Abstract
Introduction:
Human immunodeficiency virus (HIV) and malaria infections are among the major public health concerns in sub-Saharan Africa, where they are associated with high morbidity and mortality. The study was conducted to assess the occurrence and clinical features of HIV and malaria in co-infected individuals in Osun State, Nigeria.
Methods:
The study was cross-sectional, which involved 422 participants who were administered structured questionnaires for socio-demographic and clinical data. Venous blood was collected for malaria parasite detection and count from One hundred and seventy-four HIV seropositive individuals. They were re-examined clinically for HIV diagnosis, CD4+ T cell counts, and packed cell volume (PCV).
Results:
The mean age of the participants was 28.48 ± 15.38 while the overall predominance of malaria among the HIV-positive patients was 11.5% (20/174). The malaria prevalence was significantly higher in female patients (P = 0.0088) and occupational status among students (P = 0.0001). Malaria/HIV co-infected patients had a significantly lower mean value of PCV (P = 0.0001), CD4+ cell count (0.0001), and temperature (0.0001) compared to HIV-infected patients having no malaria.
Conclusion:
The study showed that females had relatively higher malaria infection compared to their male counterparts. To achieve better management of HIV patients against malaria infection, proper preventive measures, antiretroviral therapy (ART), and chemoprophylaxis are a useful strategy to put in place. Also, the monitoring of CD4+ cell count, viral load, and some hematology indices on a regular basis is crucial.
Keywords: Co-infection, HIV, malaria, Nigeria, plasmodium falciparum
Introduction
The human immunodeficiency virus (HIV) and Plasmodium species are pathogens responsible for two of the most prevalent infectious diseases in the world.[1] Both pathogens, principally Plasmodium falciparum, are the cause of significant stimulation and disorder of the immune system.[2] The dual infections have been reported as the life-threatening health problems of developing countries, including Nigeria, accounting for more than 2 million annual deaths globally.[3] Significant mortality has been reported as a result of opportunistic infections such as malaria and arrays of other pathogenic diseases faced by people living with HIV.[4]
The integral part of the universal anti-retroviral therapy (ART) program in developing countries, as recommended by the World Health Organization, is the administration of cotrimoxazole (CTX) prophylaxis treatment care for HIV-infected individuals.[5] This practice remains a key policy for the prevention of opportunistic infections among HIV-infected individuals, including Plasmodium. CTX has significantly decreased bacterial infections, HIV-related deaths, and hospital-acquired infections.[6] Preventive measures such as insecticide-treated bed nets[6] have been reported to reduce the risk of malaria in people living with HIV/AIDs. Remarkably, termination of CTX among HIV-infected individuals receiving ART has resulted in progressive increases in parasite intensity and malaria incidence.[7]
Reports have shown that in most clinical cases of malaria, anemia is a prognostic factor most commonly encountered hematological abnormality in HIV and malaria-infected individuals.[8,9,10,11,12,13,14,15,16] In most sub-Saharan African countries, where the burden of HIV and malaria co-infection is incidentally high, evaluation of clinical indices should be considered. Therefore, this study was performed to determine the occurrence and clinical features of HIV and malaria co-infected individuals in Osun State, Nigeria.
Methods
Study area
Osun State is located in the tropical zone of South Western Nigeria. The language of the majority of the people of Osun State is Yoruba, but this is however broken into scores of dialects. It is landlocked and occupies a landmass of 9,251 square kilometers and a population of 3,423,535.[17] The area experiences two seasons, the dry season (November–March) and the rainy season (April–October).[18] The mean daily temperature varies from 30°C to 34°C, and the mean annual rainfall is about 1400 mm. Malaria transmission occurs throughout the year, with the peak at the beginning and end of the rainy season.[19]
Study population
HIV-infected patients were registered, diagnosed with HIV infection, and further sought treatment at IHVN clinic, Uniosun Teaching Hospital Osogbo, General Hospital Ede, and OAUTHC Wesley Guild Hospital Ilesa. The participants included a control group of HIV seronegative individuals at the General Out-patient Department (GOPD). Only those who had given their consent for blood collection and answered the questionnaire were enrolled in the study.
Selection criteria
Inclusion criteria
Confirmed HIV-infected patients, duly registered into the anti-retroviral treatment (ART) program, presenting with uncomplicated falciparum malaria with the presence of fever (³ 37.5°C) in accordance with the WHO criteria.
Exclusion Criteria
The exclusion criteria were the patients that have been on anti-malarial medication for two weeks prior to the period of blood collection, as well as the patients that neither showed interest nor gave consent.
Sample size determination
Sample size was determined using.[20]
Sample size 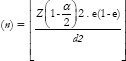
Where n = Sample size
Z = Z score, i.e., Standard normal variate at 5% type 1 error (P < 0.05)
e = Standard deviation or expected proportion in population based on previous studies
d = Margin of error or absolute error (5%)
Z = 1.96
e = 0.5.
The sample size obtained was 422. A total of 174 HIV seropositive individuals who sought care at the study locations were recruited in the study.
Ethical clearance
The study was approved by the Uniosun Teaching Hospital (LTH/EC/201/11/243) and Osun State Ministry of Health Ethical Committee Osogbo (OSHREC/PRS/569T/41) before this research was carried out. Written informed consents were obtained from patients prior to recruitment into this study. Consent for the children was provided by the parents/guardians while some of the children participants provided the assents by nodding and thumb printing.
Structured questionnaire
A questionnaire in local (Yoruba) and official (English) languages, was included in the basic socio-demographics of the study subjects as well as their knowledge and prevention of malaria. For consistency of thought, two independent translators were engaged; one translated English to Yoruba, the other, who had no prior copy of English, was tasked to translate the local language back into English. Individuals were given unique codes on the questionnaires and their laboratory specimens.
Determination of axillary temperature and weight
The weight was determined using a weighing scale, and the axillary temperature was also determined before enrolment using a digital clinical thermometer. The tip of the thermometer was inserted under the armpit of the included subjects, and the numeric value of axillary temperature was recorded.[21]
Detection and parasite density of malaria parasite
Laboratory investigation was conducted using 2 ml of blood sample was spread at an angle 30°C with a clean spreader to form a thin blood smear with a tail at the end of the film and 6 ml of blood for a thick film. The smear was thoroughly allowed to dry, fixed with methanol, and Giemsa stain stock diluted in 1:10 in buffered distilled water was used for both films for 10 minutes. The stained smears were washed off with buffered water (pH 7.2), allowed to air-dry, and examined microscopically under x100 (oil immersion) objective of an Olympus CX 22 light microscope (Olympus Optical Co. Ltd., China).[22]
The slide was considered positive if asexual forms/gametocytes of any Plasmodium species were observed in the blood films. The corresponding thin films were observed to determine the species of Plasmodium present. Malaria parasites were counted against 200 white blood cells (WBCs) in the thick film. The parasite density was recorded as the number of asexual parasites per microlitre of blood.[23]
HIV diagnosis
HIV test was conducted using determine Rapid Test kit (Abott Laboratories, Co., Ltd. Minato-Ku, Tokyo Japan) was used to test the supposed HIV negative subjects for HIV-1. Test results were read after 15 min from corresponding colour changes on the strip according to the manufacturer’s guidelines and instructions. All HIVpositive results by the test kit were confirmed using UNI GOLD or STAT PAK concurrently according to the serial algorithm of the Federal government of Nigeria.[24] Both test kits are immuno-chromatographic rapid tests for the qualitative detection of antibodies specific to HIV in human serum, plasma, or whole blood.
CD4 count estimation (Partec cyflow technique)
The CD4+ T- lymphocyte count of the study population was evaluated using flow cytometry. (Partec, GmBH, Germany).[25] 20 ml of CD4+ - PE monoclonal antibody was put in labelled Partec (Rohren) tubes, and 20 ml of well-mixed ethylenediamine tetraacetic acid (EDTA) blood was added. This content was mixed together several times for 2 min, and incubated in the dark for 15 min at room temperature with intermittent mixing every 5 min. After incubation, 800 ml of CD4 diluting buffer was added to each preparation, mixed properly before being analysed on the cyflow counter as described by the equipment manufacturer.[25] CD4 cell counts were categorized as very low or advanced stage (<250 cell/ml), 250–350 cell//ml (low), lower normal (350–500 cell/ml) and higher normal or asymptomatic stage (³500 cell/ml).[26]
Packed cell volume (PCV) and Haemoglobin concentration (Hb)
The packed cell volume (PCV) and haemoglobin concentration (Hb) were estimated with Sysmex XT- 21N Haematology Analyser Automated machine (Sysmex Corporation, Japan 2012 Model) with strict adherence to the manufacturer instruction.[27] Anaemia was defined as Hb <11.0 g/dl and further classified as described by Cheesbrough (2010) as severe (Hb <7 g/dl), moderate (Hb between 7.0 g/dl and 10.0 g/dl), and mild (>10.0 g/dl and <11 g/dl).[28,29]
Statistical analysis
After validation, data analysis was done using GraphPad Prism 5 (GraphPad Software Inc. USA) to generate means, standard deviation, median, and frequency distributions. The significant difference between groups was determined using a T-test for continuous variables such as age and parasitaemia that were normally distributed. Analysis of variance was used to investigate the relationship between the variables. The association between categorical variables was tested using the Chi-square test (<2). Statistical significance was defined as a P value < 0.05.
Results
A total of 174 participants with a mean age of 28.48 ± 5.38 were enrolled in the study. The study population comprised nearly two-third females and one-third males, distributed across six different age groups. Almost half of the study population were married (48.9%), and 40.8% were single, while the remaining (10.4%) were considered divorced/widowed. The greater proportion of the studied population had completed some level of education from primary to tertiary levels. A major proportion of the studied participants was self-employed, while about 13.3% were civil servants, students were found to be 27.6%, and 8.6% were unemployed, as presented in Table 1. The place of residence showed that more participants were residing in rural than in urban settings [Table 1].
Table 1.
Socio-demographic characteristics of the respondents
| Variable | Frequency | Percentage (%) |
|---|---|---|
| Age group (years) | ||
| 1-5 | 40 | 23.0 |
| 6-12 | 31 | 17.8 |
| 13-18 | 20 | 11.5 |
| 19-25 | 24 | 13.8 |
| 26-45 | 40 | 23.0 |
| >45 | 19 | 10.9 |
| Sex | ||
| Male | 67 | 38.5 |
| Female | 107 | 61.5 |
| Marital Status | ||
| Single | 71 | 40.8 |
| Married | 85 | 48.9 |
| Divorced/Widowed | 18 | 10.4 |
| Place of residence | ||
| Rural | 99 | 56.9 |
| Urban | 75 | 43.1 |
| Level of Education | ||
| No formal Education | 22 | 12.6 |
| Primary Education | 56 | 32.2 |
| Secondary education | 54 | 31.0 |
| Tertiary | 42 | 24.1 |
| Occupation | ||
| Civil Servant | 23 | 13.3 |
| Business | 29 | 16.7 |
| Artisan | 59 | 33.9 |
| Unemployed | 15 | 8.6 |
| Student | 48 | 27.6 |
The level of anaemia among the malaria-infected participants was 38.5% anaemic while 61.5% non-anaemic for malaria non-infected individuals. Thirty-one out of 174 (17.8%) of the study participants were malaria positive. The proportion of participants who used insecticides treated nets (ITNs) was higher (114 (81.0%)) compared to their counterparts that utilized insecticide sprays (105 (60.3%)). One hundred and thirty-two (75.9%) were on daily cotrimoxazole prophylaxis. The number of participants with a CD4 cell count less than 200 was 31.0% higher than the CD4 cell count of greater than or equal to 500 of 12.6%, as shown in Table 2.
Table 2.
Frequency of all variables among the respondents
| Variable | Frequency | Percentage (%) |
|---|---|---|
| Anemic Status | ||
| Anaemic | 67 | 38.5 |
| Non-anaemic | 107 | 61.5 |
| Malaria | ||
| Positive | 31 | 17.8 |
| Negative | 143 | 85.1 |
| Use of Insecticides treated Nets | ||
| Yes | 141 | 81.0 |
| No | 33 | 19.0 |
| Use of Insecticides Spray | ||
| Yes | 105 | 60.3 |
| No | 69 | 39.7 |
| CD4 T cell count (cells/µl) | ||
| <200 | 54 | 31.0 |
| 200-300 | 48 | 27.6 |
| 301-499 | 50 | 28.7 |
| ≥500 | 22 | 12.6 |
| On prophylaxis Cotrimoxazole | ||
| Yes | 132 | 75.9 |
| No | 42 | 24.1 |
Comparison of Socio-demographic characteristics of HIV positive individuals with malaria
The age group of 26–45 years was recorded with the highest percentage of malaria infection (10 (32.2%)). The age group, place of residence, and level of education showed no significant difference in the prevalence of HIV and malaria co-infection. The female participants (20 (64.5%)) had a higher malaria positive than male counterpart (11 (35.5%)) with a significant difference among the gender (χ2 = 6.864; df = 1; P < 0.0088) as shown in Table 2. The occurrence of malaria infection was higher among the students while the unemployed had the least malaria positive with a significant difference between occupation and malaria (χ2 = 29.29; df = 4; P < 0.0001). A total of 125 (71.8%) were observed to be under ART treatment while the remaining were not on ART treatment. Nineteen (61.3%) had anaemia, while twelve (38.7%) were non-anaemic. However, the difference was not statistically significant (χ2 = 1.636; df = 2; P < 0.4413) [Table 3].
Table 3.
Comparison of Socio-demographic characteristics of HIV positive individuals with Malaria
| Variables | Malaria Negative | Malaria Positive | Df | χ2 | P |
|---|---|---|---|---|---|
| Age | |||||
| 1-5 | 15 (10.5) | 7 (22.6) | 5 | 6.976 | 0.2224 |
| 6-12 | 12 (8.4) | 5 (16.1) | |||
| 13-18 | 10 (6.9) | 4 (12.9) | |||
| 19-25 | 17 (11.9) | 3 (9.7) | |||
| 26-45 | 39 (27.2) | 10 (32.2) | |||
| >45 | 50 (35.0) | 2 (6.5) | |||
| Sex | |||||
| Male | 52 (36.4) | 11 (35.5) | 1 | 6.864 | 0.0088* |
| Female | 91 (63.6) | 20 (64.5) | |||
| Place of Residence | |||||
| Rural | 72 (50.4) | 19 (61.3) | 1 | 3.155 | 0.0757 |
| Urban | 71 (49.7) | 12 (38.7) | |||
| Level of Education | |||||
| No Education | 27 (18.9) | 6 (19.4) | 3 | 6.748 | 0.0804 |
| Primary | 51 (35.7) | 7 (22.6) | |||
| Secondary | 45 (31.5) | 7 (22.0) | |||
| Tertiary | 20 (14.0) | 11 (35.5) | |||
| Occupation | |||||
| Civil Servant | 23 (16.1) | 4 (12.9) | 4 | 29.29 | 0.0001* |
| Business | 20 (14.0) | 6 (19.3) | 1 | ||
| Artisans | 34 (23.8) | 7 (22.6) | 2 | ||
| Unemployed | 19 (13.3) | 3 (9.7) | |||
| Students | 47 (32.9) | 11 (35.8) | |||
| Use of ART | |||||
| Yes | 116 (66.7) | 9 (29.0) | 0.4176 | 0.5181 | |
| No | 27 (15.5) | 22 (71.0) | |||
| Anemic Status | |||||
| Anemic | 48 (33.6) | 19 (61.3) | 2.116 | 0.3413 | |
| Non-anemic | 95 (66.4) | 12 (38.7) |
*=Significant value
In this study, the percentage of HIV and malaria co-infection was 31 (17.8%) in a total of 174 detected by blood smear microscopy, a gold-standard method [Figure 1].
Figure 1.
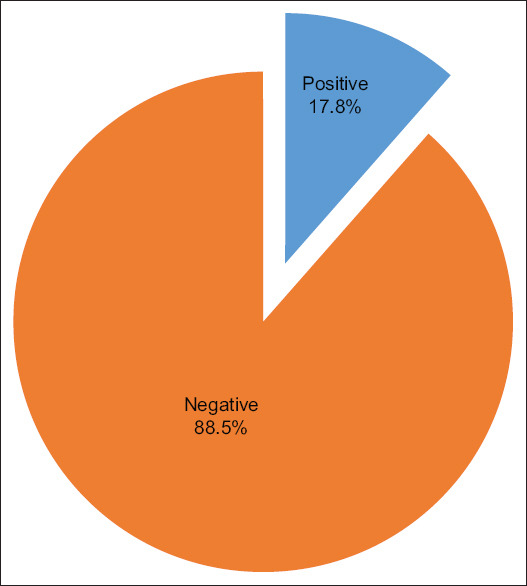
Prevalence of malaria among HIV positive participants
Effect of usage of preventive measures on the spread of malaria parasite
The use of insecticide-treated nets was significant with malaria among HIV-infected participants (χ2 = 4.054, P < 0.044) as shown in Table 4. Participants that adhere to good environmental sanitation were further observed to be significant as a preventive measure against malaria parasite among the HIV-infected individuals (χ2 = 6.881, P < 0.046).
Table 4.
Association between preventive measures and malaria among HIV positive participants
| Preventive measures | Malaria +ve |
Malaria -ve |
df | χ2 | P |
|---|---|---|---|---|---|
| Insecticide Treated Nets (ITNs) | |||||
| Yes | 8 (25.8) | 97 (67.8) | 2 | 4.054 | 0.044* |
| No | 23 (74.2) | 46 (32.2) | |||
| Insecticide Spray | |||||
| Yes | 10 (32.3) | 131 (91.6) | |||
| No | 21 (67.7) | 41 (8.4) | 2 | 5.240 | 0.014 |
| Window/door nets | |||||
| Yes | 27 (87.1) | 123 (86.0) | |||
| No | 4 (12.9) | 20 (14.0) | 2 | 1.240 | 0.265 |
| Mosquito repellent Creams. | |||||
| Yes | 26 (83.9) | 103 (72.0) | |||
| No | 5 (16.1) | 40 (28.0) | 2 | 1.283 | 0.319 |
| Environmental Sanitation | |||||
| Yes | 6 (19.4) | 85 (59.4) | |||
| No | 25 (80.7) | 58 (40.5) | 2 | 6.881 | 0.046* |
Clinical features, CD4 and PCV of respondents with malaria status
The body temperature was significantly higher in HIV and malaria co-infected participants compared to HIV mono-infected counterparts. The CD4 T-cell count was observed to be significantly lower in the HIV individuals diagnosed to be positive for malaria. Likewise, packed cell volume was significantly lower in HIV-positive participants with malaria infection as shown in [Tables 4 and 5].
Table 5.
Physiological feature, CD4, and PCV of respondents with malaria status
| Variables | Malaria -ve/HIV pos. | Malaria +ve/HIV pos. | t | P |
|---|---|---|---|---|
| Mean±SD | Mean±SD | |||
| Weight (kg) | 53.14±1.87 | 52.42±0.05 | 1.006 | 0.315 |
| Temp. (0°C) | 37.14±0.06 | 39.21±0.11 | 15.14 | <0.0001* |
| CD4 Count | 325.98±9.24 | 203.09±6.30 | 7.512 | <0.0001* |
| PCV (%) | 34.32±0.21 | 29.26±0.30 | 14.92 | <0.0001* |
Discussion
HIV and malaria co-infection may occur simultaneously in individuals and constitute two of the most devastating global health problems in sub-Sahara Africa, including Nigeria. This study was designed as a cross-sectional study to determine the occurrence and clinical features of HIV and malaria co-infected patients attending clinics in the study population. Of the species of Plasmodium that were tested, only P. falciparum was found to be present in this study.
The burden of malaria in HIV-positive participants in this study was 17.8% of the total study cohort, conforming to a similar study conducted in Osogbo by Ojurongbe et al., 2014[9] where a prevalence of 18.5% was recorded. Although the 17.8% is lower, it is indicative that the transmission rate of malaria is gradually declining, which could be due to religious preventive adherence by the population and government policy programmes initiated in the control and elimination of malaria, such as campaign on a prompt diagnosis of malaria with the use of rapid diagnostic test (RDTs), the use of Artemisinin- combination therapy according to WHO recommendation,[30,31] alertness on the regular use of long-lasting insecticides nets and free supply of ART and CTX recommended for HIV-infected patients in Africa which have proffered protection as well as improving health management of HIV/AIDs infected individuals.[30,31,32]
In comparison with studies conducted in other region of the country, 17.8% from this study is higher than 14.2% and 14% reported among HIV patients in Uyo[33]; 16.2% in North Central Nigeria[34]; and lower than 22.9% malaria co-infection prevalence by Gumel et al.[35]; 24% in Jos[36]; 56.8% in Keffi[37]; 59.2% in Kaduna.[38] Other studies around the world showed as 14% among HIV seropositive patients in Cameroon[30]; 36% in South Africa[39] and 61.7% in Mozambique.[40]
Among the HIV-positive people with malaria, it was observed that sex and occupation influenced the prevalence of malaria in this study. HIV-positive females with malaria co-infection were higher than their male counterparts. This is similar to the report of Tay et al.,[16] who also described a higher level of parasitemia in females in contrast to their male counterparts; Bello and Ishaleku,[41] from Keffi; and Amadi et al.[33] in Uyo, Nigeria. This marked difference may be due to the female engaging in household chores while staying outside during the mosquitoes’ active biting hours. On the contrary, Akinbo et al.[42] reported a statistically significant higher prevalence in males than in females. A study in Nasarawa showed that male participants showed a higher prevalence than females, reported by Dikwa et al.[43] and Okokon et al.[44] A study conducted in Kano reported an undifferentiated pattern and frequency between both sexes.[8] The highest type of occupation in which co-infection of malaria and HIV occurred was observed among students. The parasite caused by the occupation observed among the students could be due to poor environmental sanitation and their careless attitude in following preventive measures against Plasmodium infection.
The participants using anti-retroviral drugs (ART) displayed low parasitemia (29.0%) than non-ART observers (71.0%). This is in line with a study conducted by Gennano et al.,[45] and Sandie et al.,[30] which reported a higher prevalence of malaria in HIV-positive individuals who are not on ART than those on ART. This could be as a result of the reformation of their immune system connected with the drugs given to them, signifying the effectiveness of ART as well as the administration of cotrimoxazole as the component of their chemotherapy, which is known to have certain anti-malarial elements, thus lowering the occurrence of malaria in HIV-infected individuals. This is coherent with findings report on the protective capacity of CTX with other preventive measures among people living with HIV.[36]
Finding from the study revealed that a greater number of participants using insecticide-treated nets are over and above other preventive measures practiced. This could be attributed to a religious adherence to the use of preventive measures, which serves as an obstacle to mosquitoes and so prevents the spread of the parasites. This result is in line with the finding in Kenya[46] that reported that the use of ITNs greatly reduces the chance of mosquito contact and malaria transmission. Among HIV-infected participants, those who practice more than one preventive measure against malaria stand a chance of absolute protection, which helps in combating malaria transmission and infection.
Good environmental sanitation was statistically significant (P = 0.009) among study participants. Among those who adopted good sanitation practices, the malaria positivity was significantly lower in the HIV-infected participants (19.4%) than those who did not practise it. This result is consistent with a study in Cameroon where a high prevalence of malaria was reported among school children who had bushes around their residential homes.[47] The low parasitemia recorded in this study may be a result of the effort of the government enforcing periodic environmental sanitation, most importantly in urban areas due to heavy overcrowding and dirty surroundings.
The HIV and malaria co-infected participants displayed high body temperature compared to uninfected HIV counterparts. This finding confirms the previous studies done in Jos Nigeria,[44] there was a strong correlation between body temperature and malaria and HIV co-infection. The rise in temperature could have resulted from the tumult in the schizogonic cycles due to heterogeneity of the malaria parasite; hence the immune system is stimulated to secrete TNF-a, IL-1, and other body cytokines, which causes resistance that leads to up-regulation of endothelial cells that generate febrile paroxysm.[48,49]
The low mean value of PCV in malaria-infected HIV individuals was observed in this study. Although there is no scientific evidence that malaria is associated with anaemia, malaria stimulated anaemia could be a result of lysis of red blood cells (RBCs), which accelerates the clearance of parasitized and non-parasitized RBCs,[50,51] as well as complications attached to some anti-retrovirals taken by HIV-infected individuals, which have been documented to cause anaemia in conjunction with the destruction of red blood cells by Plasmodium malaria, thus decreasing PCV and hemoglobin concentrations. This is in line with the findings of Bawah et al.,[52] who reported higher rates of malaria-inducing anemia among children in Ho municipality. Also, studies conducted in Ghana by Sakzabre et al.,[53] and in Nigeria by Osaro et al.[54] linked anaemia to malaria parasitism. This contrasts with the findings in Oshogbo, Nigeria, which stated that there is no association between malaria parasites and anaemia.[9]
The mean CD4 of malaria-infected HIV individual was low compared to the uninfected HIV counterpart, which showed a significant difference as similarly observed by Ojurongbe et al. and Tay et al.[9,16] This could be due to the associated decrease in CD4 among those infected with HIV. However, several studies have separated malaria from a low CD4 count among HIV-infected individuals.[9,55] Although, it is not quite the same as Njunda et al.,[56] where malaria was higher in CD4 below 200 cells/mL.
Conclusion
In conclusion, malaria parasites prevalence was found to be high in HIV-positive individuals among the study population. Immune recovery of HIV-positive patients being monitored by the change in CD4 count may be affected by malaria co-infection, especially in a recurrent malaria attack and compliance with ART regimen. Therefore, to achieve better management of HIV-positive patients against malaria infection, primary health care physicians and healthcare providers should harness the use of preventive measures and chemotherapy as a useful strategy to mitigate this public health challenge. Also, early malaria and HIV diagnosis, monitoring of CD4 count, viral load, and haematological indices on a regular basis is crucial. In addition, concerted efforts by primary care physicians should be channeled to completely eradicate HIV and malaria co-infection through public enlightenment and sensitization.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We are sincerely grateful to the Osun State Ministry of Health and Uniosun Teaching Hospital ethical committee for their permission and cooperation as well as the participants (both children and adults) who took part in the study.
References
- 1.UNAIDS. HIV epidemic and response estimates, global and by region, 2010 and 2015 Global AIDS Update. 2016. [Last accessed on 2022 Mar 10]. Available: www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf .
- 2.Alemu A, Shiferaw Y, Addis Z, Mathewos B, Birhan W. Effect of malaria on HIV/AIDS transmission and progression. Parasit Vectors. 2013;6:18. doi: 10.1186/1756-3305-6-18. doi: 10.1186/1756-3305-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organisation (WHO). Malaria in HIV/AIDS Patients. Geneva: WHO; 2017. [Last accessed on 2022 Mar 14]. Available from: https:// www.who.int/malaria/areas/high_risk_groups/HIV/AIDs patients/en/ [Google Scholar]
- 4.Anywaine Z, Levin J, Kasirye R, Lutaakome JK, Abaasa A, Nunn A, et al. Discontinuing cotrimoxazole preventive therapy in HIV-infected adults who are stable on antiretroviral treatment in Uganda (COSTOP): A randomised placebo controlled trial. PLoS ONE. 2018;13:e0206907. doi: 10.1371/journal.pone.0206907. doi: 10.1371/journal. pone. 0206907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. World Health Organization. 2018. [Last accessed on 2022 Mar 10]. p. 155. Available from: https://www.who.int/publications/i/item/9789241549684 .
- 6.Duguma N, Kiya GT, Maleko WA, Bimerew LG. Hematological parameters abnormalities and associated factors in HIV-positive adults before and after highly active antiretroviral treatment in Goba Referral Hospital, southeast Ethiopia: A cross-sectional study. SAGE Open Med. 2021;9:1–12. doi: 10.1177/20503121211020175. doi: 10.1177/20503121211020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manyando C, Njunju EM, D'Alessandro U, Geertruyden JPV. Safety and efficacy of co-trimoxazole for treatment and prevention of plasmodium falciparum malaria: A systematic review. PLoS One. 2013;8:e56916. doi: 10.1371/journal.pone.0056916. doi: 10.1371/journal.pone. 0056916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jegede FE, Oyeyi TI, Abdulrahman SA, Mbah HA. Malaria parasite density as a predictor of hematological parameter changes among HIV infected adults attending two antiretroviral treatment clinics in Kano, Northwest Nigeria. J Trop Med 2020. 2020:9. doi: 10.1155/2020/3210585. doi: 10.1155/2020/3210585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ojurongbe O, Oyeniran O, Alli O, Taiwo S, Ojurongbe T, Olowe A, et al. Prevalence of plasmodium falciparum parasitaemia and its correlation with haematological parameters among HIV-Positive individuals in Nigeria. J Trop Med Hyg. 2014;2014:161284. doi: 10.1155/2014/161284. doi: 10.1155/2014/161284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanyaolu AO, Fagbenro-Beyioku AF, Oyibo WA, Badaru OS, Onyeabor OS, Nnaemeka CI. Malaria and HIV co-infection and their effect on haemoglobin levels from three healthcare institutions in Lagos, Southwest Nigeria. Afr Health Sci. 2013;13:295–300. doi: 10.4314/ahs.v13i2.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beletew B, Mengesha A, Mohammed Ahmed M, Fitwi A, Wudu M. Determinants of anemia among HIV-positive children on highly active antiretroviral therapy attending hospitals of North Wollo Zone, Amhara Region, Ethiopia: A case-control study 2020. 2020 doi: 10.1155/2020/3720572. 3720572. doi: 10.1155/2020/3720572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasirye RP, Grosskurth H, Munderi P, Levin J, Anywaine Z, Nunn A, et al. Effect of antiretroviral therapy on malaria incidence in HIV-infected Ugandan adults. AIDS. 2017;31:577–82. doi: 10.1097/QAD.0000000000001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenhalgh S, Ndeffo M, Galvani AP, Parikh S. The epidemiological impact of HIV antiretroviral therapy on malaria in children. AIDS. 2015;29:473–82. doi: 10.1097/QAD.0000000000000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naing C, Sandhu NK, Wai VN. The effect of malaria and HIV co-infection on anemia. Medicine. 2016;95:e3205. doi: 10.1097/MD.0000000000003205. doi: 10.1097/MD.0000000000003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ageru TA, Koyra MM, Gidebo KD, Abiso TL. Anemia and its associated factors among adult people living with human immunodeficiency virus at Wolaita Sodo University teaching referral hospital. PLoS One. 2019;14:e0221853. doi: 10.1371/journal.pone.0221853. doi: 10.1371/journal.pone.0221853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tay S, Badu K, Mensah AA, Gbedema SY. The prevalence of malaria among HIV seropositive individuals and the impact of the co-infection on their hemoglobin levels. Ann Clin Microbiol Antimicrob. 2015;14 doi: 10.1186/s12941-015-0064-6. doi: 10.1186/s12941-015-0064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bamidele JO, Ntaji MI, Oladele EA, Bamimore OK. Community participation in malaria control in Olorunda local Government area, Osun State, Southwest, and Nigeria. Afr J Infect Dis. 2012;6:24–8. doi: 10.4314/ajid.v6i2.1. doi: 10.4314/ajid.v612.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ojurongbe O, Akindele AA, Adeleke MA, Oyedeji MO, Adedokun SA, Ojo JF, et al. Co-endemicity of loiasis and Onchocerciasis in rain forest communities in southwestern, Nigeria. PLOS Neglect Trop Dis. 2015;9:e0003633. doi: 10.1371/journal.pntd.0003633. doi: 10.1371/journal.pntd. 0003633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ojo DA. Malaria in endemic area of Ogun State, Nigeria investigation of perceptions and practices among the residents and health providers. Pak J Soc Sci. 2005;3:564–71. [Google Scholar]
- 20.Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med. 2013;35:121–6. doi: 10.4103/0253-7176.116232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ten RN, Sumbele IUN, Kimbi HK, Ojong ST, Kimbi HK. Malaria parasitaemia, anaemia and malnutrition in less than 15 years residing in different altitudes along the slope of Mount cameroom: Prevalence, intensity and risk factors. Malaria J. 2018;17:336. doi: 10.1186/s12936-018-2492-1. doi: 10.1186/s12936-018-2492-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olympus LED microscope CX 22. Instruction manual olympus cooporation. 2013;1:24. Pulisher, Olympus Europa SE &CO PG. [Google Scholar]
- 23.Malaria Parasite Counting: Malaria Microscopy Standard Operating Procedure- MM-SOP-09. World Health Organization. 2016 [Google Scholar]
- 24.Federal Ministry of Health. Standard Operating Procedure for HIV testing Services. [Last accessed on 2016 Jun 08]. Available from: http://www.unaids.org/sites/default/files/media_asser/global-AIDS-update-2016_en.pdf .
- 25.Cyflow Partec. Manual UISOP CyFlow space. Instrument Equipmemt Manual. 2010 Publisher 2010 Partec GmbH Otto-Hahn-Str. 32 D-48161 Münster Germany. [Google Scholar]
- 26.Afolabi JK, Fadeyi A, Desalu OO, Durotoye IA, Fawibe AE, Adeboye MAN, et al. Normal CD4 cell counts range among healthy Nigerians population in Ilorin. J Int Assoc Prov AIDS Care (JIACPAC) 2017:359–65. doi: 10.1177/2325957414530472. [DOI] [PubMed] [Google Scholar]
- 27.Analyzer AH. Instructions for use. Sysmex Stand Oper Proced Lab Users. 2012:0–5. [Google Scholar]
- 28.Cheesbrough M. District Laboratory Practice in Tropical Countries Part 1 and 2. Cambridge Low Price Editions. Publisher Cambridge University Press. 2010:240–1. Cambridge University Press The Edinburgh Building, Cambridge CB2 8RU, UK First published in print format ISBN-13 978-0-521-67630-4 ISBN-13 978-0-511-34935-5. [Google Scholar]
- 29.Sumbele IUN, Sama SO, Kimbi HK, Taiwe GS. Malaria, moderate to severe anaemia, and malarial anaemia in children at presentation to hospital in the mount Cameroon area: A cross-sectional study. Anemia 2016. 2016 doi: 10.1155/2016/5725634. 5725634. doi: 10.1155/2016/5725634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandie SM, Sumbele IUN, Tasah MM, Kimbi HK. Malaria parasite prevalence and haematological parameters in HIV seropositive patients attending the regional hospital Limbe, Cameroon: A hospital-based cross-sectional study. BMC Infect Dis. 2019;19:988. doi: 10.1186/s12879-019-4629-4. doi: 10.1186/s12879-019-4629-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. Global Malaria Programme: Guidelines for the Treatment of Malaria. 3 ed. Geneva, Switzerland: World Health Organization; 2019. [Google Scholar]
- 32.Tagoe DNA, Boachie J. Assessment of the impact of malaria on CD4+T cells and haemoglobin levels of HIVmalaria co-infected patients. J Infect Dev Ctries. 2012;6:660–3. doi: 10.3855/jidc.2124. [DOI] [PubMed] [Google Scholar]
- 33.Amadi CP, Ikon GM, Inyang UC. Current prevalence of falciparum malarial infection among HIV patients on highly active antiretroviral therapy in University of Uyo teaching Hospital, Uyo, Nigeria. Int J Res Med Sci. 2018;6:2916–22. [Google Scholar]
- 34.Inyama PU, Omalu ICJ, Anyanwu GL, Idenniyi KA, Pam DD. Plasmodium falciparum and plasmodium malariae among HIV-infected individuals in North Central Nigeria. Int J Appl Biol Res. 2016;7:27–36. [Google Scholar]
- 35.Gumel SD, Ibrahim A, Olayinka AT, Ibrahim MS, Balogun MS, Dahiru A, et al. HIV-malaria co-infection and its determinants among patients attending antiretroviral treatment clinic in Zaria, Kaduna state, Nigeria. J Interv Epidemiol Public Health. 2021;4:2. Available from: https://www.afenet-journal.net/content/article/4/2/full/ [Google Scholar]
- 36.Iroezindu MO, Agaba EI, Okeke EN, Daniyam CA, Isa SE, Akindigh MT. Relationship between fever and malaria parasiteamia in Adults. Does HIV Infection make any difference? J Med Tropics. 2012;14:103–8. [Google Scholar]
- 37.Yohanna J, Oti V, Amuta E, Philip A, Anizoba L. Plasmodium falciparum infection among febrile patients attending a tertiary healthcare facility in central Nigeria: Prevalence haematologic and sociodemographic factors. Int J Trop Dis. 2019;2:1–6. Available from: https://pdfs.semanticscholar.org/5781/635b1083ec086e1b2efaf63c56ee5a973347.pdf . [Google Scholar]
- 38.Abioye JOK, Abdullahi DK, Ako AA. Prevalence of malaria among HIV patients in 44 Nigeria Army reference hospital Kaduna (44 NARHK) Int J Adv Biol Res. 2014;4:412–5. [Google Scholar]
- 39.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saracino A, Nacarapa EA, Massinga EA, Martinelli D, Scacchetti M, de Oliveira C, et al. Prevalence and clinical features of HIV and malaria co-infection in hospitalized adults in Beira, Mozambique. Malaria J. 2012;11:241. doi: 10.1186/1475-2875-11-241. doi: 10.1186/1475-2875-11-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bello B, Ishaleku D. Prevalence of malaria infection among people living with HIV/AIDS at federal medical center Keffi (Nassarawa State), Nigeria. J Adv Microbiol. 2018;11:1–6. doi: 10.9734/JAMB/2018/43154. [Google Scholar]
- 42.Akinbo FO, Anate PJ, Akinbo DB, Omoregie R, Okoosi S, Abdulsalami A, et al. Prevalence of malaria among HIV patients on highly active antiretroviral therapy in Kogi State, North Central Nigeria. Ann Niger Med. 2016;10:11–5. [Google Scholar]
- 43.Dikwa KB, Yahaya UA, Maikaje DB, Suleiman AB. Genetic diversity of plasmodium falciparum Isolated from symptomatic and asymptomatic individuals in parts of Kaduna metropolis, Kaduna state, Nigeria. Am J Microbiol Res. 2020;8:83–92. [Google Scholar]
- 44.Okokon II, Ubong AU, Kenneth OI, Anthony AI. Climate and plasmodium falciparum infection on the Jos Plateau, Nigeria. Int J Microbiol Biotechnol. 2017;2:161–5. [Google Scholar]
- 45.Gennaro D, Marotta FC, Pizzol FC, Chhaganlal D, Monno K, Putoto L, et al. Prevalence and predictors of Malaria in human immunodeficiency virus infected patients in Beira, Mozambique. Int J Environ Res Public Health. 2018;15:2032. doi: 10.3390/ijerph15092032. doi: 10.3390/ijerph15092032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atieli H, Zhou G, Afrane Y, Lee M-C, Mwanzo I, Githeko A, et al. Insecticide treated net (ITN) ownership, usage and malaria transmission in the highlands of western Kenya. Parasit Vectors. 2011;4:113. doi: 10.1186/1756-3305-4-113. doi: 10.1186/1756-3305-4-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inah SA, Uwadiegwu Z, Eko JE, Inah JA. Environmental sanitation practices on malaria control and prevention in abi local government area, Cross River State, Nigeria. Asian J Med Health. 2017;6:1–12. [Google Scholar]
- 48.Oyeniran OA, Ajayi OO, Afolabi AY, Oladipo EK, Adepeju AA. Comparison of rapid diagnostic tests and Microscopy for malaria. Afr J Exp Microbiol. 2014;15:158–74. [Google Scholar]
- 49.Punnath K, Dayanand KK, Chandrashekar VN, Achur RN, Kakkilaya SB, Ghosh SK, et al. Association between inflammatory cytokine levels and thrombocytopenia during Plasmodium falciparum and P. vivax infections in south-western coastal region of India. Malaria Res Treat. 2019;2019:4296523. doi: 10.1155/2019/4296523. doi: 10.1155/2019/4296523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kotepui M, Piwkham D, PhunPhuech B, Phiwklam N, Chupeerach C, Duangmano S. Effects of malaria parasite density on blood cell parameters. PLoS One. 2015;10:e0121057. doi: 10.1371/journal.pone.0121057. doi: 10.1371/journal.pone. 0121057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sahle T, Yemane T, Gedefaw L. Effect of malaria infection on hematological profiles of people living with Human immunodeficiency virus in Gambella, southwest Ethiopia. BMC Hematol. 2017;17:2. doi: 10.1186/s12878-017-0072-1. doi: 10.1186/s 12878-017-0072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bawah AT, Nyakpo KT, Ussher FA, Alidu H, Dzogbo JJ, Agbemenya S, et al. Hematological profile of children under five years with malaria at the Ho Municipality of Ghana. Edorium J Pediatr. 2018;2:100004P05AB2018. doi: 10.5348/100004P05AB2018OA. [Google Scholar]
- 53.Sakzabre D, Asiamah EA, Akorsu EE, Abaka-Yawson A, Dika ND, Kwasie DA, et al. Haematological profile of adults with malaria parasitaemia visiting the volta regional hospital, Ghana. AdvHematol 2020. 2020 doi: 10.1155/2020/9369758. 9369758. doi: 10.1155/2020/9369758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Osaro E, Abdulrahaman A, Erhabor T. Effects of malaria parasitaemia on some haematological parameters of pregnant women of African descent in specialist hospital Sokoto, North Western Nigeria. JOJ Nurse Health Care. 2019;10:555795. doi: 10.19080/JOJNHC.2019.10.555795. [Google Scholar]
- 55.Tchinda GG, Atashili J, Achidi EA, Kamga HL, Njunda AL, Ndumbe PM. Impact of malaria on hematological parameters in people living with HIV/AIDS attending the laquintinie hospital in Douala, Cameroon. PLoS One. 2012;7:e40553. doi: 10.1371/journal.pone.0040553. doi: 10.1371/journal.pone. 0040553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Njunda AL, Njumkeng C, Nsagha SD, Assob JC, Kwenti TE. The prevalence of malaria in people living with HIV in Yaounde, Cameroon. BMC Public Health. 2016;16:964. doi: 10.1186/s12889-016-3647-z. doi: 10.1186/s12889-016-3647-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


