Abstract
The etiology of cognitive dysfunction associated with Alzheimer’s disease (AD) and dementia is multifactorial. Yet, mechanistic interactions among key neurobiological factors linked to AD pathology are unclear. This study tested the effect of interactions between cerebrovascular function, individual genotype, and structural brain pathology on response inhibition performance, an early and sensitive indicator of cognitive executive dysfunction with aging.
We quantified cerebrovascular response (CVR) to moderate-intensity aerobic exercise using transcranial doppler ultrasound and global amyloid-beta (Aβ) deposition using positron emission tomography in a group of cognitively normal older adults genotyped as APOE4 carriers and noncarriers. We quantified response inhibition during a cognitive Stroop test.
Individuals with blunted CVR possessed greater Aβ deposition. There was CVR-by-carrier status-by-Aβ interaction on response inhibition. Blunted CVR was associated with impaired response inhibition specifically in carriers. Despite having greater Aβ deposition, carriers with higher CVR demonstrated better response inhibition.
Cerebrovascular interactions with individual genotype and structural brain pathology may provide a physiologically-informed target for precision-medicine approaches for early treatment and prevention of cognitive dysfunction with aging.
Keywords: cardiovascular, cognition, executive function, Stroop, Alzheimer’s disease, transcranial Doppler, amyloid plaque, APOE
1. Introduction
Among a growing older adult population, the development of cognitive dysfunction is common but poorly understood. Profound individual differences in aging trajectories allow some older adults to maintain cognitive performance to the end of life while others suffer the debilitating loss of cognitive function associated with Alzheimer’s disease (AD) and related dementias (ADRD). The field of aging neurobiology increasingly recognizes cognitive decline with aging as having a mixed pathology and multifactorial etiology (Iturria-Medina et al., 2016, 2017); thus, a trans-disciplinary approach is imperative to enhance our understanding of the diverse and interacting mechanisms leading to ADRD. In the present study, we provide an initial characterization of interactions among key neurobiological factors that have been identified as significant contributors to the development of cognitive dysfunction and AD, including cerebrovascular function (Iadecola, 2004; Xie et al., 2016; Ouellette and Lacoste, 2021), structural brain pathology (Jack et al., 2010; Hampel, 2013), and individual genotype (Heffernan et al., 2016). Further, we investigate the behavioral relevance of these interactions on response inhibition performance in cognitively normal older adults.
Early detection of pathologic disease processes is imperative as a first step towards the development of effective prevention strategies and interventions for age-related neuropathologies, in particular those involving vascular aging (e.g. AD, vascular dementia, endothelial dysfunction, vascular Parkinsonism) (Pantsiou et al., 2018). One of the earliest behavioral manifestations of mild cognitive impairment (MCI) that can progress to dementia has been identified as impaired cognitive executive function (Hutchison et al., 2010; Kirova et al., 2015). Specifically, the ability to suppress an undesired default or automatic response in the presence of interfering stimuli, or response inhibition, can distinguish between older adults who are cognitively normal versus MCI (Hutchison et al., 2010). Additionally, cortical activity dysfunction during response inhibition may underpin cognitive interference in motor behavior (Palmer et al., 2021), a common observation in older adults with MCI and early-stage AD (Coelho et al., 2012; Kirova et al., 2015), and older adults at high genetic risk for AD (Whitson et al., 2018). Yet the neurobiologic and physiologic mechanisms that contribute to the early-stage development of impaired response inhibition behavior remain elusive. Such knowledge could be leveraged towards the development of prevention strategies for clinical syndromes in the early stages of MCI, AD and related dementias.
Cerebrovascular pathology is commonly detected in older adults who present with behavioral deficits associated with clinical AD syndrome (Lorius et al., 2015; Wolters Frank J. et al., 2017; Sweeney et al., 2018; Bracko et al., 2021) and is linked to biomarkers of brain pathology, particularly the deposition of amyloid-beta (Zlokovic et al., 2005; Sisante et al., 2019; Solis et al., 2020). The dynamic characterization of cerebral blood flow response under conditions of physiologic stress enables the assessment of the cerebrovascular regulatory response to a wide variety of physiological inputs common in daily activities (Ferguson, 2014), including altered perfusion pressure, arterial blood gas, neural activity, and brain metabolism (Smith and Ainslie, 2017). Indeed, cerebrovascular response under such conditions of physiologic stress (e.g. sit-to-stand positional changes, aerobic exercise, heat stress, hypoxia) appear to play an important role in maintaining brain metabolism and function with aging (Bundo et al., 2002; Ogoh and Ainslie, 2009a, 2009b; Sato et al., 2011; Ogoh et al., 2013; Steinback and Poulin, 2016), as the effects of repeated transient disruption of blood, glucose, and oxygen supply to brain tissue accumulate over time (Tarumi and Zhang, 2018). Impaired cerebral blood flow can promote ischemic microlesions (Iadecola, 2004), and alter blood-brain barrier trafficking of amyloid-beta (Aβ) (Zlokovic et al., 2005), slowing the clearance of Aβ and promoting its accumulation in the brain.
Using transcranial Doppler ultrasound (TCD) to assess cerebral artery blood flow velocity, our group previously found that older adults demonstrate a blunted cerebrovascular response (CVR) during an acute bout of moderate-intensity aerobic exercise (Sisante et al., 2019; Alwatban et al., 2020), which may serve as an early indicator of cerebrovascular dysfunction in preclinical older adult populations (Alwatban et al., 2020). Further, CVR was associated with elevated levels of Aβ deposition, a hallmark of AD, in the brains of cognitively normal older adults (Sisante et al., 2019). Interestingly, cerebrovascular assessments performed at rest have not been able to discriminate cognitive status (Xie et al., 2016) or Aβ levels (Sisante et al., 2019) in these preclinical older adult populations, suggesting that acute aerobic exercise assessment paradigms may enhance the detection of subtle impairments in vascular function in the early stages of disease. Consistent with this theory, dysfunctional cerebral blood flow has been detected in older adults with MCI (Xie et al., 2016) and precedes the onset of dementia, implicating its early mechanistic role in cognitive decline with aging (Iadecola, 2004). However, despite the apparent role of CVR dysfunction in the preclinical stages of brain pathology, the interactions between CVR and other key neurobiological factors linked to early-stage cognitive impairments and AD pathology remain unknown.
Possession of Apolipoprotein E4 (APOE4), the strongest known genetic risk factor for sporadic Alzheimer’s disease (Heffernan et al., 2016), is strongly linked to cerebrovascular dysfunction (Montagne et al., 2020). Cognitively normal older adults with an APOE4 allele demonstrate a greater decline in cerebral blood flow with aging compared to noncarriers (Thambisetty et al., 2010), and an earlier blood-brain barrier breakdown that precipitates subsequent cognitive decline (Montagne et al., 2020). Recently, our group found that APOE4 carriers with lowest resting conductance in middle cerebral artery blood flow had the greatest Aβ deposition, a relationship that was not present in noncarriers (Kaufman et al., 2021b). Yet, in the context of early-stage cognitive impairments, the behavioral relevance of APOE4 genotype in the presence of multifactorial aging pathologies among a heterogenous older adult population is poorly understood.
In this study, we aimed to 1) investigate the interactive effect of APOE4 carrier status on the relationship between CVR to exercise and Aβ deposition and 2) test whether interactions between APOE4 carrier status, CVR, and Aβ deposition affect cognitive performance involving response inhibition in a group of cognitively normal older adults. We hypothesized that 1) older adults with the most robust CVR to a bout of moderate-intensity aerobic exercise would have the lowest levels of Aβ deposition, with the strongest effect in APOE4 carriers, and 2) higher CVR would attenuate the negative effect of Aβ deposition on response inhibition performance, particularly in APOE4 carriers.
2. Materials and Methods
2.1. Participants
We selected a subset of 70 older adults from a well-characterized data registry of 125 cognitively normal older adults from the University of Kansas Alzheimer’s Disease Research Center (AHA 16GRNT30450008 (SB)) (Alwatban et al., 2020; Perdomo et al., 2020; Liu et al.). Our recruitment efforts within this cohort included those individuals with complete datasets for cerebrovascular function assessment, genetic APOE profiling, and structural neuroimaging for Aβ deposition. Inclusion criteria were (1) between 65 and 90 years old, (2) normal cognition with the absence of clinical dementia or cognitive impairment and (3) physical ability to exercise. Exclusion criteria were (1) insulin-dependent diabetes; (2) peripheral neuropathy; (3) active coronary artery disease (angina, myocardial infarction) within 2 years that, in the investigator’s opinion, could pose a safety risk with exercise, unless cleared by the participant’s physician; (4) congestive heart failure; (5) the presence of 1 or 2 APOE2 alleles. The experimental protocol was approved by the University of Kansas Institutional Review Board (IRB#: STUDY00001444) and all participants provided written informed consent.
2.2. Acute bout of aerobic exercise on a recumbent stepper
Participants underwent a bout of moderate-intensity aerobic exercise on a recumbent stepper in an experimental protocol previously described in detail (Billinger et al., 2017; Ward et al., 2018; Witte et al., 2019). Briefly, participants arrived to the laboratory between 7:30–9:00am after abstaining from caffeine for 12 hours, physical activity for 24 hours, and consuming a large meal for 2 hours. The exercise testing room was quiet, dimly lit, and temperature controlled between 22 and 24 degrees Celsius. Following 8 minutes of quiet rest seated on the recumbent stepper (NuStep T5XR), participants began an exercise familiarization bout to determine target power. Following an 8-minute seated rest recording, participants began exercising on the recumbent stepper, maintaining a step rate of 120 steps-per-minute and beginning at a resistance of 40W. The work resistance was increased until the participant reached the target heart rage range of 40–60% of age-predicted heart rate reserve and maintained this target heart rate for one continuous minute (Billinger et al., 2017; Sisante et al., 2019). The participant then maintained exercise at this moderate-intensity hear rate zone for 8 minutes.
2.3. Cerebro- and cardiovascular assessment and analyses
During rest and exercise bouts, we measured left cerebral blood flow velocity using transcranial Doppler ultrasound (TCD) with a 2-MHz probe (RobotoC2MD, Multigon Industries) placed over the temporal window. If the left MCA signal was not obtainable, the right MCA was used. We used a 5-lead electrocardiogram for continuous heart rate monitoring. All data were sampled at 500 Hz and acquired through an analog to digital data acquisition unit (NI-USB-6212, National Instruments) and custom written software operating in MATLAB (The Mathworks Inc). MCAv, MAP and end tidal CO2 were synchronized across the cardiac cycle (Billinger et al., 2017; Ward et al., 2018; Sisante et al., 2019; Perdomo et al., 2020). Data with R-to-R intervals greater than 5 Hz or changes in peak blood flow velocity greater than 10 cm/s in a single cardiac cycle were considered not physiologically real and censored. Acquisitions with more than 15% of data points censored were discarded. Experimenters were blinded to Aβ deposition level, cognitive function, and APOE carrier status.
The mean MCA blood flow velocity was calculated during the 8-minute baseline rest and 8-minute moderate-intensity exercise bout. We calculated cerebrovascular response (CVR) as the difference between mean MCA blood flow velocity during exercise and mean MCA blood flow velocity at rest (Sisante et al., 2019). Across all participants (n=70), we calculated an average intra-trial coefficient of variation for mean MCAv under resting conditions of 8.0% and the moderate-intensity aerobic exercise condition of 9.1%.
2.4. Structural neuroimaging of amyloid-beta (Aβ) deposition
Participants underwent Florbetapir PET scans on a GE Discovery ST-16 PET/CT scanner at approximately 50 minutes after administration of intravenous florbetapir 18F-AV45 (370 MBq). Continuous acquisition of two 5-minute PET brain frames were summed and attenuation corrected (Vidoni et al., 2021). The global standardized update value ratio (SUVR) was calculated using standard procedures described previously (Liu et al.) to determine global Aβ deposition for each participant. Individuals were classified as having elevated Aβ deposition with global Aβ levels > 1.1, a threshold previously identified as a sensitive detector of neural plaques (Clark et al., 2012; Joshi et al., 2012) and that is associated cerebrovascular dysfunction in older adults (Sisante et al., 2019).
2.5. APOE genotyping
Whole blood was drawn and stored frozen at −80 degrees Celsius prior to genetic analyses using a Taqman single nucleotide polymorphism (SNP) allelic discrimination assay (ThermoFisher) to determine APOE genotype. Taqman probes were used to determine APOE4, APOE3, and APOE2 alleles to the two APOE-defining SNPs, rs429358 (C_3084793_20) and rs7412 (C_904973_10) (Kaufman et al., 2021c; Vidoni et al., 2021). Individuals were classified as APOE4 carrier in the presence of 1 or 2 APOE4 alleles (e.g. E3/E4, E4/E4). Individuals with homozygous E3 (e.g. E3/E3) were classified as a noncarriers. Because APOE2 is associated with reduced risk for Alzheimer’s disease, all APOE2 carriers, whether homozygous or paired with a different APOE allele), were excluded from analyses in the present study (Whitson et al., 2018; Kaufman et al., 2021a).
2.6. Cognitive behavior assessment
2.6.1. Clinical neuropsychological test battery
The Uniform Data Set (UDS) neuropsychological test battery and the Clinical Dementia Rating (CDR) scale employed by the United States Alzheimer’s Disease Research Center network (Morris, 1993; Monsell et al., 2016) were performed for all participants on a separate day. All participants completed a standard in-person clinical and cognitive evaluation, during which the clinical CDR was performed by a trained clinician and the neuropsychological test battery by a trained psychometrist. Clinical and cognitive data were reviewed and finalized at a consensus diagnostic conference (Graves et al., 2015). All participants in the present analysis were rated as CDR = 0 and cognitively normal. Participant also completed a Mini-Mental State Exam (MMSE) (Folstein et al., 1975) (Table 1).
Table 1.
Participant characteristics.
| All (n=70) |
APOE4 (+) (n=20) |
APOE4 (−) (n=50) |
|
|---|---|---|---|
| Age (years) | 71±5 [65–87] |
71±7 [65–87] |
71±5 [65–81] |
| Workload (W/kg BW) | 0.80±.0.27 [0.20–1.46] |
0.81±.0.39 [0.20–1.46] |
0.80±.0.21 [0.24–1.17] |
| Gender | F=47 M=23 |
F=13 M=7 |
F=34 M=16 |
| MMSE | 28.6±1.8 [22.0–30.0] |
28.6±1.4 [25.0–30.0] |
28.7±2.0 [22.0–30.0] |
| Aβ Deposition | 1.09±.17 [0.86 to 1.6] |
1.16±.17 [ 0.98 to 1.6] |
1.07±.17 [0.86 to 1.6] |
| CVR | 5.55±5.29 [−13.00 to 21.88] |
5.18±4.58 [−3.57 to 13.78] |
5.69±5.58 [−13.00 to 21.88] |
| Stroop ratio | 0.52±.12 [0.29 to 1.0] |
0.51±.12 [0.33 to 0.82] |
0.53±.17 [0.29 to 1.00] |
MMSE = Mini-Mental State Exam; Aβ = amyloid-beta; CVR = cerebrovascular response to moderate-intensity aerobic exercise; Aβ = amyloid-beta; [Range]; W= Watts at moderate intensity exercise workload; kg BW = kilograms of body weight. Values are depicted as mean ± SD.
2.6.2. Response Inhibition Performance
Participants completed a Stroop test during which they were presented with three conditions: 1) Stroop word reading, where color words were printed in black ink, 2) Stroop color naming, in which rectangular color patches were shown, and 3) Stroop interference, in which color words were printed in an incongruent ink color and participants were instructed to ignore the word and state the color of the ink. Each of the three conditions was presented individually in a 45-second trial. The raw number of correct responses was recorded within the time limit for each condition.
The difference in the time for naming the colors in which the words are printed and the same colors printed in rectangles is the measure of the interference of conflicting word stimuli upon naming colors, standardized for speed differences between people (raw number of word interference on color /raw number of control color squares read) (Stroop, J, 1935).
We calculated response inhibition performance as the Stroop ratio, where:
A Stroop ratio = 1.0 reflects perfect response inhibition performance and no interference of conflicting word stimuli. Here, the participant perfectly inhibited interfering word stimuli during the Stroop interference condition, with no slowing of response time relative to the control Stroop color condition. A Stroop ratio = 0.0 reflects poor response inhibition performance and complete failure to inhibit conflicting word stimuli on a color naming task. Here, the participant was unable to state any correct responses during the Stroop interference condition. Additionally, in the absence of response inhibition, we quantified a cognitive processing speed control condition as the Stroop color naming score normalized to the test time duration of 45 seconds.
2.7. Statistical analyses
We tested for normality and heterogeneity of variance of all data used for analyses using Kolmogorov-Smirnov and Levene’s tests, respectively. To test the effect of APOE4 carrier status on the relationship between CVR and Aβ, we performed two-way moderated multiple linear regression analysis after controlling for participant age and workload at moderate intensity exercise normalized to participant body weight. In an exploratory analyses, we tested whether individuals classified as possessing elevated global Aβ levels (>1.1 SUVR) displayed differential relationships between CVR and Aβ deposition in each APOE4 carriers and noncarriers.
To test for interactions between APOE4 carrier status, CVR, and Aβ deposition on response inhibition performance, we performed a three-way multiple linear regression analyses after controlling for participant age. Specifically included in the three-way model were CVR, APOE4 carrier status, Aβ deposition, and the interaction CVR × carrier status × Aβ deposition. The relationship between Aβ deposition and response inhibition performance was compared for participants across the range of CVR to aerobic exercise. Additionally, we tested the behavioral specificity for the predictive ability of interactions between CVR, APOE4 carrier status, and Aβ deposition by testing the three-way multiple regression model against the cognitive processing speed control condition.
In an exploratory analysis, we tested response inhibition performance in APOE4 carriers and noncarriers as a function of CVR classification. Here, we dichotomized participants by the group median CVR value and performed a two-way analysis of variance (ANOVA). All analyses were performed using SPSS version 25 with an a priori level of significance set to 0.05.
3. Results
Among the 70 total participants included in analyses (67% female, 29% APOE4 carriers, 71±5 yo) (Table 1), there were no significant differences between APOE4 carriers and noncarriers in response inhibition performance (p=.301), age (p=.94), normalized workload (p=.966), gender distribution (p=.723), MMSE (p=.916), or CVR to aerobic exercise (p=.716) in carriers and noncarriers. APOE4 carriers had greater levels of Aβ deposition compared to noncarriers (p=.02).
3.1. Relationship between cerebrovascular function and amyloid beta and effect of APOE4
After controlling for age and normalized workload, the two-way multiple regression model significantly predicted Aβ deposition, F5,69=4.05, p=0.003, R2=0.24, adjusted R2=0.18. Regression coefficients and standard errors are detailed in Table 2. A higher CVR predicted lower Aβ deposition across all participants (p=.018) (Figure 1). Though APOE4 carriers showed a stronger effect of CVR on Aβ deposition compared to noncarriers, the difference of this relationship was not significant (t=−0.50, p=.618). There was a main effect of APOE carrier status on Aβ deposition in the model, with APOE4 carriers having greater Aβ deposition than noncarriers (p=.041) (Table 2).
Table 2.
Two-way moderated multiple regression analysis testing the effect of APOE4 carrier status on the relationship between CVR and Aβ deposition after controlling for age, N = 70; Regression model predicting Aβ deposition, p=.003.
| Variable | B | SEB | β | p-value |
|---|---|---|---|---|
| Intercept | 0.794 | 0.278 | .006* | |
| Age | 0.004 | 0.004 | 0.138 | .227 |
| Workload (W/ kg BW) | −0.054 | 0.075 | −0.084 | .474 |
| CVR | −0.056 | 0.023 | −0.308 | .018* |
| APOE4 carrier status (+) | 0.087 | 0.042 | 0.227 | .041* |
| Interaction APOE4 carrier status (+) × CVR | −0.025 | 0.050 | −0.063 | .618 |
Aβ = amyloid-beta; CVR = cerebrovascular response to moderate-intensity aerobic exercise; SEB = standard error of the coefficient; B = unstandardized regression coefficient; β = standardized coefficient;
p<.05
Figure 1.
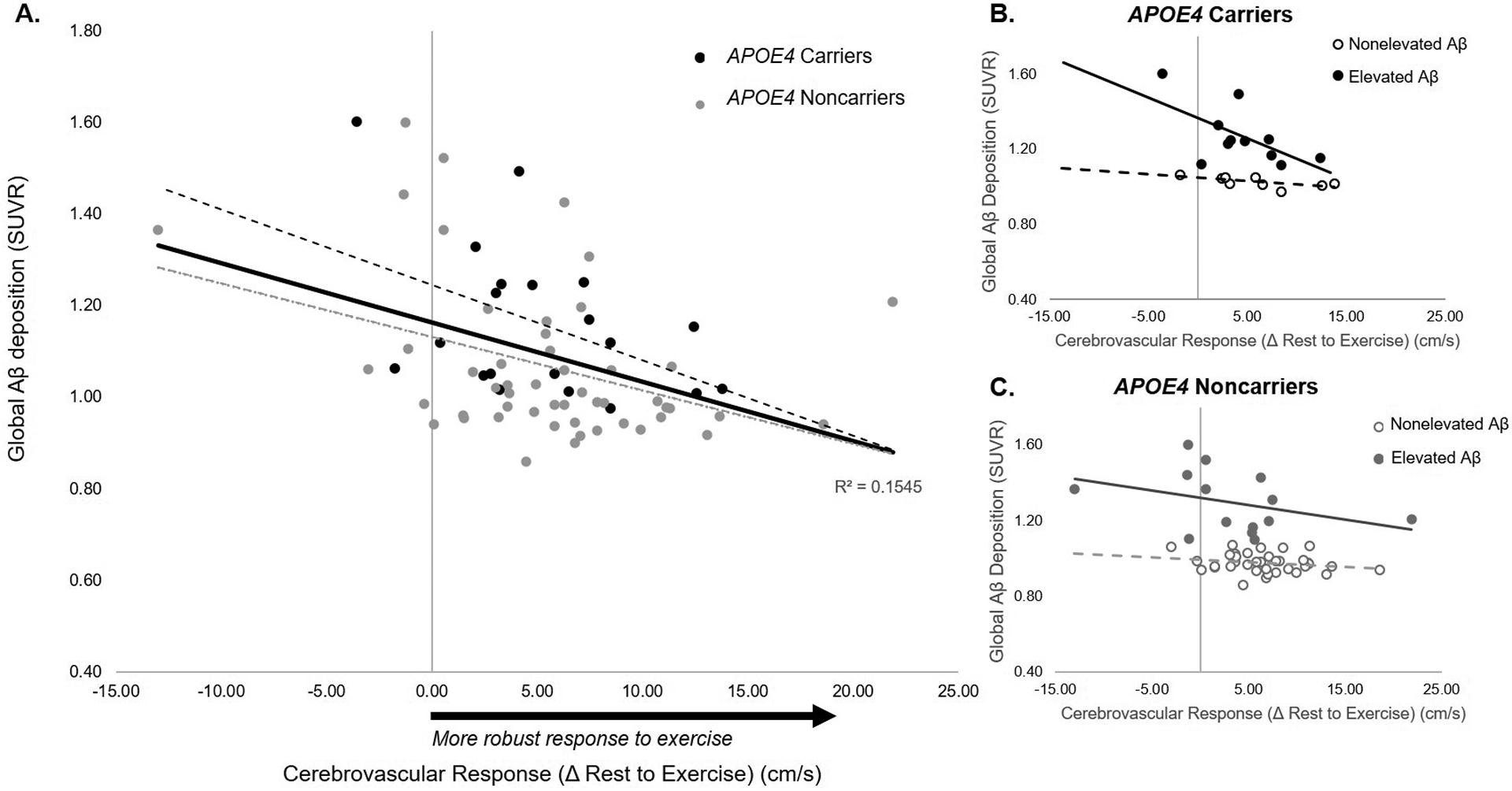
Relationship between cerebrovascular response to moderate-intensity exercise and global amyloid-beta (Aβ) burden. A higher CVR predicted lower Aβ deposition across all participants (p=.013) (A). In APOE4 carriers, individuals classified as having elevated Aβ (solid line) demonstrated a stronger negative effect of CVR on Aβ deposition compared to APOE4 carriers classified as nonelevated Aβ (broken line) (p=.032) (B). In APOE4 noncarriers, there were no significant differences in the effect of CVR on Aβ deposition between individuals with elevated versus nonelevated Aβ deposition (p=.272) (C).
Exploratory analyses revealed that the negative association between CVR and Aβ deposition was driven by individuals classified as having elevated Aβ levels (>1.1), with the strongest effect in APOE4 carriers (Figure 1 B&C). We found differential relationships between CVR and Aβ deposition in APOE4 carriers with elevated versus nonelevated Aβ levels(CVR- by- Aβ interaction, t=−2.39, p=.032) (Figure 1B), in which APOE4 carriers classified as having elevated Aβ demonstrated a stronger negative effect of CVR on Aβ deposition compared to APOE4 carriers classified as nonelevated Aβ (Figure 1B). In APOE4 noncarriers, individuals with elevated Aβ levels also showed a stronger negative association between CVR and Aβ deposition compared to those with nonelevated Aβ levels (Figure 1C), but differences in this effect were not significant (CVR- by- Aβ interaction, t=−1.11, p=.272).
3.2. Interactions between cerebrovascular function, amyloid-beta, and APOE genotype as predictors of response inhibition behavior
After controlling for age and normalized workload, the multiple regression model significantly predicted response inhibition performance, F6,69=2.92, p=.014, R2=0.22, adjusted R2=0.14. Regression coefficients and standard errors are detailed in Table 3. There was a significant three-way interaction between CVR, APOE carrier status, and Aβ deposition (t=2.68, p=.010), indicating differences in the predictive value of Aβ deposition and CVR on response inhibition performance for APOE4 carriers and noncarriers. Specifically, APOE4 carriers with higher CVR demonstrated better response inhibition performance (p<.001), while noncarriers showed no significant effect of CVR (p=.112) (Figure 2). As illustrated in Figure 3A & B, APOE4 carrier status moderated the interactive effect of CVR and Aβ deposition on response inhibition.The model predicted that APOE4 carriers who achieved a high CVR would show a positive relationship between Aβ deposition and response inhibition, where carriers with a robust CVR to an acute bout of aerobic exercise were predicted to perform better on the response inhibition task despite having high levels of Aβ deposition. In contrast, the model predicted that Aβ deposition would have no effect on response inhibition performance in APOE4 carriers who had lower CVR (Figure 3A). Noncarriers showed a lower effect of CVR on the relationship between Aβ deposition and response inhibition, where older adult noncarrier performance was independent of their CVR to the exercise bout (Figure 3B).
Table 3.
Three-way multiple regression analysis results to predict response inhibition performance after controlling for age, N = 70; Regression Model, p=.014.
| Variable | B | SEB | β | p-value |
|---|---|---|---|---|
| Intercept | 0.674 | 0.207 | .002* | |
| Age | −0.006 | 0.003 | −0.257 | .033* |
| Workload (W/kg BW) | 0.004 | 0.52 | 0.009 | .942 |
| CVR | −0.002 | 0.003 | −0.080 | .557 |
| APOE4 carrier status (+) | −0.130 | 0.044 | −0.509 | .004* |
| Aβ deposition | 0.236 | 0.085 | 0.356 | .007* |
| Interaction APOE4 carrier status (+) * Aβ * CVR | 0.015 | 0.005 | 0.485 | .001* |
Aβ = amyloid-beta; CVR = cerebrovascular response to moderate-intensity aerobic exercise; SEB = standard error of the coefficient; B = unstandardized regression coefficient; β = standardized coefficient;
p<.05
Figure 2.
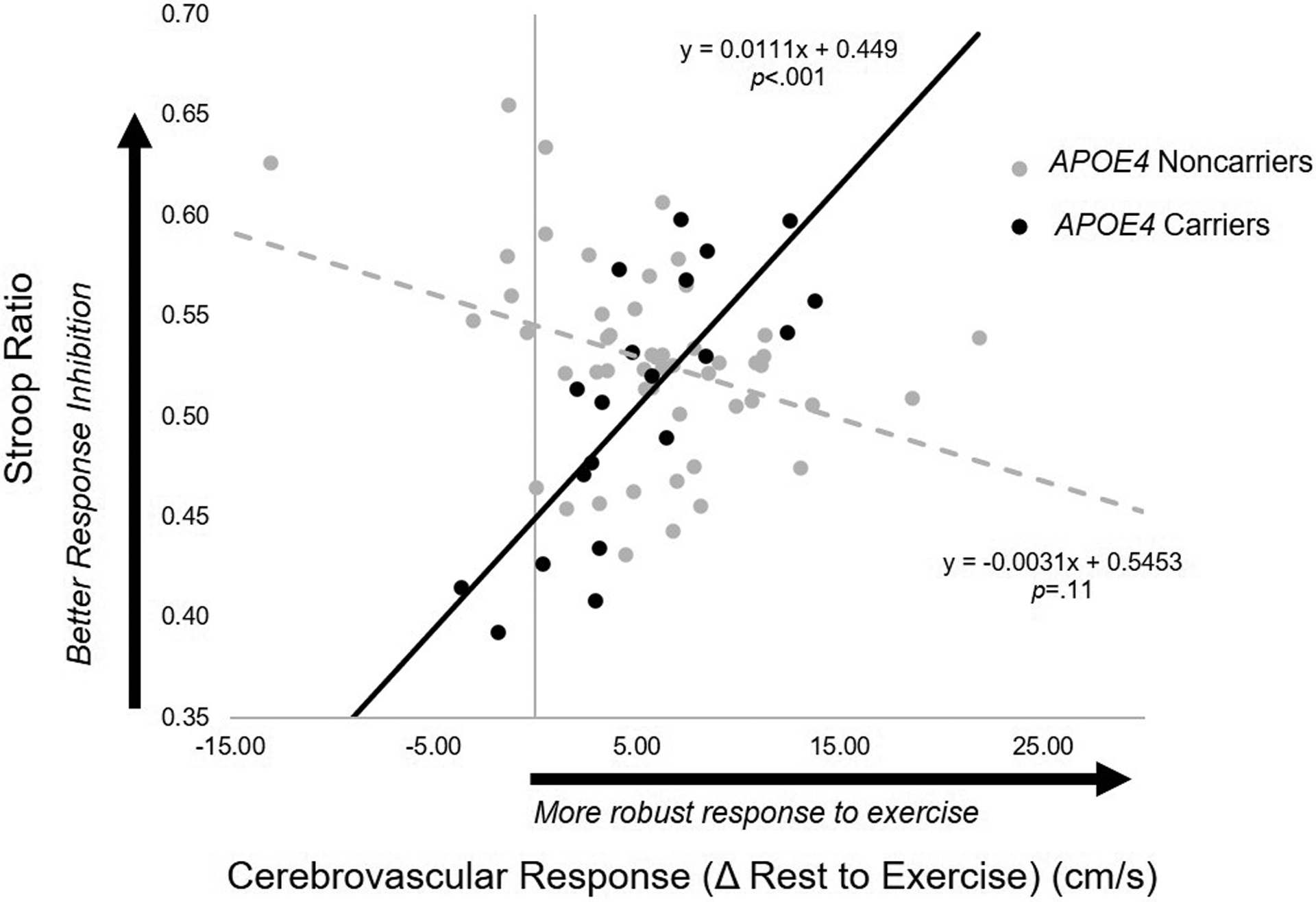
Association between cerebrovascular response to moderate-intensity aerobic exercise and response inhibition performance predicted from the regression model in APOE4 carriers (n=20) (solid line) and noncarriers (n=50) (broken line). In APOE4 carriers, greater cerebrovascular response to exercise was associated with better performance on the Stroop response inhibition task (p<.001). In noncarriers, no relationship was observed (p=.112).
Results for this regression model are detailed in Table 3.
Figure 3.
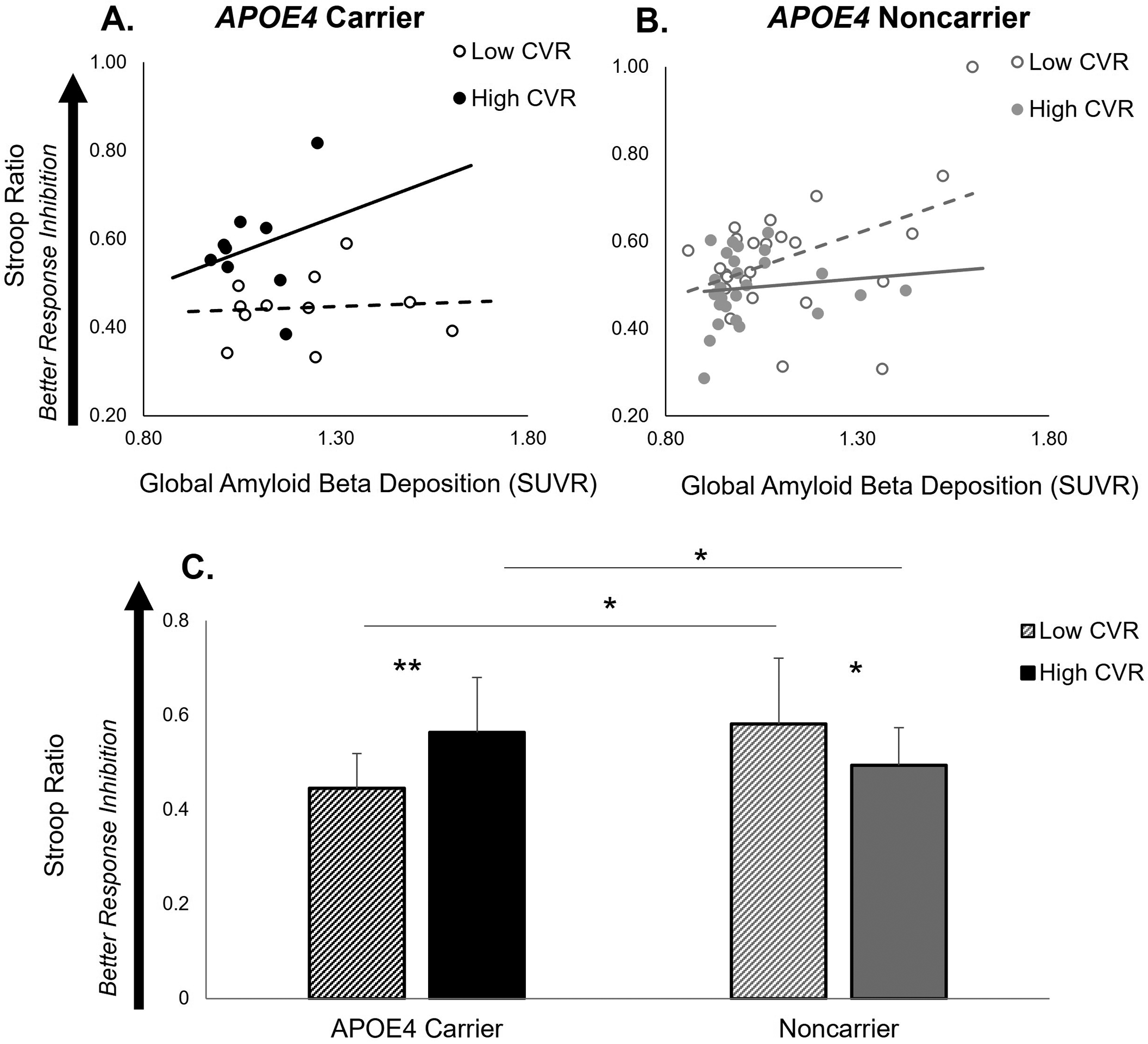
Association between Aβ deposition and response inhibition performance illustrated as a function of cerebrovascular response (CVR) to moderate-intensity aerobic exercise in APOE4 carriers (black color) (n=20) (A) and noncarriers (grey color) (n=50) (B). APOE4 carriers with high CVR (≥ 5.7cm/s) (solid line) showed a positive relationship between Aβ deposition and response inhibition performance, while APOE4 carriers with low CVR (< 5.7cm/s) (broken line) showed no positive effect. CVR also showed a lower effect on response inhibition in noncarriers. Results for this regression model are detailed in Table 3. (C) Response inhibition performance in APOE4 carriers and noncarriers with low and high CVR. There was a significant APOE4 carrier group × CVR interaction. APOE4 carriers with high CVR had higher response inhibition performance compared to APOE4 carriers with low CVR (p=.005) and noncarriers (p=.017). Among individuals with low CVR, APOE4 carriers had lower response inhibition performance compared to noncarriers (p=.013). Within the noncarrier group, noncarriers with low CVR had higher response inhibition performance compared to those with high CVR (p=.034). *p<.05, **p<.01.
CVR is illustrated as a median (5.7cm/s) split of the group, where High CVR ≥ 5.7cm/s; Low CVR < 5.7cm/s.
When participants were dichotomized by the group median CVR value (5.7 cm/s), there was a significant APOE4 carrier group × CVR interaction ((F3,69)=12.84, p=.001) on response inhibition performance (Figure 3C). Post-hoc analyses revealed that APOE4 carriers with a high CVR ( ≥ 5.7cm/s) demonstrated higher response inhibition performance compared to APOE4 carriers with low CVR (< 5.7cm/s) (t= −3.19, p=.005) and noncarriers with high CVR (t=−2.51, p=.017). Among participants with low CVR, APOE4 carriers had poorer response inhibition performance compared to noncarriers (t=2.64, p=.013). Within the noncarrier group, noncarriers with low CVR had higher response inhibition performance compared to those with high CVR (t=2.18, p=.034) (Figure 3C).
3.3. Specificity of physiologic interactions for response inhibition
Controlling for age and normalized workload, the three-way multiple regression model (factors: CVR, APOE4 carrier status, and Aβ deposition) failed to predict cognitive processing speed (F6,69=1.825, p=.108, R2=0.148, adjusted R2=0.067). Specifically, the main effect of CVR remained the only significant predictor in this three-way model (t= 2.230, p=.029), while no interactive effects (p=.159), effects of Aβ deposition (p=.949), or effects of APOE4 carrier status (p=.758) were observed (Supplementary Figure S1).
4. Discussion
Findings of the present study shed light on the complex neurobiological interactions of factors known to influence the development of cognitive dysfunction with aging. Our results provide novel evidence for the behavioral significance of interactions between cerebrovascular function, individual genotype, and structural brain pathology. Despite having higher Aβ deposition, we found that APOE4 carriers who had a more robust CVR to exercise had higher response inhibition performance, an effect that was not present in noncarriers. These findings imply higher cerebrovascular function may serve a neuroprotective role for the preservation of cognitive executive function specifically in APOE4 carriers, possibly through increased resiliency to Aβ deposition with aging. Building upon previous research, our findings support an individualized framework gleaned from a multifactorial approach for detection of subtle impairments in cognitive behavior prior to the onset of clinical syndrome associated with AD and age-related neurodegenerative disorders. Together, these results may be informative for targeted precision-medicine approaches for early intervention and prevention of declines in cognitive executive function with aging.
4.1. Cerebrovascular response (CVR) to exercise is linked to amyloid beta (Aβ) deposition in the absence of clinical cognitive dysfunction
Our findings support the assessment of CVR to acute aerobic exercise as a useful functional biomarker of structural brain pathology in the absence of clinically detectible impairments in cognitive function. In the present study, we found that cognitively normal older adults with blunted CVR to aerobic exercise demonstrated greater Aβ deposition (Figure 1A), a finding consistent with previous work from our laboratory (Sisante et al., 2019) across a larger cohort of older adults. This finding also supports previous research implicating cerebrovascular assessments performed under conditions of physiologic stress may be sensitive enough to detect the first signs of dysfunction in the modulation of cerebral blood flow that may be salient to subtle changes in cognitive function in preclinical older adult populations. When Aβ was viewed as a continuous variable, no group differences in the association between CVR and Aβ deposition were detected (Figure 1A). Interestingly, when participants were classified by the presence of clinically relevant levels of elevated Aβ (>1.1 SUVR) (Clark et al., 2012; Joshi et al., 2012), we observed an interactive effect in the relationship between CVR and AB deposition in APOE4 carriers (Figure 1B). Here, APOE4 carriers with elevated Aβ deposition were unable to achieve a robust CVR to exercise, an effect that was not present in noncarriers. Together, these findings provide evidence that the strongest physiologic interactions between CVR and Aβ deposition are present in a subgroup of individuals who carry the APOE4 allele and possess elevated levels of Aβ deposition. The identification of this subgroup of individuals may provide a useful target for precision medicine approaches aimed at modulating cerebrovascular or structural brain metrics to influence brain health and function with aging.
Our findings implicate common neurobiological mechanisms that contribute to cerebrovascular dysfunction and structural brain pathology in the early stages of brain aging. In particular, APOE4 carriers demonstrate the strongest negative relationship between CVR to exercise and Aβ deposition (Figure 1A&B) that is consistent with a mechanistic link between CV dysfunction and deposition of Aβ in the aging brain. However, the causal nature of this relationship remains to be tested. For example, the presence of Aβ may blunt the CVR to exercise through increased resistance of blood flow and perfusion to cortical tissues as blood flow velocity increases during aerobic exercise (Niwa et al., 2002; Iadecola, 2004). Consistent with this theory, increasing Aβ levels in the brains of rodents can reduce cerebral blood flow (i.e. elevated Aβ led to reduced blood flow to the brain) (Suo et al., 1998). Conversely, poor cerebrovascular function may alter blood-brain barrier trafficking of Aβ, slowing the clearance of Aβ and promoting its accumulation in the brain (Zlokovic et al., 2005). This theory is also supported by animal research, where bilateral carotid artery stenosis significantly increased Aβ levels (i.e. blunting cerebral blood flow led to increased Aβ deposition) (Yamada et al., 2011). The later may also be supported by evidence that habitual aerobic exercise appears to have no effect on Aβ deposition in older adults (Vidoni et al., 2021), yet can improve cerebrovascular health (Thomas et al., 2013; Whitaker et al., 2020) and slow or, in some cases, reverse the development of cognitive impairment in older adults (Zhang et al., 2020). These observations may explain the neuroprotective effect of aerobic exercise against AD and related dementias (Tarumi and Zhang, 2018). Future research aimed at modulating each of these variables (e.g. aerobic exercise interventions that improve CVR) will provide insight into the directionality of the relationship between cerebrovascular function and structural brain pathology.
4.2. Behavioral significance of interactions between individual genotype, cerebrovascular function, and brain structure
Importantly, our findings provide evidence for the behavioral significance of interactions between individual genotype, cerebrovascular function, and brain structure. We found that cerebrovascular function interacts with individual APOE genotype and Aβ deposition to influence an individual older adult’s ability to inhibit a default and undesired response (Figure 2 and Figure 3A&B). Our results are consistent with accumulating evidence within the field of aging neurobiology and the increasing recognition of the mixed pathology and multifactorial etiology that contributes to cognitive decline with aging (Iturria-Medina et al., 2016, 2017). Clinically, it is well known that an individual who has structural brain biomarker evidence of AD pathology (e.g. high levels of Aβ deposition) may paradoxically present with the absence of any behavioral indicator associated with AD clinical syndrome. Notably, vascular pathology and individual genotype (e.g. possession of the APOE4 allele) are frequently detected in the typical clinical presentation of AD (Iadecola, 2004; Xie et al., 2016; Ouellette and Lacoste, 2021), and can significantly increase an individual’s risk for developing dementias (Heffernan et al., 2016). Building upon our current knowledge of each of these factors individually, our present results provide unique insight into interactions between these key neurobiological factors that can be gleaned from an interdisciplinary and multifactorial assessment of AD and related dementia risk factors. This rich multifactorial approach offers a powerful characterization of factors potentially influencing the early development of cognitive behavioral impairment that could have clinically meaningful implications for intervention, treatment, and prevention of AD and related dementia in preclinical older adult populations.
4.3. Role of cerebrovascular health as a function of individual genotype in the preservation of brain behavioral function with aging
Our results suggest cerebrovascular function plays a key role in the early behavioral manifestations of cognitive executive dysfunction in older adults who carry the APOE4 allele. The three-way interaction model found that older adult APOE4 carriers with blunted CVR to exercise demonstrated poorer response inhibition performance, while CVR did not predict behavioral performance in noncarriers (Figure 2). The characterization of these interactions builds upon previous findings from our laboratory, in which we demonstrated measures poor cardiovascular health negatively influenced the CVR to exercise preferentially in APOE4 carriers (Kaufman et al., 2021c). Our findings are also consistent with previous evidence demonstrating a stronger effect of cardiovascular health on cognitive performance metrics in APOE4 carriers (Zade et al., 2010; Caselli et al., 2011; Shaaban et al., 2019). Specifically within a behavioral context, a key novel finding of the present study identifies the unique relationship between cerebrovascular response and response inhibition performance in APOE4 carriers compared to noncarriers. The positive relationship between CVR and response inhibition performance supports that higher cerebrovascular regulatory function may serve a unique neuroprotective function in early brain aging processes affecting cognition in APOE4 carriers, while playing less of a role in noncarriers. Interestingly, Kaufman et al. (2021a) revealed that cerebrovascular dysfunction in APOE4 carriers was differentially modifiable through an aerobic exercise intervention compared to noncarriers, with older adult APOE4 carriers showing greater improvements in cerebral blood flow following a year-long exercise intervention (Kaufman et al., 2021a). Despite the strong ties between physical fitness and cerebrovascular health, we found that these physiologic interactions were able to predict cognitive behavior even when controlling for normalized workload at a matched heart rate range, a proxy metric for physical fitness (Table 3). Interestingly, previous research by Ward et al (Ward et al., 2018) found that older and younger adults who were matched to identical workloads during moderate intensity exercise demonstrated differences in cardiovascular responsiveness to the acute exercise bout, suggesting an influence of aging on these physiologic interactions. Together, these findings support that cerebrovascular response during aerobic exercise provides unique insight into mechanisms of brain health and behavior that are not available from physical fitness assessments alone and may be useful to guide approaches aimed at preventing and reversing cognitive decline in aging populations.
4.4. Genetic mechanisms influencing interactions between cerebrovascular function and structural brain pathology in the context of cognitive behavior
Possession of an APOE4 allele may reveal unique genetic mechanisms that influence interactions between cerebrovascular response and structural brain pathology to influence cognitive behavior in older adults. Specifically, we found differential effects of CVR on the relationship between Aβ and response inhibition in APOE4 carriers and noncarriers (Figure 3A&B). In APOE4 carriers, individuals with a higher CVR and greater Aβ deposition had higher response inhibition performance, an effect not observed in APOE4 carriers with low CVR or noncarriers (Figure 3A&B). Further, APOE4 carriers with high CVR outperformed both carriers with low CVR and noncarriers on the response inhibition task (Figure 3C). In the face of age-related neuropathology, some individuals appear to utilize a neurologic “reserve” that enables behavioral compensation and attenuates cognitive decline (Stern et al., 2019). Findings in the present study provide preliminary evidence that the neuroprotective effect of high CVR in APOE4 carriers may act mechanistically through increased resilience to age-related structural pathology reflected in Aβ deposition, potentially supporting a functional neurologic “reserve” in the aging brains of these individuals. We also found that, among participants with low CVR, APOE4 carriers had poorer response inhibition compared to noncarriers (Figure 3C). This suggests that cerebrovascular health not only serves a specialized protective effect to maintain cognitive function, but may additionally attenuate the rate of cognitive decline that is accelerated in APOE4 carriers (Caselli et al., 2004, 2007). It is conceivable that such differences in the resiliency effect of cerebrovascular function between APOE4 carriers and noncarriers may overlap with the neurobiological mechanisms that predispose APOE4 carriers to a higher genetic risk for the development of AD in the later stages of aging (Farrer et al., 1997; Heffernan et al., 2016). Further, our findings that CVR has a greater effect on cognitive function in APOE4 carriers compared to noncarriers supports the notion of different etiologies and pathologic disease processes leading to the development of AD for each APOE4 carriers and noncarriers (Emrani et al., 2020), particularly that cerebrovascular dysfunction may play a preferential role in dementia pathogenesis for APOE4 carriers (Høgh et al., 2001; Montagne et al., 2020). These preliminary findings motivate future research in a larger cohort of heterogeneous older adults that may additionally include APOE2 carriers, for whom the effect of resiliency to aging processes has been most consistently observed and reported (Farrer et al., 1997; James et al., 2017).
In the present study, the limited range of participants who possessed high levels of Aβ deposition (e.g. >1.1 SUVR) may limit the generalization of these findings to older adults with the more advanced signatures of structural brain pathology. For example, the low representation of participants with both high Aβ deposition and high CVR (as illustrated in Figure 3 A&B), could be explained by the fact that high Aβ deposition in these individuals may lead to cerebrovascular dysfunction, impeding their ability to achieve robust CVR during exercise. As such, a plausible alternative theory would be that individuals in this cohort of cognitively normal older adults with high Aβ deposition (and low CVR) may possess a neuroprotective resilience against the development of MCI in the presence of high levels of Aβ deposition, enabling their inclusion in the present study. Indeed, the expansion of the range of cognitive abilities to include those older adults with MCI may increase the upper range of AB deposition particularly in APOE4 carriers. Expansion of the range of cognitive ability among participants could potentially equalize the differences in levels of Aβ deposition between groups (see Table 1) and even lead to an inverse relationship between Aβ deposition and response inhibition performance, in contrast to the positive relationship observed in Figure 3A. Future studies that include older adults with MCI may expand and equalize the range of Aβ deposition across groups and elucidate the presence of these interactive relationships and salience to cognitive behavior.
While high cerebrovascular function demonstrated a neuroprotective effect for APOE4 carriers, an interesting pattern in our data suggests that noncarriers may possess greater resiliency to potential negative effects of low CVR and high Aβ deposition. While APOE4 carriers with high CVR had higher response inhibition performance, noncarriers with low CVR tended to have higher response inhibition performance than noncarriers with high CVR, though the relationship of this effect did not meet our a priori adopted level of significance (Figure 2) and showed a lower magnitude of effect compared to the contrasting effect observed in APOE4 carriers (Figure 3C). Previous studies found that, compared to cognitively normal APOE4 carriers, noncarriers showed differences in cortical neural function (Leuthold et al., 2013) and had greater adaptive processes to repeated trauma (James et al., 2017). Although the mechanisms are not yet fully understood, these interactive functional and structural processes appear to increase neuroprotection and promote brain resilience to specific disease processes in noncarriers, including the development of MCI (Pa et al., 2009), posttraumatic stress disorder (Peterson et al., 2015), and recovery from traumatic brain injury (Zhou et al., 2008). A greater ability to repair and protect against neuronal damage offered by the noncarrier-specific genotypic interaction (Mahley and Rall, 2000) may similarly increase noncarriers’ resilience to the chronic neurotoxic effects poor cerebrovascular function on both the accumulation of Aβ in the brain (Figure 1) and cognitive performance (Figure 2 and Figure 3) compared to APOE4 carriers in the present study. This notion of increased resilience to low cerebrovascular function in noncarriers may be further supported by the larger range of CVR, particularly in the lower range of response, present in noncarriers compared to APOE4 carriers in the present study (Table 1), Notably, our exclusion of older adults with MCI would have biased the selection of only individuals with such genotypic resiliency (i.e. excluding those with low CVR and high Aβ without this protective adaptive characteristic, which could have effectively narrowed the range of CVR in APOE4 carriers in the present study). The present participant selection criteria may explain poorer response inhibition performance in noncarriers with low Aβ and high CVR (Figure 3B & C). Future research in animal models may elucidate our mechanistic understanding of risk and resilience associated with individual APOE genotype and further identify epigenetic pathways underpinning individual-specific physiologic stress responses for neuronal repair and protection in the context of cerebrovascular health and cognitive dysfunction with aging.
4.5. Cerebrovascular dysfunction affects neural networks involved in response inhibition in the early stages of cognitive dysfunction with aging
Our hypothesis-driven analyses in the present study focused on the neurobiological factors influencing a highly specific aspect of cognition involving inhibition of a default and undesired response (Stroop, J, 1935; Hutchison et al., 2010). While interactions between CVR, APOE4 genotype, and Aβ deposition were predictive of response inhibition performance (Table 2), the failure of these physiologic interactions to predict general cognitive processing speed implicates their functional neural specificity (Wessel and Aron, 2017; Wessel et al., 2019). The present findings are in line with previous research supporting the specificity of cerebrovascular function and individual APOE genotype (Whitson et al., 2018) effects on response inhibition behavior that engages inhibitory neural networks within the prefrontal cortex (PFC) (Wessel and Aron, 2017; Wessel et al., 2019). In neurologically-intact young adults, differences in acute cerebrovascular changes within the frontal cortical regions could be dissociated during a challenging Stroop task; here, participants with a greater frontal cortical hemodynamic response demonstrated higher response inhibition performance (Gratton et al., 2020). In aging populations, previous studies have demonstrated n that cortical inhibitory function is one of the earliest neural mechanisms affected by aging processes in cognitively normal older adults (Nielson et al., 2002; Heise et al., 2013; Levin et al., 2014; Rossiter et al., 2014; Legon et al., 2016), which may explain the presence of impaired response inhibition in older adults with absent clinical cognitive syndrome in the present study. Interestingly, PFC brain regions may preferentially benefit from the therapeutic effects of aerobic exercise interventions on cognition (Duchesne et al., 2015; Levin and Netz, 2015), supporting the robust interactive effect of cerebrovascular function on response inhibition performance in the present study. Taken together, these findings may inform the development of individualized approaches for therapeutic noninvasive brain stimulation (e.g. neuromodulation of PFC brain regions in preclinical APOE4 carriers) for the treatment and prevention of cognitive dysfunction with aging (Hsu et al., 2015).
4.6. Limitations
When no signal in the left MCA could be detected, we used the right MCA response to aerobic exercise in the present study, as previous results from our laboratory found no systematic differences in cerebrovascular kinetic responses to aerobic exercise between left and right MCA (Billinger et al., 2017). However, others have reported differences between left versus right sided hemisphere pulsatility (Atwi et al., 2020). Whether measurements of cerebrovascular function between left and right MCA can be substituted interchangeably remains to be tested in a larger study across more diverse aging populations, and should be considered carefully in the interpretation of the results of the present study.
Assessment of CVR during a validated acute moderate-intensity aerobic exercise paradigm (Billinger et al., 2008, 2017) is behaviorally relevant to activities commonly faced by older adults in the community (e.g. ascending a flight of stairs). It remains to be seen how the CVR to other types of acute physiologic stressors compare to an aerobic exercise paradigm. It is possible that the high demand of the cerebrovascular regulatory response required for brain blood flow during a bout of moderate-intensity aerobic exercise is an earlier and more sensitive detector for autonomic nervous system dysfunction with aging, and could provide unique physiologic information independent of gravity-dependent activities. Alternatively, the temporal demand required for rapid cerebrovascular response during a behavior such as a sit-to-stand positional transfer may reveal insight into autonomic nervous system dysfunction with aging not available during activities with prolonged duration responses such as aerobic exercise. Future research is warranted to compare paradigms involving physiologic stressors that are behaviorally relevant to older adults.
It is important to consider the limitations of the statistical modeling approach used in the present study within the context of the relatively modest sample size. In particular, the higher levels of AB deposition in the APOE4 carrier group compared to the noncarrier group (difference magnitude of 0.07 SUVR, see Table 1) could influence the present results; future studies that increase the heterogeneity of cognitive inclusion criteria and in a larger cohort of older adults could help to mitigate these limitations. Given the novelty of the present dataset, our dichotomization of CVR using the group median provides a first step towards better understanding the influence of CVR on other physiologic and behavioral metrics. While providing an interesting illustration of the relationship between Aβ deposition and response inhibition performance in individuals with high versus low CVR to exercise (Figure 3 A&B), this sample-based classification method is likely sub-optimal in comparison to a physiologically-informed CVR classification method. Notably, given the significant interaction of CVR as a continuous variable with APOE4 carrier status and Aβ deposition for the prediction of response inhibition performance, we cannot rule out the possibility that CVR may not possess a behaviorally salient threshold. As such, we expect that these results will guide future studies with larger sample sizes that will be more adequately powered to determine whether the presence of a physiologically-based threshold for CVR classification exists for the detection of interactive physiologic effects on cognitive behavior. The speed dependence of the Stroop task in the present study could influence results compared to other methods of assessing response inhibitory control (e.g. The Tower of Hanoi task, the WCST, and the Iowa Gambling task). Thus, results of the present study may not be generalizable to generalized inhibitory control outside of this temporal context.
In the present study, we do not account for individual chronotype, which can have an effect on physical and cognitive performance (Facer-Childs et al., 2018). While all participants performed CVR assessment in the morning, diurnal variation and sleep pattern could have influenced cognitive performance (Song et al., 2019), as the time of cognitive testing was not controlled in the present study.
Sex as a biological variable has been shown to have complex interactions with each APOE genotype and cardiovascular function with aging (Farrer et al., 1997; Riedel et al., 2016; Ward et al., 2018; Vermunt et al., 2019). In the present study, there were no differences in gender distribution between APOE4 carrier groups (Table 1). However, these findings together with previous research motivate an in-depth exploration of the effect of sex and interactions with key physiologic factors known to influence the development of structural brain pathology and early cognitive impairment with aging.
5. Conclusions
Interactions between cerebrovascular function, individual genotype, and structural brain pathology may offer a useful and sensitive biomarker for early preclinical behavioral manifestations of cognitive impairment in older adults. Additionally, cerebrovascular response to aerobic exercise may provide a physiologically-informed target for precision-medicine approaches aimed at attenuating negative effects of structural brain pathology and preventing age-related declines in cognitive executive function. Future research may reveal that individuals with APOE4 carrier genotype show the highest therapeutic benefit for such intervention approaches.
Supplementary Material
Interactions between cerebrovascular function, APOE4 genotype, and amyloid-beta as predictors of cognitive processing speed
Supplementary FigureS1. Cerebrovascular response to moderate-intensity aerobic exercise plotted against cognitive processing speed performance in APOE4 carriers (n=20) (black line) and noncarriers (n=50) (grey line). The regression model failed to predict cognitive processing speed in the Stroop color response control condition (p=.108), suggesting the CVR-by-APOE4 carrier status-by-Aβ deposition interactive effects are specific to cognitive response inhibition (see Figure 2).
Results for this regression model are detailed the text of Results 3.3.
Highlights.
Interactions between CVR, APOE genotype, and Aβ are relevant to cognitive behavior.
Blunted CVR to exercise is associated with impaired response inhibition specifically in APOE4 carriers.
APOE4 carriers with more robust CVR have higher response inhibition performance, despite having greater Aβ deposition.
Assessment of multifactorial neurobiological variables offers an early and sensitive biomarker of cognitive behavioral dysfunction with aging.
Funding
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development and the National Institute on Aging of the National Institutes of Health [NIH F32HD096816 (JP), P30 AG072973, P30AG035982, R01 AG043962, UL1TR000001], the American Heart Association [16GRNT30450008 (SB)], and the Georgia Holland Endowment Fund. Gifts from Frank and Evangeline Thompson (JMB), The Ann and Gary Dickinson Family Charitable Foundation, John and Marny Sherman, Brad and Libby Bergman supported amyloid measurement infrastructure. Lilly Pharmaceuticals provided a grant to support F18-AV45 doses and partial scan costs. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or any other funding agency.
Abbreviations:
- CVR
cerebrovascular response
- Aβ
amyloid-beta
- APOE4
Apolipoprotein E allele 4
- AD
Alzheimer’s Disease
- MCI
mild cognitive impairment
- ANOVA
analysis of variance
- SD
standard deviation
Footnotes
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data Availability Statement
The datasets generated and analyzed supporting the conclusions of this article will be made available by the authors upon request, without undue reservation.
References
- Alwatban MR, Liu Y, Perdomo SJ, Ward JL, Vidoni ED, Burns JM, et al. (2020). TCD Cerebral Hemodynamic Changes during Moderate-Intensity Exercise in Older Adults. Journal of Neuroimaging 30, 76–81. doi: 10.1111/jon.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwi S, Robertson AD, Theyers AE, Ramirez J, Swartz RH, Marzolini S, et al. (2020). Cardiac-Related Pulsatility in the Insula Is Directly Associated With Middle Cerebral Artery Pulsatility Index. Journal of Magnetic Resonance Imaging 51, 1454–1462. doi: 10.1002/jmri.26950. [DOI] [PubMed] [Google Scholar]
- Billinger SA, Craig JC, Kwapiszeski SJ, Sisante J-FV, Vidoni ED, Maletsky R, et al. (2017). Dynamics of middle cerebral artery blood flow velocity during moderate-intensity exercise. Journal of Applied Physiology 122, 1125–1133. doi: 10.1152/japplphysiol.00995.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billinger SA, Loudon JK, and Gajewski BJ (2008). Validity of a total body recumbent stepper exercise test to assess cardiorespiratory fitness. J Strength Cond Res 22, 1556–1562. doi: 10.1519/JSC.0b013e3181739dd7. [DOI] [PubMed] [Google Scholar]
- Bracko O, Hernández JCC, Park L, Nishimura N, and Schaffer CB (2021). Causes and consequences of baseline cerebral blood flow reductions in Alzheimer’s disease: Journal of Cerebral Blood Flow & Metabolism. doi: 10.1177/0271678X20982383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundo M, Inao S, Nakamura A, Kato T, Ito K, Tadokoro M, et al. (2002). Changes of neural activity correlate with the severity of cortical ischemia in patients with unilateral major cerebral artery occlusion. Stroke 33, 61–66. doi: 10.1161/hs0102.101816. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Locke DEC, Sabbagh MN, Ahern GL, Rapcsak SZ, et al. (2011). Cerebrovascular risk factors and preclinical memory decline in healthy APOE ε4 homozygotes. Neurology 76, 1078–1084. doi: 10.1212/WNL.0b013e318211c3ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Reiman EM, Locke DEC, Hutton ML, Hentz JG, Hoffman-Snyder C, et al. (2007). Cognitive domain decline in healthy apolipoprotein E epsilon4 homozygotes before the diagnosis of mild cognitive impairment. Arch Neurol 64, 1306–1311. doi: 10.1001/archneur.64.9.1306. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Reiman EM, Osborne D, Hentz JG, Baxter LC, Hernandez JL, et al. (2004). Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology 62, 1990–1995. doi: 10.1212/01.wnl.0000129533.26544.bf. [DOI] [PubMed] [Google Scholar]
- Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, et al. (2012). Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol 11, 669–678. doi: 10.1016/S1474-4422(12)70142-4. [DOI] [PubMed] [Google Scholar]
- Coelho F. G. de M., Stella F, de Andrade LP, Barbieri FA, Santos-Galduróz RF, Gobbi S, et al. (2012). Gait and risk of falls associated with frontal cognitive functions at different stages of Alzheimer’s disease. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 19, 644–656. doi: 10.1080/13825585.2012.661398. [DOI] [PubMed] [Google Scholar]
- Duchesne C, Lungu O, Nadeau A, Robillard ME, Boré A, Bobeuf F, et al. (2015). Enhancing both motor and cognitive functioning in Parkinson’s disease: Aerobic exercise as a rehabilitative intervention. Brain Cogn 99, 68–77. doi: 10.1016/j.bandc.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Emrani S, Arain HA, DeMarshall C, and Nuriel T (2020). APOE4 is associated with cognitive and pathological heterogeneity in patients with Alzheimer’s disease: a systematic review. Alzheimers Res Ther 12, 141. doi: 10.1186/s13195-020-00712-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facer-Childs ER, Boiling S, and Balanos GM (2018). The effects of time of day and chronotype on cognitive and physical performance in healthy volunteers. Sports Med Open 4, 47. doi: 10.1186/s40798-018-0162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. (1997). Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 278, 1349–1356. [PubMed] [Google Scholar]
- Ferguson B (2014). ACSM’s Guidelines for Exercise Testing and Prescription 9th Ed. 2014. J Can Chiropr Assoc 58, 328. [Google Scholar]
- Folstein MF, Folstein SE, and McHugh PR (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12, 189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gratton G, Weaver SR, Burley CV, Low KA, Maclin EL, Johns PW, et al. (2020). Dietary flavanols improve cerebral cortical oxygenation and cognition in healthy adults. Sci Rep 10, 19409. doi: 10.1038/s41598-020-76160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves RS, Mahnken JD, Swerdlow RH, Burns JM, Price C, Amstein B, et al. (2015). Open-source, Rapid Reporting of Dementia Evaluations. J Registry Manag 42, 111–114. [PMC free article] [PubMed] [Google Scholar]
- Hampel H (2013). Amyloid-β and cognition in aging and Alzheimer’s disease: molecular and neurophysiological mechanisms. J Alzheimers Dis 33 Suppl 1, S79–86. doi: 10.3233/JAD-2012-129003. [DOI] [PubMed] [Google Scholar]
- Heffernan AL, Chidgey C, Peng P, Masters CL, and Roberts BR (2016). The Neurobiology and Age-Related Prevalence of the ε4 Allele of Apolipoprotein E in Alzheimer’s Disease Cohorts. J Mol Neurosci 60, 316–324. doi: 10.1007/s12031-016-0804-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise K-F, Zimerman M, Hoppe J, Gerloff C, Wegscheider K, and Hummel FC (2013). The Aging Motor System as a Model for Plastic Changes of GABA-Mediated Intracortical Inhibition and Their Behavioral Relevance. J. Neurosci 33, 9039–9049. doi: 10.1523/JNEUROSCI.4094-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høgh P, Knudsen GM, Kjaer KH, Jørgensen OS, Paulson OB, and Waldemar G (2001). Single photon emission computed tomography and apolipoprotein E in Alzheimer’s disease: impact of the epsilon4 allele on regional cerebral blood flow. J Geriatr Psychiatry Neurol 14, 42–51. doi: 10.1177/089198870101400110. [DOI] [PubMed] [Google Scholar]
- Hsu W-Y, Ku Y, Zanto TP, and Gazzaley A (2015). Effects of noninvasive brain stimulation on cognitive function in healthy aging and Alzheimer’s disease: a systematic review and meta-analysis. Neurobiol Aging 36, 2348–2359. doi: 10.1016/j.neurobiolaging.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison KA, Balota DA, and Duchek JM (2010). The Utility of Stroop Task Switching as a Marker for Early Stage Alzheimer’s Disease. Psychol Aging 25, 545–559. doi: 10.1037/a0018498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C (2004). Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci 5, 347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Iturria-Medina Y, Carbonell FM, Sotero RC, Chouinard-Decorte F, Evans AC, and Alzheimer’s Disease Neuroimaging Initiative (2017). Multifactorial causal model of brain (dis)organization and therapeutic intervention: Application to Alzheimer’s disease. Neuroimage 152, 60–77. doi: 10.1016/j.neuroimage.2017.02.058. [DOI] [PubMed] [Google Scholar]
- Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Pérez JM, and Evans AC (2016). Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nature Communications 7, 11934. doi: 10.1038/ncomms11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Wiste HJ, Vemuri P, Weigand SD, Senjem ML, Zeng G, et al. (2010). Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer’s disease. Brain 133, 3336–3348. doi: 10.1093/brain/awq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James LM, Engdahl BE, and Georgopoulos AP (2017). Apolipoprotein E: the resilience gene. Exp Brain Res 235, 1853–1859. doi: 10.1007/s00221-017-4941-4. [DOI] [PubMed] [Google Scholar]
- Joshi AD, Pontecorvo MJ, Clark CM, Carpenter AP, Jennings DL, Sadowsky CH, et al. (2012). Performance characteristics of amyloid PET with florbetapir F 18 in patients with alzheimer’s disease and cognitively normal subjects. J Nucl Med 53, 378–384. doi: 10.2967/jnumed.111.090340. [DOI] [PubMed] [Google Scholar]
- Kaufman CS, Honea RA, Pleen J, Lepping RJ, Watts A, Morris JK, et al. (2021a). Aerobic exercise improves hippocampal blood flow for hypertensive Apolipoprotein E4 carriers. J Cereb Blood Flow Metab 41, 2026–2037. doi: 10.1177/0271678X21990342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman CS, Morris JK, Vidoni ED, Burns JM, and Billinger SA (2021b). Apolipoprotein E4 moderates the association between vascular risk factors & brain pathology. Journal of Alzheimer Disease & Associated Disorders. doi:epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman CS, Morris JK, Vidoni ED, Burns JM, and Billinger SA (2021c). Apolipoprotein E4 Moderates the Association Between Vascular Risk Factors and Brain Pathology. Alzheimer Dis Assoc Disord. doi: 10.1097/WAD.0000000000000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirova A-M, Bays RB, and Lagalwar S (2015). Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer’s disease. Biomed Res Int 2015, 748212. doi: 10.1155/2015/748212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legon W, Punzell S, Dowlati E, Adams SE, Stiles AB, and Moran RJ (2016). Altered Prefrontal Excitation/Inhibition Balance and Prefrontal Output: Markers of Aging in Human Memory Networks. Cereb Cortex 26, 4315–4326. doi: 10.1093/cercor/bhv200. [DOI] [PubMed] [Google Scholar]
- Leuthold AC, Mahan MY, Stanwyck JJ, Georgopoulos A, and Georgopoulos AP (2013). The number of cysteine residues per mole in apolipoprotein E affects systematically synchronous neural interactions in women’s healthy brains. Exp Brain Res 226, 525–536. doi: 10.1007/s00221-013-3464-x. [DOI] [PubMed] [Google Scholar]
- Levin O, Fujiyama H, Boisgontier MP, Swinnen SP, and Summers JJ (2014). Aging and motor inhibition: a converging perspective provided by brain stimulation and imaging approaches. Neuroscience and Biobehavioral Reviews 43, 100–117. doi: 10.1016/j.neubiorev.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Levin O, and Netz Y (2015). Aerobic training as a means to enhance inhibition: what’s yet to be studied? European Review of Aging and Physical Activity: Official Journal of the European Group for Research into Elderly and Physical Activity 12, 14. doi: 10.1186/s11556-015-0160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Perdomo SJ, Ward J, Vidoni ED, Sisante JF, Kirkendoll K, et al. Vascular Health is Associated with Amyloid-β in Cognitively Normal Older Adults. J Alzheimers Dis 70, 467–475. doi: 10.3233/JAD-181268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorius N, Locascio JJ, Rentz DM, Johnson KA, Sperling RA, Viswanathan A, et al. (2015). Vascular disease and risk factors are associated with cognitive decline in the Alzheimer’s disease spectrum. Alzheimer Dis Assoc Disord 29, 18–25. doi: 10.1097/WAD.0000000000000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, and Rall SC (2000). Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet 1, 507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- Monsell SE, Dodge HH, Zhou X-H, Bu Y, Besser LM, Mock C, et al. (2016). Results From the NACC Uniform Data Set Neuropsychological Battery Crosswalk Study. Alzheimer Dis Assoc Disord 30, 134–139. doi: 10.1097/WAD.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A, Nation DA, Sagare AP, Barisano G, Sweeney MD, Chakhoyan A, et al. (2020). APOE4 leads to blood–brain barrier dysfunction predicting cognitive decline. Nature 581, 71–76. doi: 10.1038/s41586-020-2247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC (1993). The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43, 2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Langenecker SA, and Garavan H (2002). Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychology and Aging 17, 56–71. doi: 10.1037//0882-7974.17.1.56. [DOI] [PubMed] [Google Scholar]
- Niwa K, Kazama K, Younkin L, Younkin SG, Carlson GA, and Iadecola C (2002). Cerebrovascular autoregulation is profoundly impaired in mice overexpressing amyloid precursor protein. Am J Physiol Heart Circ Physiol 283, H315–323. doi: 10.1152/ajpheart.00022.2002. [DOI] [PubMed] [Google Scholar]
- Ogoh S, and Ainslie PN (2009a). Cerebral blood flow during exercise: mechanisms of regulation. J Appl Physiol (1985) 107, 1370–1380. doi: 10.1152/japplphysiol.00573.2009. [DOI] [PubMed] [Google Scholar]
- Ogoh S, and Ainslie PN (2009b). Regulatory mechanisms of cerebral blood flow during exercise: new concepts. Exerc Sport Sci Rev 37, 123–129. doi: 10.1097/JES.0b013e3181aa64d7. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Sato K, Okazaki K, Miyamoto T, Hirasawa A, Morimoto K, et al. (2013). Blood flow distribution during heat stress: cerebral and systemic blood flow. J Cereb Blood Flow Metab 33, 1915–1920. doi: 10.1038/jcbfm.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette J, and Lacoste B (2021). From Neurodevelopmental to Neurodegenerative Disorders: The Vascular Continuum. Front Aging Neurosci 13, 749026. doi: 10.3389/fnagi.2021.749026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pa J, Boxer A, Chao LL, Gazzaley A, Freeman K, Kramer J, et al. (2009). Clinical-neuroimaging characteristics of dysexecutive mild cognitive impairment. Ann Neurol 65, 414–423. doi: 10.1002/ana.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JA, Payne AM, Ting LH, and Borich MR (2021). Cortical engagement metrics during reactive balance are associated with distinct aspects of balance behavior in older adults. Front. Aging Neurosci 13. doi: 10.3389/fnagi.2021.684743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantsiou K, Sfakianaki O, Papaliagkas V, Savvoulidou D, Costa V, Papantoniou G, et al. (2018). Inhibitory Control, Task/Rule Switching, and Cognitive Planning in Vascular Dementia: Are There Any Differences From Vascular Aging? Front Aging Neurosci 10, 330. doi: 10.3389/fnagi.2018.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdomo SJ, Ward J, Liu Y, Vidoni ED, Sisante JF, Kirkendoll K, et al. (2020). Cardiovascular disease risk is associated with middle cerebral artery blood flow velocity in older adults. Cardiopulm Phys Ther J 31, 38–46. doi: 10.1097/cpt.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CK, James LM, Anders SL, Engdahl BE, and Georgopoulos AP (2015). The Number of Cysteine Residues per Mole in Apolipoprotein E Is Associated With the Severity of PTSD Re-Experiencing Symptoms. J Neuropsychiatry Clin Neurosci 27, 157–161. doi: 10.1176/appi.neuropsych.13090205. [DOI] [PubMed] [Google Scholar]
- Riedel BC, Thompson PM, and Brinton RD (2016). Age, APOE and sex: Triad of risk of Alzheimer’s disease. J Steroid Biochem Mol Biol 160, 134–147. doi: 10.1016/j.jsbmb.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter HE, Davis EM, Clark EV, Boudrias M-H, and Ward NS (2014). Beta oscillations reflect changes in motor cortex inhibition in healthy ageing. NeuroImage 91, 360–365. doi: 10.1016/j.neuroimage.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Ogoh S, Hirasawa A, Oue A, and Sadamoto T (2011). The distribution of blood flow in the carotid and vertebral arteries during dynamic exercise in humans. J Physiol 589, 2847–2856. doi: 10.1113/jphysiol.2010.204461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaaban CE, Jia Y, Chang C-CH, and Ganguli M (2019). Independent and joint effects of vascular and cardiometabolic risk factor pairs for risk of all-cause dementia: a prospective population-based study. Int Psychogeriatr 31, 1421–1432. doi: 10.1017/S1041610219001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisante J-FV, Vidoni ED, Kirkendoll K, Ward J, Liu Y, Kwapiszeski S, et al. (2019). Blunted cerebrovascular response is associated with elevated beta-amyloid. J Cereb Blood Flow Metab 39, 89–96. doi: 10.1177/0271678X17732449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, and Ainslie PN (2017). Regulation of cerebral blood flow and metabolism during exercise. Experimental Physiology 102, 1356–1371. doi: 10.1113/EP086249. [DOI] [PubMed] [Google Scholar]
- Solis E, Hascup KN, and Hascup ER (2020). Alzheimer’s Disease: The Link Between Amyloid-β and Neurovascular Dysfunction. J Alzheimers Dis 76, 1179–1198. doi: 10.3233/JAD-200473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Feng P, Wu X, Li B, Su Y, Liu Y, et al. (2019). Individual Differences in the Neural Basis of Response Inhibition After Sleep Deprivation Are Mediated by Chronotype. Frontiers in Neurology 10. Available at: https://www.frontiersin.org/article/10.3389/fneur.2019.00514 [Accessed January 15, 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinback CD, and Poulin MJ (2016). Influence of Hypoxia on Cerebral Blood Flow Regulation in Humans. Adv Exp Med Biol 903, 131–144. doi: 10.1007/978-1-4899-7678-9_9. [DOI] [PubMed] [Google Scholar]
- Stern Y, Barnes CA, Grady C, Jones RN, and Raz N (2019). Brain reserve, cognitive reserve, compensation, and maintenance: operationalization, validity, and mechanisms of cognitive resilience. Neurobiology of Aging 83, 124–129. doi: 10.1016/j.neurobiolaging.2019.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop J (1935). Studies of interference in serial verbal reactions. J Exp Psychol, 643–62. doi: 10.1037/h0054651. [DOI] [Google Scholar]
- Suo Z, Humphrey J, Kundtz A, Sethi F, Placzek A, Crawford F, et al. (1998). Soluble Alzheimers beta-amyloid constricts the cerebral vasculature in vivo. Neurosci Lett 257, 77–80. doi: 10.1016/s0304-3940(98)00814-3. [DOI] [PubMed] [Google Scholar]
- Sweeney MD, Kisler K, Montagne A, Toga AW, and Zlokovic BV (2018). The role of brain vasculature in neurodegenerative disorders. Nature Neuroscience 21, 1318–1331. doi: 10.1038/s41593-018-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarumi T, and Zhang R (2018). Cerebral blood flow in normal aging adults: cardiovascular determinants, clinical implications, and aerobic fitness. Journal of Neurochemistry 144, 595–608. doi: 10.1111/jnc.14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thambisetty M, Beason-Held L, An Y, Kraut MA, and Resnick SM (2010). APOE epsilon4 genotype and longitudinal changes in cerebral blood flow in normal aging. Arch Neurol 67, 93–98. doi: 10.1001/archneurol.2009.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas BP, Yezhuvath US, Tseng BY, Liu P, Levine BD, Zhang R, et al. (2013). Life-long aerobic exercise preserved baseline cerebral blood flow but reduced vascular reactivity to CO2. Journal of magnetic resonance imaging: JMRI 38, 1177–1183. doi: 10.1002/jmri.24090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermunt L, Sikkes SAM, van den Hout A, Handels R, Bos I, van der Flier WM, et al. (2019). Duration of preclinical, prodromal, and dementia stages of Alzheimer’s disease in relation to age, sex, and APOE genotype. Alzheimers Dement 15, 888–898. doi: 10.1016/j.jalz.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidoni ED, Morris JK, Watts A, Perry M, Clutton J, Sciver AV, et al. (2021). Effect of aerobic exercise on amyloid accumulation in preclinical Alzheimer’s: A 1-year randomized controlled trial. PLOS ONE 16, e0244893. doi: 10.1371/journal.pone.0244893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JL, Craig JC, Liu Y, Vidoni ED, Maletsky R, Poole DC, et al. (2018). Effect of healthy aging and sex on middle cerebral artery blood velocity dynamics during moderate-intensity exercise. Am J Physiol Heart Circ Physiol 315, H492–H501. doi: 10.1152/ajpheart.00129.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, and Aron AR (2017). On the Globality of Motor Suppression: Unexpected Events and Their Influence on Behavior and Cognition. Neuron 93, 259–280. doi: 10.1016/j.neuron.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, Waller DA, and Greenlee JD (2019). Non-selective inhibition of inappropriate motor-tendencies during response-conflict by a fronto-subthalamic mechanism. eLife 8. doi: 10.7554/eLife.42959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker AA, Alwatban M, Freemyer A, Perales-Puchalt J, and Billinger SA (2020). Effects of high intensity interval exercise on cerebrovascular function: A systematic review. PLOS ONE 15, e0241248. doi: 10.1371/journal.pone.0241248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitson HE, Potter GG, Feld JA, Plassman BL, Reynolds K, Sloane R, et al. (2018). Dual-Task Gait and Alzheimer’s Disease Genetic Risk in Cognitively Normal Adults: A Pilot Study. J Alzheimers Dis 64, 1137–1148. doi: 10.3233/JAD-180016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte E, Liu Y, Ward JL, Kempf KS, Whitaker A, Vidoni ED, et al. (2019). Exercise Intensity and Middle Cerebral Artery Dynamics in Humans. Respir Physiol Neurobiol 262, 32–39. doi: 10.1016/j.resp.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters Frank J, Zonneveld Hazel I, Hofman Albert, van der Lugt Aad, Koudstaal Peter J., Vernooij Meike W., et al. (2017). Cerebral Perfusion and the Risk of Dementia. Circulation 136, 719–728. doi: 10.1161/CIRCULATIONAHA.117.027448. [DOI] [PubMed] [Google Scholar]
- Xie L, Dolui S, Das SR, Stockbower GE, Daffner M, Rao H, et al. (2016). A brain stress test: Cerebral perfusion during memory encoding in mild cognitive impairment. Neuroimage Clin 11, 388–397. doi: 10.1016/j.nicl.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Ihara M, Okamoto Y, Maki T, Washida K, Kitamura A, et al. (2011). The Influence of Chronic Cerebral Hypoperfusion on Cognitive Function and Amyloid β Metabolism in APP Overexpressing Mice. PLoS One 6, e16567. doi: 10.1371/journal.pone.0016567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zade D, Beiser A, McGlinchey R, Au R, Seshadri S, Palumbo C, et al. (2010). Interactive Effects of Apolipoprotein E Type 4 Genotype and Cerebrovascular Risk on Neuropsychological Performance and Structural Brain Changes. Journal of Stroke and Cerebrovascular Diseases 19, 261–268. doi: 10.1016/j.jstrokecerebrovasdis.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Lu Y, Zhao X, Zhang Q, and Li L (2020). Aerobic Exercise Attenuates Neurodegeneration and Promotes Functional Recovery – Why It Matters For Neurorehabilitation & Neural Repair. Neurochemistry International, 104862. doi: 10.1016/j.neuint.2020.104862. [DOI] [PubMed] [Google Scholar]
- Zhou W, Xu D, Peng X, Zhang Q, Jia J, and Crutcher KA (2008). Meta-analysis of APOE4 allele and outcome after traumatic brain injury. J Neurotrauma 25, 279–290. doi: 10.1089/neu.2007.0489. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, Deane R, Sallstrom J, Chow N, and Miano JM (2005). Neurovascular pathways and Alzheimer amyloid beta-peptide. Brain Pathol 15, 78–83. doi: 10.1111/j.1750-3639.2005.tb00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Interactions between cerebrovascular function, APOE4 genotype, and amyloid-beta as predictors of cognitive processing speed
Supplementary FigureS1. Cerebrovascular response to moderate-intensity aerobic exercise plotted against cognitive processing speed performance in APOE4 carriers (n=20) (black line) and noncarriers (n=50) (grey line). The regression model failed to predict cognitive processing speed in the Stroop color response control condition (p=.108), suggesting the CVR-by-APOE4 carrier status-by-Aβ deposition interactive effects are specific to cognitive response inhibition (see Figure 2).
Results for this regression model are detailed the text of Results 3.3.
Data Availability Statement
The datasets generated and analyzed supporting the conclusions of this article will be made available by the authors upon request, without undue reservation.


