Abstract
Blood–testis barrier is body’s innate mechanism to defend germ cells by creating a physical and immunological barrier. But some viral infections are known to evade it. As ACE2 and TMPRSS2 are present all over the body including male reproductive tract, it is worth exploring how coronavirus disease (COVID-19) could possibly affect male fertility. A review of literature was done using search engines like PubMed, Medline, Google Scholar, etc., and all the latest articles up to May 2021 were considered. Some studies have substantiated the presence of orchitis in COVID patients using semen and tissue samples. Though most studies report the absence of virus in testis, involvement of seminiferous tubules has been seen in pathological analysis suggesting defective spermatogenesis. This can be primarily attributed to inflammation and increased vascular permeability. Other factors that could affect male fertility are fever, autoimmune response, drugs, and erectile dysfunction. Male fertility is an important aspect of health care and must be looked into. Further studies can be done to understand host immunity towards SARS-CoV-2 in the testis. It will be worthwhile to know whether viral orchitis and its sequelae are acute or chronic in nature, and if they are reversible. Effect of the virus on female reproductive tract can also be assessed further. Counselling can be given to affected/recovering patients along with correct selection of drugs to prevent these long-term complications.
Keywords: ACE2, COVID-19, germ cells, male infertility, spermatogenesis, TMPRSS2
Introduction
COVID-19 has led to unprecedented morbidity and mortality worldwide. Its pathogenesis includes several mechanisms like cytokine storm, dysregulation of Renin Angiotensin Aldosterone system (RAAS), microvascular thrombosis, increased vascular permeability, antibody dependent enhancement, hypoxaemia and secondary sepsis leading to acute respiratory distress syndrome (ARDS), respiratory failure, sudden cardiac arrest, and multi-organ damage.[1,2,3] The virus SARS-CoV-2 binds to ACE2 receptor, which triggers protease TMPRSS2 receptor to cleave S spike protein of the virus and lets it enter the cells as well as infect them. The reason why the virus affects so many organs is the presence of ACE2 receptor in different parts of the body including blood vessels, heart, lungs, kidney, etc.[1]
Though many studies have focused on clinical aspects of COVID, not many studies have explored the long-term sequelae of SARS-CoV-2 specifically in the male reproductive system. ACE 2 receptors are known to exist in the male reproductive system in Spermatogonia, Leydig, and Sertoli cells.[4] TMPRSS2 is also highly expressed in the epithelium of prostate and prostate luminal cells along with Leydig cells. It is regulated by androgens and is a component of seminal fluid.[5,6] Hence there is a high possibility that SARS-CoV-2 can affect male reproductive system, which has been discussed in this review article.
Evidence of Involvement of Male Reproductive Tract
In 2020, first known case of orchi-epididymitis was reported in a boy with COVID.[7] In another study, 19% (6 out of 34) patients had scrotal discomfort due to viral orchitis around the time of infection but there was no evidence of SARS CoV-2 in the semen samples collected after a month.[8] Cellular changes were found in Sertoli cells suggesting seminiferous tubular injury, decrease in Leydig cells, and mild lymphocytic inflammation in a study with no evidence of SARS-CoV-2 in the testis.[9] One research reported that 8 out of 12 patients had normal semen quality with no evidence of virus in any semen sample whereas LH was increased with low testosterone in these COVID patients.[10] Low testosterone (total and free) has been found in COVID patients and is linked with ICU transfers and mortality.[11,12,13] On the other hand, there is some evidence that suggests presence of virus in 6 out of 38 individuals, mostly in the acute phase of disease as compared to recovering patients.[14] It has been suggested that COVID-induced vasculitis, coagulation abnormalities, and segmental vascularization of testis might attribute to orchitis like findings.[15]
In a study done on 100 patients, erectile dysfunction (ED) was found to be more prevalent in COVID positive patients.[16] It has been attributed to endothelial damage causing impaired vascular integrity, psychological distress, subclinical hypogonadism due to testicular damage and cardiorespiratory complications.[17]
Theories of Pathogenesis of COVID in Testis
Normally, blood-testis barrier (BTB) creates a physiological, anatomical, and immunological divide in the seminiferous tubules and segregates it into two compartments—basal and adluminal.[18] Blood–testis barrier creates an immune privileged apical compartment for spermatogenesis to occur, preventing an attack of individual’s immunity against germ cells.[19] Certain viruses (eg Mumps, Zika) have the ability to penetrate the barrier as the viral load increases, and cause inflammation, apoptosis, free radical damage resulting in orchitis, thus affecting male fertility.[20,21] Though Sertoli cells have immunosuppressive action and reduce testicular damage due to viruses, inflammation could temporarily affect blood testis barrier.[4]
Several mechanisms could contribute in pathogenesis [Figure 1].
Figure 1.
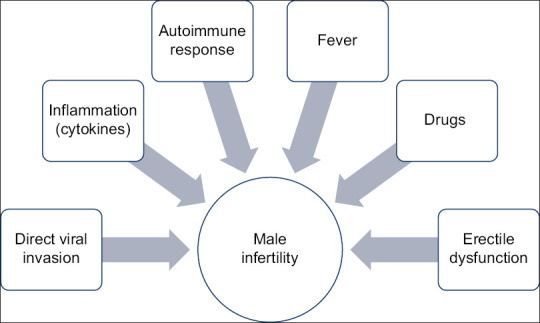
Factors causing male infertility in COVID patients
It has been seen that downregulation of ACE 2 receptors may not contribute to infertility per se but direct invasion of virus by ACE 2 receptors as well indirect inflammatory response may affect male reproductive health.[22] Although ACE 2 participates in spermatogenesis, it has been observed that ACE2 null male mice are fertile suggesting that other rescue mechanisms like testis ACE1 and ACE3 come into play in ACE deficient mice.[23,24] However, in SARS infection ACE 2 expression can be increased thus causing dysfunction of cells due to the viral invasion.[25] Production of excessive cytokines like IL-1a, IL-1b, IL-6, and TNF-a due to viral infection can compromise blood testis barrier by affecting endothelial integrity and vascular permeability, thus impairing spermatogenesis.[26] Autoimmune response could be seen due to proinflammatory cytokines.[25] Testosterone production could be reduced due to general immune response mediated by T lymphocytes and macrophages.[27] Fever may also be detrimental to male fertility as spermatocytes and spermatids are sensitive to temperature.[28] Certain drugs like ribavirin, lopinavir/ritonavir, glucocorticoids, chloroquine phosphate, etc., have been found to affect spermatogenesis in rat studies.[29,30,31]
Future Directions and Implications
SARS-CoV-2 can affect male fertility. Many studies have shown lack of virus in the semen samples of COVID patients, however there is growing evidence of impaired spermatogenesis and ED in COVID patients. This poses doubts on whether the virus is able to evade the blood testis barrier by itself or affects male reproductive system indirectly by inflammatory response. What is the role of Sertoli cells/host defence in preventing viral invasion? Is orchitis a transient effect of acute infection that shall subside with time or more chronic in nature? Are these effects reversible? Another relevant area of research could be about recommended drugs and their dosage that could be considered safe to prevent these complications. Further studies can be done on female reproductive system as well. In this COVID pandemic, male fertility is an important aspect that must not be ignored. It can be suggested that suitable advice on conception and assisted reproductive techniques must be given to couples/recovering patients as a part of therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LF. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–74. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGonagle D, O'Donnell JS, Sharif K, Emery P, Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020;2:e437–45. doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z, Xu X. scRNA-seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS-CoV-2 infection in spermatogonia, leydig and sertoli cells. Cells. 2020;9:920. doi: 10.3390/cells9040920. doi: 10.3390/cells9040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y-W, Lee M-S, Lucht A, Chou F-P, Huang W, Havighurst TC, et al. TMPRSS2, a serine protease expressed in the prostate on the apical surface of luminal epithelial cells and released into semen in prostasomes, is misregulated in prostate cancer cells. Am J Pathol. 2010;176:2986–96. doi: 10.2353/ajpath.2010.090665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucas JM, True L, Hawley S, Matsumura M, Morrissey C, Vessella R, et al. The androgen-regulated type II serine protease TMPRSS2 is differentially expressed and mislocalized in prostate adenocarcinoma. J Pathol. 2008;215:118–25. doi: 10.1002/path.2330. [DOI] [PubMed] [Google Scholar]
- 7.Gagliardi L, Bertacca C, Centenari C, Merusi I, Parolo E, Ragazzo V, et al. Orchiepididymitis in a boy with COVID-19. Pediatr Infect Dis J. 2020;39:e200–2. doi: 10.1097/INF.0000000000002769. [DOI] [PubMed] [Google Scholar]
- 8.Pan F, Xiao X, Guo J, Song Y, Li H, Patel DP, et al. No evidence of severe acute respiratory syndrome-coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril. 2020;113:1135–9. doi: 10.1016/j.fertnstert.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang M, Chen S, Huang B, Zhong J-M, Su H, Chen Y-J, et al. Pathological findings in the testes of COVID-19 patients: Clinical implications. Eur Urol Focus. 2020;6:1124–9. doi: 10.1016/j.euf.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma L, Xie W, Li D, Shi L, Ye G, Mao Y, et al. Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J Med Virol. 2021;93:456–62. doi: 10.1002/jmv.26259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rastrelli G, Di Stasi V, Inglese F, Beccaria M, Garuti M, Di Costanzo D, et al. Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology. 2021;9:88–98. doi: 10.1111/andr.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Çayan S, Uğuz M, Saylam B, Akbay E. Effect of serum total testosterone and its relationship with other laboratory parameters on the prognosis of coronavirus disease 2019 (COVID-19) in SARS-CoV-2, infected male patients: A cohort study. Aging Male Off J Int Soc Study Aging Male. 2020;23:1493–503. doi: 10.1080/13685538.2020.1807930. [DOI] [PubMed] [Google Scholar]
- 13.Schroeder M, Tuku B, Jarczak D, Nierhaus A, Bai T, Jacobsen H, et al. The majority of male patients with COVID-19 present low testosterone levels on admission to intensive care in Hamburg, Germany: A retrospective cohort study. medRxiv. 2020 doi: 10.1101/2020.05.07.20073817. [Google Scholar]
- 14.Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open. 2020;3:e208292. doi: 10.1001/jamanetworkopen.2020.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corona G, Baldi E, Isidori AM, Paoli D, Pallotti F, De Santis L, et al. SARS-CoV-2 infection, male fertility and sperm cryopreservation: A position statement of the Italian society of andrology and sexual medicine (SIAMS) (SocietàItaliana di Andrologia e Medicina della Sessualità) J Endocrinol Invest. 2020;43:1153–7. doi: 10.1007/s40618-020-01290-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sansone A, Mollaioli D, Ciocca G, Colonnello E, Limoncin E, Balercia G, et al. ” Mask up to keep it up” : Preliminary evidence of the association between erectile dysfunction and COVID-19. Andrology. 2021;9:1053–9. doi: 10.1111/andr.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sansone A, Mollaioli D, Ciocca G, Limoncin E, Colonnello E, Vena W, et al. Addressing male sexual and reproductive health in the wake of COVID-19 outbreak. J Endocrinol Invest. 2021;44:223–31. doi: 10.1007/s40618-020-01350-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olaniyan OT, Dare A, Okotie GE, Adetunji CO, Ibitoye BO, Bamidele OJ, et al. Testis and blood-testis barrier in Covid-19 infestation: Role of angiotensin-converting enzyme 2 in male infertility. J Basic Clin Physiol Pharmacol. 2020;31 doi: 10.1515/jbcpp-2020-0156. doi: 10.1515/jbcpp-2020-0156. [DOI] [PubMed] [Google Scholar]
- 19.Cheng CY, Mruk DD. The blood-testis barrier and its implications for male contraception. Pharmacol Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubin S, Eckhaus M, Rennick LJ, Bamford CGG, Duprex WP. Molecular biology, pathogenesis and pathology of mumps virus. J Pathol. 2015;235:242–52. doi: 10.1002/path.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meinhardt A. Infection: A new threat on the horizon-Zika virus and male fertility. Nat Rev Urol. 2017;14:135–6. doi: 10.1038/nrurol.2016.265. [DOI] [PubMed] [Google Scholar]
- 22.Abobaker A, Raba AA. Does COVID-19 affect male fertility? World J Urol. 2021;39:975–6. doi: 10.1007/s00345-020-03208-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramaraj P, Kessler SP, Colmenares C, Sen GC. Selective restoration of male fertility in mice lacking angiotensin-converting enzymes by sperm-specific expression of the testicular isozyme. J Clin Invest. 1998;102:371–8. doi: 10.1172/JCI3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–8. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Qi L, Chi X, Yang J, Wei X, Gong E, et al. Orchitis: A complication of severe acute respiratory syndrome (SARS) 1. Biol Reprod. 2006;74:410–6. doi: 10.1095/biolreprod.105.044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guazzone VA, Jacobo P, Theas MS, Lustig L. Cytokines and chemokines in testicular inflammation: A brief review. Microsc Res Tech. 2009;72:620–8. doi: 10.1002/jemt.20704. [DOI] [PubMed] [Google Scholar]
- 27.Hedger MP, Meinhardt A. Cytokines and the immune-testicular axis. J Reprod Immunol. 2003;58:1–26. doi: 10.1016/s0165-0378(02)00060-8. [DOI] [PubMed] [Google Scholar]
- 28.Jung A, Schuppe HC, Schill WB. [Fever as etiology of temporary infertility in the man. Hautarzt Z Dermatol Venerol Verwandte Geb. 2001;52:1090–3. doi: 10.1007/s001050170018. [DOI] [PubMed] [Google Scholar]
- 29.Almasry SM, Hassan ZA, Elsaed WM, Elbastawisy YM. Structural evaluation of the peritubular sheath of rat's testes after administration of ribavirin: A possible impact on the testicular function. Int J Immunopathol Pharmacol. 2017;30:282–96. doi: 10.1177/0394632017726261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adaramoye OA, Akanni OO, Adewumi OM, Owumi SE. Lopinavir/Ritonavir, an antiretroviral drug, lowers sperm quality and induces testicular oxidative damage in rats. Tokai J Exp Clin Med. 2015;40:51–7. [PubMed] [Google Scholar]
- 31.Asuquo OR, Igiri AO, Olawoyin OO, Eyong EU. Correlation of histological and histometric changes in rats testes treated with chloroquine phosphate. Niger J Physiol Sci Off Publ Physiol Soc Niger. 2007;22:135–9. doi: 10.4314/njps.v22i1-2.54885. [DOI] [PubMed] [Google Scholar]


