Abstract
Fixed drug eruption is a cutaneous drug reaction which recurs at the same site when the individual is exposed to the causative drug, characterized by single or multiple round sharply demarcated erythematous‐to‐violaceous patches. Here, we report a patient with generalized non‐bullous fixed drug eruption following mRNA‐based Pfizer‐BioNTech COVID‐19 vaccine.
Keywords: drug rash; Exanthem; fixed drug eruption, COVID‐19 vaccine; hyperpigmentation; mRNA vaccine; skin
Short abstract
Fixed drug eruption following COVID‐19 vaccination has been reported on rare occasions which includes bullous and non‐bullous lesions. The presented case adds to this list a “multiple non‐bullous” presentation of FDE following mRNA COVID‐19 vaccine.
1. INTRODUCTION
Fixed drug eruption (FDE) is a cutaneous drug reaction which recurs at the same site when the individual is exposed to the causative drug. The eruption is characterized by single or multiple round sharply demarcated erythematous‐to‐violaceous patches and rarely with generalized bullae resembling Stevens–Johnson syndrome or toxic epidermal necrolysis. The erythematous‐to‐violaceous hue usually improves over several days, resulting in a characteristic round hyperpigmented patch. 1 Although the culprit agent is usually a medication, such as a sulfa antibiotic or a nonsteroidal anti‐inflammatory drug (NSAID), FDE has also been reported following vaccinations such as the influenza, yellow fever, and human papilloma virus vaccines. 1 , 2 , 3
To date, only five cases of FDE following COVID‐19 vaccination have been reported, 3 following mRNA and 2 after adenoviral vector‐based vaccines (Table 1). 4 , 5 , 6 , 7 , 8 Here, we report a patient with generalized non‐bullous FDE following vaccination with the mRNA‐based Pfizer‐BioNTech COVID‐19 vaccine.
TABLE 1.
Patient characteristics of the reported cases of fixed drug eruption following COVID‐19 vaccination
| Nationality | Thailand 4 | Guyan 5 | Malta 6 | Tunisia 7 | France 8 |
|---|---|---|---|---|---|
| Gender | Male | Male | Female | Female | Female |
| Age | 74 | 66 | 26 | 40 | 54 |
| Time when lesions started to appear | 25 h after the 1st dose | Within 24 h of receiving the 2nd dose | 15 days after the 1st dose; subsequent lesion appeared 14 days after the 2nd dose |
3 days after the 1st dose Subsequent lesions appeared 7 days after the 1st dose |
24 h after the 1st dose; subsequent lesion appeared 4 days after the 2nd dose |
| COVID‐19 vaccine type | Adenoviral‐vectored | mRNA | mRNA | Adenoviral‐vectored | mRNA |
| Associated symptoms | None reported | Fever, myalgia | None reported | None reported | None reported |
| Past medical history | End stage renal disease, Atrial fibrillation, Ischemic stroke | Hypertension, hyperlipidemia, diabetes, chronic kidney disease, coronary artery disease, idiopathic hypothyroidism | None reported | None reported | Seasonal allergic rhinitis |
| Medication history | None reported | Amlodipine, aspirin, brimonidine solution, clopidogrel, famotidine, furosemide, gabapentin, insulin aspart and glargine, levothyroxine, lisinopril, metoprolol, pravastatin, tamsulosin |
Hydroxyzine prior to receiving the 2nd dose of vaccine. Topical 1% hydrocortisone and terbinafine applied on the 2nd lesion was ineffective |
None reported | None reported |
| Skin Examination | Multiple, well‐defined, round to oval, erythematous to violaceous plaques with central dusky appearances and bullous formation | Extensive areas of painful violaceous patches many of which developed into large flaccid bullae |
1st lesion: mildly pruritic, erythematous, annular patch 7 cm below the injection site in the same arm, developing central erosion surrounded by a halo over 2 days, then resolved spontaneously 2nd lesion was a similar patch although with prominent vesiculation at the center which eventually ruptured and scabbed |
A well‐defined bullous erythematous plaque sur‐rounding the injection site, plus two other lesions on the upper and lower limbs | A 3–4 cm‐well‐defined erythematous patch |
| Location | Trunk and both extremities | Abdomen, buttocks, and lower extremities | Same arm in which injection had been done | Upper and lower limbs | Left wrist |
| Labs | Unremarkable |
Hyperkalemia, hyperglycemia, mild transaminitis, serum CK of 347, and an incidental finding of mild AKI on CKD. Normal serum Ab to bullous pemphigoid |
Not discussed in the article | Not discussed in the article | Not discussed in the article |
| Pathology | Subepidermal separation with superficial and deep perivascular inflammatory cell infiltration plus mixed inflammatory cells infiltrate composing of lymphohistiocytes and numerous eosinophils. Melanophages were seen in the upper dermis | Full‐thickness epidermal necrosis and a very sparse lymphocytic inflammatory infiltrate |
Overall, lichenoid interface dermatitis was found Patchy lymphohistiocytic infiltrate in the upper dermis around skin adnexal structures, lymphocytic infiltration of the basal layer of epidermis, basal cell vacuolar damage, Civatte body formation and pigment incontinence, dermal‐epidermal junction clefting |
Epidermal necrosis, bullae formation, superficial and deep perivascular mixed infiltrate of lymphocytes, neutrophils and eosinophils, and dermal melanophages were detected | Acute vacuolar interface changes and keratinocyte necrosis with superficial perivascular infiltrate of lymphocytes |
| Final diagnosis | Generalized bullous fixed drug eruption | Extensive bullous fixed drug eruption | Fixed drug eruption | Generalized bullous fixed drug eruption | Fixed drug eruption |
| Treatment | Topical 0.25% desoximetasone cream | Ibuprofen and high‐dose oral prednisone, bullae drainage followed by mupirocin and Vaseline for wound care | Not discussed in the article | Potent topical corticosteroids and hydroxyzine | Topical beclomethasone dipropionate |
| Outcome | Lesions gradually resolved within 2 weeks with subsequent post‐inflammatory hyperpigmentation | Discharged from hospital after 7 days with prednisone taper and outpatient follow‐up recommendation | Not discussed in the article | Resolution of the lesion after a few days of treatment with subsequent development of post‐inflammatory hyperpigmentation | Clearance of the lesion in 21 days without residual hyperpigmentation |
2. CASE PRESENTATION
A 50‐year‐old female patient presented to the dermatology clinic for her initial visit, with a concern of multiple dark spots on her skin. The patient reported that approximately 1 year prior to presentation, she noticed an erythematous tender patch on her left upper back 2 days following her second dose of the Pfizer‐BioNTech COVID‐19 mRNA vaccine. Of note, both doses of the vaccine had been administrated in her right arm. Over the next several days, the lesion gradually lost its erythematous hue and became a nontender round hyperpigmented patch. Approximately 1 month later, she noticed similar tender erythematous plaques gradually developing on her trunk and upper extremities. These lesions similarly progressed to nontender hyperpigmented patches over the next several days. She continued to develop new lesions over the course of 9 months. No bullous lesions were reported.
Her past medical history was notable for breast cancer treated with left mastectomy and chemoradiation 3 years prior to presentation. Additionally, she started taking amlodipine for hypertension 1 week prior to presentation. She denied taking any other medications both at the time of presentation and for the past year, including over‐the‐counter medications such as acetaminophen or NSAIDs. She reported a history of pruritus after taking a sulfa antibiotic many years prior to presentation, but she did not remember the name of that medication. She denied any history of vaccine allergies. Her family history was noncontributory.
On examination, multiple round hyperpigmented patches ranging from 2 to 8 cm in size were seen on the left upper back (Figure 1A), right forearm, right and left anterior chest wall (Figure 1B), left abdomen, and left axilla. The remainder of the cutaneous and mucosal examination was unremarkable. A punch biopsy was obtained from the round hyperpigmented patch on the left upper back. Histopathological examination was notable for post‐inflammatory pigmentary alteration with rare eosinophils consistent with a resolving FDE (Figures 2A,B). The patient was reassured and advised to weigh the potential benefits and harms prior to receiving any booster doses of the COVID‐19 vaccine.
FIGURE 1.
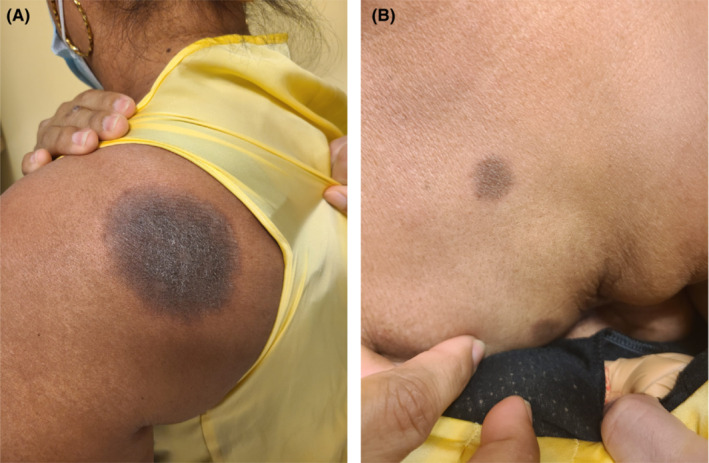
(A, B) Fixed drug eruption: Sharply demarcated round hyperpigmented patches on the left upper back and left anterior chest wall
FIGURE 2.
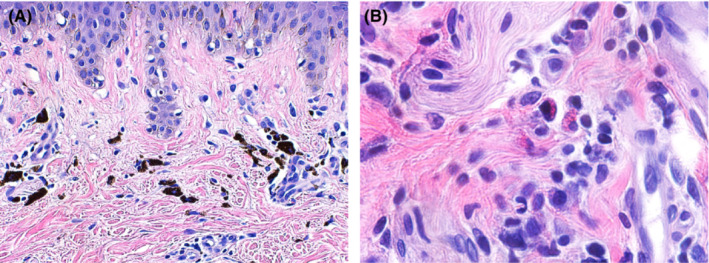
(A) Fixed drug eruption: Dense aggregate of melanophages in the superficial dermis. (B) Fixed drug eruption: Sparse perivascular infiltrate with eosinophils
3. DISCUSSION
COVID‐19 vaccination has been associated with an array of cutaneous adverse effects. Essentially, we may encounter two different categories of reactions: the flare of preexisting dermatoses and the development of new reactions. Local reactions are the most common type of reactions which are usually seen following the injection of mRNA‐based vaccine and less commonly after adenoviral vectored vaccines. Other types of reactions include filler reactions (e.g., through hyaluronic acid‐driven CD8+ T‐cell‐medicated granuloma‐forming immunoreactivity), exanthemas, vascular lesions, and urticarial. Less commonly, erythromelalgia, pityriasis rosea‐like rash, and varicella zoster reactivation may be seen following COVID‐19 vaccination. 9 FDE following COVID‐19 vaccination has only been reported in a handful of cases. 10
The cutaneous reactions following COVID‐19 vaccinations have been reported more in women. However, whether it is a real distribution predilection or merely a publication/selection bias is yet to be determined. 9 Most of the cutaneous reactions are mild with rapid resolution and will not be a barrier for the second/booster dose injection. In case of moderate‐to‐severe reactions; however, administration of a different vaccine may be considered for booster doses to prevent the relapse of cutaneous reactions. 9 Finally, COVID‐19 vaccine is contraindicated only in people with a severe allergic reaction to a previous COVID‐19 vaccine dose or a known (diagnosed) allergy to a component of the vaccine. 11
The pathogenesis of FDE following COVID‐19 vaccination has yet to be determined. One potential mechanism is an immune response to the polyethylene glycol excipient of the mRNA vaccine. 8 Similarly, a reaction to ChAdOx1 nCoV‐19 virotopes in adenoviral vectored vaccines 4 has been proposed as a possible mechanism. Notably, polysorbate 80, the vaccine excipient, does not seem to be the culprit allergen. 4 , 7
FDE following COVID‐19 vaccination appears to have a very low incidence rate, and its outcome is generally favorable. However, our case illustrates the potential for prolonged cosmetic sequelae following FDE secondary to the Pfizer‐BioNTech COVID‐19 vaccination. As such, a clear conversation about the risk of FDE should be included in the informed consent discussion when weighing the risks and benefits of COVID‐19 vaccination and booster doses.
AUTHOR CONTRIBUTIONS
Soodeh Kabir and Eric J. Feit involved in data collection, drafting the manuscript, and final approval of the manuscript. Edward R Heilman involved in revising the manuscript and final approval of the manuscript.
FUNDING INFORMATION
This article has no funding source.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
CONSENT
A written consent for publication of all patient's photographs and medical information was obtained from the patient.
ACKNOWLEDGMENT
None.
Kabir S, Feit EJ, Heilman ER. Generalized fixed drug eruption following Pfizer‐BioNtech COVID‐19 vaccination. Clin Case Rep. 2022;10:e06684. doi: 10.1002/ccr3.6684
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Shiohara T. Fixed drug eruption. In: Post TW, ed. UpToDate. UpToDate Inc. Accessed April 26, 2022. https://www.uptodate.com/contents/fixed‐drug‐eruption?search=Shiohara%20T.%20Fixed%20drug%20eruption.%20&source=search_result&selectedTitle=1~139&usage_type=default&display_rank=1 [Google Scholar]
- 2. Byrd RC, Mournighan KJ, Baca‐Atlas M, Helton MR, Sun NZ, Siegel MB. Generalized bullous fixed‐drug eruption secondary to the influenza vaccine. JAAD Case Rep. 2018;4(9):953‐955. doi: 10.1016/j.jdcr.2018.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pyl J, Aerts O, Siozopoulou V, et al. Bullous fixed drug eruption following human papilloma virus vaccination. J Eur Acad Dermatol Venereol. 2020;34(11):e697‐e698. doi: 10.1111/jdv.16377 [DOI] [PubMed] [Google Scholar]
- 4. Wantavornprasert K, Noppakun N, Klaewsongkram J, Rerknimitr P. Generalized bullous fixed drug eruption after Oxford‐AstraZeneca (ChAdOx1 nCoV‐19) vaccination. Clin Exp Dermatol. 2022;47(2):428‐432. doi: 10.1111/ced.14926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kong J, Cuevas‐Castillo F, Nassar M, et al. Bullous drug eruption after second dose of mRNA‐1273 (Moderna) COVID‐19 vaccine: case report. J Infect Public Health. 2021;14(10):1392‐1394. doi: 10.1016/j.jiph.2021.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mintoff D, Pisani D, Betts A, Scerri L. SARS‐CoV‐2 mRNA vaccine‐associated fixed drug eruption. J Eur Acad Dermatol Venereol. 2021;35(9):e560‐e563. doi: 10.1111/jdv.17390 [DOI] [PubMed] [Google Scholar]
- 7. Ben Salem C, Khelif A, Sahnoun D, Ghariani N, Sriha B, Denguezli M. Another case of generalized bullous fixed drug eruption following an adenoviral vector‐based COVID‐19 vaccine (ChAdOx1 nCov‐19). J Eur Acad Dermatol Venereol. 2022;36:e516‐e517. doi: 10.1111/jdv.18059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lellig E, Mouton‐Faivre C, Abs D, Bursztejn AC. Fixed drug eruption after Pfizer‐BioNTech COVID‐19 vaccine: a case report. J Allergy Clin Immunol Pract. 2022;10:1922‐1923. doi: 10.1016/j.jaip.2022.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bellinato F, Maurelli M, Gisondi P, Girolomoni G. Cutaneous adverse reactions associated with SARS‐CoV‐2 vaccines. J Clin Med. 2021;10(22):5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seirafianpour F, Pourriyahi H, Gholizadeh Mesgarha M, Pour Mohammad A, Shaka Z, Goodarzi A. A systematic review on mucocutaneous presentations after COVID‐19 vaccination and expert recommendations about vaccination of important immune‐mediated dermatologic disorders. Dermatol Ther. 2022;35:e15461. doi: 10.1111/dth.15461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. https://www.cdc.gov/vaccines/covid‐19/downloads/pre‐vaccination‐guidelines.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


