Abstract
Background and Objectives:
Pityriasis versicolor is a common fungal infection of the skin which leads to the formation of scaly and discoloured small lesions on skin. The main objective of this study is to describe clinical and mycological characteristics and the predisposing factors in patients with pityriasis versicolor.
Material and Methods:
In this prospective, observational, hospital-based cross-sectional study, patients of all ages with clinically suspected lesions of pityriasis versicolor were included. After detailed history and thorough clinical examination, skin scrapings were examined with 10% potassium hydroxide (KOH) under light microscope. The scrapings were also subjected to culture examination.
Results:
A total of 113 patients [78 (69.0%) male; 35 (31%) female] were included in the study. A total of 87 (76.9%) patients were from rural area. Outdoor occupation and positive family history of pityriasis versicolor was present in 65 (57.5%) and 38 (33.6%) patients, respectively. Recurrent episodes were reported by 66 (60%) patients. Excessive sweating and oily skin were seen in 36 (31.8%) and 24 (21.1%) patients, respectively. History of occlusive clothing was present in 22 (19.4%) patients. Chest, back, and shoulders were affected in 36 (31.8%), 22 (19.4%), and 08 (07.0%) patients, respectively. Hypopigmented lesions were seen in 97 (85.8%) patients. Patches and macules were observed in 60 (53.1%) and 53 (46.9%) patients, respectively. A total of 27 (23.8%) patients reported mild prutitus. A total of 79 (69.9%) patients were KOH positive and culture negative, whereas 26 (23.0%) patients were KOH as well as culture positive. Eight (7.0%) patients were both KOH and culture negative.
Conclusion:
Pytiriasis versicolor is more common in young adults and males with the most common presentation of hypopigmentation lesions. In our study population, presentation with large patches was more common than macular lesions. Pruritus was more in patients with large patches than those with macules.
Keywords: Hypopigmentation, KOH, pityriasis versicolor, rural population, symptoms
Introduction
Pityriasis versicolor, known as tinea versicolor or Peter Elam’s disease, is a common skin disorder observed in clinical practice by dermatologists and primary care physicians.[1] In this superficial fungal infectious disease caused by Malassezia, a lipophilic yeast, patients often present with scaly lesions on different parts of body, either hypopigmented or hyperpigmented in nature. Erythema and itching are other associated symptoms.[1,2,3] Presence of itching has been suggested to be based on the type of lesion, severity of condition, site of the lesions, and other associated factors such as sweat or exposure to sunlight.[1] The disease is most commonly observed in young adult people.[3,4] Many species of the fungus Malassezia are observed in skin of healthy people as part of normal microbiota. Of these, Malassezia furfur, Malassezia globosa and Malassezia sympodialis are primarily associated with pityriasis versicolor.[5,6]
Precipitating factors such as the use of oily preparations and humidity can increase the risk of the disease. Occurrence of this disease is more common during summer.[1,4]
Diagnosis of pityriasis versicolor is based on the clinical findings and confirmed by potassium hydroxide examination (KOH) of skin scrapping and Wood’s lamp examination.[1] Early diagnosis and treatment is important considering risk of psychopathological symptoms such as anxiety and depression and impaired quality of life in these patients.[7] Several topical and systemic treatment options are available for its treatment.[2]
Insufficient studies from India reporting clinico epidemiology of p. versicolor creates a knowledge gap which needs to be fulfilled. Therefore, this study is an attempt to fill existing research gap by providing recent data on epidemiology and mycology of p. versicolor from Northern India. Data generation will also help family physicians to provide effective primary care in terms of better clinical suspicion of p. versicolor and to take effective diagnostic and therapeutic measures at the earliest.
Objective
The objective of this study was to describe clinical and mycological characteristics and the predisposing factors in patients with pityriasis versicolor.
Material and Methods
This prospective, observational, hospital-based cross-sectional study was conducted in collaboration of Dermatology and Microbiology departments of a teaching tertiary care hospital over a period of 1 year. Approval was taken from institutional ethical committee prior to the commencement of the study.
Patients of all ages with clinically suspected lesions of pityriasis versicolor attending outpatient department were included. Patients with diabetes mellitus, other chronic diseases and as well as those on immunosuppressive drugs with suspected pityriasis versicolor lesions were also included. Patients who received oral treatment in the last 30 days for similar skin lesions were excluded from the study.
After explaining study procedure and any anticipated risks and benefits of the study, informed written consent was taken from each patient who volunteered to participate in the study. Details were asked about onset and duration of disease, recurrent episodes, concurrent symptoms, treatment taken in past (>30 days) for same disease and presence of similar lesions in other family members. Thorough clinical examination was done and type of lesions, colour of lesions and site of lesions were recorded. Status of personal hygiene, occupation, any chronic systemic illness present and family details was also recorded.
Skin scrapings were collected from active edge of the discoloured areas of skin by using a sterile blade and smears were prepared with 10% KOH for direct microscopic examination under 10× and 40× magnification power. Spagghetti and meatball appearance on KOH wet mount was considered as positive for microscopy. The second part of the scrapings was inoculated on two slants of Sabouraud’s dextrose agar with and without cyclohexamide with olive oil overlayer and incubated at 37°C for 4 weeks. The inoculated slants were observed at frequent intervals for suspected growth of Malassezia. If growth appeared, it was confirmed microscopically by lactophenol cotton blue mount.
Statistical analysis
The results are expressed using descriptive statistics. Categorical data are presented as frequency and percentages and continuous data are presented as mean and standard deviation. Median and standard error of mean are also provided for appropriate variables.
Results
A total of 113 clinically suspected patients of pityriasis versicolor infection were recruited. Out of 113, 78 (69.0%) were male and 35 (31%) were female patients. Maximum number of cases [56 (49.5%)] belonged to 21 to 30 years age group followed by 11 to 20 years [30 (26.5%)] and 31 to 40 years age group [14 (12.3%)] [Table 1].
Table 1.
Baseline characteristics of patients with suspected pityriasis versicolor
| Parameter | n (%) |
|---|---|
| Gender n (%) | |
| Male | 78 (69.0%) |
| Female | 35 (31.0%) |
| Mean age in years | 25.8 |
| Age range: n (%) | |
| 0-10 years | 02 (1.7%) |
| 11-20 years | 30 (26.5%) |
| 21-30 years | 56 (49.5%) |
| 31-40 years | 14 (12.3%) |
| 41-50 years | 04 (3.5%) |
| 51-60 years | 06 (5.3%) |
| 61-70 years | 01 (0.8%) |
| Residence n (%) | |
| Rural | 87 (76.9%) |
| Urban | 26 (23.0%) |
| Duration of illness n (%) | |
| 1 month-1 year | 34 (30%) |
| 1 year-3 years | 79 (69.9%) |
| Outdoor occupation | 65 (57.5%) |
| Family history of P. versicolor n (%) | 38 (33.6%) |
| Treatment taken in past more than 3 months for same illness | 32 (29.1%) |
| Recurrent episodes | 66 (60%) |
| Diabetes mellitus | 09 (7.9%) |
A total of 87 (76.9%) patients were from rural area and 79 (69.9%) patients had history of illness since 1 to 3 years. Outdoor occupation and positive family history of P. versicolor was present in 65 (57.5%) and 38 (33.6%) patients, respectively. A total of 32 (29.1%) patients reported taking treatment in past more than 3 months. Recurrent episodes were reported by 66 (60%) patients [Table 1]. Excessive sweating and oily skin were seen in 36 (31.8%) and 24 (21.1%) patients, respectively. History of occlusive clothing was present in 22 (19.4%) patients [Table 2]. Chest, back, and shoulders were affected in 36 (31.8%), 22 (19.4%), and 08 (07.0%) patients, respectively. Hypopigmented lesions were seen in 97 (85.8%) of the cases [Table 3] and lesions in the form of patches and macules were observed in 60 (53.1%) and 53 (46.9%) of the patients respectively. Figure 1 shows large hypopigmented patches on back.
Table 2.
Clinically relevant history
| History | n (%) |
|---|---|
| Excessive sweating | 36 (31.8%) |
| Oily skin | 24 (21.2%) |
| Occlusive clothing | 22 (19.4%) |
Table 3.
Clinical characteristics of P. versicolor
| Parameter | n (%) |
|---|---|
| Affected body part n (%) | |
| Chest | 36 (31.8%) |
| Back | 22 (19.4%) |
| Shoulders | 08 (07.0%) |
| Abdomen | 07 (06.2%) |
| Neck | 10 (08.8%) |
| Upper arms | 06 (05.3%) |
| Face | 05 (04.4%) |
| All body | 19 (16.8%) |
| Colour of lesions | |
| Hypopigmented | 97 (85.8%) |
| Hyperpigmented | 16 (14.1%) |
| Nature of lesions | |
| Patches | 60 (53.1%) |
| Macules | 53 (46.9%) |
| Presence of mild pruritus | 27 (23.8%) |
Figure 1.

Image showing large hypopigmented lesions on back in Pityriasis versicolor
A total of 27 (23.8%) patients reported mild pruritus. Table 4 shows mean and median number of lesions observed on various parts of body areas. A total of 79 (69.9%) patients were KOH positive and culture negative, whereas 26 (23.0%) patients were KOH as well as culture positive. Eight (7.0%) patients were both KOH and culture negative [Figure 2]. By using clinical suspicion as gold standard, sensitivity of KOH and culture was 92.9% (confidence interval 0.95–0.05) and 23% (confidence interval 0.95–0.17), respectively. Figure 3 showed spaghetti and meat balls appearance on KOH microscopy.
Table 4.
Descriptive statistics of various lesions
| Statistical variables | Lesions | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Neck | Face | Shoulders | Chest | Back | Upper arms | Neck and shoulder | |
| Mean | 1.15 | 1.15 | 2.00 | 1.05 | 1.15 | 1.35 | 1.25 |
| Std. Deviation | 0.366 | 0.366 | 0.000 | 0.224 | 0.366 | 0.489 | 0.444 |
| Median | 1.00 | 1.00 | 2.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Std. Error of Mean | 0.082 | 0.082 | 0.000 | 0.050 | 0.082 | 0.109 | 0.099 |
Figure 2.
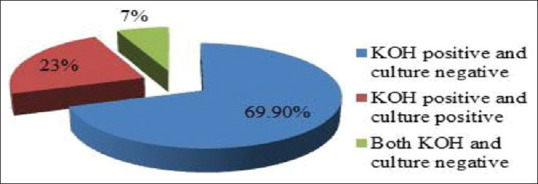
Mycological characteristics of P. versicolor
Figure 3.
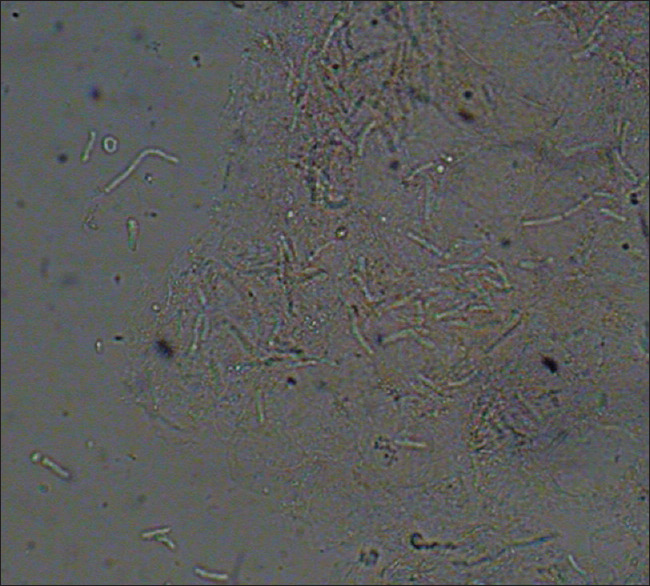
Image showing Spaghetti and meatball appearance in pityriasis versicolor under KOH microscopy
Discussion
In this study, we examined epidemiological, clinical and mycological characteristics of 113 patients with suspected pityriasis versicolor infection. In our study, the most commonly affected age group was from 21 to 30 years of age which coincides with other studies.[8,9,10,11] This is explained by the role of sebum and hormone in young adults. Increased activity of pilosebaceous glands at puberty leads to increased sebum production and thus provides a lipid rich environment for the growth of Malassezia species. Sebaceous glands activity start decreasing after third decade of life, hence, older age groups become less susceptible.[9]
Our study population was predominated by male population in accordance with others.[8,9,12] However, in another study, females from fishery community had higher rate of infection than males.[13] We observed females presenting with face involvement more commonly whereas males had more lesions on chest and abdomen. Several studies have reported upper trunk as the most commonly involved body part for P. versicolor such as Ghosh et al. (chest 48.2% and back 41.8%),[3] Rao et al. (neck 71.6%, back 70%, and chest 58.3%),[4] Morais et al. (upper trunk 80.2%)[14] and Pallai et al. (chest 55%, neck 24%, and back 15.5%).[15] Considering presence of pilosebaceous glands more on upper trunk, this area is more susceptible to infection. Unusual face involvement in females may be explained by behaviour of reporting to healthcare professionals. Since the condition is asymptomatic in most cases, the females often do not report to healthcare professionals especially when lesions occur on covered parts of body. They do not seem to pay heed to cosmetic problem unless it occurs on face.
In our study, frequency of hypopigmented lesions (85.8%) was more common than hyperpigmented lesions (14.1%). This is in agreement with most studies such as Morais et al.,[14] Ghosh et al.,[3] Rao et al.[4] and Krishnan and Thapa[10] described hypopigmentation in 62.9%, 81.83%, 75% and 84% of study cases, respectively. According to Gupta et al.,[2] in Indians, 75% to 85% of the lesions are hypopigmented, 5% to 15% are mixed and the rest of the cases are hyperpigmented. Difference in the morphological appearance may be related to differences in the climate and skin colour of people.[9] Role of azelaic acid produced by Malassezia during metabolism produces cytotoxic effect on melanocytes and melanogenesis has been suggested to play a role in the development of hypopigmentation.[16]
In our study, lesions in the form of patches (53.1%) were more common than scattered macules (46.9%). However, other authors have reported generalised macular pattern as the most common presentation.[3,4,10] Most of our study subjects had disease longer than 3 years of duration (69.9%). Due to longer duration of the disease, the individual macular lesions increases in size and join together to form large irregular patches. At the stage of large patches, the disease is more noticeable and causes more discomfort. This could be a reason for more patients visited with patches in our study.
Pruritus was present in 57.5% of patients in this study. Other researchers such as Ghosh et al.,[3] Rao et al.[4] and Morais et al.[14] have also reported pruritus in 47.27%, 30% and 50% of patients, respectively.
Observation related to the history of recurrent episodes in our study was in agreement with others. A study from Brazil reported recurrent episodes in 55.2% of the patients.[17] Various predisposing factors for recurrence and chronicity of the disease was evaluated in study subjects. Excessive sweating was present in 31.8% of the patients, oily skin was present in 21.2% patients, occlusive clothing were worn by 19.4% and chronic systemic illness like diabetes mellitus was present in 7.9% of the cases. Among these predisposing factors, excessive sweating was significantly associated with recurrences (Chi-square statistic 8.1601, P value. 004 significant). Approximately three-fourth of our study population had agriculture and livestock keeping profession, both of which demands more outdoor working under high temperature and harsh climatic conditions of this region, leading to prolonged and excessive sweating and more recurrences. Patient education and counselling related to lifestyle related risk factors may be useful to reduce the risk of recurrence.
Positive family history was present in almost one-third cases in our study. Other authors have also reported presence of positive family history in many patients with p. versicolor suggesting role of hereditary factors which need to be explored further.[4,12,14,15,16,18]
Out of 113 clinically suspected cases, 92.9% were mycologically positive for the fungus by KOH microscopy (characteristic spaghetti and meat balls seen), of which 24.7% were positive by culture indicating high sensitivity of microscopy over culture.
A study from Punjab reported positive microscopy in 89.2% of the clinically suspected cases and growth of Malassezia in 83% of the samples.[11] Similarly, Chaudhary et al.[19] and Kavitha et al.[12] also showed higher rate of positive microscopy than culture. Kindo et al.[20] reported growth of Malassezia in 68.7% of the microscopically confirmed cases which is higher than the current study (23%).
Lower rate of isolation of Malassezia from culture in present study as compared to other studies may be explained by no use of enriched and selective media for Malassezia, such as modified Dixon’s agar and peptone glucose yeast extract medium containing glycerol monostearate due to non-availability. Second, we did not exclude patients who used topical antifungal or steroid cream on the day of visit to hospital.
Based on our findings, we recommend KOH microscopy as a useful screening test for p. versicolor. It is a simple and rapid test and independent on disease prevalence. Thus, microscopy can be used by primary care physicians as a point of care test to diagnose p. versicolor.
Conclusion
Pityriasis versicolor is more prevalent in young adults and in males with the most common presentation of hypopigmentation lesions. Unlike other study, in our study population, presentation with large patches was more common than macular lesions. Pruritus was more in patients with large patches than those with macules. Hyperhidrosis was an important predisposing factor for recurrence and chronicity of disease. Further investigations are needed to establish relationship between pruritus and extent of involvement as well as between hyperhidrosis and recurrence of disease.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kaushik A, Pinto HP, Bhat RM, Sukumar D, Srinath MK. A study of the prevalence and precipitating factors of pruritus in pityriasis versicolor. Indian Dermatol Online J. 2014;5:223–4. doi: 10.4103/2229-5178.131141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta AK, Kogan N, Batra R. Pityriasis versicolor: A review of pharmacological treatment options. Expert OpinPharmacother. 2005;6:165–78. doi: 10.1517/14656566.6.2.165. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh SK, Dey SK, Saha I, Barbhuiya JN, Ghosh A, Roy AK. Pityriasis versicolor: A clinicomycological and epidemiological study from a tertiary care hospital. Indian J Dermatol. 2008;53:182–5. doi: 10.4103/0019-5154.44791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao GS, Kuruvilla M, Kumar P, Vinod V. Clinico- epidermiological studies on tinea versicolor. Indian J Dermatol VenereolLeprol. 2002;68:208–9. [PubMed] [Google Scholar]
- 5.DeAngelis YM, Saunders CW, Johnstone KR, Reeder NL, Coleman CG, Kaczvinsky JR, Jr, et al. Isolation and expression of a Malassezia globosalipase gene, LIP1. J Invest Dermatol. 2007;127:2138–46. doi: 10.1038/sj.jid.5700844. [DOI] [PubMed] [Google Scholar]
- 6.Tarazooie B, Kordbacheh P, Zaini F, Zomorodian K, Saadat F, Zeraati H, et al. Study of the distribution of Malassezia species in patients with pityriasis versicolor and healthy individuals in Tehran, Iran. BMC Dermatol. 2004;4:5. doi: 10.1186/1471-5945-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaymak Y, Taner E. Anxiety and depression in patients with pityriasis rosea compared to patients with tinea versicolor. Dermatol Nurs. 2008;20:367–70. [PubMed] [Google Scholar]
- 8.Tabaseera N, Kuchangi N, Swaroop MR. Clinico-epidemiological study of pityriasis versicolor in a rural tertiary care hospital. Int J Res Med Sci. 2014;2:1438–40. [Google Scholar]
- 9.Kambil SM. A clinical and epidemiological study of pityriasis versicolor. Int J Sci Stud. 2017;5:155–9. [Google Scholar]
- 10.Krishnan A, Thapa DM. Morphological and pigmentary variations of tinea versicolor in South Indian patients. Indian J Dermatol. 2003;48:83–6. [Google Scholar]
- 11.Kaur M, Narang T, Bala M, Gupte S, Aggarwal P, Manhas A. Study of the distribution of Malassezia species in patients with pityriasis versicolor and healthy individuals in tertiary care hospital, Punjab. Indian J Microbiol. 2013;31:270–4. doi: 10.4103/0255-0857.115636. [DOI] [PubMed] [Google Scholar]
- 12.Kavitha K, Usha MG, Murugesh, Chandrashekar NR. Distribution of Malassezia species in patients with pityriasis versicolor and healthy individuals in South India. J Evid Based Med Health. 2016;3:1627–31. [Google Scholar]
- 13.Acosta ME, Perfetti CDJ. Clinical-epidemiological aspects of pityriasis versicolor (PV) in a fishing community of the semiarid region in Falcon State, Venezuela. Rev IberoamMicol. 2004;21:191–4. [PubMed] [Google Scholar]
- 14.Morais PM, Cunha MGS, Frota MZM. Clinical aspects of patients with pityriasis versicolor seen at a referral center for tropical dermatology in Manaus, Amazonas, Brazil. An Bras Dermatol. 2010;85:797–803. doi: 10.1590/s0365-05962010000600004. [DOI] [PubMed] [Google Scholar]
- 15.Pallai RT, Balakrishnan A, Elizabeth, Sourabh AP. Clinical, epidemiological and mycological study of tinea versicolor. JEvol Med Dent Sci. 2014;3:10796–803. [Google Scholar]
- 16.Snekavalli R, Madhu R, Ramesh A, Janaki C, Dhanalakshmi UR. Clinico epidemiological and mycological study of pityriasis versicolor. Int J Res Med Sci. 2018;6:1963–70. [Google Scholar]
- 17.Santana JO, Azevedo FLA, Campos Filho PC. Pityriasis versicolor: Clinical-epidemiological characterization of patients in the urban area of Buerarema-BA, Brazil. An Bras Dermatol. 2013;88:216–21. doi: 10.1590/S0365-05962013000200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zenab MG, El-Gothamy A review of pityriasis versicolor. J Egypt Wom Dermatol. 2004;1:36–43. [Google Scholar]
- 19.Chaudhary R, Singh S, Banerjee T, Tilak R. Prevalence of different Malassezia species in pityriasis versicolor in central India. Indian J Dermatol VenereolLeprol. 2010;76:159–64. doi: 10.4103/0378-6323.60566. [DOI] [PubMed] [Google Scholar]
- 20.Kindo AJ, Sophia SK, Kalyani J, Anandan S. Identification of Malasseziaspecies. Indian J Med Microbiol. 2004;22:179–81. [PubMed] [Google Scholar]


