Abstract
STUDY QUESTION
Can rapamycin improve the developmental competence of human oocytes during the IVM process?
SUMMARY ANSWER
Rapamycin at 10 nM could markedly improve the developmental competence of human oocytes undergoing IVM.
WHAT IS KNOWN ALREADY
Embryos derived from oocytes that mature in vitro have lower developmental competence than sibling embryos derived from oocytes matured in vivo. Rapamycin was shown to effectively improve IVM outcomes in mammalian oocytes; however, its effects on IVM of human oocytes have not been investigated.
STUDY DESIGN, SIZE, DURATION
In 2021, donated immature oocytes (n = 202) from 80 infertile couples receiving ICSI were included in a control group, and 156 oocytes from 72 couples were included in a rapamycin group. The oocytes underwent IVM with 10 nM rapamycin or without (control) rapamycin, followed by insemination by ICSI and embryo culture.
PARTICIPANTS/MATERIALS, SETTING, METHODS
The germinal vesicle breakdown (GVBD), maturation, normal fertilization, high-quality embryo (HQE) and blastocyst formation rates were calculated to evaluate the developmental competence of IVM oocytes, and fluorescence staining was used to assess DNA damage levels of oocytes in both groups. Whole-genome amplification and DNA sequencing were performed to analyze chromosome euploidy in embryos derived from the rapamycin group.
MAIN RESULTS AND THE ROLE OF CHANCE
The baseline characteristics of patients who donated oocytes for the two experimental groups were similar. In the control group, GVBD happened in 135 (66.8%) oocytes, and the maturation rate reached 52.5% at 24 h and 63.4% at 48 h. In the rapamycin group, 143 (91.7%) oocytes underwent GVBD, and the maturation rate reached 60.3% at 24 h and 82.7% at 48 h. Following ICSI, more HQEs were obtained in the rapamycin group versus control (34.2% versus 22.1%, respectively, P = 0.040), although with comparable fertilization rates in the two groups. In addition, the levels of histone γH2AX in oocytes cultured with 10 nM rapamycin were markedly decreased, compared with those in the control group (0.3 ± 0.0 versus 0.6 ± 0.1, respectively, P = 0.048). Embryos with normal karyotype could be obtained from oocytes cultured with rapamycin.
LIMITATIONS, REASONS FOR CAUTION
Our preliminary results indicated that the addition of rapamycin during human oocyte IVM did not cause extra aneuploidy. However, this safety evaluation of rapamycin treatment was based on limited samples and more data are needed before possible application in the clinic.
WIDER IMPLICATIONS OF THE FINDINGS
In the current study, 10 nM rapamycin was applied in the IVM process of human oocytes for the first time and showed positive effects, providing new insights for potentially improving IVM outcomes in the clinic. There were subtle differences between the results presented here on human oocytes and our previous studies on mouse oocytes, indicating the necessity of more research on human samples.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by the research grants from National Key Research and Development Project (2018YFC1002103) and Health Commission of Hubei Province scientific research project (WJ2021M110). All authors declared no conflicts of interest.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: DNA damage, human oocyte, in vitro maturation, IVM, oocyte quality, rapamycin
WHAT DOES THIS MEAN FOR PATIENTS?
During assisted reproduction cycles, for in vitro fertilization of eggs (oocytes), we need to collect mature oocytes from the ovaries after hormone stimulation. However, some of the oocytes obtained in this way are immature and cannot be fertilized without further development outside the body (in vitro). By using IVM technology, these oocytes that would otherwise be discarded can be maintained in culture dishes until they reach maturity, providing patients with more available oocytes and embryos.
Studies have shown that the embryos derived from IVM oocytes do not develop as well as embryos derived from oocytes that have matured within the ovary itself (in vivo), and therefore, we need to improve the IVM outcomes by optimizing the culture conditions.
In this study, we added rapamycin (a drug already used in clinical practice: for example, to help avoid transplant rejection) to the IVM culture medium surrounding the oocytes and observed its effects. The results suggested that rapamycin could increase the oocyte maturation rate, improve the developmental potential of oocytes and reduce the accumulation of DNA damage during the IVM process. Our encouraging findings suggest that the addition of rapamycin during egg maturation outside the body could eventually result in more healthy embryos available for transfer. More studies must now be carried out to confirm this.
Introduction
Human oocyte IVM technology has a long history. The earliest reported human IVF technology was based on IVM oocytes (Rock and Menkin, 1944; Menkin and Rock, 1948). Although in 1965 Edwards et al, established a culture system supporting the in vitro nuclear maturation of human oocytes within 36 h (Edwards, 1965a,b), it was not until 1991 that the first human birth from IVM oocytes was obtained (Cha et al., 1991). Since then, the research on IVM technology and its clinical application have gradually increased. IVM technology was applied to avoid ovarian hyperstimulation syndrome in patients with PCOS and was also used as a tool to explore the specific mechanism of oocyte nucleocytoplasmic maturation (Delvigne and Rozenberg, 2002). The diversity of research around IVM technology has led to various definitions of ‘IVM’. Traditional IVM refers to obtaining immature cumulus–oocyte complexes (COCs) from antral follicles in the natural state and their culture to metaphase II phase, while rescue IVM refers to culturing oocytes that failed to mature in vivo during conventional controlled ovarian hyperstimulation (COH) cycles (De Vos et al., 2016; Lee et al., 2016; Escrich et al., 2018b).
However, it is known that embryos derived from IVM oocytes show lower developmental competence than sibling embryos derived from oocytes matured in vivo, regardless of IVM type (Shu et al., 2007; Escrich et al., 2018a). A recent review summarized two main ways to improve the developmental competence of IVM oocytes, by: mimicking the in vivo environment of oocyte maturation, including keeping the structural integrity of COCs; maintaining meiotic arrest by increasing the concentration of cAMP in oocytes to ensure the synchronization of cytoplasm and nuclear development; and adding growth factors that are present in the follicular environment in vivo, such as epidermal growth factor peptide, to the IVM culture medium, so as to improve the quality of IVM oocytes (De Vos et al., 2021). Nevertheless, as well as losing follicular support, oocytes cultured in vitro also suffered from metabolic disorders, oxidative stress and increased DNA double-strand breaks (DSBs) or chromosome segregation (Zhao et al., 2019). Minimizing the damage to oocytes caused by the in vitro environment is also an important method of improving IVM outcomes.
Rapamycin is a macrolide metabolite produced by Streptomyces hygroscopicus, which was first identified in 1975, with antifungal and immunosuppressive effects (Sehgal et al., 1975; Collier, 1989). The mammalian target of rapamycin (mTOR) is sensitive to various environmental factors, such as nutrients and growth factors, and regulates important processes, such as cell growth, metabolism, differentiation, aging and autophagy (Long et al., 2004). Some previous studies have reported positive effects of rapamycin on the IVM process for oocytes from pigs and bovines, and the mechanism was claimed to be the autophagy-activation function of rapamycin: following the activation of autophagy in oocytes of the rapamycin group, mRNA transcription level of the apoptosis-promoting gene BAX was reduced, while that of the anti-apoptosis gene BCL-XL was increased. Moreover, addition of the autophagy inhibitor 3-methyladenine (3-MA) significantly reduced the oocyte maturation rate, also indicating the importance of autophagic activity for oocyte maturation in vitro (Song et al., 2014; Lee et al., 2015; Kohata-Ono et al., 2019; Kordowitzki et al., 2020). In our recent study of mouse oocyte IVM, 10 nM rapamycin was confirmed to reduce reactive oxygen species levels by promoting the expression of Nrf2 (a master transcription factor for many antioxidant genes and phase II detoxifying enzymes) and improve DNA damage repair (DDR) ability by enhancing the activity of several DDR-associated genes, including ataxia telangiectasia mutated (Atm), ataxia telangiectasia and Rad3 related (Atr) and DNA protein kinase (DNA-PK, encoded by Prkdc), thereby improving the quality and developmental potential of mouse oocytes (Yang et al., 2022). However, the effects of rapamycin on IVM of human oocytes have not been investigated.
Therefore, the purpose of the current study is to explore the impact of rapamycin on the maturity and developmental competence of human oocytes after IVM, assess its protective effect on DNA integrity and undertake a preliminary evaluation of its safety by analyzing embryo karyotype.
Materials and methods
Study population
Couples undergoing ICSI owing to male-factor infertility from June to December 2021 in our center were registered. The ICSI cycles in which immature germinal vesicle (GV) oocytes were available after denudation were included. Patients with morphologically abnormal oocytes, normal sperm morphology rate <2%, normal fertilization rate (presence of two pronuclei) of in vivo matured oocytes <25%, patients undergoing testicular sperm aspiration or percutaneous/microsurgical epididymal sperm aspiration and patients in preimplantation genetic testing cycles were excluded. The included patients were randomly divided into a control group and a rapamycin group.
The original study was approved by the Ethical Committee of Tongji Hospital, Tongji Medicine College, Huazhong University of Science and Technology (TJ-IRB20201209). Each of the couples had given written informed consent before the cycle start for the donation of their immature oocytes and sperm for research use.
Oocyte collection and in vitro culture
COH was undertaken as previously described (Xu et al., 2018; Geng et al., 2019). Briefly, pituitary suppression was achieved by injection of a GnRH agonist starting in the mid-luteal phase of the previous cycle or GnRH antagonist starting with the appearance of follicles measuring 13–14 mm in diameter. The dosage and duration of recombinant FSH were adjusted based on individual ovarian responses. When two to three leading follicles reached a mean diameter of 18 mm, recombinant hCG was injected (i.m.) and COCs were retrieved by guided transvaginal ultrasound 36–38 h after the hCG trigger. The COCs were cultured for 2–3 h in IVF medium (Vitrolife, Stegomore, Sweden) under standard culture conditions (37°C and 6% CO2 in air), after which cumulus cells were removed by mechanical stripping in 80 IU hyaluronidase (Vitrolife, Stegomore, Sweden) to assess the maturity of oocytes.
After denudation, the oocytes with a GV structure were collected and cultured for up to 48 h in G1-plus medium (Vitrolife, Stegomore, Sweden) with or without 10 nM rapamycin (MedChemExpress, New Jersey, USA) in an Embryo Scope (Vitrolife, Stegomore, Sweden) incubator (37°C, 6% CO2 and 5% O2) equipped with a time-lapse monitoring system. All the screened GV oocytes were put into the time-lapse system to set a starting time point at around 12 noon, and the dynamic parameters of oocyte nuclear maturation were recorded, including the time of germinal vesicle breakdown (GVBD) and first polar body (PB1) extrusion. ICSI was subsequently performed on the in vitro matured oocytes using sperm from the patient’s husband.
Embryo culture and evaluation following ICSI
After ICSI, oocytes from both groups were continuously cultured in G1-plus medium under time-lapse monitoring. A fertilization check was performed 16–18 h after insemination and the presence of two pronuclei was regarded as normal fertilization. The fertilized zygotes were cultured to the cleavage stage until Day 3, followed by 2 or 3 days of culture in the G2-plus medium (Vitrolife, Stegomore, Sweden) to the blastocyst (BC) stage. Embryo development was assessed on Day 2 and Day 3 based on the number of blastomeres, the degree of fragmentation and the blastomere symmetry. High-quality embryos (HQE) were defined as normally fertilized oocytes with no less than six blastomeres, fragmentation <20% and symmetrical blastomeres on Day 3.
Immunofluorescence analyses
For the assessment of DNA damage levels, we additionally collected 21 in vitro matured oocytes for fluorescence staining (12 from the control group and 9 from the rapamycin group). The oocytes matured with or without rapamycin were: fixed in 4% paraformaldehyde (Servicebio, G1101) for 1 h; permeabilized and blocked for 1 h in 3% bovine serum albumin (BSA) containing 0.1% Triton X-100 (Solarbio, T8200); washed three times in 1% BSA and incubated overnight at 4 °C with primary antibodies (1:200 diluted anti-rabbit phospho-histone H2AX antibody, CST, 9718S); and washed three times in 1% BSA then incubated for 1 h in goat anti-rabbit IgG labeled with cyanine 3 (1:200, Servicebio, GB21303). Then, the oocytes were incubated for 10 min with 4 μg/ml Hoechst 33258 (Servicebio, G1011). The stained oocytes were mounted onto glass slides and examined under a fluorescence microscope (Carl Zeiss, Oberkochen, Germany).
For the measurement of immunofluorescence intensity of γH2AX, all images of the two groups were taken using the digital microscope software Zen (Carl Zeiss, Oberkochen, Germany), using fixed microscopic parameters. The images were analyzed with ImageJ software (NIH, Bethesda, MD, USA).
Whole-genome amplification and DNA sequencing
A preliminary analysis of the safety of rapamycin was carried out by determining BC karyotype, to assess the feasibility of future clinical application of rapamycin. The detailed protocol has been described previously (Zhu et al., 2021). Three BC samples were chosen from the rapamycin IVM group and analyzed by whole-embryo aneuploidy analysis. Whole-genome amplification of the embryos was achieved using the multiple annealing- and looping-based amplification cycles method, and this was followed by next-generation sequencing.
Statistical analyses
Data were analyzed and presented using SPSS software 22.0 (IBM, Amunk, New York, USA) and Graphpad Prism 8.0 (GraphPad Software, San Diego, CA, USA). Continuous data were analyzed with Student’s t-test and expressed as mean ± SEM if normally distributed, or with Kruskal–Wallis nonparametric method and expressed as median (interquartile range [IQR]) if not normally distributed. Categorical data were presented as the number of cases and frequency (percentage), with a Chi-square test to assess between-group differences. Wald P-values were two-sided; P < 0.05 was considered to be statistically significant.
Results
Rapamycin improved the maturation rate of human IVM oocytes
Donated GV oocytes (n = 202) from 80 infertile couples receiving ICSI were included in the control group, and 156 GV oocytes from 72 couples were included in the rapamycin group. The baseline clinical characteristics of the females, including age, BMI, infertility type and duration, basal FSH level, antral follicle count, COH protocol and numbers of oocytes obtained and matured, were generally consistent between the two groups, as shown in Table I.
Table I.
Baseline characteristics of patients who donated oocytes for IVM.
| Patient characteristics | Control group | Rapamycin group | P level |
|---|---|---|---|
| Age,a years | 31.3 ± 0.5 | 31.9 ± 0.5 | 0.390 |
| BMI,a kg/m2 | 21.4 ± 0.3 | 22.1 ± 0.4 | 0.142 |
| Infertility type,b n (%) | 0.894 | ||
| Primary | 57 (71.3) | 52 (72.2) | |
| Secondary | 23 (28.7) | 20 (27.8) | |
| Infertility duration,c years | 3 (2-5) | 2.5 (2-4) | 0.569 |
| Basal serum FSH level,c mIU/ml | 6.6 (5.8-7.7) | 7.0 (6.0-8.3) | 0.223 |
| Antral follicle counta | 14.7 ± 0.8 | 13.4 ± 0.8 | 0.230 |
| Ovarian stimulation protocol,b n (%) | 0.126 | ||
| GnRH agonist protocol | 41 (51.2) | 28 (38.9) | |
| GnRH antagonist protocol | 39 (48.8) | 44 (61.1) | |
| Oocytes obtained,a n | 16.2 ± 1.0 | 14.8 ± 0.7 | 0.245 |
| Mature oocytes obtained,a n | 11.4 ± 0.7 | 11.0 ± 0.6 | 0.682 |
Student’s t-test.
Chi-square test.
Kruskal–Wallis nonparametric test.
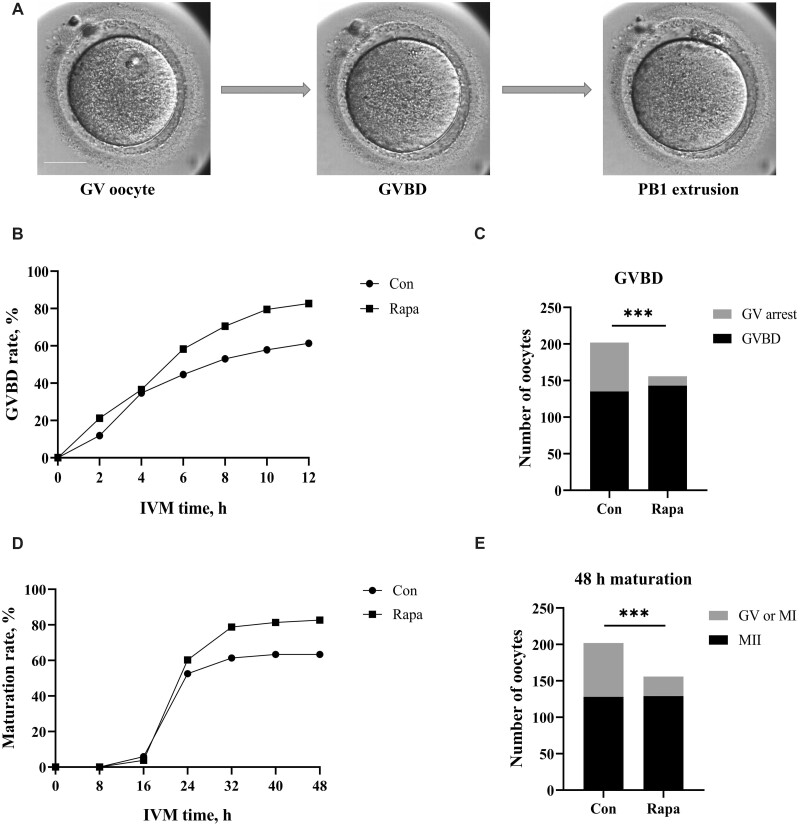
During IVM, the developmental processes of every oocyte were recorded by the time-lapse system (Fig. 1A). At each time point, the GVBD rate of oocytes in the rapamycin group was always higher than that in the control group (Fig. 1B) and finally, 135 (66.8%) oocytes in the control group and 143 (91.7%) oocytes in the rapamycin group underwent GVBD (Fig. 1C). The GVBD rate in the rapamycin group was significantly higher than in the control group (P < 0.001). Overall, the 24 h maturation rate of 202 GV oocytes in the control group was 52.5%, while that of 156 GV oocytes in the rapamycin group was 60.3%, with no significant difference between the two groups (P = 0.142). However, after further culture, the maturation rate of oocytes in the rapamycin group significantly exceeded that in the control group, and the 48 h maturation rate reached 82.7% and 63.4% (P < 0.001), respectively (Fig. 1D and E).
Figure 1.
IVM outcomes for human oocytes in the control and rapamycin groups. (A) A human oocyte undergoing the IVM process (captured by time-lapse monitoring system). GV, germinal vesicle; GVBD, germinal vesicle breakdown; PB1, first polar body. Scale bar, 50 μm. (B) GVBD rates of oocytes during 12 h of culture for the control group (n = 202) and the 10-nM rapamycin group (n = 156). (C) Comparison of the proportion of oocytes undergoing GVBD between groups (Chi-square test). (D) Maturation rates of oocytes over 48 h of culture for the control group (n = 202) and the 10-nM rapamycin group (n = 156). (E) Comparison of the proportion of oocytes that matured within 48 h between groups (Chi-square test). Con, control; Rapa, rapamycin; MII, metaphase II. ***P < 0.001.
Rapamycin enhanced the developmental competence of human IVM oocytes
To assess the developmental competence of the IVM oocytes, oocytes matured in vitro were collected for insemination and continued culture. There were no significant differences in the normal fertilization rate and BC formation rate of IVM oocytes between the rapamycin group and the control group (normal fertilization rate: 52.3% versus 44.3%, P = 0.223; BC formation rate: 9.0% versus 10.7%, P = 0.674), but the HQE rate in the rapamycin group was significantly increased (34.2% versus 22.1%, P = 0.040). In addition, oocytes that matured within 24 h and within 24–48 h were assessed separately, and the results suggested that rapamycin could significantly increase the HQE rate of IVM oocytes matured within 24 h (37.2% versus 23.6%, P = 0.036). For oocytes that matured at 24–48 h, no statistically significant differences between groups were observed, as shown in Table II.
Table II.
Subsequent development of in vitro matured oocytes from both groups.
| Development evaluation | Control group | Rapamycin group | P level |
|---|---|---|---|
| Overall situation | |||
| No. of oocytes undergoing ICSI | 122 | 111 | |
| Normal fertilization,* n (%) | 54 (44.3) | 58 (52.3) | 0.223 |
| High-quality embryo, n (%) | 27 (22.1) | 38 (34.2) | 0.040 |
| Blastocyst formation, n (%) | 13 (10.7) | 10 (9.0) | 0.674 |
| Oocytes mature within 24 h | |||
| No. of oocytes undergoing ICSI | 106 | 94 | |
| Normal fertilization, n (%) | 52 (49.1) | 52 (55.3) | 0.376 |
| High-quality embryo, n (%) | 25 (23.6) | 35 (37.2) | 0.036 |
| Blastocyst formation, n (%) | 13 (12.3) | 9 (9.6) | 0.544 |
| Oocytes matured within 24–48 h | |||
| No. of oocytes undergoing ICSI | 16 | 17 | |
| Normal fertilization, n (%) | 2 (12.5) | 6 (35.3) | 0.225 |
| High-quality embryo, n (%) | 2 (12.5) | 3 (17.6) | 0.685 |
| Blastocyst formation, n (%) | 0 (0.0) | 1 (5.9) | 0.332 |
The presence of two pronuclei was regarded as normal fertilization. A Chi-square test was used to compare the difference between groups.
Rapamycin rescued DNA damage in human oocytes during IVM
Considering our previous experimental results in mice: that 10 nM rapamycin could decrease DNA damage levels in IVM oocytes (Yang et al., 2022), we collected some human oocytes after IVM from the two groups for measurement of the histone γH2AX, which in response to DSBs provides a basis for the sensitive detection of DNA damage (Mah et al., 2010). By counting the number of γH2AX foci after fluorescence staining or measuring its fluorescence intensity, the level of DNA damage in oocytes could be detected and compared. The results suggested that the γH2AX fluorescence intensity of oocytes was 0.6 ± 0.1 in the control group (n = 12) and 0.3 ± 0.0 in the rapamycin group (n = 9), and the difference was statistically significant (P = 0.048) (Fig. 2).
Figure 2.
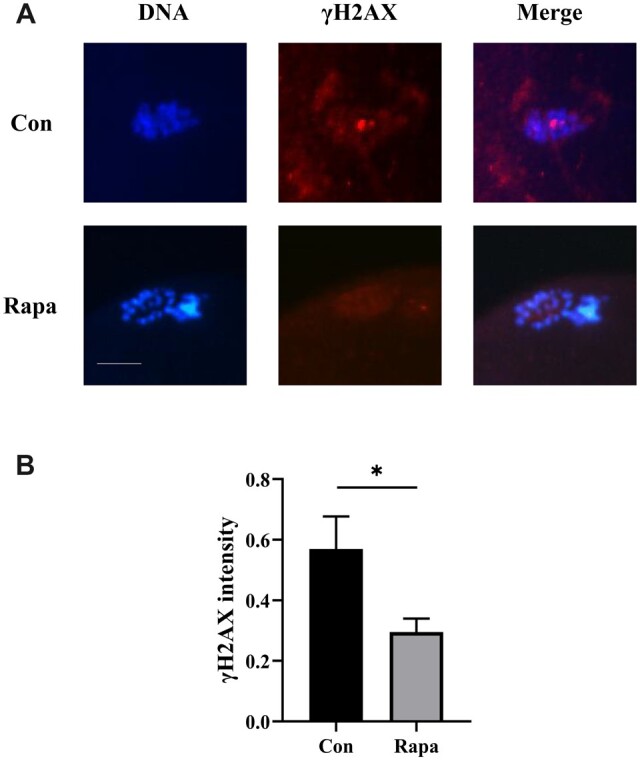
Effects of rapamycin on the DNA damage levels in human oocytes matured in vitro. (A) The fluorescence images of DNA and histone γH2AX in oocytes after IVM (with or without 10 nM rapamycin). Scale bar, 25 μm. (B) Comparison of the intensity of γH2AX normalized to the mean DNA intensity between the control group (n = 12) and the rapamycin group (n = 9) by Student’s t-test. Con, control; Rapa, rapamycin. *P < 0.05.
Oocytes cultured with rapamycin formed embryos with normal karyotype
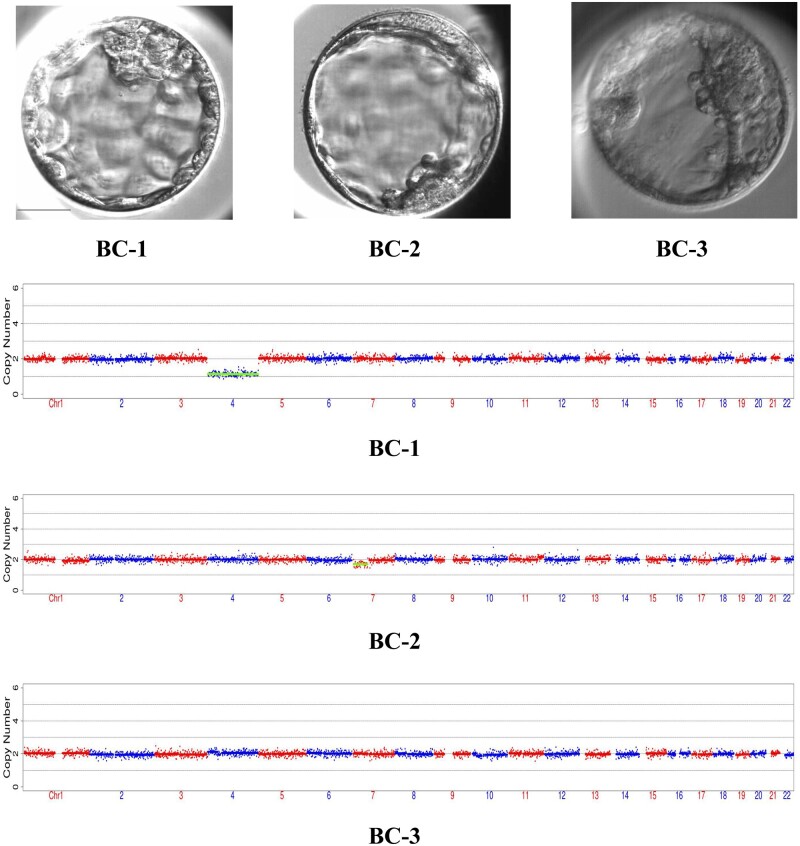
To detect potential aneuploidies of the resulting embryos as a preliminary exploration of the safety of rapamycin, three BCs from the rapamycin group were selected for whole-embryo aneuploidy analysis. Rapamycin seemed to positively affect the qualities of BCs, as the morphological scores of the three selected BCs were 4AA, 4BC and 4BC, based on the Gardner grading system (Gardner and Schoolcraft, 1999). The chromosome euploidy analyses suggested that IVM oocytes cultured with rapamycin can form normal embryos: the karyotypes of BC-1, BC-2 and BC-3 were 45, XN, −4(×1); 46, XN, −7p(×1, mos, ∼30%); and 46, XN, respectively (Fig. 3).
Figure 3.
The morphology and karyotype profile of blastocysts derived from human oocytes cultured with rapamycin. Three blastocysts (top row) were selected for whole-embryos aneuploidy analysis. The karyotypes of these three embryos were 45, XN, −4(×1); 46, XN, −7p(×1, mos, ∼30%); and 46, XN, respectively. BC, blastocysts. Scale bar, 50 μm.
Discussion
In the current study, we aimed to evaluate the effects of rapamycin on IVM of human oocytes by comparing the maturation rate, subsequent development and DNA damage levels of the oocytes cultured with or without rapamycin. The results suggested that 10 nM rapamycin could significantly increase the GVBD rate and 48 h maturation rate, improve the developmental competence of oocytes and reduce the accumulation of DNA damage during IVM process.
The effects of rapamycin on the developmental competences of IVM oocytes in different species have been investigated in previous studies. Yang et al. (2009) added rapamycin at concentrations of 0.5–10 μg/μl (i.e. 0.55–10.94 mM) to the IVM system of mouse oocytes and found that the GVBD rate and PB1 extrusion rate were significantly reduced when the concentration reached or exceeded 3.3 μg/μl. Later studies, however, reached opposite conclusions. Several studies demonstrated that 1 nM rapamycin increased the maturation rate and improved the subsequent development of IVM porcine oocytes, especially the poor-quality oocytes (Song et al., 2014; Lee et al., 2015; Kohata-Ono et al., 2019). Kordowitzki et al. (2020) added 0.1, 1, 10 or 100 nM rapamycin to the IVM medium of bovine oocytes and found that the maturation rate was the highest in the 1 nM group and the lowest in the 100 nM group, but the maturation, cleavage and BC formation rates of bovine IVM oocytes were not significantly different between the 1 nM rapamycin group and the control group. In another study, bovine COCs were divided into high-quality and low-quality groups according to morphological evaluation. The results showed that 100 nM rapamycin in IVM medium significantly increased the cleavage rate of low-quality bovine COCs after maturation and fertilization, but not the BC formation rate (Li et al. 2020). The inconsistency of the results may be attributed to the different species and rapamycin concentrations used in each study. Our recent study confirmed the ameliorative effect of 10 nM rapamycin on the IVM process in mice based on concentration gradient experiments (Yang et al., 2022). In the current study, 10 nM rapamycin was applied during the IVM process of human oocytes for the first time and showed similar positive effects.
Interestingly, there were still subtle differences between the experimental results of human oocytes and mice oocytes. In this study, we found that 10 nM rapamycin significantly increased the GVBD rate of human immature oocytes cultured in vitro, indicating that 10 nM rapamycin may promote meiotic resumption, which was not obvious in the mouse experiments (Yang et al., 2022). The GV oocytes of most mammals, including mice, would spontaneously resume meiosis soon after removal from the inhibitory environment in follicles. Although human immature oocytes also underwent GVBD, the incidence was significantly lower than that of other mammals (Edwards, 1965b). Previous studies have shown that rapamycin at high concentrations inhibited meiotic resumption and reduced the maturation rate of mouse oocytes (Yang et al., 2009), but there was no direct evidence that rapamycin affected key factors inducing meiosis in oocytes, including cAMP, maturation promoting factor (MPF), and other cyclins. More studies on human samples are needed to further investigate the relation between the degree of mTOR inhibition and meiotic resumption of IVM oocytes.
After ICSI, regardless of the rapamycin intervention, the normal fertilization rate, HQE rate and BC formation rate of human oocytes matured within 24 h were higher than those of oocytes matured within 24–48 h, which is consistent with the results in our previous research (Yang et al., 2021). For the human oocytes matured within 24 h, rapamycin treatment could increase the HQE rate, but not the normal fertilization rate and BC formation rate. This result differed from the conclusions of previous studies (Song et al., 2014; Lee et al., 2015; Kohata-Ono et al., 2019; Kordowitzki et al., 2020; Li et al., 2020), in which the cleavage rate and BC formation rate were the main evaluating indicators of developmental competence, and the increased BC formation rate turned out to be the most significant positive effect of rapamycin, especially during IVM of porcine oocytes (Song et al., 2014; Lee et al., 2015; Kohata-Ono et al., 2019). In addition to species differences, the specificity of samples in these studies might account for the inconsistency of results: increased BC formation rates were only observed in the morphologically poor oocytes and oocytes derived from medium follicles (Lee et al., 2015; Kohata-Ono et al., 2019). Notably, no significant effect of rapamycin on developmental competence of oocytes matured within 24–48 h was observed. The reason may be related to the intrinsic low level of MPF, the DNA damage, abnormal mitochondrial function and metabolic disorders caused by extended culture of oocytes that matured in 24–48 h. Rapamycin (10 nM) has been shown to alleviate DNA damage to some extent but other changes were not improved. Considering the limited number of 24–48 h matured oocytes undergoing ICSI, studies with larger sample sizes are needed to draw a more reliable conclusion.
The improvement of embryo quality by rapamycin might be attributed to its effect on the DNA damage levels in IVM oocytes. DNA damage may cause chromatin disarrangement or even fragmentation, which leads to oocyte developmental disorders (Bennabi et al., 2016; Coticchio et al., 2015; Oktay et al., 2015), and increasing evidence showed that the mTORC1 pathway was involved in the DNA damage response (Ma et al., 2018). ATM, DNA-PK and ATR are three proteins from the phosphatidylinositol 3-kinase-related kinase family, which are recruited to the site of DNA damage and subsequently phosphorylate H2AX, activate cell checkpoints and coordinate DNA repair through homologous recombination or non-homologous end joining (NHEJ) (Sancar et al., 2004; Matsuoka et al., 2007; Toulany et al., 2012). Shen and Houghton (2013) have demonstrated, in the context of childhood sarcoma, that mTOR signaling suppressed Atm mRNA via the S6K pathway, and inhibition of mTOR enhanced the expression of Atm. Also, exposure of cancer cells to rapamycin can increase DNA-PK activity and promote NHEJ (Li et al., 2013). While most research about the relation between mTOR and DNA damage has focused on the fields of anti-tumor, tumor drug resistance and aging, our recent study has confirmed in oocytes that the inhibition of mTOR by 10 nM rapamycin during IVM could increase the expression of these DDR-associated genes (Atm, Atr and Prkdc), improved the DNA repair capacity of the oocytes and thus prevented the accumulation of DNA damage (Yang et al., 2022). In the current study, the protective effect of rapamycin on DNA integrity during IVM was further reinforced in human oocytes, providing a new insight into possibilities for improving the IVM outcomes in the clinic.
Importantly, the safety of rapamycin should not be ignored before its final application in the clinic. The aneuploidy rates of embryos derived from IVM human oocytes varied greatly in previous studies, ranging from 28% to 60% (Requena et al., 2009; Zhang et al., 2010; Li et al., 2021). In our study, although based on limited samples, the preliminary results indicated that the addition of rapamycin did not cause extra aneuploidy and could provide embryos with normal karyotype for clinical practice. In the future, we will further explore the mechanism by which rapamycin intervenes in meiotic progression and evaluate its safety in a larger sample by other methods, such as single-cell RNA-sequencing, DNA methylation level detection and health assessment of animal offspring, so as to provide additional evidence in support of possible clinical application.
In conclusion, our study provides preliminary data on the effects of 10 nM rapamycin that support its potential for improving IVM outcomes in human oocytes, mainly by increasing the GVBD rate, the 48 h maturation rate and the HQE rate after ICSI. The specific mechanism(s) involved might include reducing DNA damage, and simultaneously promoting meiotic resumption and progression. However, the safety of rapamycin on human IVM oocytes needs to be further investigated.
Acknowledgments
We would like to express our heartfelt gratitude to Yikon Genomics for the technical support in the process of whole-genome amplification and DNA sequencing.
Authors’ roles
Q.Y., L.Z. and J.H. conducted the experiment; Q.Y., Q.X., M.W., Z.L. and J.L. collected and analyzed the data; Q.Y. and L.Z. wrote the article; and Q.Y., L.Z. and L.J. were responsible for the concept and study design. All authors contributed to the interpretation, discussion and editing of the article. All authors have read and approved the article.
Funding
This work was supported by research grants from National Key Research and Development Project (2018YFC1002103) and Health Commission of Hubei Province scientific research project (WJ2021M110).
Conflict of interest
None declared.
Contributor Information
Qiyu Yang, Reproductive Medicine Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Qingsong Xi, Reproductive Medicine Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Meng Wang, Reproductive Medicine Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Jing Liu, Reproductive Medicine Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Zhou Li, Reproductive Medicine Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Juan Hu, Reproductive Medicine Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Lei Jin, Reproductive Medicine Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Lixia Zhu, Reproductive Medicine Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Data Availability
The data underlying this article are available in the article.
References
- Bennabi I, Terret ME, Verlhac MH.. Meiotic spindle assembly and chromosome segregation in oocytes. J Cell Biol 2016;215:611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha KY, Koo JJ, Ko JJ, Choi DH, Han SY, Yoon TK.. Pregnancy after in vitro fertilization of human follicular oocytes collected from nonstimulated cycles, their culture in vitro and their transfer in a donor oocyte program. Fertil Steril 1991;55:109–113. [DOI] [PubMed] [Google Scholar]
- Collier SJ. Immunosuppressive drugs. Curr Opin Immunol 1989;2:854–858. [DOI] [PubMed] [Google Scholar]
- Coticchio G, Dal Canto M, Guglielmo MC, Albertini DF, Mignini Renzini M, Merola M, Lain M, Sottocornola M, De Ponti E, Fadini R.. Double-strand DNA breaks and repair response in human immature oocytes and their relevance to meiotic resumption. J Assist Reprod Genet 2015;32:1509–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos M, Grynberg M, Ho TM, Yuan Y, Albertini DF, Gilchrist RB.. Perspectives on the development and future of oocyte IVM in clinical practice. J Assist Reprod Genet 2021;38:1265–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos M, Smitz J, Thompson JG, Gilchrist RB.. The definition of IVM is clear-variations need defining. Hum Reprod 2016;31:2411–2415. [DOI] [PubMed] [Google Scholar]
- Delvigne A, Rozenberg S.. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Hum Reprod Update 2002;8:559–577. [DOI] [PubMed] [Google Scholar]
- Edwards RG. Maturation in vitro of human ovarian oöcytes. Lancet 1965a;2:926–929. [DOI] [PubMed] [Google Scholar]
- Edwards RG. Maturation in vitro of mouse, sheep, cow, pig, rhesus monkey and human ovarian oocytes. Nature 1965b;208:349–351. [DOI] [PubMed] [Google Scholar]
- Escrich L, Galiana Y, Grau N, Insua F, Soler N, Pellicer A, Escribá MJ.. Do immature and mature sibling oocytes recovered from stimulated cycles have the same reproductive potential? Reprod Biomed Online 2018a;37:667–676. [DOI] [PubMed] [Google Scholar]
- Escrich L, Pellicer A, Meseguer M.. Let's rescue oocytes: in vitro maturation 2.0 is coming. Fertil Steril 2018b;110:638–639. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Schoolcraft WB.. In vitro culture of human blastocyst. In: Jansen R, Mortimer D (eds). Towards Reproductive Certainty: Infertility and Genetics beyond. Carnforth: Parthenon Press, 1999,377–388. [Google Scholar]
- Geng Y, Xun Y, Hu S, Lai Q, Jin L.. GnRH antagonist versus follicular-phase single-dose GnRH agonist protocol in patients of normal ovarian responses during controlled ovarian stimulation. Gynecol Endocrinol 2019;35:309–313. [DOI] [PubMed] [Google Scholar]
- Kohata-Ono C, Wakai T, Funahashi H.. The autophagic inducer and inhibitor display different activities on the meiotic and developmental competencies of porcine oocytes derived from small and medium follicles. J Reprod Dev 2019;65:527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordowitzki P, Hamdi M, Derevyanko A, Rizos D, Blasco M.. The effect of rapamycin on bovine oocyte maturation success and metaphase telomere length maintenance. Aging (Albany NY) 2020;12:7576–7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Barad DH, Kushnir VA, Shohat-Tal A, Lazzaroni-Tealdi E, Wu YG, Gleicher N.. Rescue in vitro maturation (IVM) of immature oocytes in stimulated cycles in women with low functional ovarian reserve (LFOR). Endocrine 2016;52:165–171. [DOI] [PubMed] [Google Scholar]
- Lee J, Park JI, Yun JI, Lee Y, Yong H, Lee ST, Park CK, Hyun SH, Lee GS, Lee E.. Rapamycin treatment during in vitro maturation of oocytes improves embryonic development after parthenogenesis and somatic cell nuclear transfer in pigs. J Vet Sci 2015;16:373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Balboula AZ, Aboelenain M, Fujii T, Moriyasu S, Bai H, Kawahara M, Takahashi M.. Effect of autophagy induction and cathepsin B inhibition on developmental competence of poor quality bovine oocytes. J Reprod Dev 2020;66:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chen J, Sun T, Zhang S, Jiao T, Chian RC, Li Y, Xu Y.. Chromosome aneuploidy analysis in embryos derived from in vivo and in vitro matured human oocytes. J Transl Med 2021;19:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang X, Yue P, Tao H, Ramalingam SS, Owonikoko TK, Deng X, Wang Y, Fu H, Khuri FR. et al. Protein phosphatase 2A and DNA-dependent protein kinase are involved in mediating rapamycin-induced Akt phosphorylation. J Biol Chem 2013;288:13215–13224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long X, Müller F, Avruch J.. TOR action in mammalian cells and in Caenorhabditis elegans. Curr Top Microbiol Immunol 2004;279:115–138. [DOI] [PubMed] [Google Scholar]
- Mah LJ, El-Osta A, Karagiannis TC.. gammaH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia 2010;24:679–686. [DOI] [PubMed] [Google Scholar]
- Ma Y, Vassetzky Y, Dokudovskaya S.. mTORC1 pathway in DNA damage response. Biochim Biophys Acta Mol Cell Res 2018;1865:1293–1311. [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y. et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 2007;316:1160–1166. [DOI] [PubMed] [Google Scholar]
- Menkin MF, Rock J.. In vitro fertilization and cleavage of human ovarian eggs. Am J Obstet Gynecol 1948;55:440–452. [DOI] [PubMed] [Google Scholar]
- Oktay K, Turan V, Titus S, Stobezki R, Liu L.. BRCA mutations, DNA repair deficiency, and ovarian aging. Biol Reprod 2015;93:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requena A, Bronet F, Guillén A, Agudo D, Bou C, García-Velasco JA.. The impact of in-vitro maturation of oocytes on aneuploidy rate. Reprod Biomed Online 2009;18:777–783. [DOI] [PubMed] [Google Scholar]
- Rock J, Menkin MF.. In vitro fertilization and cleavage of human ovarian eggs. Science 1944;100:105–107. [DOI] [PubMed] [Google Scholar]
- Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K, Linn S.. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem 2004;73:39–85. [DOI] [PubMed] [Google Scholar]
- Sehgal SN, Baker H, Vézina C.. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiot (Tokyo) 1975;28:727–732. [DOI] [PubMed] [Google Scholar]
- Shen C, Houghton PJ.. The mTOR pathway negatively controls ATM by up-regulating miRNAs. Proc Natl Acad Sci U S A 2013;110:11869–11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Gebhardt J, Watt J, Lyon J, Dasig D, Behr B.. Fertilization, embryo development, and clinical outcome of immature oocytes from stimulated intracytoplasmic sperm injection cycles. Fertil Steril 2007;87:1022–1027. [DOI] [PubMed] [Google Scholar]
- Song BS, Kim JS, Kim YH, Sim BW, Yoon SB, Cha JJ, Choi SA, Yang HJ, Mun SE, Park YH. et al. Induction of autophagy during in vitro maturation improves the nuclear and cytoplasmic maturation of porcine oocytes. Reprod Fertil Dev 2014;26:974–981. [DOI] [PubMed] [Google Scholar]
- Toulany M, Lee KJ, Fattah KR, Lin YF, Fehrenbacher B, Schaller M, Chen BP, Chen DJ, Rodemann HP.. Akt promotes post-irradiation survival of human tumor cells through initiation, progression, and termination of DNA-PKcs-dependent DNA double-strand break repair. Mol Cancer Res 2012;10:945–957. [DOI] [PubMed] [Google Scholar]
- Xu B, Chen Y, Geerts D, Yue J, Li Z, Zhu G, Jin L.. Cumulative live birth rates in more than 3,000 patients with poor ovarian response: a 15-year survey of final in vitro fertilization outcome. Fertil Steril 2018;109:1051–1059. [DOI] [PubMed] [Google Scholar]
- Yang CR, Wei YC, Zhang Y, Zheng KJ, Li N, Yan YQ.. The expression and effect of mTOR during mouse oocyte maturation. Prog Biochem Biophys 2009;36:1334–1339. [Google Scholar]
- Yang Q, Xi Q, Wang M, Long R, Hu J, Li Z, Ren X, Zhu L, Jin L.. Rapamycin improves the quality and developmental competence of mice oocytes by promoting DNA damage repair during in vitro maturation. Reprod Biol Endocrinol 2022;20:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Zhu L, Wang M, Huang B, Li Z, Hu J, Xi Q, Liu J, Jin L.. Analysis of maturation dynamics and developmental competence of in vitro matured oocytes under time-lapse monitoring. Reprod Biol Endocrinol 2021;19:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Ata B, Son WY, Buckett WM, Tan SL, Ao A.. Chromosome abnormality rates in human embryos obtained from in-vitro maturation and IVF treatment cycles. Reprod Biomed Online 2010;21:552–559. [DOI] [PubMed] [Google Scholar]
- Zhao H, Li T, Zhao Y, Tan T, Liu C, Liu Y, Chang L, Huang N, Li C, Fan Y. et al. Single-cell transcriptomics of human oocytes: environment-driven metabolic competition and compensatory mechanisms during oocyte maturation. Antioxid Redox Signal 2019;30:542–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Li J, Wang M, Fang Z, Zheng F, Li Z, Jin L.. Normalized mitochondrial DNA copy number can optimize pregnancy outcome prediction in IVF. Reprod Sci 2021;28:1439–1446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article.