, topical anti-viral and oral anti-viral, ganciclovir. He responded to this treatment.[9]
Corneal graft rejection
A 35-year-old male complained of diminution of vision in the left eye within two days of COVID-19 vaccination (ChAdOx1, viral vector vaccine). He had a history of repeat PKP of the same eye. A diagnosis of acute graft rejection post vaccination was made. An improvement of visual acuity and corneal graft clarity was achieved with topical and systemic immunosuppression therapy.[10]
A 44-year-old woman presented with features of acute corneal graft rejection after 25 years of corneal transplantation for keratoconus. She had received BNT162b2 vaccine (Comirnaty, BioNTech/Pfizer) 13 days ago. She was put under regular follow-up. In a span of 6 months, the inflammation in the cornea appeared twice. A diagnosis of biphasic corneal graft rejection was established.[11] Supplementation of Vitamin D was given in view of deficiency in this case. She was put on standard regimen of topical therapy as well.
A 71-year-old male patient had a Descemet membrane endothelial keratoplasty (DMEK) procedure of the right eye for endothelial decompensation post phacoemulsification surgery. He had a history of coronary artery disease, high blood pressure and smoking. He had no complaints for five months post surgery. Afterwards, he got his first shot of the BNT162b2 mRNA vaccine. After 7 days, he suddenly developed painless diminution of vision in his right eye. Acute endothelial graft rejection was diagnosed. He was put on topical steroid, dexamethasone, two-hourly and oral antiviral, valacyclovir 1000 mg TID.[12]
Uveal tissue inflammation
Reactivation of VKH
A 43- year-old woman had an episode of severe initial-onset Vogt–Koyanagi–Harada (VKH) disease. Steroids and non-steroidal immunosuppressants (mycophenolic acid and cyclosporine) were used to control the disease process. Additional infliximab infusions were also used as indocyanine green angiography (ICGA) which showed the persistence of subclinical choroiditis. There was no inflammation, no subclinical disease and absence of sunset glow fundus under infliximab therapy alone for the last 6 years. However, the second dose of Pfizer anti-SARS-CoV-2 vaccination led to severe resurgence after 6 weeks with exudative retinal detachments. Oral prednisone (1 mg/kg) and a new loading scheme of infliximab therapy were started to control the disease process.[13]
Anterior Uveitis or Posterior Uveitis or Scleritis
Seventy patients, at 40 multinational centers over a duration of 3 months complained of ocular inflammatory events within 14 days after COVID-19 vaccination. Most commonly reported cases were as follows:- Anterior uveitis (41), Posterior Uveitis (9), Scleritis (7). The data related to age and gender were missing in 8 cases. The mean time between onset of inflammation and first dose of vaccination was 5 days while it was 6 days in case of second dose. Previous episodes of ocular inflammatory events were seen in 36 patients. Topical corticosteroids were used in most of the patients. Sixty-five patients had unaffected final vision, whereas 5 patients had reduced visual acuity. Nummular corneal lesion in one patient, cystoid macular edema in two patients and macular scarring in two patients were seen. Although the causality was not proven, a temporal association is suggested between vaccination and ocular events. Most of the inflammatory episodes were mild and visual outcome was good. The most common ocular inflammatory event observed after COVID-19 vaccination was anterior uveitis. More than 50% of these patients had similar episodes in the past. The Pfizer vaccine was most commonly associated with these inflammatory events. This association was probably related to the number of the administered doses.[14]
Bilateral posterior uveitis (Choroiditis)
A 50-year-old woman presented with bilateral blurred vision and visual distortion five days after vaccination with the inactivated COVID-19 vaccine. She was diagnosed with bilateral posterior uveitis (choroiditis). Upon administration of local and systemic steroids, the symptoms improved markedly 5 weeks later.[15]
Reactivation of prior infection or inflammation
At an ocular immunology unit in Italy, episodes of ocular inflammation and reactivation in patients after COVID-19 vaccination between January 2021 and October 2021 were analyzed. Three herpetic keratitis, two anterior scleritis, five anterior uveitis (AU), two pars planitis, three multiple evanescent white dot syndromes (MEWDS), two VKH disease reactivations, three toxoplasma retinochoroiditis, two retinal vasculitis, one bilateral panuveitis in new-onset Behcet’s disease, one acute macular neuro-retinopathy (AMN), five retinal vein occlusions (RVO), three activations of quiescent choroidal neovascularization (CNV) secondary to myopia or uveitis, one central serous chorioretinopathy (CSCR), and one non-arteritic ischemic optic neuropathy (NAION) were recorded. Mean duration between vaccination and onset of ocular complication was 9.4 days (range 1–30 days). Twenty-three cases post BNT162b2 mRNA vaccination, seven post ChAdOx1 nCoV-19 vaccination, three post mRNA-1273 vaccination, and one post Ad26.COV2 vaccination were noted. Most of the patients developed ocular complications after the second dose of the vaccine.[16]
AZOOR and AAION
A 79-year-old female complained of sudden loss of vision in both eyes. She received the second shot of recombinant mRNA vaccine (Pfizer) two days ago before loss of vision. A diagnosis of bilateral arteritic anterior ischemic optic neuropathy (AAION) was established after temporal artery biopsy. She was put on subcutaneous tocilizumab 162 mg weekly. Her ESR, CRP, and IL-6 were monitored regularly.[17]
A 33-year-old healthy female, after receiving the second shot of recombinant mRNA vaccine (Moderna) 10 days ago, turned up with a progressive nasal field defect in the left eye and flashes in both eyes. High ESR (25) and CRP (19) levels were recorded on laboratory investigation. A diagnosis of unilateral acute zonal occult outer retinopathy (AZOOR) in her left eye was established. An intravitreal dexamethasone was implanted in the left eye.[17]
Anterior uveitis
A 23-year-old male presented with red eye, pain and photophobia and diminution of vision in left eye after receiving his second dose of BNT162b2 vaccine 14 days ago. The ocular inflammatory signs disappeared and vision was completely restored after a 10-day treatment course of topical steroids and cycloplegic eye drops.[18]
Retinopathy
AMN
A 27-year-old female complained of disturbances in vision in her left eye. She had flu-like symptoms on the day of vaccination with the first shot of the AstraZeneca vaccine. Resolution of symptoms happened two days later. It was followed by appearance of a left paracentral scotoma. Her best-corrected visual acuity (BCVA) was 20/20. She was on oral contraceptive (combined ethinyl estradiol and desogestrel). A paracentral scotoma in the upper temporal quadrant of the left eye was noted on threshold perimetry. Fundus examination of the left eye showed a teardrop-shaped macular lesion nasal to the fovea. It was better depicted on ocular coherence tomography (OCT). A diagnosis of acute macular neuro-retinopathy (AMN) was established.[19]
PAMM and AMN
A 35-year-old male patient complained of blurred vision and black spots in the visual field. He had taken the second dose of Covishield vaccine one month ago. His vision was 6/6 in both eyes. On examination, normal findings were seen in both anterior and posterior segments. Optical coherence tomogram depicted multiple hyperreflective lesions in the nerve fiber layer with back shadowing. OCT further showed hyperreflective spots in the ganglion cell layer and an intact inner segment/outer segment junction and outer plexiform layer with focal loss of external limiting membrane. A diagnosis of paracentral acute middle maculopathy (PAMM) and acute macular neuro-retinopathy (AMN) was established. He was put under observation.[20]
Disc edema and CSCR
A 41-year-old female complained of blurred vision in the right eye and a defect in inferior visual field in the left eye. She had her first COVID-19 vaccine (Vaxzevria) two days ago. She had no systemic disease. Vision was 20/20 and 20/30 in the right and left eye, respectively. Disc edema in the right eye and a dome-shape serous detachment over the upper arcade in the left eye were detected on fundus examination and fundus fluorescein angiogram (FFA). With a diagnosis of idiopathic optic disc edema in the right eye and central serous chorioretinopathy (CSCR) in the left eye, she was put under regular follow-up without any invasive treatment. A complete resolution was seen in both eyes at three months follow-up.[21]
Retinal vascular events
Six patients reported different retinal vascular events after receiving vaccination with the mRNA vaccine Comirnaty® (BioNTech®), the mRNA vaccine Spikevax® (Moderna®), and the adenoviral-vectored vaccine ChAdOx1 nCoV-19 (AstraZeneca®).
A 38-year-old healthy male patient was diagnosed with branch retinal arterial occlusion four days after receiving his second shot of Comirnaty®. An 81-year-old female patient presented with combined arterial and venous occlusion in her right eye twelve days after the second shot of Comirnaty®. A 40-year-old male patient presented with venous stasis retinopathy in his left eye five days after his first shot of Comirnaty®. A 67-year-old male presented with non-arteritic anterior ischemic optic neuropathy in his right eye four days after getting the first shot of Vaxzevria®. A 32-year-old man was diagnosed with circumscribed nerve fiber infarction two days after receiving the second shot of Spikevax®. A 21-year-old female patient presented with bilateral acute macular neuro-retinopathy three days after getting the first shot of Vaxzevria®.[22]
A tertiary referral center from Abu Dhabi reported ocular complications within 15 days following administration of first of two doses of inactivated COVID-19 vaccine [Sinopharm]. One episcleritis, two anterior scleritis, one paracentral acute middle maculopathy, two acute macular neuro-retinopathy and one subretinal fluid (forme fruste of central serous chorioretinopathy) were recorded post vaccination.[23]
Neuropathy
Bell’s Palsy
A healthy 37-year-old white Caucasian male received the first injection of the mRNA Vaccine BNT162b2 on 8 January 2021. From the 11th, he started complaining of left-sided latero- cervical pain irradiating ipsilaterally to the mastoid, ear, and retro-maxillary region. On 13 January, he presented with a left-sided facial droop accompanied by reduced mobility (paresis). On examination, flattening of forehead’s skin and marionette line (labial-buccal sulcus) ipsilaterally, mild fattening of the nasolabial fold, lagophthalmos and mild labial hypomobility were recorded. This was accompanied by a moderate Bell’s sign. It was clinically diagnosed as Bell’s palsy. The timing and mode of onset of the palsy strongly suggests that it was associated with BNT162b2 vaccine injection though a causal relationship could not be established.[24]
Abducens nerve palsy
A healthy 23-year-old male suddenly developed double vision along with severe headache. He had received his first shot of COVID-19 vaccine (Covishield) one week ago. His BCVA remained unaffected. On cover test, a 40 PD left esotropia was noted along with limited abduction of the left eye. Two similar episodes had happened in the past. The first episode of the left eye sixth nerve palsy occurred five years ago following a fever. Complete resolution took place in two months after Botox injection in medial rectus of the left eye. After two years of this episode, the second episode in the left eye happened after chickenpox infection. This time resolution period was of three months.[25]
Optic neuritis
A 19-year-old female developed optic neuritis after single dose of Ad26.COV2. S vaccine 1 week ago. It responded to steroid therapy. On evaluation, left amaurosis, afferent pupillary defect and papillitis on fundoscopy of the left eye were noted. It was clinically diagnosed as inflammatory optic neuritis.[26]
Non-arteritic anterior ischemic optic neuropathy
A 55-year-old woman complained of disturbance in the inferior visual field of the right eye for four days. She had the first shot of Pfizer-BioNTech COVID-19 vaccine seven days ago. A diffuse swelling, more prominent above, of the optic disc, was seen in the right eye on fundus examination. An inferior altitudinal visual field defect with I/2 isopter was noted on Goldmann visual field testing in the right eye. Non-arteritic anterior ischemic optic neuropathy (NA-AION) was diagnosed despite of the absence of a typical complete inferior visual field defect.[27]
Multiple cranial nerve palsies
A 29 -year- old immunocompetent male presented with double vision and Bell’s palsy six days after getting first shot of Pfizer-BioNTech COVID-19 vaccine. On neurological examination, multiple cranial nerve palsies on left side were noted. He had partial 3rd nerve palsy (with no ptosis), partial 6th nerve palsy, hypoesthesia in middle and lower 5th nerve distribution and 7th nerve palsy. CSF cytology and biochemical analysis and serial Gadolinium-enhanced MRI were done. It ruled out any tumor, vascular cause or demyelinating disease. Screening for autoimmune disease, systemic inflammatory disease and granulomatosis was negative. Tuberculosis was ruled out. Gadolinium-enhanced magnetic resonance imaging (MRI) showed enhancement of 7th, 5th and 3rd nerve. Symptoms responded to 1 g of methyl prednisolone for 5 days. After 1 month, only minimal 7th nerve palsy was observed.[28]
Miller Fisher syndrome
A healthy 24 -year- old female complained of binocular horizontal diplopia. She had her first shot of Pfizer COVID-19 vaccine 18 days ago. Anti-GQ1b antibody was positive on anti-ganglioside testing. A patient of acute ophthalmoplegia is tested for this. Its titer is an indicator of clinical severity particularly for ophthalmoplegia. Miller Fisher syndrome is a variant of Guillain-Barre syndrome seen rarely. It is characterized by a triad of areflexia, ataxia and ophthalmoplegia. After treating with intravenous immunoglobulins for five days (2 g per kg of body weight), clinical improvement was seen in three weeks.[29]
Superior ophthalmic vein thrombosis
A 55-year-old woman was admitted with conjunctival congestion, double vision, and retro- orbital pain after receiving her first shot of vaccine, ChAdOx1 nCoV-19, ten days ago. She had flu-like symptoms and fever on the night after vaccination. She had similar symptoms seven days later. On examination, binocular diplopia was noted at vertical and right lateral gaze. Bilateral visual acuity was 0·85. No contrast filling, bilateral high T2 signal intensity of the superior ophthalmic vein was detected on MRI leading to a diagnosis of Superior ophthalmic vein thrombosis.[30] Secondary immune thrombocytopenia (ITP) was confirmed on laboratory investigations. She was on heparin, still she developed ischemic stroke with complaints of a transient, mild, right-sided hemiparesis, and aphasia. MRI findings were consistent with the diagnosis. Thus, after receiving the ChAdOx1nCoV-19 vaccination, the previously healthy patient developed marked flu-like symptoms, two rare disorders-bilateral superior ophthalmic vein thrombosis (SOVT) and ITP, and an ischemic stroke. A causal relationship was indicated.
Thyroid eye disease
A 50 -year- old female complained of blurred vision, watering, irritation, pain and bulging out of both eyes, left more than right, for two months. These symptoms started three days after getting her second shot of BNT162b2 vaccine. She had a history of controlled Graves’ disease with no ophthalmopathy. On presentation, positive clinical signs (clinical activity score 5), elevated thyroid stimulating immunoglobulin levels, enlarged inferior and medial recti muscle (left more than right) on CT scan of orbit established a diagnosis of Thyroid Eye Disease (TED). Intravenous Teprotumumab was administered. After getting the second dose, relieve in congestion and reduction in proptosis by 1 mm in right eye and by 3 mm in left eye were noted.[31]
Acute hemorrhagic conjunctivitis in a Systemic Lupus Erythematosus patient
A 28-year-old female with diagnosed systemic lupus erythematosus (SLE) and under treatment received inactivated COVID-19 vaccine. In three days, she developed conjunctival congestion, itching, lid edema in her left eye and severe headache. Acute hemorrhagic conjunctivitis was suspected. Levofloxacin eye drop was started. On aggravation of signs and symptoms, diclofenac sodium eye drop and ganciclovir eye gel were added. Flumilone eye drop was added after relief of congestion and edema. Patient developed similar mild symptoms in right eye as well on the eighth day of presentation. On the tenth day, lesions faded.[32]
Discussion
COVID-19 vaccines included in our study were mRNA vaccines (BNT162b2 and mRNA-1273) and adenoviral vector vaccines (ChAdOx1—chimpanzee adenovirus Y25 and Ad26.COV2. S—human adenovirus 26) and whole-cell inactivated virus vaccine (BBV152, CoronaVac, BBIBP-Cor V and one non-categorized inactive COVID-19 vaccine).
The mRNA vaccines contain codon-optimized sequences for spike protein synthesis and use the authentic signal sequence for its biosynthesis.
Adenoviral vector vaccines use replication-incompetent adenovirus as a vector to deliver the DNA signal sequence of spike protein.
Pathomechanism of ocular adverse events in our study
The proposed hypothesis associated with different ocular adverse events are discussed herein.
Eyelid edema
A hypothesis points towards possibility of reactivation of an autoimmune response triggered by the mRNA vaccine leading to eye lid edema and erythema.[7]
Eyelid purpuric lesions
BNT162b2 mRNA vaccine might induce a mild and localized form of vaccine-induced microangiopathy leading to purpuric and ecchymotic lesions on eyelids.[8]
Symptomatic and asymptomatic thrombocytopenia following BNT162b2 vaccination have been reported.[33]
Vaccine-induced thrombocytopenic thrombosis (VITT), a new term coined for life-threatening condition following ChAdOx1 vaccination was also reported.[34]
Herpes simplex keratitis or Herpes zoster corneal endotheliitis
Neurotropic herpes simplex nirus [HSV] and Varicella Zoster Virus [VZV] are known to remain lifelong in the host.
Vaccine-induced immunomodulation, like decreased cellular immunity immediately after vaccination, can trigger their reactivation.[35] It might lead to herpes simplex keratitis and herpes zoster endotheliitis.
Corneal graft rejection
The cornea is considered to be immunologically privileged. It is attributed to its avascularity and absence of lymph vessels. Major histocompatibility complex (MHC) class II antigen-presenting cells are also absent which is the basis of acceptance of the allogenic donor tissue by recipient corneal bed without rejection.[36] However, vaccination triggers increased generalized immunological response. Subsequent rejection of allograft follows due to immunological reaction towards it. Induction of Class II MHC complex antigens in all layers of the cornea is seen post vaccination. The similar pathomechanism of rejection is seen with influenza vaccine.[37,38]
The case of dual corneal graft rejection post vaccination with concomitant vitamin D deficiency highlights the immunomodulatory role of vitamin D, need of longer follow up in corneal graft rejection cases, and risk-benefit ratio and timing of second dose of vaccination. Authors proposed preventive check and integration of vitamin D before the vaccine administration.[11]
It has been proposed to increase dosage of topical steroids in cases with corneal grafts before they opt for any type of COVID-19 vaccination.[39]
In view of association between COVID-19 vaccination and graft rejection, a time gap of three to six months between the second dose and elective corneal transplant is suggested.[40]
Uveal tissue inflammation
The following are the proposed pathways of pathomechanism of uveal tissue inflammation post SARS-CoV-2 vaccination: (1) uveal peptides and vaccine peptide fragments similarities leading to molecular mimicry; (2) antigen-specific cell and antibody-mediated hypersensitivity reactions; (3) adjuvants in the vaccines causes inflammatory damage which stimulates innate immunity through endosolic or cytoplasmic nucleic acid receptors.[41,42,43,44,45]
Reactivation of herpetic keratouveitis
Patients with previous herpetic keratitis or keratouveitis can have reactivation of the disease following COVID-19 vaccination. It is proposed that herpes reactivation is due to alteration in the immune status. Lymphocyte exhaustion is a type of altered immunological profile. Here comes the role of prophylactic antiviral therapy with oral valacyclovir in high-risk patients with several previous episodes of herpetic uveitis.[46]
AZOOR and AAION
An association between the mRNA COVID-19 vaccination and ocular inflammatory disease of autoimmune etiology was highlighted by these two cases.[17]
Several pro-inflammatory pathways can be activated through mRNA vaccines before translation. Type I interferon, nuclear translation of the transcription factor and nuclear factor (NF)- kB are included in this activated pathway.[47]
The basis of immune-mediated diseases is the activation of these pathways, more so in genetically predisposed patients such as young females.[48,49]
Anterior uveitis
Molecular mimicry, inflammatory damage induced by adjuvants such as aluminum salts, direct viral infection (applicable to live and attenuated vaccines) are different mechanisms hypothesized for aforementioned events.[41]
AMN
Ischemia in the deep retinal capillary plexus or choriocapillaris is the potential pathophysiological mechanism although the exact cause is unknown.[50] Recent infection, febrile illness and use of oral contraceptives are major associated factors.
PAMM and AMN
It was hypothesized that the development of PAMM and AMN was due to vasculitis changes leading to ischemia of deep capillary plexus.[20]
Abducens nerve palsy
Ambiguity persists about the site of cellular injury and pathophysiological mechanism in 6th nerve palsy, although there is temporal association between immunization and abducens nerve palsy. It is proposed that a neurotropic effect of the infectious agent, demyelination of para-infectious etiology, sectarian arteritis or microinfarction, and an immune-mediated reaction are the possible etiologies of the abducens nerve palsy post vaccination.[25]
Optic neuritis
Optic neuritis is the most common demyelinating condition associated with vaccination. It includes hepatitis A and B, influenza, pneumococcal vaccine, measles, rabies, human papilloma virus and bacillus Calmette–Guérin (BCG).[51,52]
Proposed hypothesis says that a dysregulated immunological reaction could be triggered by the active component of the Ad26.COV2. S vaccine (adenovirus type 26 vector containing the DNA of spike) and the specific B cell response targeting the vector-based vaccine. This leads to the adverse reactions. This cross reactivity is related to several genetic and environmental factors, such as aberrant major histocompatibility complex (MHC) class II antigen presentation to autoreactive T cells.[53]
NAION
The ambiguity about consequential or coincidental development of NA-AION after COVID-19 vaccination persists. Though the temporal relationship between the two points towards vasculopathy on the microvascular network of optic nerve head and inflammatory or immune mediated component behind the onset of NA-AION.[27]
Multiple cranial nerve palsies
It has been suggested to observe closely for neurological side effects in post-vaccination cases.[28] More such no of cases needs to be reported to comprehend and to establish definite causal relationship.
Miller Fisher syndrome
Abundance of ganglioside GQ1b in cranial nerves innervating extraocular muscles, and in presynaptic neuromuscular junction, and studies supporting anti- GQ1b Ig antibodies having pathogenic effect at neuromuscular junction implicates a dysregulated immune response as the underlying cause.[54,55]
Superior ophthalmic vein thrombosis
The thrombotic events like bilateral SOVT may occur in the context of thrombocytopenia.[30]
Thyroid eye disease
In absence of any other known etiology, stress or altered physiological or other factors known to cause thyroiditis in this case, a temporal association between vaccine and appearance of TED points towards immune stimulation and autoimmune etiology.[31] Thyroid, like many other endocrine organs, and SARS-CoV-2 share similar functional receptor, angiotensin -converting enzyme 2.[56] Possibility of molecular mimicry and first mRNA vaccine, COVID -19 vaccine, in clinical use in humans explains the probable underlying path mechanism.
Systemic lupus erythematosus
Ocular hyperemia is a known complication in acute episodes of SLE exacerbation.[57] Overactive immune system following COVID-19 vaccination and increased specific auto-antibodies and certain factors in the serum might have led to this injury.[58]
Outcome
In our study, BNT162b2 (m RNA vaccine) had a maximum number of 82 reported ocular adverse events followed by ChAdOx1 (adenoviral vector vaccine) with 32 in number [Figure 4].
Figure 4.
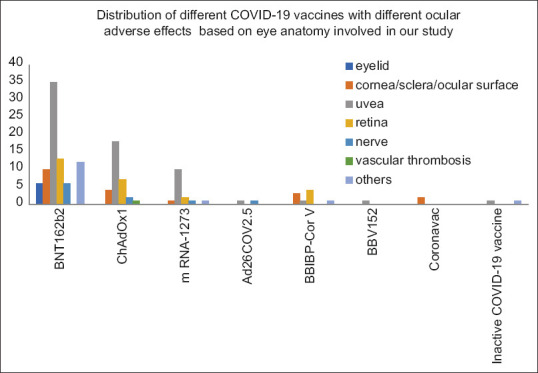
Compound bar diagram showing adverse outcome in different parts of eye with different types of COVID-19 vaccines
Uvea-related adverse events were maximum in our study, i.e., 67 in number, followed by retina related adverse effects, that is, 26 in number.
Statistical analysis was done by grouping vaccines into three groups, that is, mRNA, adenoviral vector vaccine, and whole-cell inactivated virus vaccine on one hand, and ocular adverse events into seven groups based on eye anatomy involved on the other hand. “Others” group involved miscellaneous ocular adverse events.
As per the available studies, the side effects related to the eyelid, cornea, ocular surface, sclera, uvea, retina and nerve were most dominant as a result of different types of vaccination. The side effects related to “others” accounted for 11% of side effects.
It was observed that the side effect related to the eyelid was seen in about 6% of cases who had taken mRNA vaccine whereas it was none in other categories such as adeno viral vector and whole cell. However, it was not found to be statistically significant (P = 0.18)
While observing the side effects related to the cornea, it was observed that about 11% had side effects in mRNA type of vaccine where as it was seen in 12.5% and 36% in adenoviral vector and whole cell in active type, respectively, which was seen as statistically significant (P = 0.04).
The uvea-related side effects were seen in 45% of mRNA vaccines whereas it was as high as 56% in adeno viral and 36% in whole cell in activated type vaccine. The results did not express statistical significance (P = 0.38).
The retinal related issues were seen in 15 cases administered with mRNA vaccine whereas 7 out of 34 in adenoviral and 4 out of 14 in whole cell (P = 0.67).
The nerve-related issues were observed in 7.4%, 8.6% and 28.6%, respectively, in three types of vaccines which was statistically significant.
The vessel-related issues were none in mRNA and whole cell type of vaccine as compared to 2.9% in adeno viral type of vaccine. No statistical significance was observed (P = 0.34).
The side effects categorized as “others” (including TED and SLE related ocular events) were observed in 14.4% in mRNA category as compared to 12.5% in whole cell type of vaccine while it was none in adeno viral (P = 0.09).
The comparisons were carried out using Chi-squared test or Fisher’s exact test.
Reporting bias
Testi et al.[14] did not report the individual types of adverse events included in the “others” category with type of vaccines.
Maleki et al.[17] reported one patient of bilateral AAION and one patient of bilateral AZOOR, though it was grouped as only two ocular adverse events in our study.
Vinzamuri et al.[20] reported bilateral PAMM and AMN in a patient which was counted as one adverse event in our study and grouped under the “retina” subgroup.
Lee et al.[21] reported disc edema in right eye and CSCR in left eye of the same patient. In our calculation, it was taken as one adverse event only and grouped under “retina” subgroup.
Pichi et al.[23] did not describe 4 cases included in their study affecting gender calculation in our study.
Limitation
Our review has several limitations. First, it included only case reports, brief communication, letters to editors and case series with significant reporting bias, some missing, and few incomplete data. Hence, the result cannot be generalized to all COVID-19 patients. Second, all studies lacked the control arm and the sample size was very small.
Adverse effects of approved COVID-19 vaccines on the eye as reported are quite infrequent, transient and treatable, if diagnosed early and prompt treatment instituted. Serious adverse effects related to retinal vascular occlusions and corneal graft rejections call for further multicentric studies, prompt reporting and formulation of a protocol for proper management of these cases. Decisions on timing of vaccination in such cases and also delay in interventional elective surgeries like PKP should be emphasized.
Both general physicians and ophthalmologists should make themselves aware of possibilities of adverse effects of COVID-19 vaccination on the eye. Their role in educating general populations about this is of great value so that patients seek timely consultation and intervention as and when required. Though the reports of ophthalmic adverse events of COVID-19 vaccination are being reported worldwide, its incidence rate is less than the prevalence rate of ophthalmic manifestations of COVID-19.[59] These fewer infrequent adverse events should not deter the mass vaccination program globally though a vigilant approach to diagnosis and treatment them is also a must.
Conclusions
With emergence of newer mutated strains of coronaviruses and the third wave of the pandemic worldwide, mass campaigns to vaccinate the world’s population is the need of the hour.
Most of the ocular adverse effects of vaccines are transient and resolve without any sequelae except for cases of retinal and ophthalmic vascular occlusions and corneal graft rejections.
An emphasis on close follow -up and a need to delay vaccination and modification of therapy to control flare-up of signs and symptoms in certain subpopulations, Graves’ disease (autoimmune etiology), pre-existing uveal inflammation and corneal graft cases is warranted.
COVID-19 vaccines are a boon in our armamentarium to restore normalcy globally. With newer vaccines in the pipeline and probable endemicity of COVID-19 infection, large multicentric and longitudinal studies are required in future to establish definite association of vaccines to ocular adverse events. This would help in management protocols to combat COVID-19 vaccination induced ocular adverse effects.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1. [Last accessed on 2022 Jan 25]. Available from: http://www.cdc.gov/coronavirus/2019ncov/vaccines/differentvaccines/Moderna.html.2022 .
- 2. [Last accessed on 2022 Jan 25]. Available from: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccine .
- 3. [Last accessed on 2022 Jan 25]. Available from: https://www.mohfw.gov.in/covid_vaccination/
- 4.Moher D, Liberati A, Tetzlaff J, Altman D G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.JAMA Network Open—Instructions for Authors: Ratings of the Quality of the Evidence. [Last accessed on 2022 Jan 25]. Available from: https://jamanetwork.com/journals/jamanetworkopen/pages/instructions-for-authors#SecRatingsofQuality .
- 6.The Centre for Evidence-Based Medicine. [Last accessed on 2022 Jan 25]. Available from: https://www.cebm.net/
- 7.Austria QM, Lelli GJ, Segal KL, Godfrey KJ. Transient eyelid edema following COVID-19 vaccination. Ophthalmic Plast Reconstr Surg. 2021;37:501–2. doi: 10.1097/IOP.0000000000002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazzatenta C, Piccolo V, Pace G, Romano I, Argenziano G, Bassi A. Purpuric lesions on the eyelids developed after BNT162b2 mRNA COVID-19 vaccine: Another piece of SARS-CoV-2 skin puzzle? J Eur Acad Dermatol Venereol. 2021;35:e543–5. doi: 10.1111/jdv.17340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li S, Jia X, Yu F, Wang Q, Zhang T, Yuan J. Herpetic keratitis preceded by COVID- 19 vaccination. Vaccines. 2021;9:1394. doi: 10.3390/vaccines9121394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parmar DP, Garde PV, Shah SM, Bhole PK. Acute graft rejection in a high-risk corneal transplant following COVID -19 vaccination: A case report. Indian J Ophthalmol. 2021;69:3757–8. doi: 10.4103/ijo.IJO_2515_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nioi M, d'Aloja E, Fossarello M, Napoli PE. Dual corneal-graft rejection after mRNA vaccine (BNT162b2) for COVID-19 during the first six months of follow-up: Case report, state of the art and ethical concern. Vaccines. 2021;9:1274. doi: 10.3390/vaccines9111274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crnej A, Khoueir Z, Cherfan G, Saad A. Acute corneal endothelial graft rejection following COVID-19 vaccination. J Fr Ophthalmol. 2021;44:e445–7. doi: 10.1016/j.jfo.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papasavvas I, Herbort C P. Reactivation of Vogt-Koyanagi-Harada disease under control for more than 6 years following anti-SARS-CoV-2 vaccination. J Ophthalmic Inflamm Infect. 2021;11:21. doi: 10.1186/s12348-021-00251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Testi I, Brandão-de-Resende C, Agrawal R, Pavesio C COVID-19 Vaccination Ocular Inflammatory Events Study Group. Ocular inflammatory events following COVID-19 vaccination: A multinational case series. J Ophthal Inflamm Infect. 2022;12:4. doi: 10.1186/s12348-021-00275-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan L, Zhang Y, Cui Y, Wu X. Bilateral uveitis after inoculation with COVID-19 vaccine: A case report. Int J Infect Dis. 2021;113:116–8. doi: 10.1016/j.ijid.2021.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolletta E, Iannetta D, Mastrofilippo V, De Simone L, Gozzi F, Croci S, et al. Uveitis and other ocular complications following COVID-19 vaccination. J Clin Med. 2021;10:5960. doi: 10.3390/jcm10245960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maleki A, Look-Why S, Manhapra A, Foster CS. COVID-19 recombinant mRNA vaccines and serious ocular inflammatory side effects: Real or coincidence? J Ophthalmic Vis Res. 2021;16:490–501. doi: 10.18502/jovr.v16i3.9443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renisi G, Lombardi A, Stanzione M, Invernizzi A, Bandera A, Gori A. Anterior uveitis onset after bnt162b2 vaccination: Is this just a coincidence? Int J Infect Dis. 2021;110:95–7. doi: 10.1016/j.ijid.2021.07.035. [DOI] [PubMed] [Google Scholar]
- 19.Bøhler AD, Strøm ME, Sandvig KU, Moe MC, Jørstad ØK. Acute macular neuroretinopathy following COVID-19 vaccination. Eye. 2022;36:644–5. doi: 10.1038/s41433-021-01610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinzamuri S, Pradeep TG, Kotian R. Bilateral paracentral acute middle maculopathy and acute macular neuroretinopathy following COVID-19 vaccination. Indian J Ophthalmol. 2021;69:2862–4. doi: 10.4103/ijo.IJO_1333_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee DY, Wu H-J, Cheng K-C, Chang Y-C. Disc edema in one eye and central serous chorioretinopathy in the other eye shortly after AstraZeneca COVID-19 vaccination. Kaohsiung J Med Sci. 2022;38:283–5. doi: 10.1002/kjm2.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girbardt C, Busch C, Al-Sheikh M, Gunzinger JM, Invernizzi A, Xhepa A, et al. Retinal vascular events after mRNA and adenoviral-vectored COVID-19 vaccines—A case series. Vaccines. 2021;9:1349. doi: 10.3390/vaccines9111349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pichi F, Aljneibi S, Neri P, Hay S, Dackiw C, Ghazi NG. Association of ocular adverse events with inactivated COVID-19 vaccination in patients in Abu Dhabi. JAMA Ophthalmol. 2021;139:1131–5. doi: 10.1001/jamaophthalmol.2021.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colella G, Orlandi M, Cirillo N. Bell's palsy following COVID-19 vaccination. J Neurol. 2021;268:3589–91. doi: 10.1007/s00415-021-10462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pawar N, Ravindran M, Padmavathy S, Chakrabarty S. Acute abducens nerve palsy after COVID-19 vaccination in a young adult. Indian J Ophthalmol. 2021;69:3764–6. doi: 10.4103/ijo.IJO_1968_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García-Estrada C, Gómez-Figueroa E, Alban L, Arias-Cárdenas A. Optic neuritis after COVID-19 vaccine application. Clin Exp Neuroimmunol. 2021 doi: 10.1111/cen3.12682. doi: 10.1111/cen3.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsukii R, Kasuya Y, Makino S. Nonarteritic anterior ischemic optic neuropathy following COVID-19 vaccination: Consequence or coincidence. Case Rep Ophthalmol Med. 2021;5126254 doi: 10.1155/2021/5126254. doi: 10.1155/2021/5126254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manea MM, Dragoş D, Enache L, Sirbu AG, Tuta S. Multiple cranial nerve palsies following COVID-19 vaccination—Case report. Acta Neurol Scand. 2021 doi: 10.1111/ane.13548. doi: 10.1111/ane. 13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abičić A, Adamec I, Habek M. Miller Fisher syndrome following Pfizer COVID-19 vaccine. Neurol Sci. 2022;43:1495–7. doi: 10.1007/s10072-021-05776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bayas A, Menacher M, Christ M, Behrens L, Rank A, Naumann M. Bilateral superior ophthalmic vein thrombosis, ischemic stroke, and immune thrombocytopenia after ChAdOx1 nCoV-19 vaccination. Lancet. 2021;e11:397. doi: 10.1016/S0140-6736(21)00872-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubinstein Tal J. Thyroid eye disease following COVID-19 vaccine in a patient with a history Graves'disease: A case report. Ophthalmic Plast Reconstr Surg. 2021;37:221–3. doi: 10.1097/IOP.0000000000002059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin W, Tang Y, Wen C. An ocular adverse event in temporal association with COVID-19 vaccination in a patient with systemic lupus erythematosus: A case report. Hum Vaccin Immunother. 2021;17:4102–4. doi: 10.1080/21645515.2021.1976036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee EJ, Cines DB, Gernsheimer T, Kessler C, Michel M, Tarantino MD, et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021;96:534–7. doi: 10.1002/ajh.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdO×1 nCov-19 vaccination [published online ahead of print, 2021 Apr 9] N Engl J Med. 2021;10:1056. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rand KH, Rasmussen LE, Pollard RB, Arvin A, Merigan TC. Cellular immunity and herpesvirus infections in cardiac transplant patients. N Engl J Med. 1977;296:1372–7. doi: 10.1056/NEJM197706162962402. [DOI] [PubMed] [Google Scholar]
- 36.Wertheim MS, Keel M, Cook SD, Tole DM. Corneal transplant rejection following influenza vaccination. BrJ Ophthalmol. 2006;90:925. doi: 10.1136/bjo.2006.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinemann TL, Koffler BH, Jennings CD. Corneal allograft rejection following immunization. Am J Ophthalmol. 1988;106:575–8. doi: 10.1016/0002-9394(88)90588-0. [DOI] [PubMed] [Google Scholar]
- 38.Solomon A, Frucht-Pery J. Bilateral simultaneous corneal graft rejection after influenza vaccination. Am J Ophthalmol. 1996;121:708–9. doi: 10.1016/s0002-9394(14)70638-5. [DOI] [PubMed] [Google Scholar]
- 39.Lockington D, Lee B, Jeng BH, Larkin DFP, Hjortdal J. Survey of corneal surgeons'attitudes regarding keratoplasty rejection risk associated with vaccinations. Cornea. 2021;40:1541–7. doi: 10.1097/ICO.0000000000002662. [DOI] [PubMed] [Google Scholar]
- 40.Rallis KI, Ting DSJ, Said DG, Dua HS. Corneal graft rejection following COVID-19 vaccine. Eye. 2022;36:1319–20. doi: 10.1038/s41433-021-01671-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cunningham ET, Jr, Moorthy RS, Fraunfelder FW, Zierhut M. Vaccine associated uveitis. OculImmunol Inflamm. 2019;27:517–20. doi: 10.1080/09273948.2019.1626188. [DOI] [PubMed] [Google Scholar]
- 42.Watad A, De Marco G, Mahajna H, Druyan A, Eltity M, Hijazi N, et al. Immune-mediated disease flares or new- onset disease in 27 subjects following mRNA/DNA SARSCoV-2 vaccination. Vaccines. 2021;9:435. doi: 10.3390/vaccines9050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teijaro JR, Farber DL. COVID-19 vaccines: Modes of immune activation and future challenges. Nat Rev Immunol. 2021;21:195–7. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodero MP, Crow YJ. Type I interferon-mediated monogenic autoinflammation: The type I interferonopathies, a conceptual overview. J Exp Med. 2016;213:2527–38. doi: 10.1084/jem.20161596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cunningham ET, Jr, Moorthy RS. Vaccine-associated posterior uveitis. Retina. 2020;40:595–8. doi: 10.1097/IAE.0000000000002816. [DOI] [PubMed] [Google Scholar]
- 46.Triantafyllidis KK, Giannos P, Mian IT, Kyrtsonis G, Kechagias KS. Varicella zoster virus reactivation following COVID-19 vaccination: A systematic review of case reports. Vaccines. 2021;9:1013. doi: 10.3390/vaccines9091013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reikine S, Nguyen JB, Modis Y. Pattern recognition and signaling mechanisms of RIG-I and MDA5. Front Immunol. 2014;5:342. doi: 10.3389/fimmu.2014.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pelka K, Shibata T, Miyake K, Latz E. Nucleic acid-sensing TLRs and autoimmunity: Novel insights from structural and cell biology. Immunol Rev. 2016;269:60–75. doi: 10.1111/imr.12375. [DOI] [PubMed] [Google Scholar]
- 49.Schurz H, Salie M, Tromp G, Hoal EG, Kinnear CJ, Möller M. The X chromosome and sex-specific effects in infectious disease susceptibility. Hum Genomics. 2019;13:2. doi: 10.1186/s40246-018-0185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhavsar KV, Lin S, Rahimy E, Joseph A, Freund KB, Sarraf D. Acute macular neuroretinopathy: A comprehensive review of the literature. Surv Ophthalmol. 2016;61:538–65. doi: 10.1016/j.survophthal.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 51.Cheng JY, Margo CE. Ocular adverse events following vaccination: Overview and update. Surv Ophthalmol. 2021 doi: 10.1016/j.survophthal.2021.04.001. doi: 10.1016/j.survophthal.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 52.Karussis D, Petrou P. The spectrum of post-vaccination inflammatory CNS demyelinating syndromes. Autoimmun Rev. 2014;13:215–24. doi: 10.1016/j.autrev.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Segal Y, Shoenfeld Y. Vaccine-induced autoimmunity: The role of molecular mimicry and immune cross-reaction. Cell Mol Immunol. 2018;15:586–94. doi: 10.1038/cmi.2017.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lo YL. Clinical and immunological spectrum of the Miller Fisher syndrome. Muscle Nerve. 2007;36:615–27. doi: 10.1002/mus.20835. [DOI] [PubMed] [Google Scholar]
- 55.Biswas S, Ghosh R, Mandal A, Pandit A, Roy D, Sengupta S, et al. COVID-19 induced miller fisher syndrome presenting with autonomic dysfunction: A unique case report and review of literature. Neurohospitalist. 2021;17:1–6. doi: 10.1177/19418744211016709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pal R, Banerjee M. COVID-19 and the endocrine system: Exploring the unexplored. J Endocrinol Invest. 2020;43:1027–31. doi: 10.1007/s40618-020-01276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silpa-Archa S, Lee JJ, Foster CS. Ocular manifestations in systemic lupus erythematosus. Br J Ophthalmol. 2016;100:135–41. doi: 10.1136/bjophthalmol-2015-306629. [DOI] [PubMed] [Google Scholar]
- 58.Liu H, Zhang J, Zhou P, Sun H, Katsarou M, Drakoulis N. Exploration of vascular adhesion protein-1 expression in patients with conjunctivitis associated systemic lupus erythematosus using 2D-DIGE. Exp Ther Med. 2019;18:5072–7. doi: 10.3892/etm.2019.8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sopp N M, Sharda V. An eye on COVID-19: A meta-analysis of positive conjunctival reverse transcriptase-polymerase chain reaction and SARS-CoV-2 conjunctivitis prevalence. Optom Vis Sci. 2021;98:429–36. doi: 10.1097/OPX.0000000000001687. [DOI] [PMC free article] [PubMed] [Google Scholar]