Abstract
Interest in the use of percutaneous left ventricular assist devices (p-LVADs) for patients undergoing high-risk percutaneous coronary intervention (PCI) is growing rapidly. The Impella™ (Abiomed Inc.) is a catheter-based continuous micro-axial flow pump that preserves haemodynamic support during high-risk PCI. Anticoagulation is required to counteract the activation of the coagulation system by the patient’s procoagulant state and the foreign-body surface of the pump. Excessive anticoagulation and the effect of dual antiplatelet therapy (DAPT) increase the risk of bleeding. Inadequate anticoagulation leads to thrombus formation and device dysfunction. The precarious balance between bleeding and thrombosis in patients with p-LVAD support is often the primary reason that patients’ outcomes are jeopardized. In this chapter, we will discuss anticoagulation strategies and anticoagulant management in the setting of protected PCI. This includes anticoagulant therapy with unfractionated heparin, direct thrombin inhibitors, DAPT, purge blockage prevention by bicarbonate-based purge solution, and monitoring by activated clotting time, partial thromboplastin time, as well as anti-factor Xa levels. Here, we provide a standardized approach to the management of peri-interventional anticoagulation in patients undergoing protected PCI.
Keywords: Purge solution, Percutaneous left ventricular assist devices, Anticoagulation strategies, Unfractionated heparin, Bicarbonate-based purge solution
Introduction
Micro-axial flow pump support during protected percutaneous coronary intervention (PCI) carries both advantages and risks.1 Anticoagulation, aimed at finding the optimal trade-off between thrombotic and bleeding complications, is among the most critical risk modulators (Table 1). In this perspective, acute limb thromboembolism and bleeding requiring transfusion accounted for 27.6% and 21.8% of the vascular complications observed in a real-world analysis of more than 30,000 cases treated with percutaneous left ventricular assist devices (p-LVADs).1 The rate of vascular and bleeding complications was significantly lower in prospective cohort studies following standardized protocols and less potent antithrombotic strategies.1–3 While inadequate anticoagulation carries a high risk for the patient,4 none of these studies investigated anticoagulation management in detail. Therefore, meticulous (anti)coagulation management during protected PCI is the key in reducing procedure associated complications in this critically ill patient group.
Table 1.
The role of antithrombotic therapy on percutaneous left ventricular assist devices
| The role of antithrombotic therapy in p-LVAD |
|---|
|
|
|
|
|
|
This chapter summarizes current evidence and provides a rational and pragmatic approach to this crucial aspect of p-LVAD support.
Bleeding, thrombotic complications, and haemolysis during protected percutaneous coronary intervention
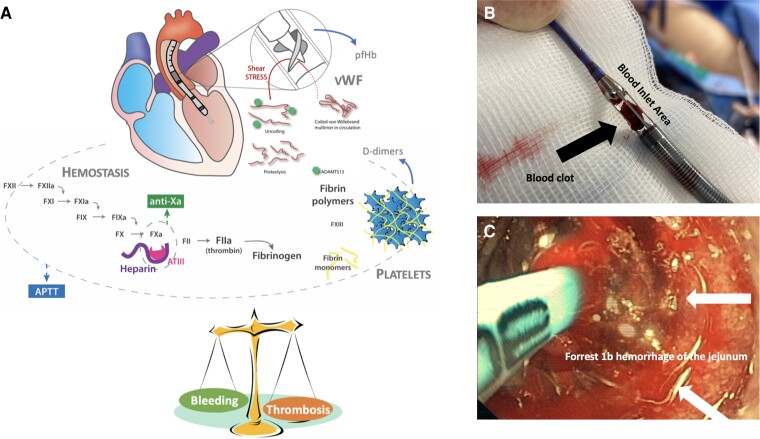
Bleeding and vascular complications are frequent with short-term mechanical circulatory support and increase mortality. Impella™ (Abiomed Inc.) is a catheter-based continuous micro-axial flow pump that drains blood from the ventricle and expels it into the ascending aorta or pulmonary artery. Anticoagulation, usually with intravenous (IV) unfractionated heparin (UFH), is required to counteract the activation of the coagulation system by the shear stress and the foreign-body surface of the pump. In more detail, Impella™-devices require anticoagulation to prevent purge blockage, which may result in severe haemolysis and pump failure and thrombus formation, which may result in systemic thromboembolism. In order to prevent thrombus formation inside the pump, the Impella™ technology relies on a purge solution that runs opposite to blood flow, preventing blood from entering the Impella™ motor. The pump bearings are additionally protected by either heparinization or addition of low-dose sodium bicarbonate to the purge. On the other hand, to prevent thrombus formation on the catheter surface, a target level of systemic anticoagulation should be achieved5 (Figure 1A). Inadequate anticoagulation leads to thrombus formation and device dysfunction (Figure 1B). Conversely, excessive anticoagulation and the effect of dual antiplatelet therapy (DAPT) increase the risk of bleeding (Figure 1C). Moreover, the shear stress and the continuous flow may lead to proteolysis of the high molecular weight von Willebrand factor5 resulting in an acquired von Willebrand syndrome (AVWS) within 24 h of starting the p-LVAD5 further aggravating the bleeding risk6 (Figure 1A). Most studies investigated bleeding risk focused on Impella™ support during cardiogenic shock (CS).4,7–9 A recent meta-analysis of 17 observational retrospective studies, including 3933 patients with CS on p-LVAD support, reported a major bleeding rate of 15.2% (defined as BARC >2, TIMI Major, or GUSTO severe bleeding). Bleeding is associated with prolonged inotropic and ventilatory support and increases the risk of death.5,10 Non-modifiable risk factors associated with increased p-LVAD-related vascular complications and major bleeding include older age, female gender, obesity, previous hypertension, and peripheral arterial disease.8,11 As one or more of these factors is often present in patients with p-LVAD, standardized peri- and post-interventional management of anticoagulation is essential/critical.
Figure 1.
(A) Haemostasis/unfractionated heparin is monitored via parallel measurements of activated partial thromboplastin time/anti-factor Xa. Thrombosis is measured indirectly via D-dimer levels (product of fibrinolysis). Shear stress-induced acquired von Willebrand factor is measured via von Willebrand factor antigen and functional von Willebrand testing. Haemolysis is measured via plasma-free haemoglobin levels5 and/or bilirubin. (B) Thrombus formation in the blood inlet area of the Impella® devices. (C) Forrest 1b haemorrhage of the jejunum in a patient with Impella™ Support (>72 h) and excessive anticoagulation, as typically seen in the acquired von Willebrand syndrome. vWF, von Willebrand Factor; pfHb, plasma-free haemoglobin.
Another important complication during p-LVAD support is the occurrence of haemolysis (Figure 1A). Haemolysis may occur more frequently if the Impella™ cannot be removed within the first 72 h and is frequently caused by high-shear stress environments around the device, which can be enhanced by suboptimal position of the pump and low volume in the ventricle leading to suction. While it may occur immediately after pump activation, ‘seeking’ the right intraventricular pump position can often resolve the issue. Severe haemolysis not only causes (severe) kidney damage, but also increased vascular resistance and creates a prothrombotic state of the patient.5 Although its incidence has decreased over time due to technical improvements, it is still observed in 5–63% of cases with significant variations possibly related to differences in device management.5,12 Haemolysis is defined by a recent consensus document in patients on p-LVAD as a plasma-free haemoglobin (pfHb) concentration >20 mg/dL or a serum LDH level >2.5 times the normal upper range after more than 72 h post-insertion.5,13 Therefore, if prolonged p-LVAD support after protected PCI is necessary, it is recommended to measure LDH levels daily with the integration of pfHb according to local availability.5 In case of haemolysis, p-LVAD positioning should immediately be checked with bedside echocardiography, and stringent pfHb/LDH follow-up is required. In most cases, haemolysis can be resolved by pump repositioning, lowering the pump output or cardiac preload optimization.14 However, if pump repositioning and cardiac preload optimization fail to resolve the problem, this may suggest pump thrombosis and pump exchange and/or plasmapheresis could be the only solutions.5
Anticoagulant strategies in patients undergoing protected percutaneous coronary intervention
Anticoagulation during p-LVAD-supported protected PCI may present with several challenges, including concomitant DAPT, access site complications, urgent procedures, heparin-induced-thrombocytopaenia (HIT) and in rare cases UFH allergy. While anticoagulation is most commonly pursued with UFH,12 some centres favour IV direct thrombin inhibitors (DTIs, e.g. bivalirudin or argatroban) because of their shorter half-life and safety in case of HIT.5,6,15 Recent data suggest that the use of a bicarbonate-based purge solution (BBPS) is an alternative to heparin in Impella™ purge fluid to prevent purge blockage.16–18
The role of the purge solution and anticoagulation with unfractionated heparin
In order to prevent blood from penetrating inside the motor pump, the device automatically sets and adjusts the purge flow rate (2–30 mL/h) to achieve a purge pressure of 300–1100 mmHg. A 25–50 units/mL of UFH in D5W as the initial purge solution is suggested.19 Due to the automatic adjustments of purge flow rate, which the operator cannot directly modify, patients are exposed to a variable hourly dose of purge flow and thus UFH. However, as previously stated, Impella™ devices require anticoagulation not only to prevent thrombus formation inside the motor pump, but also on the catheter surface in critically ill patients that are already in a prothrombotic state. Heparinized purge solution may be sufficient to achieve systemic anticoagulation goals alone. However, when systemic anticoagulation goals are not achieved with heparinized purge solution, simultaneous systemic administration of IV UFH is required.20
When the systemic effect of anticoagulation is excessive even after cessation of IV UFH or in patients who have lower anticoagulation needs (i.e. patients with a lower body surface area, coagulation disorders), a simple countermeasure is to reduce the heparin concentration in the purge solution (e.g. from 50 to 25 UI/mL). A more elaborated alternative is to increase the glucose concentration of the purge solution up to 20–40%. In fact, the higher viscosity of the solution allows achievement of the target purge pressure with lower purge flow rates, resulting in lower UFH dose administration5,19,21 and increased risk of purge obstruction. Further, increasing purge solution viscosity might be considered when a purge pressure of at least 300 mmHg cannot be achieved, but the consequences on UFH dose administration should be carefully weighted. In case of prolonged Impella™ support after protected PCI the purge rate should be frequently checked because significant changes in flow rate (and thus UFH administration) may occur.5
In case of HIT, a parenteral DTI (bivalirudin, argatroban) is the first choice. A survey conducted at 182 centres in the USA showed that 25% of the centres use a pure dextrose purge solution in case of HIT and 58% add argatroban or bivalirudin to the purge solution.22 However, robust evidence to support use of a DTI in this setting is lacking15,23,24 and it might even be detrimental.16,24,25
Running Impella™ devices without anticoagulation in the purge solution are not recommended and should be considered off-label use. However, BBPS has been proposed in a study using benchtop and animal models as an alternative to a heparin-based purge solution for maintaining pump purge performance.18 This was confirmed in a study of 316 patients using BBPS [25 mEq in 1 L 5% dextrose in water (D5W)] as purge solution. The maximum duration of p-LVAD support using this strategy was 40 days and the purge pressure and flow remained stable while on BBPS.17 These data suggest that BBPS may replace current purge solutions, simplify systemic anticoagulation management, support heparin-intolerant patients, and represent a bailout strategy in uncontrolled bleeding in critically ill.17,18
Periprocedural anticoagulation monitoring during protected percutaneous coronary intervention
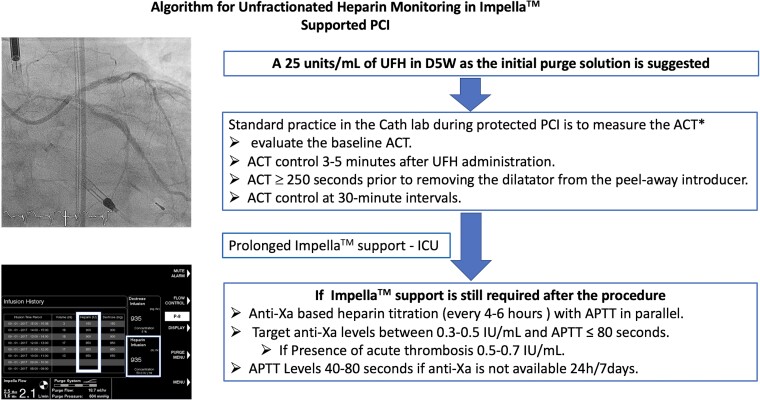
The coagulation system in critically ill patients on p-LVAD-support is complex and no one UFH monitoring test will give the physician the right answer.26 Currently, there are no prospective randomized controlled trials available comparing anticoagulation strategies based on activated clotting time (ACT), partial thromboplastin time (APTT), and anti-factor Xa level (anti-Xa levels) in patients on micro-axial flow pump support (Central Illustration). Standard practice in the cathlab during protected PCI is to measure the ACT because of its fast and bedside availability and because ACT is useful to evaluate the effect of an UFH-bolus. However, the feasibility of APTT and anti-Xa bedside point-of-care devices should be urgently explored in the cathlab setting. It is recommended to evaluate the baseline ACT prior to starting UFH infusion. However, the response to anticoagulation with UFH is highly variable, especially in critically ill patients.5,27 It is recommended to keep the ACT ≥250 s prior to removing the dilatator from the peel-away introducer. This will help prevent thrombus formation in the Impella catheter, causing an early pump failure.20,28 If the patient is receiving a GP IIb–IIIa inhibitor, ACT should be 200 s or above before the dilatator can be removed.20 The authors recommend achieving an ACT of 250 s during coronary intervention. If p-LVAD support is still required after the procedure anti-factor Xa level (anti-Xa) and/or activated partial thromboplastin time (APTT) should be used to monitor anticoagulation in p-LVAD-supported patients (Central Illustration). Currently, no general recommendations are available for APTT goals. Recent data suggest that an APTT of 55–80 may be sufficient to prevent thromboembolic events.19,29–33 or even lower.34 In a pilot analysis, low-dose heparin (APTT 40–60 s or anti-Xa 0.2–0.3) was shown to be associated with less bleeding without more ischaemic events in 114 LV Impella™ patients with CS.34
Central Illustration.
Algorithm for unfractionated heparin monitoring in Impella™. Supported PCI. *If available, the use of APTT/anti-Xa point of care devices is recommended. UFH, unfractionated heparin; PCI, percutaneous coronary intervention; ACT, activated clotting time; ICU, intensive care unit; APTT, activated partial prothromboplastin time.
Several factors may influence APTT in response to UFH administration. These include fibrinogen or antithrombin depletion caused by acute inflammation, factor VIII depletion caused by AVWS, or liver failure and factor XI/XII depletion caused by plastic surface adherence.5 Anti-Xa measurement has the advantage of not being influenced by these factors. In a large cohort of hospitalized patients treated with UFH, patients with high APTT as compared with anti-Xa values appeared to be at increased risk of adverse outcomes, even associated with increased 30-day mortality.35 Therefore, Vandenbriele et al.5 recommend an anti-Xa guided UFH dosing protocol to control the thrombotic risk with parallel APTT-measurements to lower the bleeding risk in p-LVAD-supported critically ill patients. Target anti-Xa levels should be between 0.3 and 0.5 IU/mL. In case of an acute thrombosis, escalation to 0.5–0.7 IU/mL should be considered.5 If anti-Xa is not available 24/7, it should be performed at least once daily.
Due to the various patient-related factors influencing the UFH effect and need for UFH and the different commercially available ACT/APTT tests, a general recommendation regarding dosage and dose adjustment is complex. This must be individually tailored on the patient and disease profile. These findings further confirm the importance of multidisciplinary team approach between (interventional) cardiologist, intensivist, haematologists, and the coagulation lab. Here, local standards should be developed.
Formula for initial calculation of unfractionated heparin infusion rate
In order to perform the dose adjustments required to achieve the desired levels of anticoagulation, it is essential to account for the Impella™-delivered UFH dose, which is listed in the ‘infusion history’ screen of the controller. The total UFH dose administered to the patient is the sum of the UFH dose delivered through the purge fluid and the IV UFH dose.
An ease-of-use approach for calculating the initial UFH dose is reported by Vandenbriele et al.5
For example: purge rate calculation assumes pump use of 25 U/mL UFH for a patient weighing 75 kg and a purge rate of 20 mL/h:
If the protocol specifies use of heparin 10 U/kg/h to maintain an acceptable anticoagulation goal the total UFH concentration would be calculated as: 10 U/kg/h × 75 kg = 750 U/h. The maximum value of 1800 U/h should not be exceeded.
Purge rate: 20 mL/h (25 U/mL): 20 mL/h × 25 U/mL = 500 U/h.
Systemic (IV) UFH rate = total UFH − purge UFH = 750 U/h − 500 U/h = 250 U/h of IV UFH.
Anticoagulation with direct thrombin inhibitors
Unfractionated heparin has a highly negative ionic charge and plays a significant role in protecting the purge gaps; non-heparin anticoagulants lacking ionic charge should generally be avoided in the purge solution.16 Although successful use of DTI has been described in case series, there is evidence that low purge flows with rising purge pressure, pump failure, and/or thrombosis with DTI-based purge solutions can occur.15,16,22–25,36–38 Therefore, BBPS (25 mEq per 1000 mL in 5% of dextrose; same highly negative charge as UFH) may be preferred in scenarios when a heparin-based purge solution is not feasible, or HIT is present.39 Here, the additional IV administration of a DTI is recommended. Initial IV dosage of argatroban is left to the decision of the physician and local standard operating procedures. In a small case series, Blum et al.23 describe that an APTT 1.5–3 times the baseline seems to be safe.
Bivalirudin can also be used for IV anticoagulation in patients with HIT.22,36,39 The recommended dose of bivalirudin for patients undergoing PCI is an IV bolus of 0.75 mg/kg body weight followed immediately by an IV infusion of 1.75 mg/kg body weight/h.40 As there are no dose recommendations regarding anticoagulation with bivalirudin and the use of Impella™, we recommend starting with the above dosage and adjusting the dose (APTT 50–80 s36).
Dual antiplatelet therapy in patients undergoing protected percutaneous coronary intervention
Dual antiplatelet therapy, consisting of aspirin and a P2Y12-receptor inhibitor, is the cornerstone treatment for patients undergoing elective PCI.40 However, due to the increased risk of bleeding and access side complications, there are several things to keep in mind in the context of protected PCI. For elective PCI in stable coronary artery disease the P2Y12 inhibitor clopidogrel is recommended. The guidelines recommend a pre-treatment with clopidogrel (600 mg loading dose) in addition to aspirin before elective stenting.40 This recommendation should also be considered for protected PCI due to the concomitant need of therapeutic anticoagulation therapy. In patients on a maintenance dose of 75 mg clopidogrel, a new loading dose of 600 mg is at the discretion of the interventional cardiologist and should be chosen based on the risk–benefit ratio (bleeding risk vs. coronary ischaemia).
In the setting of an acute coronary syndrome (ACS), the more potent oral P2Y12-receptor inhibitors ticagrelor and prasugrel have both been shown to be more effective than clopidogrel.41,42 In case of protected PCI without ACS prasugrel or ticagrelor may only be considered in selected patients for specific high-risk situations of elective stenting (e.g. complex left main PCI) or in patients with a history of stent thrombosis on clopidogrel treatment.40 However, based on the results of the ACCOAST trial, prasugrel should not be used upfront in patients with unknown coronary anatomy.43 Because the antiplatelet effect of oral P2Y12-receptor inhibitors may be delayed in patients in CS (slower absorption, slower metabolism), crushing ticagrelor or prasugrel tablets may result in more rapid absorption of the drug and more rapid and potent antiplatelet activity compared with whole-tablet ingestion.5,44
Furthermore, the use of cangrelor may be discussed in individual cases. Cangrelor is a directly reversible (recovery of platelet function within ≈ 60 min) and short-acting (half-life of 3–6 min) P2Y12 inhibitor.40 The administration of cangrelor is recommended by a bolus of 30 mg/kg i.v. followed by 4 mg/kg/min infusion for at least 2 h or duration of procedure, whichever is longer.40 The use of an IV antiplatelet agent offers several advantages, including rapid onset of action, rapid return of platelet function after discontinuation, and ease of administration.5 However, data in the context of protected PCI or bleeding risk are lacking. After the acute phase, cangrelor can be switched to ticagrelor because they act at different sites on the ADP receptor.5
Management of bleeding complication in patients on left ventricular assist device
Bleeding at the access site is frequent and should initially be treated with light compression and optimization of the fixation (adapting the device skin level with an underlying gauze) and stitching the incision of the sheath. Checking the distal pulse is mandatory to detect ischaemia at an early stage.5 Furthermore, bleeding from the ear, nose, and throat area can frequently occur. However, preventive measures (e.g. avoidance of nasogastric tubes) and local measures (tranexamic acid-/diluted adrenalin-soaked gauze; intranasal balloon occlusion) can often resolve the problem. Lowering the anticoagulation level should only be considered if the initial local bleeding control therapies fail. If uncontrollable and life-threatening bleeding occurs, stopping UFH should be considered. Unfractionated heparin infusion stop should be as short as possible to prevent complications such as device thrombosis, purge blockage or peripheral embolism. To avoid device thrombosis the pump speed should be maximized.5 In addition, purge solution might be changed to bicarbonate solutions, as described above. If restarting anticoagulation is not an option, the possibility of early p-LVAD weaning should be considered.5
Conclusions
A standardized approach to the management of peri-interventional anticoagulation is essential. In most cases, anticoagulation is performed with UFH. In case of HIT use of a BBPS is an alternative to heparin in Impella™ purge fluid and systemic therapy with DTI should be considered. Anticoagulation should be adjusted on the basis of ACT in the cath lab or APTT/anti-Xa point of care devices if available. An anti-Xa guided UFH-protocol with APTT-measurements in parallel should be foreseen in case of prolonged Impella™ support. The frequent (re)calculation of the total UFH dose (systemic + purge solution) as well as the body weight-adapted dose adjustment is highly recommended.
Acknowledgements
This manuscript is one of eight manuscripts published as a Supplement to address best practices for Impella protected PCI. JetPub Scientific Communications, LLC, supported by funding from Abiomed Europe GmbH, provided editorial assistance to the authors during preparation of this manuscript.
Contributor Information
Jürgen Leick, Heartcenter Trier, Krankenhaus der Barmherzigen Brüder Trier, Nordallee 1, 54296 Trier, Germany.
Oliver Grottke, Department of Anaesthesiology, RWTH Aachen University Hospital, 52074 Aachen, Germany.
Mehmet Oezkur, Department of Cardiovascular Surgery, University Hospital Mainz, 55131 Mainz, Germany.
Norman Mangner, Department of Internal Medicine and Cardiology, Herzzentrum Dresden, Technische Universitaet Dresden, Dresden, Germany.
Tommaso Sanna, Institute of Cardiology, Fondazione Policlinico A. Gemelli IRCCS, Università Cattolica del Sacro Cuore, 00168 Rome, Italy.
Fadi Al Rashid, Department of Cardiology and Vascular Medicine, West German Heart and Vascular Center, Medical Faculty, University Hospital Essen, 45147 Essen, Germany.
Christophe Vandenbriele, Department of Cardiovascular Sciences, University Hospitals Leuven, 3000 Leuven, Belgium; Royal Brompton and Harefield NHS Foundation Trust, SW36LP London, UK.
Funding
This work has been supported by the Abiomed Europe GmbH to cover publication costs as well as professional language editing of each manuscript. No individual fees were paid to the authors in the generation of this publication. This paper was published as part of a supplement financially supported by Abiomed GmbH.
Data availability
No new data were generated or analysed in support of this research.
References
- 1. Patel N, Sharma A, Dalia T, Rali A, Earnest M, Tadros P, et al. Vascular complications associated with percutaneous left ventricular assist device placement: a 10-year US perspective. Catheter Cardiovasc Interv 2020;95:309–316. [DOI] [PubMed] [Google Scholar]
- 2. O’Neill WW, Anderson M, Burkhoff D, Grines CL, Kapur NK, Lansky AJ, et al. Improved outcomes in patients with severely depressed LVEF undergoing percutaneous coronary intervention with contemporary practices. Am Heart J 2022;248:139–149. [DOI] [PubMed] [Google Scholar]
- 3. O’Neill WW, Kleiman NS, Moses J, Henriques JP, Dixon S, Massaro J, et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation 2012;126:1717–1727. [DOI] [PubMed] [Google Scholar]
- 4. Chieffo A, Ancona MB, Burzotta F, Pazzanese V, Briguori C, Trani C, et al. Collaborators . Observational multicentre registry of patients treated with IMPella mechanical circulatory support device in ITaly: the IMP-IT registry. EuroIntervention 2020; 15:e1343–e1350. [DOI] [PubMed] [Google Scholar]
- 5. Vandenbriele C, Arachchillage DJ, Frederiks P, Giustino G, Gorog DA, Gramegna M, et al. Anticoagulation for percutaneous ventricular assist device-supported cardiogenic shock: JACC review topic of the week. J Am Coll Cardiol 2022;79:1949–1962. [DOI] [PubMed] [Google Scholar]
- 6. Vandenbriele C, Vanassche T, Price S. Why we need safer anticoagulant strategies for patients on short-term percutaneous mechanical circulatory support. Intensive Care Med 2020;46:771–774. [DOI] [PubMed] [Google Scholar]
- 7. Burzotta F, Russo G, Ribichini F, Piccoli A, D’Amario D, Paraggio L, et al. Long-term outcomes of extent of revascularization in complex high risk and indicated patients undergoing Impella-protected percutaneous coronary intervention: report from the Roma-Verona Registry. J Interv Cardiol 2019;2019:5243913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iannaccone M, Albani S, Giannini F, Colangelo S, Boccuzzi GG, Garbo R, et al. Short term outcomes of Impella in cardiogenic shock: a review and meta-analysis of observational studies. Int J Cardiol 2021;324:44–51. [DOI] [PubMed] [Google Scholar]
- 9. Thiele H, Jobs A, Ouweneel DM, Henriques JPS, Seyfarth M, Desch S, et al. Percutaneous short-term active mechanical support devices in cardiogenic shock: a systematic review and collaborative meta-analysis of randomized trials. Eur Heart J 2017;38:3523–3531. [DOI] [PubMed] [Google Scholar]
- 10. Freund A, Jobs A, Lurz P, Feistritzer HJ, de Waha-Thiele S, Meyer-Saraei R, et al. Frequency and impact of bleeding on outcome in patients with cardiogenic shock. JACC Cardiovasc Interv 2020;13:1182–1193. [DOI] [PubMed] [Google Scholar]
- 11. Pahuja M, Ranka S, Chehab O, Mishra T, Akintoye E, Adegbala O, et al. Incidence and clinical outcomes of bleeding complications and acute limb ischemia in STEMI and cardiogenic shock. Catheter Cardiovasc Interv 2021;97:1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Balthazar T, Vandenbriele C, Verbrugge FH, Den Uil C, Engstrom A, Janssens S, et al. Managing patients with short-term mechanical circulatory support: JACC review topic of the week. J Am Coll Cardiol 2021;77:1243–1256. [DOI] [PubMed] [Google Scholar]
- 13. Kormos RL, Antonides CFJ, Goldstein DJ, Cowger JA, Starling RC, Kirklin JK, et al. Updated definitions of adverse events for trials and registries of mechanical circulatory support: a consensus statement of the mechanical circulatory support academic research consortium. J Heart Lung Transplant 2020;39:735–750. [DOI] [PubMed] [Google Scholar]
- 14. Esposito ML, Morine KJ, Annamalai SK, O’Kelly R, Aghili N, Pedicini R, et al. Increased plasma-free hemoglobin levels identify hemolysis in patients with cardiogenic shock and a trans valvular micro-axial flow pump. Artif Organs 2019;43:125–131. [DOI] [PubMed] [Google Scholar]
- 15. Fabrizio C, Levito MN, Rivosecchi R, Bashline M, Slocum B, Kilic A, et al. Outcomes of systemic anticoagulation with bivalirudin for Impella 5.0. Int J Artif Organs 2021;44:681–686. [DOI] [PubMed] [Google Scholar]
- 16. Beavers CJ, DiDomenico RJ, Dunn SP, Cox J, To L, Weeks P, et al. Optimizing anticoagulation for patients receiving Impella support. Pharmacotherapy 2021;41:932–942. [DOI] [PubMed] [Google Scholar]
- 17. Beavers CJ, Dunn SP, DiDomenico RJ, Moretz J, Jennings DL. Bicarbonate-based purge solution during Impella support: a growing alternative. J Am Coll Cardiol 2022;79:633–633. [Google Scholar]
- 18. Gilman V, Das S, Popovsky M, McMinn S, Wolf F, Berth S.. Bicarbonate as an alternative to heparin in Impella purge fluid: understanding the biochemical basis. Paper presented at: ASAIO 66th Annual Conference; 10–12 June 2021; Washington, DC, 2021.
- 19. Succar L, Sulaica EM, Donahue KR, Wanat MA. Management of anticoagulation with Impella(R) percutaneous ventricular assist devices and review of new literature. J Thromb Thrombolysis 2019;48:284–291. [DOI] [PubMed] [Google Scholar]
- 20. Abiomed . Impella 2.5, 5.0, LD, and CP system instructions for use and clinical reference manual. Abiomed, 2020.
- 21. Dietrich JN, Kazmi H. Bleeding risks in patients on percutaneous ventricular assist devices receiving two different dextrose concentrations of heparinized purge solution: a case series. J Pharm Pract 2019;32:464–469. [DOI] [PubMed] [Google Scholar]
- 22. Reed BN, DiDomenico RJ, Allender JE, Coons JC, Cox JF, Johnson D, et al. Survey of anticoagulation practices with the Impella percutaneous ventricular assist device at high-volume centers. J Interv Cardiol 2019;2019:3791307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blum EC, Martz CR, Selektor Y, Nemeh H, Smith ZR, To L. Anticoagulation of percutaneous ventricular assist device using argatroban-based purge solution: a case series. J Pharm Pract 2018;31:514–518. [DOI] [PubMed] [Google Scholar]
- 24. Hohlfelder B, Militello MA, Tong MZ, Soltesz EG, Wanek MR. Anticoagulation with temporary Impella device in patients with heparin-induced thrombocytopenia: a case series. Int J Artif Organs 2021;44:367–370. [DOI] [PubMed] [Google Scholar]
- 25. Succar L, Donahue KR, Varnado S, Kim JH. Use of tissue plasminogen activator alteplase for suspected Impella thrombosis. Pharmacotherapy 2020;40:169–173. [DOI] [PubMed] [Google Scholar]
- 26. Kanji R, Vandenbriele C, Arachchillage DRJ, Price S, Gorog DA. Optimal tests to minimise bleeding and ischaemic complications in patients on extracorporeal membrane oxygenation. Thromb Haemost 2022;122:480–491. [DOI] [PubMed] [Google Scholar]
- 27. Arachchillage DRJ, Kamani F, Deplano S, Banya W, Laffan M. Should we abandon the APTT for monitoring unfractionated heparin? Thromb Res 2017;157:157–161. [DOI] [PubMed] [Google Scholar]
- 28. Abiomed . Anticoagulation therapy with Impella® HeparinInfusion. Abiomed data on file, 2020.
- 29. Burzotta F, Trani C, Doshi SN, Townend J, van Geuns RJ, Hunziker P, et al. Impella ventricular support in clinical practice: collaborative viewpoint from a European expert user group. Int J Cardiol 2015;201:684–691. [DOI] [PubMed] [Google Scholar]
- 30. Jennings DL, Nemerovski CW, Kalus JS. Effective anticoagulation for a percutaneous ventricular assist device using a heparin-based purge solution. Ann Pharmacother 2013;47:1364–1367. [DOI] [PubMed] [Google Scholar]
- 31. Jennings DL, Nemerovski CW, Khandelwal A. Extended use of a percutaneous left-ventricular assist device without a heparin-based purge solution. Am J Health Syst Pharm 2010;67:1825–1828. [DOI] [PubMed] [Google Scholar]
- 32. Seyfarth M, Sibbing D, Bauer I, Frohlich G, Bott-Flugel L, Byrne R, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol 2008;52:1584–1588. [DOI] [PubMed] [Google Scholar]
- 33. Jiang C, Stuart M, Makowski C, Jennings DL, To L. Safety and efficacy of a percutaneously inserted ventricular support device purge solution Heparin 25 U/mL. Ann Pharmacother 2021;55:174–180. [DOI] [PubMed] [Google Scholar]
- 34. Vandenbriele C, Dannenberg L, Monteagudo-Vela M, Balthazar T, Metzen D, Voss F, et al. Optimal antithrombotic regimen in patients with cardiogenic shock on Impella™ mechanical support: less might be more. Eur Heart J 2020;41(Suppl 2): 1843. [Google Scholar]
- 35. Price EA, Jin J, Nguyen HM, Krishnan G, Bowen R, Zehnder JL. Discordant aPTT and anti-Xa values and outcomes in hospitalized patients treated with intravenous unfractionated heparin. Ann Pharmacother 2013;47:151–158. [DOI] [PubMed] [Google Scholar]
- 36. Kazmi H, Milkovits AE. Use of systemic bivalirudin and an anticoagulant-free purge solution in a percutaneous left ventricular assist device in a patient with heparin-induced thrombocytopenia. J Pharm Pract 2021;34:662–664. [DOI] [PubMed] [Google Scholar]
- 37. Laliberte B, Reed BN. Use of an argatroban-based purge solution in a percutaneous ventricular assist device. Am J Health Syst Pharm 2017;74:e163–e169. [DOI] [PubMed] [Google Scholar]
- 38. Mir T, Changal KH, Smith A, Ambreen S. Argatroban as purge solution in patients with heparin-induced thrombocytopenia on an Impella device, a case review. Am J Ther 2020;28:e763–e765. [DOI] [PubMed] [Google Scholar]
- 39. Szymanski TW, Weeks PA, Lee Y, Kumar S, Castillo B, Kar B, Gregoric ID. Anticoagulation of Impella with a bivalirudin purge solution. ASAIO J 2020;66:e117–e120. [DOI] [PubMed] [Google Scholar]
- 40. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. Group ESCSD . 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165.30165437 [Google Scholar]
- 41. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 42. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Investigators T-T . Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001–2015. [DOI] [PubMed] [Google Scholar]
- 43. Montalescot G, Bolognese L, Dudek D, Goldstein P, Hamm C, Tanguay JF, et al. ACCOAST Investigators . Pretreatment with prasugrel in non-ST-segment elevation acute coronary syndromes. N Engl J Med 2013;369:999–1010. [DOI] [PubMed] [Google Scholar]
- 44. Parodi G, Xanthopoulou I, Bellandi B, Gkizas V, Valenti R, Karanikas S, et al. Ticagrelor crushed tablets administration in STEMI patients: the MOJITO study. J Am Coll Cardiol 2015;65:511–512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.