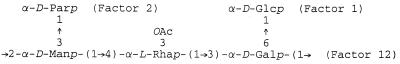Abstract
Salmonella enterica serovar Paratyphi A O-specific polysaccharide (O-SP) was activated with 1-cyano-4-dimethylaminopyridinium tetrafluoroborate (CDAP) and bound to tetanus toxoid (TT) with adipic acid dihydrazide as a linker (SPA-TT1) or directly (SPA-TT2). In mice, these two conjugates elicited high levels of immunoglobulin G (IgG) anti-lipopolysaccharide (LPS) in serum with bactericidal activity (E. Konadu, J. Shiloach, D. A. Bryla, J. B. Robbins, and S. C. Szu, Infect. Immun. 64:2709–2715, 1996). The safety and immunogenicity of the two conjugates were then evaluated sequentially in Vietnamese adults, teenagers, and 2- to 4-year-old children. None of the vaccinees experienced significant side effects, and all had preexisting LPS antibodies. At 4 weeks after injection, there were significant increases of the geometric mean IgG and IgM anti-LPS levels in the adults and teenagers: both conjugates elicited a greater than fourfold rise in the IgG anti-LPS level in serum in ≥80% of the volunteers. SPA-TT2 elicited slightly higher, though not statistically significantly, levels of IgG anti-LPS than did SPA-TT1 in these age groups. Accordingly, only SPA-TT2 was evaluated in the 2- to 4-year-old children. On a random basis, one or two injections were administered 6 weeks apart to the children. No significant side effects were observed, and the levels of preexisting anti-LPS in serum were similar in children of all ages. A significant rise in the IgG anti-LPS titer was elicited by the first injection (P = 0.0001); a second injection did not elicit a booster response. Representative sera from all groups had bactericidal activity that could be adsorbed by S. enterica serovar Paratyphi A LPS.
Enteric fever, with its septicemia and complications, is caused by Salmonella serogroups A, B, C, and D. Although reported throughout the world several decades ago (2, 13), Salmonella enterica serovar Paratyphi A seems to be confined to Southeast Asia, where it is the second most common cause of enteric fever, accounting for about 10% of cases (1, 3, 19, 22, 25, 28, 29, 31, 36, 37, 39–43, 46–48, 55, 57, 62, 63, 66). Despite the frequency and severity of enteric fevers and efforts to control the diseases, there is no licensed vaccine for nontyphoidal salmonellae. TAB vaccine, composed of inactivated Salmonella, was removed as a licensed product because efficacy could not be verified for groups A and B (17, 20, 26, 59).
Both S. enterica serovars Typhi and Paratyphi are inhabitants and pathogens of humans only, and they can be considered clones (11, 21, 24, 32, 45, 49, 53, 58). The lipopolysaccharide (LPS) of nontyphoidal Salmonella is both an essential virulence factor and a protective antigen (50). Animal experiments on the structurally related serogroup B and clinical results with Vi capsular polysaccharide of S. enterica serovar Typhi were the basis for our prediction that a critical level of serum immunoglobulin G (IgG) to the O-specific polysaccharide (O-SP) will confer protection against S. enterica serovar Paratyphi A (10, 15, 16, 50, 58, 64). Accordingly, we developed O-SP conjugates of S. enterica serovar Paratyphi A designed to elicit IgG LPS antibodies in serum (35).
The O-SP of S. enterica serovar Paratyphi A is composed of a trisaccharide backbone with a branch of d-paratose from the C-3 of α-d-mannose; C-3 of the adjacent α-l-rhamnose is partially O acetylated (8, 9, 23, 27) (Fig. 1).
FIG. 1.
Structure of S. enterica serovar Paratyphi O-SP.
S. enterica serovar Paratyphi A O-SP required O-acetyl groups to elicit serum LPS antibody with bactericidal activity in mice (33). O-SP was activated with 1-cyano-4-dimethylaminopyridinium tetrafluoroborate (CDAP) at neutral pH and bound either directly or through an adipic acid hydrazide linker to the protein (33, 35, 38). In mice, S. enterica serovar paratyphi A conjugates induced LPS antibody with group-specific bactericidal activity against serogroup A.
We describe a clinical evaluation of S. paratyphi A O-SP, bound to tetanus toxoid (TT) directly or through an adipic acid hydrazide spacer, in adults, teenagers, and 2- to 4-year-old children.
MATERIALS AND METHODS
Investigational vaccines.
The O-SP was prepared as described previously and had a molar ratio of 0.71 O-acetyl groups per repeating unit of polysaccharide (33). Pasteur-Mérieux Serum et Vaccins, Lyon, France, provided TT (lot GYA). Conjugates of the O-SP of S. enterica serovar Paratyphi A bound to TT through a linker, adipic acid dihydrazide (SPA-TT1; lot 64811), or directly (SPA-TT2, lot 64812) have been described previously (13, 35). SPA-TT1 contained 30.2 μg of protein per ml and 48.6 μg of polysaccharide per ml, and SPA-TT2 contained 40.7 μg of protein per ml and 51.3 μg of polysaccharide per ml; the dose for both conjugates was 0.5 ml. Both lots passed the Food and Drug Administration requirements for sterility, pyrogenicity, and general safety.
Study protocol.
The investigation was approved by the Ministry of Health of Vietnam; the Institutional Review Board of the National Institute of Child Health and Human Development, National Institutes of Health (protocol OH96-CH-N023); the Office of Protection from Research Risks; and the Food and Drug Administration (BB IND 6530). Informed consent was obtained from adult volunteers and from parents or guardians of vaccinees younger than 18 years.
The site was Cao Lânh District, Dong Thap Province, of the Mekong Delta region of southern Vietnam. Twenty healthy adults were randomly assigned to receive one 0.5-ml injection of either SPA-TT1 or SPA-TT2 in the deltoid muscle. The vaccinees were examined at 30 min and at 6, 24, and 48 h following immunication. Reactions at the immunization sites were inspected, and the temperatures of the volunteers were measured; these findings were recorded. Side effects were defined as a fever of >38.5°C, erythema of >2.5 cm, or induration of >2 cm within 48 h of the injection. Sera were obtained before and at 6 and 26 weeks after vaccination and stored at −70°C. When it was confirmed that adults did not experience significant side effects, 108 teenagers between 13 and 17 years old were injected with one of these two conjugates according to the same protocol. The IgG LPS antibody titers in serum in the two groups were assayed and SPA-TT2, which elicited higher geometric mean (GM) IgG LPS antibody levels in serum, was chosen for evaluation in the 2- to 4-year-old children. A total of 110 children between 2 and 4 years old were injected with SPA-TT2. Half of the children were randomly assigned to receive a second dose 6 weeks later. Vaccinees were observed for adverse reactions at 30 min and 6, 24, and 48 h after each injection. Sera were collected before each injection and at 6, 10, and 26 weeks after the first injection.
LPS antibodies in serum.
Sera were analyzed for IgG and IgM LPS antibodies by enzyme-linked immunosorbent assay (ELISA) (35). Goat anti-human IgG or IgM conjugated to alkaline phosphatase (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) was the secondary antibody. The results are expressed in ELISA units (EU) as a percentage of a high-titer serum from an adult vaccinee. A response was defined as at least a fourfold rise in titer compared to the level at time zero. Bactericidal activity was assayed as described previously (34). Antibodies to TT were measured by ELISA with a human tetanus immune globulin, titrated for its neutralizing activity, as a reference (14). Results for TT antibodies are expressed in antitoxin units.
Statistical analyses.
All antibody levels are expressed as GM. Levels below the sensitivity of the ELISA (0.1 EU) were assigned the value of one-half of that level (0.05 EU). Comparison of GMs was performed by either a paired or unpaired t test or the Wilcoxon test when appropriate.
RESULTS
Clinical observations.
No significant side effects were reported for any of the three age groups.
LPS antibody levels in serum. (i) Prevaccination.
All vaccinees had preexisting IgG and IgM antibodies in serum. The adults and 13- to 17-year-old teenagers had similar levels that were significantly higher than those in the 2- to 4-year-old children (1.46, 1.70, and 0.85 EU, respectively, for IgG [P < 0.05]; 17.5, 22.6, and 6.80, EU, respectively, for IgM [P = 0.0001]).
(ii) Adults.
At 6 weeks after injection, 75% of the adults responded with at least a fourfold rise in the IgG anti-LPS titer (Table 1). Recipients of SPA-TT2 had higher IgG anti-LPS levels than did recipients of SPA-TT1 (27.3 and 9.82 EU, respectively [not significant]) at 42 days following injection. These levels fell about threefold at 180 days but were still higher than the prevaccination levels (8.28 versus 1.69, and 3.73 versus 1.26 EU [P < 0.005]). Recipients of SPA-TT2 had higher levels of both IgG and IgM than did recipients of SPA-TT1 (8.28 and 3.73 EU, respectively [P = 0.03]).
TABLE 1.
LPS antibodies elicited by SPA-TT conjugates in sera of adults and teenagers
| Ig | Conjugate | Age (yr) | No. | GM LPS antibody titera (25th to 75th percentiles) (EU) on days after vaccination:
|
||
|---|---|---|---|---|---|---|
| 0 | 42 | 180 | ||||
| IgG | SPA-TT1 | 18–44 | 10 | 1.26 (0.6–2.6) | 9.82 (4.7–15.6) | 3.73 (2.67–7.10) |
| SPA-TT2 | 18–44 | 10 | 1.69 (1.0–2.9) | 27.3 (8.65–54.1) | 8.28 (9.86–10.1) | |
| SPA-TT1 | 13–17 | 56 | 1.69 (0.98–2.69) | 12.8 (6.75–22.9) | 6.72 (4.25–11.6) | |
| SPA-TT2 | 13–17 | 52 | 1.70 (1.13–2.34) | 17.4 (10.3–22.6) | 7.37 (4.80–12.0) | |
| IgM | SPA-TT1 | 18–44 | 10 | 17.3 (8.32–31.5) | 35.2 (23.7–48.9) | 18.7 (11.9–39.5) |
| SPA-TT2 | 18–44 | 10 | 17.7 (11.3–26.4) | 42.3 (25.4–74.9) | 24.1 (16.4–30.6) | |
| SPA-TT1 | 13–17 | 56 | 20.7 (14.3–28.0) | 29.6 (20.6–37.9) | 21.9 (17.2–25.4) | |
| SPA-TT2 | 13–17 | 52 | 24.9 (17.8–32.5) | 37.3 (27.1–48.6) | 23.9 (21.4–28.9) | |
Serum standard is from a high-responder vaccinee and is assigned a value of 100 EU.
Although significant, rises in the IgM anti-LPS levels induced by SPA-TT1 (35.2 versus 17.3 EU [P = 0.0004]) and by SPA-TT2 (42.3 versus 17.7 EU [P = 0.004]) 6 weeks after injection were lower than that in the IgG level. IgM anti-LPS antibody levels elicited by SPA-TT2 were only slightly higher than those elicited by SPA-TT1 (42.3 and 35.2 EU, respectively [P > 0.05]). IgM anti-LPS levels at the 180-day interval declined to levels similar to those prior to the vaccination.
(iii) Teenagers.
Conjugate-induced IgG anti-LPS levels in the 13- to 17-year-old volunteers were similar to but slightly lower than those in the adults (Table 1). At the 6-week interval, 85% responded with at least a fourfold rise in the IgG anti-LPS titer (P = 0.0001). Recipients of SPA-TT1 had a 7.6-fold rise to 12.8 EU, and recipients of SPA-TT2 elicited a 10.2-fold rise to 17.4 EU (P > 0.01). At 180 days after vaccination, the IgG anti-LPS titers had declined about 50% compared to those at 42 days: the level elicited by SPA-TT2 was only slightly higher than that elicited by the SPA-TT1 (7.37 and 6.72 EU, respectively, P > 0.05). These levels were higher than the preimmune levels (6.72 versus 1.69, and 7.37 versus 1.70 EU [P = 0.0001]).
As with IgG anti-LPS, the IgM responses in serum elicited by the two conjugates were similar to those observed in the adults. At the 42-day interval, the IgM anti-LPS titer had risen 1.4-fold (P = 0.0001) in the SPA-TT1 group and 1.5-fold (P = 0.0001) in the SPA-TT2 group. At 180 days, the IgM anti-LPS levels in the teenagers and adults were similar to each other and to the prevaccination levels.
Based on these results, only SPA-TT2 was evaluated in 2- to 4-year-old children.
(iv) Children.
About 90% of the children had at least a fourfold rise in their IgG anti-LPS titer at the 42-day interval (19.3 versus 0.91, and 16.7 versus 0.77 EU [P = 0.0001]) (Table 2). Reinjection at 42 days did not elicit a booster response: the levels 2 weeks after the second injection (11.7 versus 11.9 EU [not significant]) and 180 days after the first interval (4.08 versus 3.47 EU [not significant]) were similar in both groups. The IgG anti-LPS levels at 180 days were higher than at prevaccination (3.47 versus 0.91, and 4.08 versus 0.77 EU [P = 0.0001]).
TABLE 2.
LPS antibodies in serum of children receiving one or two injections of SPA-TT2
| Ig | No. of injections | No. of children | GM LPS antibody titera (25th to 75th percentiles) (EU) on days after vaccination:
|
|||
|---|---|---|---|---|---|---|
| 0 | 42b | 70 | 180 | |||
| IgG | 1 | 63 | 0.91 (0.49–1.56) | 19.3 (8.40–38.1) | 11.7 (6.13–21.4) | 3.47 (2.34–4.79) |
| 2 | 47 | 0.77 (0.47–1.17) | 16.7 (9.41–32.3) | 11.9 (6.57–19.8) | 4.08 (2.59–6.00) | |
| IgM | 1 | 63 | 6.65 (3.52–11.4) | 28.8 (16.3–50.4) | 22.0 (13.6–41.8) | 15.9 (10.1–21.3) |
| 2 | 47 | 7.00 (4.67–10.3) | 32.3 (16.3–48.3) | 24.7 (13.6–44.9) | 17.6 (10.3–26.2) | |
A high-responder serum was used as the standard and assigned a value of 100 EU.
Group 2 reinjected after bleeding.
IgM anti-LPS responses were slightly different from those of the teenagers and adults. The rises in IgM anti-LPS titers were slightly higher (ca. fourfold), although the levels at 42 days were similar to those in the teenagers and adults. As with IgG, there was no booster response to a second injection. At 180 days, IgM anti-LPS levels were higher in the children than in the teenagers and adults and were about twofold higher than the prevaccination levels (15.9 versus 6.65, and 17.6 versus 7.00 EU [P = 0.0001]).
(v) Summary.
GM IgG anti-LPS levels in serum in vaccinees of the three age groups are summarized in Table 3. The levels of anti-LPS in the preimmune sera were similar for the adults and teenagers; both of these groups had higher levels than did the children. Both the levels and fold rises elicited by SPA-TT2 were slightly but not significantly higher than those elicited by SPA-TT1. With the exception of SPA-TT1 in the adults, there was a gradual decline in the IgG anti-LPS levels at 180 days that was age related.
TABLE 3.
Age-related IgG levels of LPS antibody in serum before and 180 days after vaccination with SPA-TT conjugates
| Age (yr) | Conjugate | No. of injections | GM LPS antibody titer (EU) on days after vaccination:
|
Fold rise at 180 days | |||
|---|---|---|---|---|---|---|---|
| 0 | 42 | 68 | 180 | ||||
| 18–44 | SPA-TT1 | 1 | 1.26 | 9.82 | NAa | 3.73 | 3.0 |
| SPA-TT2 | 1 | 1.69 | 27.3 | NA | 8.28 | 4.9 | |
| 13–17 | SPA-TT1 | 1 | 1.69 | 12.8 | NA | 6.72 | 4.0 |
| SPA-TT2 | 1 | 1.70 | 17.4 | NA | 7.37 | 4.3 | |
| 2–4 | SPA-TT2 | 1 | 0.91 | 19.3 | 11.7 | 3.47 | 3.8 |
| SPA-TT2 | 2 | 0.77 | 16.7 | 11.9 | 4.08 | 5.3 | |
NA, not applicable.
Serum bactericidal levels.
Representative sera (10) from the three age groups were assayed for their bactericidal titer (Table 4). Two pairs of sera from the children, prevaccination and after the first vaccination, were assayed, and their bactericidal titers were compared to the IgG and IgM anti-LPS titers by ELISA. All these sera had a bactericidal activity that required complement. The bactericidal activity was roughly related to the IgG anti-LPS titer. Adsorption of these sera with the LPS of S. enterica serovar Paratyphi A removed most of the bactericidal activity (data not shown).
TABLE 4.
Bactericidal levels elicited by SPA-TT conjugates in sera of vaccinees
| Subject | Group | IgG antibody titer (EU)
|
Bactericidal titer | |
|---|---|---|---|---|
| IgG | IgM | |||
| P1004 | Adult | 73.6 | 17.0 | 1:8,000 |
| P1012 | Adult | 15.5 | 31.5 | 1:2,000 |
| P2033 | Teenager | 10.1 | 36.8 | 1:2,000 |
| P2061 | Teenager | 133 | 73.6 | 1:8,000 |
| P3002 | Child | 78.8 | 32.4 | 1:1,600 |
| P3018a | Child | 1.76 | 11.4 | 1:500 |
| p3018 | Child | 118 | 46.4 | 1:4,000 |
| p3094a | Child | 0.73 | 2.73 | 1:500 |
| p3094 | Child | 58.5 | 10.8 | 1:8,000 |
Prevaccination sample.
Anti-TT levels in serum.
All volunteers had preexisting IgG anti-TT: the children had the highest levels among the three age groups, probably due to their comparatively recent immunization with DTP. The levels at 180 days following injection were similar to those at 42 days in the adults and teenagers. In the children, the anti-TT levels at 180 days declined to about one-third the levels at 42 days but were higher than those in the adults and teenagers (3.62 and 4.10 versus 0.33 and 0.24 for teenagers and 0.48 and 0.56 for adults [P = 0.0001]). As observed for LPS antibodies, SPA-TT2 elicited slightly higher levels and fold rises of TT antibody for all three age groups than SPA-TT1 did. At 180 days, the children receiving two injections had slightly higher levels of TT antibody than did those receiving only one injection (9.8 and 7.7, respectively [P > 0.05]).
DISCUSSION
Enteric fever is a systemic infection caused by S. enterica serovar Typhi and salmonellae of groups A to D (21, 32, 53). We proposed that a critical level of IgG LPS antibody in serum could initiate complement-mediated lysis of these pathogens as they enter the intestine (50, 52, 61). Vaccination with the surface polysaccharide of S. enterica serovar Typhi (Vi) prevents typhoid fever (65). The immune moiety elicited by Vi is mainly serum IgG antibody (51). The O-SP of S. enterica serovar Paratyphi A alone is not immunogenic (hapten) due to its comparatively low molecular weight (30, 35, 60). The failure of whole-cell bacterial vaccines, such as TAB, to confer protection against group A and B salmonellae may be likened to the failure of parenterally injected inactivated gram-negative bacteria to induce high levels of IgG LPS antibodies (6, 18; T. A. Schwartzer, D. V. Alcid, V. Numsuwan, and D. J. Gocke, Letter, J. Infect. Dis. 158:1135–1136, 1988). Another explanation might have been gradual hydrolysis of O-acetyl groups of the LPSs in TAB vaccine that are essential for eliciting LPS antibody with bactericidal activity to S. enterica serovar Paratyphi A (35).
S. enterica serovar Paratyphi A is a pathogen of humans only, and we propose that conjugate-induced bactericidal antibodies will provide a correlate for protection (35, 50–52, 61). Subcutaneous injection of mice with S. enterica serovar Paratyphi A conjugates using only 1/10 of a human dose in saline elicited bactericidal antibodies specific for group A but not group B Salmonella (35). This is consistent with the immunodominant region of the group-specific antigen (factor 2), conferred by paratose, as predicted from the proposed structures of group A, B, and D O-SPs of salmonellae (8–10, 17).
Earlier, we found that O-acetyl groups on the O-specific polysaccharide of S. enterica serovar Paratyphi A are essential for its immunogenicity and that conjugation with CDAP is useful for synthesis of S. enterica serovar Paratyphi A conjugates to preserve O-acetyl epitopes (35, 38). Activation by CDAP occurs at neutral pH and does not reduce O-acetyls on the O-SP. CDAP-activated O-SP, bound directly to TT without a spacer (SPA-TT2), elicited the highest level of TT antibodies (P < 0.005) as well as slightly higher level of anti-LPS.
SPA-TT2 did not elicit a booster response in the 2- to 4-year-old group. This is in contrast to the booster response elicited in this age group by our pneumococcus type 6B-TT, Vi-rEPA, and Shigella flexneri 2a-rEPA conjugates (4, 54). There is no explanation for these differing properties of polysaccharide-protein conjugates. We plan to evaluate the S. enterica serovar Paratyphi A O-SP conjugate in infants, an age group that shows booster responses to conjugate vaccines (51).
The C-3 of rhamnose of the O-SP is partially O acetylated (27). We found a molar ratio of 0.71 O-acetyl per repeat unit (35). However, we detected two acetamido signals in the 13C nuclear magnetic resonance spectrum. The O-SP of S. typhimurium (group B) is 2-O acetylated at abequose, conferring the specificity defined as factor 5 (8, 9). Carlin et al. reported that a murine monoclonal IgA antibody to factor 5 had low protective activity against S. enterica serovar Typhimurium (10). It cannot be concluded that the O-acetyl does not have important biological activity for group B Salmonella because IgA may not protect against blood-borne infection. The failure of de-O-acetylated O-SP of S. enterica serovar Paratyphi A to induce bactericidal antibodies in mice might be due to the fact that paratose (factor 2 or group-specific antigen) is partially O acetylated. Since antibody-mediated immunity to nontyphoidal salmonellae is largely group specific and since the dideoxy sugars are essential for this specificity (8, 9, 17), the location(s) and immunologic roles of O-acetyls in O-SP of groups A, B, and D should be investigated further. O-acetyls are essential for expression of the protective action of other polysaccharides (5).
In summary, S. enterica serovar Paratyphi A O-specific polysaccharide–TT conjugates were safe and elicited IgG antibodies with bactericidal activity in the serum of adults, teenagers, and 2- to 4-year-old children. There is sufficient information about Vi (65), with S. enterica serovar Typhimurium O-SP conjugates in mice (10, 16, 64) and clinical studies with Shigella O-SP conjugates (15), to evaluate our S. enterica serovar Paratyphi A conjugates for efficacy. If effective, O-SP conjugates against group B, C, and D Salmonella should also protect against nontyphoidal enteric fever.
TABLE 5.
IgG levels of tetanus antitoxin elicited by SPA-TT conjugates in sera of volunteers
| Age (yr) | Conjugate | GM antitoxin level (25th to 75th percentiles) on days after vaccination:
|
Fold rise at 180 days | |||
|---|---|---|---|---|---|---|
| 0 | 42 | 70 | 180 | |||
| 18–44 | SPA-TT1 | 0.29 (0.12–0.59) | 0.57 (0.23–1.38) | NAa | 0.56 (0.25–1.01) | 1.9 |
| SPA-TT2 | 0.15 (0.09–0.16) | 0.33 (0.11–0.66) | NA | 0.48 (0.24–0.52) | 3.2 | |
| 13–17 | SPA-TT1 | 0.11 (0.07–0.15) | 0.24 (0.09–0.47) | NA | 0.24 (0.14–0.36) | 2.2 |
| SPA-TT2 | 0.11 (0.07–0.15) | 0.27 (0.15–0.31) | NA | 0.33 (0.20–0.50) | 3.0 | |
| 2–4 | SPA-TT2 | 0.47 (0.29–0.81) | 11.5 (8.31–20.8) | 6.73 (4.66–12.7) | 3.62 (2.54–5.19) | 7.7 |
| SPA-TT2b | 0.42 (0.25–0.73) | 8.76 (4.89–20.3) | 6.74 (3.61–17.2) | 4.10 (1.71–10.7) | 9.8 | |
NA, not applicable.
Received a second injection on day 42.
ACKNOWLEDGMENTS
We are grateful to Vee Gill and the staff of the Microbiology Branch, Clinical Center, NIH for their assistance. James C. Mond, Uniformed Services University of the Health Sciences, Bethesda, Md., and Andrew Lees, Virion Systems Inc., Rockville, Md., provided helpful advice about CDAP.
REFERENCES
- 1.Acharya G, Butler T, Ho M, Sharma P R, Tawari M, Adhikari R K, Khagda J B, Pokhrel B, Pathak U N. Treatment of typhoid fever: randomized trial of a three-day course of ceftriaxone versus a fourteen-day course of chloramphenicol. Am J Trop Med Hyg. 1995;52:162–165. doi: 10.4269/ajtmh.1995.52.162. [DOI] [PubMed] [Google Scholar]
- 2.Adeyokunnu A, Hendrickse R G. Salmonella osteomyelitis in childhood. Arch Dis Child. 1980;55:175–184. doi: 10.1136/adc.55.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anand A C, Kataria V K, Singh W, Chatterjee S K. Epidemic multiresistant enteric fever in eastern India. Lancet. 1990;335:352. doi: 10.1016/0140-6736(90)90635-i. [DOI] [PubMed] [Google Scholar]
- 4.Ashkenazi S, Passwell J H, Harlev E, Miron D, Dagan R, Farzan N, Ramon R, Majadly F, Bryla D A, Karpas A B, Robbins J B, Schneerson R the Israel Pediatric Shigella Study Group. Safety and immunogenicity of Shigella sonnei and Shigella flexneri 2a O-specific polysaccharide conjugates in adults. J Infect Dis. 1999;179:1565–1568. doi: 10.1086/314759. [DOI] [PubMed] [Google Scholar]
- 5.Avery O T, Goebel W F. Chemoimmunological studies on the soluble specific substance of pneumococcus. I. The isolation and properties of the acetyl polysaccharide of pneumococcus type 1. J Exp Med. 1933;58:731–755. doi: 10.1084/jem.58.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumgartner J D, Heumann D, Calandra T, Glauser M P. Antibodies to lipopolysaccharide after immunization of humans with rough mutant Escherichia coli. J Infect Dis. 1991;163:769–772. doi: 10.1093/infdis/163.4.769. [DOI] [PubMed] [Google Scholar]
- 7.Bhutta Z, Farooqui B J, Sturm A W. Eradication of a multiple drug resistant Salmonella paratyphi A causing meningitis with ciprofloxacin. J Infect. 1992;25:215–219. doi: 10.1016/0163-4453(92)94173-u. [DOI] [PubMed] [Google Scholar]
- 8.Bock K, Meldal M, Bundle D R, Iversen T, Garegg P J, Norberg T, Lindberg A A, Svenson S B. The conformation of Salmonella O-antigenic polysaccharide chains of serogroups A, B, and D1 predicted by semi-empirical hard-sphere (HSEA) calculations. Carbohydr Res. 1984;130:23–34. doi: 10.1016/0008-6215(84)85267-2. [DOI] [PubMed] [Google Scholar]
- 9.Bock K, Meldal M, Bundle D R, Iversen T, Mario Pinto B, Garegg P J, Kvanstrom I, Norberg T, Lindberg A A, Svenson S B. The conformation of Salmonella O-antigenic oligosaccharides of serogroup A, B, and D1 inferred from 1H- and 13C-nuclear magnetic resonance spectroscopy. Carbohydr Res. 1984;130:35–53. doi: 10.1016/0008-6215(84)85268-4. [DOI] [PubMed] [Google Scholar]
- 10.Carlin N I A, Svenson S B, Lindberg A A. Role of monoclonal O-antigen antibody epitope specificity and isotype in protection against experimental mouse typhoid. Microb Pathog. 1987;2:171–183. doi: 10.1016/0882-4010(87)90019-2. [DOI] [PubMed] [Google Scholar]
- 11.Carter P B, Collins F M. Growth of typhoid and paratyphoid bacilli in intravenously infected mice. Infect Immun. 1974;10:816–822. doi: 10.1128/iai.10.4.816-822.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chalker R B, Blaser M J. A review of human salmonellosis. III. Magnitude of Salmonella infection in the United States. Rev Infect Dis. 1988;10:111–124. doi: 10.1093/clinids/10.1.111. [DOI] [PubMed] [Google Scholar]
- 13.Chu C, Liu B, Watson D, Szu S C, Bryla D, Shiloach J, Schneerson R, Robbins J B. Preparation, characterization, and immunogenicity of conjugates composed of the O-specific polysaccharide of Shigella dysenteriae type 1 bound to tetanus toxoid. Infect Immun. 1991;59:4450–4458. doi: 10.1128/iai.59.12.4450-4458.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Claesson B O, Trollfors B, Lagergard T, Taranger J, Bryla D, Otterman G, Cramton T, Yang Y, Reimer C B, Robbins J B, Schneerson R. Clinical and immunologic responses to the capsular polysaccharide of Haemophilus influenzae type b alone or conjugated to tetanus toxoid in 18- to 23-month-old children. J Pediatr. 1988;112:695–702. doi: 10.1016/s0022-3476(88)80684-x. [DOI] [PubMed] [Google Scholar]
- 15.Cohen D, Ashkenazi S, Green M S, Gdalevich M, Robin G, Slepon R, Yavzori M, Orr N, Block C, Ashkenazi I, Shemer J, Taylor D N, Hale T L, Sadoff J C, Pavliakova D, Schneerson R, Robbins J B. Double-blind vaccine-controlled randomized efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet. 1997;349:155–159. doi: 10.1016/S0140-6736(96)06255-1. [DOI] [PubMed] [Google Scholar]
- 16.Colwell D E, Michalek S M, Briles D E, Emilio J, McGhee J R. Monoclonal antibodies to Salmonella lipopolysaccharide: anti-O-polysaccharide antibodies protect C3H mice against challenge with virulent Salmonella typhimurium. J Immunol. 1984;133:950–957. [PubMed] [Google Scholar]
- 17.Cygler M, Rose D R, Bundle D R. Recognition of a cell-surface oligosaccharide of pathogenic Salmonella by an antibody Fab fragment. Science. 1991;253:442–445. doi: 10.1126/science.1713710. [DOI] [PubMed] [Google Scholar]
- 18.DeMaria A, Johns M A, Berberich H, McCabe W R. Immunization with rough mutants of Salmonella minnesota: initial studies in human subjects. J Infect Dis. 1988;158:301–311. doi: 10.1093/infdis/158.2.301. [DOI] [PubMed] [Google Scholar]
- 19.Ebong W W. Acute osteomyelitis in Nigerians with sickle cell disease. Ann Rheum Dis. 1986;45:911–915. doi: 10.1136/ard.45.11.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards P R, Bruner D W. The occurrence and distribution of Salmonella types in the United States. J Infect Dis. 1943;72:58–61. doi: 10.1093/infdis/83.3.220. [DOI] [PubMed] [Google Scholar]
- 21.Edwards P R, Ewing W H. Identification of Enterobacteriaceae. 3rd ed. Minneapolis, Minn: Burges Publishing Company; 1972. p. 175. [Google Scholar]
- 22.Escamilla J, Florez-Ugarte H, Kilpatrick M E. Evaluation of blood clot cultures for isolation of Salmonella typhi, Salmonella paratyphi A, and Brucella melitensis. J Clin Microbiol. 1986;24:388–390. doi: 10.1128/jcm.24.3.388-390.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fouquey C, Polonsky J, Lederer E, Westphal O, Luderitz O. Synthesis of 3,6-dideoxy-d-ribo-hexose (3,6-dideoxy-d-glucose) and its identification with paratose. Nature. 1958;182:944. doi: 10.1038/182944a0. [DOI] [PubMed] [Google Scholar]
- 24.Gerichter C B. The dissemination of Salmonella typhi, Salmonella paratyphi A, and Salmonella paratyphi B through the organs of the white mouse by oral infection. J Hyg Camb. 1960;58:307–319. doi: 10.1017/s0022172400038420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hafiz S, Khan S W, Shariff R, Yazdani I, Syed Y, Hafiz T. Epidemiology of salmonellosis and its sensitivity in Karachi. J Pak Med Assoc. 1993;43:178–179. [PubMed] [Google Scholar]
- 26.Hargrett-Bean N T, Pavia A T, Tauxe R V. Salmonella isolates from humans in the United States, 1984–1986. Morbid Mortal Weekly Rep. 1988;37(SS-2):25–31. [PubMed] [Google Scholar]
- 27.Hellerqvist C G, Lindberg B, Samuelsson K, Lindberg A A. Structural studies on the O-specific side-chains of the cell-wall lipopolysaccharide from Salmonella paratyphi A var. durazzo. Acta Chem Scand. 1971;25:955–961. doi: 10.3891/acta.chem.scand.25-0955. [DOI] [PubMed] [Google Scholar]
- 28.Hien T T, Bethell D B, Hoa N T T, Wain J, Diep T S, Phi L T, Cuong B M, Duong N M, Thanh P T, Walsh A L, Day N P J, White N J. Short course of ofloxacin for treatment of multidrug-resistant typhoid. Clin Infect Dis. 1995;20:915–923. [PubMed] [Google Scholar]
- 29.Jude A, Le Minor L. Salmonella isolées en Indochine au cours d'affections typho-paratyphoidiques. Bull Soc Pathol Exot. 1948;41:129–133. [Google Scholar]
- 30.Kabat E A, Bezer A E. The effect of variation in molecular weight on the antigenicity of dextran in man. Arch Biochem Biophys. 1958;78:306–310. doi: 10.1016/0003-9861(58)90354-0. [DOI] [PubMed] [Google Scholar]
- 31.Kapil A, Sood A, Reddaiah V P, Das B, Seth P. Paratyphoid fever due to Salmonella enterica serotype paratyphi A. Emerg Infect Dis. 1997;3:407. doi: 10.3201/eid0303.970325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kauffmann F. Enterobacteriaceae. 2nd ed. Copenhagen, Denmark: Munksgaard; 1954. p. 107. [Google Scholar]
- 33.Kohn J, Wilchek M. 1-Cyano-4-dimethylamino pyridinium tetrafluoroborate as a cyanylating agent for the covalent attachment of ligand to polysaccharide resins. FEBS Lett. 1983;154:209–210. doi: 10.1016/0014-5793(83)80905-3. [DOI] [PubMed] [Google Scholar]
- 34.Konadu E, Robbins J B, Shiloach J, Bryla D A, Szu S C. Preparation, characterization, and immunological properties in mice of Escherichia coli O157 O-specific polysaccharide-protein conjugate vaccines. Infect Immun. 1994;62:5048–5054. doi: 10.1128/iai.62.11.5048-5054.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konadu E, Shiloach J, Bryla D A, Robbins J B, Szu S C. Synthesis, characterization, and immunological properties in mice of conjugates composed of detoxified lipopolysaccharide of Salmonella paratyphi A bound to tetanus toxoid, with emphasis on the role of O-acetyls. Infect Immun. 1996;64:2709–2715. doi: 10.1128/iai.64.7.2709-2715.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lasserre R, Sangalang R P, Santiago L. Three-day treatment of typhoid fever with two different doses of ceftriaxone, compared to 14-day therapy with chloramphenicol: a randomized trial. J Antimicrob Chemother. 1991;28:765–772. doi: 10.1093/jac/28.5.765. [DOI] [PubMed] [Google Scholar]
- 37.Lee S-C, Yang P-H, Shieh W-B, Lasserre R. Bacteremia due to non-typhi Salmonella: analysis of 64 cases and review. Clin Infect Dis. 1994;19:693–696. doi: 10.1093/clinids/19.4.693. [DOI] [PubMed] [Google Scholar]
- 38.Lees A, Nelson B L, Mond J J. Activation of soluble polysaccharides with 1-cyano-4-dimethylaminopyridium tetrafluoroborate for use in protein-polysaccharide conjugate vaccines and immunological reagents. Vaccine. 1996;14:190–198. doi: 10.1016/0264-410x(95)00195-7. [DOI] [PubMed] [Google Scholar]
- 39.Lepage P, Bogaerts J, van Goethem C, Ntahorutaba M, Nsengumuremyi F, Hitimana D G, Vandepitte J, Butzler J-P, Levy J. Community-acquired bacteremia in African children. Lancet. 1987;i:1458–1461. doi: 10.1016/s0140-6736(87)92207-0. [DOI] [PubMed] [Google Scholar]
- 40.Leung M. Simultaneous infection by a sensitive and a multiresistant strain of Salmonella paratyphi A. J Infect. 1995;30:181–183. doi: 10.1016/s0163-4453(95)80019-0. [DOI] [PubMed] [Google Scholar]
- 41.Mahanta J. Drug sensitivity of Salmonella paratyphi A isolated from a suspected outbreak of fever in Dulijan. J Indian Med Assoc. 1994;92:49–50. [PubMed] [Google Scholar]
- 42.Nesbitt A, Mirza N B. Salmonella septicaemias in Kenyan children. J Trop Pediatr. 1989;35:35–39. doi: 10.1093/tropej/35.1.35. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen T A, Ha H K, Nguyen T D. Typhoid fever in South Vietnam, 1990–1993. Bull Soc Pathol Exot. 1993;86:476–478. [PubMed] [Google Scholar]
- 44.Nournand A, Ziai M, Tahernia A C. Typhoid-paratyphoid fevers in infancy. Clin Pediatr. 1971;10:272–274. doi: 10.1177/000992287101000510. [DOI] [PubMed] [Google Scholar]
- 45.Ørskov F, Ørskov I. Summary of a workshop on the clone concept in the epidemiology, taxonomy, and evolution of the Enterobacteriaceae and other bacteria. J Infect Dis. 1983;148:346–357. doi: 10.1093/infdis/148.2.346. [DOI] [PubMed] [Google Scholar]
- 46.Phillips I, Wharton B. Acute bacterial infection in kwashiorkor and marasmus. Br Med J. 1968;1:407–409. doi: 10.1136/bmj.1.5589.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rao P S, Bairy I, Rao P V, Shivananda P G. Multiresistant Salmonella paratyphi ‘A’ infection in coastal Karnataka. J Assoc Physicians India. 1994;42:929. [PubMed] [Google Scholar]
- 48.Raynal J H, Fournier J. Les salmonelles à Changhaï. Med Trop. 1947;7:199–237. [Google Scholar]
- 49.Reeves M W, Evins G M, Heiba A A, Plikaytis B D, Farmer J J., III Clonal nature of Salmonella typhi and its genetic relatedness to other salmonellae as shown by multilocus enzyme electrophoresis and proposal of Salmonella bongori comb. nov. J Clin Microbiol. 1989;27:313–320. doi: 10.1128/jcm.27.2.313-320.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robbins J B, Chu C Y, Schneerson R. Hypothesis for vaccine development: protective immunity to enteric diseases caused by nontyphoidal salmonellae and shigellae may be conferred by serum IgG antibodies to the O-specific polysaccharide of their lipopolysaccharides. Clin Infect Dis. 1992;15:346–361. doi: 10.1093/clinids/15.2.346. [DOI] [PubMed] [Google Scholar]
- 51.Robbins J B, Schneerson R. Polysaccharide-protein conjugates: a new generation of vaccines. J Infect Dis. 1990;161:821–832. doi: 10.1093/infdis/161.5.821. [DOI] [PubMed] [Google Scholar]
- 52.Robbins J B, Schneerson R, Szu S C. Perspective: hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis. 1995;171:1387–1398. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- 53.Rowe B, Gross R J. Genus II. Salmonella. In: Krieg N R, Holt J G, editors. Bergey's manual of systemic bacteriology. Vol. 1. Baltimore, Md: The Williams & Wilkins Co.; 1984. pp. 423–458. [Google Scholar]
- 54.Sarnaik S, Kaplan J, Schiffman G, Bryla D A, Robbins J B, Schneerson R. Studies on pneumococcus vaccine alone or mixed with DTP and on pneumococcus type 6B and Haemophilus influenzae type b capsular polysaccharide-tetanus toxoid conjugates in 2- to 5-year-old children with sickle cell anemia. Pediatr Infect Dis. 1990;9:181–186. doi: 10.1097/00006454-199003000-00007. [DOI] [PubMed] [Google Scholar]
- 55.Schwartz E, Schlim D R, Eaton M, Jenks N, Houston R. The effect of oral and parenteral typhoid vaccination on the rate of infection with Salmonella typhi and Salmonella paratyphi A among foreigners in Nepal. Arch Intern Med. 1988;150:349–351. [PubMed] [Google Scholar]
- 56.Selander R K, Beltran P, Smith N H, Helmuth R, Rubin F A, Kopecko D J, Ferris K, Tall B D, Cravioto A, Musser J M. Evolutionary genetic relationships of clones of Salmonella serovars than cause human typhoid and other enteric fevers. Infect Immun. 1990;58:2262–2275. doi: 10.1128/iai.58.7.2262-2275.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simanjuntak C H, Paleologo F P, Punjabi N H, Darmowigoto R, Totosudirjo H, Haryanto P, Suprijanto E, Witham N D, Hoffman S L. Oral immunization against typhoid fever in Indonesia with Ty21a vaccine. Lancet. 1991;338:1055–1059. doi: 10.1016/0140-6736(91)91910-m. [DOI] [PubMed] [Google Scholar]
- 58.Svensson S B, Lindberg A A. Artificial Salmonella vaccines: Salmonella typhimurium O-antigen-specific oligosaccharide-protein conjugates elicit protective antibodies in rabbits and mice. Infect Immun. 1981;32:490–496. doi: 10.1128/iai.32.2.490-496.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Syverton J T, Ching R E, Smith A B. Typhoid and paratyphoid A in immunized military personnel. JAMA. 1946;131:507–514. doi: 10.1001/jama.1946.02870230013004. [DOI] [PubMed] [Google Scholar]
- 60.Szu S C, Li X, Schneerson R, Vickers J H, Bryla D, Robbins J B. Comparative immunogenicities of Vi polysaccharide-protein conjugates composed of cholera toxin or its B subunit as a carrier bound to high- or lower-molecular-weight Vi. Infect Immun. 1989;57:3823–3827. doi: 10.1128/iai.57.12.3823-3827.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szu S C, Gupta R, Robbins J B. Induction of serum vibriocidal antibodies by O-specific polysaccharide-protein conjugate vaccines for prevention of cholera. In: Wachsmuth I K, Blake P A, Olsvik O, editors. Vibrio cholerae. Washington, D.C.: American Society for Microbiology; 1994. pp. 381–394. [Google Scholar]
- 62.Thisyakorn U, Mansuwan P, Taylor D N. Typhoid and paratyphoid fever in 192 hospitalized children in Thailand. Am J Dis Child. 1987;141:862–865. doi: 10.1001/archpedi.1987.04460080048025. [DOI] [PubMed] [Google Scholar]
- 63.Verghese S L, Manonmani R, Balasubramanian S, Chandrasekharan S. Multi-drug resistance in salmonellae isolated from enteric fever cases at Porur—a semi urban area near Madras City. J Commun Dis. 1992;24:12–15. [PubMed] [Google Scholar]
- 64.Watson D C, Robbins J B, Szu S C. Protection of mice against Salmonella typhimurium with an O-specific polysaccharide-protein conjugate vaccine. Infect Immun. 1992;60:4679–4686. doi: 10.1128/iai.60.11.4679-4686.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.World Health Organization Expert Committee on Biologic Standardization. Requirements on Vi polysaccharide for typhoid. W H O Tech Rep Ser. 1993;43:14–32. [Google Scholar]
- 66.Zimmerman L E, Cooper M, Graber C D. Bacteriologic studies in an outbreak of salmonellosis in Korea. Am J Hyg. 1952;56:252–264. doi: 10.1093/oxfordjournals.aje.a119550. [DOI] [PubMed] [Google Scholar]