Abstract
BACKGROUND AND PURPOSE:
The presence of malformations of cortical development in patients with hereditary hemorrhagic telangiectasia has been reported on previous occasions. We evaluated a sample of adults with hereditary hemorrhagic telangiectasia for the presence of malformations of cortical development, spatial coincidence of malformations of cortical development and AVMs, and the coincidence of brain and pulmonary AVMs.
MATERIALS AND METHODS:
A total of 141 patients 18 years of age or older who were referred to the Augusta University hereditary hemorrhagic telangiectasia clinic and underwent brain MR imaging between January 19, 2018, and December 3, 2020, were identified. MR imaging examinations were reviewed retrospectively by 2 experienced neuroradiologists, and the presence of malformations of cortical development and AVMs was confirmed by consensus. Demographic and clinical information was collected for each case, including age, sex, hereditary hemorrhagic telangiectasia status by the Curacao Criteria, mutation type, presence of malformations of cortical development, presence of brain AVMs, presence of pulmonary AVMs, and a history of seizures or learning disabilities.
RESULTS:
Five of 141 (3.5%) patients with hereditary hemorrhagic telangiectasia had malformations of cortical development. Two of the 5 patients with polymicrogyria also had closed-lip schizencephaly. One of the patients had a porencephalic cavity partially lined with heterotopic GM. The incidence of spatially coincident polymicrogyria and brain AVMs was 40% (2/5 cases). Of the patients with hereditary hemorrhagic telangiectasia and malformations of cortical development, 4/5 (80%) had pulmonary AVMs and 2/5 (40%) had brain AVMs.
CONCLUSIONS:
To our knowledge, we are the first group to report the presence of schizencephaly in patients with hereditary hemorrhagic telangiectasia. The presence of schizencephaly and porencephaly lends support to the hypothesis of regional in utero cerebral hypoxic events as the etiology of malformations of cortical development in hereditary hemorrhagic telangiectasia.
Hereditary hemorrhagic telangiectasia (HHT), also known as Osler-Weber-Rendu syndrome, is an autosomal dominant disorder that results from mutations in genes related to blood vessel formation. The 2 primary forms of HHT, HHT1 and HHT2, account for up to 85% of HHT diagnoses. HHT1 is caused by mutations of the ENG gene (coding for endoglin); and mutations in the ACVRL1 gene (coding for ALK1) result in HHT2. The products of these genes are receptor proteins involved in the transforming growth factor β signaling pathway. This pathway regulates normal endothelial cell growth, migration, and proliferation. Without functioning endoglin and ALK1, vessels form abnormal connections and exhibit a tortuous and leaky endothelium that has a tendency to rupture.1
Common clinical symptoms of HHT include recurrent nosebleeds and telangiectasias of the hands, face, and oral cavities. HHT exhibits age-related penetrance with more severe symptoms seen later in life. AVMs can be found in pulmonary (40%–60%), hepatic (50%–75%), gastrointestinal (50%–78%), cerebral (10%), and spinal (1%) circulations. Complications related to CNS AVMs can present as headache, seizure, intracranial hemorrhage, or stroke.
Malformations of cortical development (MCDs) are a diverse group of neurodevelopmental disorders. Cortical development is a highly structured process that involves a complex set of precisely timed events. Disruptions in cell proliferation, neuronal migration, and postmigrational cortical organization, caused by environmental or genetic factors, can result in MCDs.2 The exact mechanisms of MCD formation are largely unknown. The clinical presentation of MCDs is highly variable and ranges from asymptomatic to intellectual disabilities and epilepsy.3 Pathologic findings are equally heterogeneous and may include excessive cortical folding, abnormal cell arrangement, and fusion of gyral surfaces.4
The presence of MCDs in patients with HHT has been reported on 3 previous occasions, once in a pediatric sample5 and twice in adult samples.6,7 The prevalence of MCDs in HHT in these studies ranged between 5% and 12%. To date, nearly all of the MCDs reported in patients with HHT have been polymicrogyrias. Unlike polymicrogyrias in general, polymicrogyrias in patients with HHT are largely unilateral, more focal, and rarely associated with symptoms.
Klostranec et al7 reported a sample of patients with HHT with MCDs that were spatially coincident with AVMs and proposed a mechanism by which endoglin impairment leads to regional hypoperfusion which, in turn, leads to impairment in neuronal migration and cortical organization. Palagallo et al5 reported spatially coincident MCDs and AVMs in one-quarter of their sample. They also found that patients with HHT and MCDs were statistically more likely to have pulmonary and brain AVMs than patients with HHT without MCDs.
In the current study, we evaluated a sample of adults with HHT for the presence of MCDs, spatial coincidence of MCDs and AVMs, and the coincidence of brain and pulmonary AVMs.
MATERIALS AND METHODS
Consent requirements were waived by the internal review board for this retrospective chart review study. A total of 141 patients 18 years of age or older who were referred to the Augusta University HHT clinic and who underwent brain MR imaging between January 19, 2018, and December 3, 2020, were identified. All MR imaging examinations were performed using a 3T field strength and included pre- and postcontrast 3D T1-weighted FSPGR images. MR imaging examinations were reviewed retrospectively by 2 board-certified radiologists who hold Certificates of Added Qualification in Neuroradiology (B.C.G. and S.E.F.) with 7 and 10 years of experience, respectively.
The presence of MCDs and AVMs was confirmed by consensus. MCDs were defined in accordance with Severino et al.8 AVMs were defined as a network of dilated vessels (ie, nidus) with dilated feeding arteries and dilated draining veins. The Curacao Criteria were used to diagnose HHT and included the following: 1) spontaneous and recurrent epistaxis, 2) mucocutaneous telangiectasias, 3) visceral AVMs, and 4) HHT diagnosis in a first-degree relative. The diagnosis of HHT was considered “definite” if 3 or 4 of these criteria were met, “possible” if 2 criteria were met, and “unlikely” if <2 criteria were met.9
Demographic and clinical information was collected from the medical record for each case, including age, sex, HHT status by the Curacao Criteria, mutation type, presence of MCDs, presence of brain AVMs, presence of pulmonary AVMs (including the presence of macroscopic pulmonary AVMs or a delayed right-to-left shunt on contrast echocardiography), and a history of seizures or learning disabilities. Descriptive and inferential statistics were calculated with StatPlus software (https://statplus.io/). The Fisher exact test was performed with a threshold of P < .05 established for statistical significance.
RESULTS
Patient demographic data are summarized in the Table. Five of 141 (3.5%) patients with HHT had MCDs. Of the 5 patients with HHT with MCDs, all 5 had polymicrogyria. All 4 patients with HHTs with polymicrogyria in whom testing was performed had endoglin mutations. Two of the 5 patients with polymicrogyria also had CSF-containing clefts lined with polymicrogyria (ie, schizencephaly), both of which were of the closed-lip variety (Figs 1 and 2). One of the patients had a porencephalic cavity partially lined with heterotopic GM (Fig 3). The other 2 cases of polymicrogyria included an 18-year-old man (Fig 4) and a 28-year-old man (Fig 5).
Summary of 141 patients
| Demographics | Summary |
|---|---|
| Sex | |
| Male | 36.9% |
| Female | 63.1% |
| Age (mean) (yr) | 45.3 |
| MCD | |
| Yes | 5 |
| No | 136 |
| HHT mutation | |
| Endoglin | 35.4% |
| ALK 1a | 27.7% |
| SMAD4a | 2.8% |
| RASA1 | 0.7% |
| Negative × 5 | 15.6% |
| Unknown/not tested | 17.7% |
| AVMs | |
| Brain AVM | 12.0% |
| Brain AVM (possible) | 2.8% |
| Pulmonary AVM (macroscopic)b | 43.3% |
| Pulmonary AVM (microscopic)b | 32.6% |
| Spinal AVM | 0.7% |
| Brain vascular malformations | |
| Developmental venous anomaly | 14.9% |
| Capillary vascular malformation, definite | 1.4% |
| Capillary vascular malformation, possible | 4.3% |
| Curacao category | |
| Definite HHT | 79.4% |
| Possible or suspected HHT | 12.1% |
| Probable | 4.3% |
| Unlikely | 4.3% |
aALK1 includes 2 variants of unknown significance; SMAD4 includes 1 variant of unknown significance.
bPulmonary AVMs were defined as macroscopic if they were definitely visible on a CT scan and microscopic if contrast echocardiography showed a Grade 1 or greater delayed shunt and the CT findings were negative.
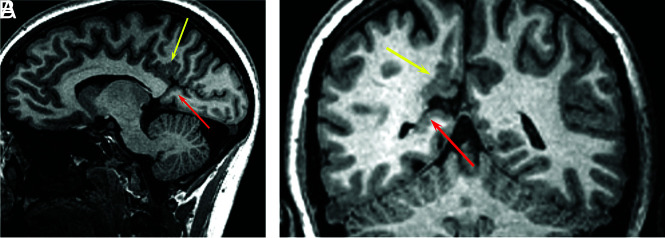
FIG 1.
A 26-year-old woman with HHT. Sagittal 3D fast-spoiled gradient recalled imaging demonstrates polymicrogyria involving the right posterior cingulate gyrus (A, yellow arrow). There is an abnormal GM-lined cleft between the calcarine sulcus and the occipital horn of the right lateral ventricle consistent with schizencephaly (A, red arrow). Coronal T1 image shows polymicrogyria (B, yellow arrow) and schizencephaly (B, red arrow).
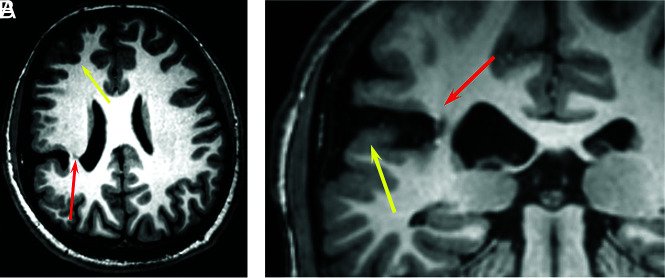
FIG 2.
A 20-year-old man with HHT. 3D volume T1 images demonstrate a closed-lip schizencephaly projecting through the right inferior parietal lobe that extends to the lateral margin of the right lateral ventricle at the junction of the posterior body and atrium (A, red arrow). There is polymicrogyria involving the adjacent frontal and parietal cortex (A, yellow arrow). Coronal T1 image shows polymicrogyria (B, yellow arrow) and schizencephaly (B, red arrow).
FIG 3.
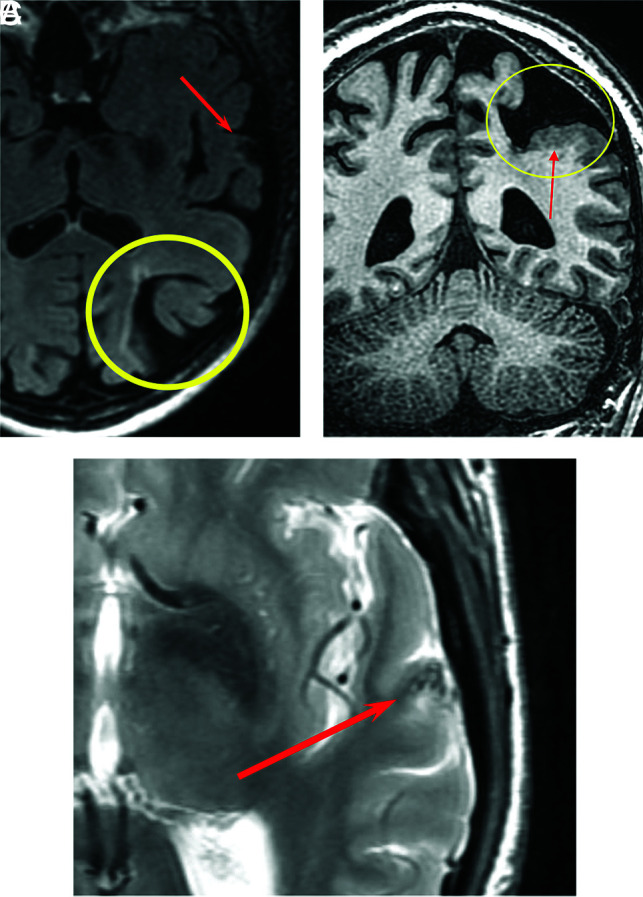
An 80-year-old man with HHT. Axial T2 FLAIR image shows porencephaly with surrounding gliosis (A, yellow circle) and an AVM in the left superior temporal gyrus (A, red arrow). Coronal volume T1 image shows porencephaly (B, yellow circle) partially lined by polymicrogyria (B, red arrow). Axial T2 FSE image shows flow voids associated with a small AVM (C, red arrow).
FIG 4.
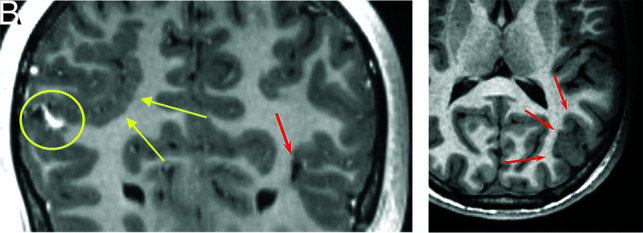
An 18-year-old man with HHT. Coronal 3D T1 postcontrast imaging shows right parietal polymicrogyria (A, yellow arrows), an AVM (A, yellow circle), and a small AVM (A, red arrow). Axial T1 image shows left parietal occipital polymicrogyria (B, red arrows).
FIG 5.
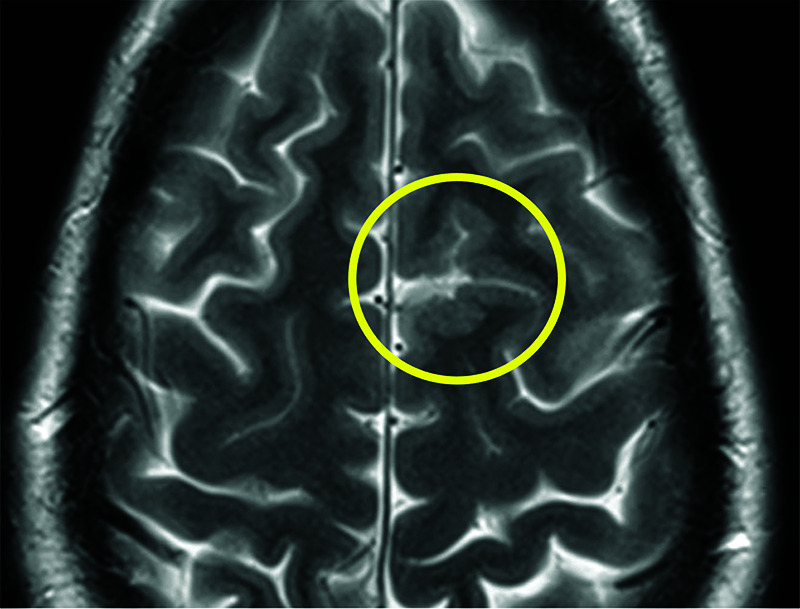
A 28-year-old man with HHT. Axial T2 FSE image shows polymicrogyria in the left superior frontal gyrus (yellow circle).
The incidence of spatially coincident polymicrogyria and brain AVMs in this sample was 40% (2/5 cases; Figs 3 and 4). Neither of the patients with schizencephaly had a spatially coincident brain AVM. Of the patients with HHT and MCDs, 4/5 (80%) had pulmonary AVMs and 2/5 (40%) had brain AVMs. Summary data for patients with HHT and polymicrogyria are seen in the Online Supplemental Data.
Patients with MCDs were not more likely to have coincident brain AVMs than patients without MCDs. Two of 5 (40%) patients with MCDs had brain AVMs, compared with 17/136 (13%) patients without MCDs (P = .11). Patients with MCDs were not more likely to have coincident pulmonary AVMs than patients without MCDs. Four of 5 (80%) patients with MCDs had pulmonary AVMs versus 103/136 (76%) patients without MCDs (P = 1.0).
Three of the patients with MCDs reported a history of migraine headaches. Two of the patients with MCDs were asymptomatic. One of the patients with polymicrogyria reported right-handed contracture and weakness. Both patients with schizencephaly reported a history of migraine headaches. None of the patients with MCDs had a history of seizures or learning disabilities.
DISCUSSION
Five of 141 (3.5%) patients in our sample of patients with HHT had MCDs, in the general range of prevalence observed by other authors examining adult patients with HHT.6,7 Polymicrogyria was present in all 5 of these patients. A novel finding in our sample was the presence of 2 patients with schizencephaly. To our knowledge, we are the first group to report the presence of schizencephaly in patients with HHT. The clefts were unilateral, lined with polymicrogyria, and closed-lip in morphology. Both patients with schizencephaly had a history of migraine headaches. Neither of these patients had a history of seizures or cognitive disabilities. Schizencephaly is now added to the growing list of MCDs reported in HHT, including polymicrogyria,5-7,10-12 nodular GM heterotopia,5 and focal cortical dysplasia.6
Polymicrogyria is thought to arise from a brain insult occurring in the late migration to early cortical organizational stages of corticogenesis.2,4 All patients with HHT with polymicrogyria reported to date, including our sample, have shown focal polymicrogyria, the pattern most commonly associated with hypoxic events and infections. Generalized polymicrogyria is most commonly associated with congenital cytomegalovirus infection and peroxisomal disorders.13 Overall, polymicrogyria is most commonly perisylvian in location and bilateral.4 All reported cases of polymicrogyria in HHT to date, including our cases, are unilateral and not strictly perisylvian in location.
Schizencephaly is widely believed to be the result of a destructive lesion, particularly prenatal ischemic injuries.4,14-17 Porencephaly and schizencephaly are both considered to result from destructive lesions that occur within different developmental timeframes. If the destructive lesion occurs within 4–6 months’ gestation, neuroglial migration is possible and polymicrogyria or GM heterotopia along the margins of the cavity will result. After 6 months’ gestation, the involved brain parenchyma is resorbed. Because neuroglial migration is no longer possible, the cavity is not lined with polymicrogyria or GM heterotopia.3,16 The presence of patients with MCDs partially outlining porencephalic cavities in the current sample and in a previous report5 may indicate that the ischemic events in these patients occurred at the transition between the late migrational and early cortical organizational stages of corticogenesis.
We were not able to replicate an earlier finding of the high rate of spatially coincident MCDs and brain AVMs.7 Spatial coincidence of brain AVMs and MCDs has been described by multiple groups.5,10-12 Only 2 of our patients had an AVM spatially related to an MCD. Palagallo et al5 reported spatial coincidence of MCDs and AVMs in only 3 of 12 patients. There are many possible explanations for these discrepant findings. First, it is possible that MCDs or AVMs were present but below current MR imaging resolution. Second, although suggestive imaging findings were not present, it is possible that spatially coincident AVMs were present and had spontaneously resolved. Third, spatially coincident AVMs may have not yet developed at the time of imaging. Finally, it is possible that spatial coincidence is an inconstant finding.
Patients with HHT with MCDs in our sample were not more likely have pulmonary AVMs than patients with HHT without MCDs. As in previous studies, all patients with HHT with MCDs in our sample who were tested had HHT1 and did not have clinically evident seizures or developmental or cognitive disabilities.
Our study has a number of limitations, first and foremost being the retrospective nature of this study. Another limitation is the small sample of patients obtained with this rare disease. Although up-to-date MR imaging protocols for HHT were used throughout the study period, it is possible that additional MCDs and AVMs may not have been detected due to limitations in current MR imaging protocols.
CONCLUSIONS
Our study replicates many of the findings of previous studies showing an association between HHT and MCDs. To our knowledge, we are the first group to report the presence of schizencephaly in patients with HHT. The presence of schizencephaly and porencephaly lends support to the hypothesis of regional in utero cerebral hypoxic events as the etiology of MCDs in HHT.
Supplementary Material
ABBREVIATIONS:
- HHT
hereditary hemorrhagic telangiectasia
- MCD
malformation of cortical development
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.McDonald J, Wooderchak-Donahue W, VanSant Webb C, et al. Hereditary hemorrhagic telangiectasia: genetics and molecular diagnostics in a new era. Front Genet 2015;6:1–8 10.3389/fgene.2015.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barkovich AJ, Guerrini R, Kuzniecky RI, et al. A developmental and genetic classification for malformations of cortical development: update 2012. Brain 2012;135:1348–69 10.1093/brain/aws019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raybaud C, Widjaja E. Development and dysgenesis of the cerebral cortex: malformations of cortical development. Neuroimaging Clin N Am 2011;21:483–543, vii 10.1016/j.nic.2011.05.014 [DOI] [PubMed] [Google Scholar]
- 4.Squier W, Jansen A. Polymicrogyria: pathology, fetal origins and mechanisms. Acta Neuropathol Commun 2014;2:80 10.1186/s40478-014-0080-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palagallo GJ, McWilliams SR, Sekarski LA, et al. The prevalence of malformations of cortical development in a pediatric hereditary hemorrhagic telangiectasia population. AJNR Am J Neuroradiol 2017;38:383–86 10.3174/ajnr.A4980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergerot JF, Dupuis-Girod S, Berthezene Y, et al. Malformations of cortical development and brain vessels in patients with hereditary haemorrhagic telangiectasia. In: Proceedings of the International Hereditary Hemorrhagic Telangiectasia Scientific Conference, Cork, Ireland; June 12–15, 2013 [Google Scholar]
- 7.Klostranec JM, Chen L, Mathur S, et al. A theory for polymicrogyria and brain arteriovenous malformations in HHT. Neurology 2019;92:34–42 10.1212/WNL.0000000000006686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Severino M, Geraldo AF, Utz N, et al. Definitions and classification of malformations of cortical development: practical guidelines. Brain 2020;143:2874–94 10.1093/brain/awaa174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faughnan ME, Mager JJ, Hetts SW, et al. Second International Guidelines for the Diagnosis and Management of Hereditary Hemorrhagic Telangiectasia. Ann Intern Med 2020;173:989–1001 10.7326/M20-1443 [DOI] [PubMed] [Google Scholar]
- 10.Abe T, Singer RJ, Marks MP, et al. Arterial vascular abnormality accompanying cerebral cortical dysplasia. AJNR Am J Neuroradiol 1997;18:144–46 [PMC free article] [PubMed] [Google Scholar]
- 11.Shankar JJ, Banerjee ST, Hogan M, et al. A rare case of cerebral cortical dysplasia with arterial vascular dysplasia. Can J Neurol Sci 2009;36:757–60 10.1017/s0317167100008398 [DOI] [PubMed] [Google Scholar]
- 12.Villa D, Cinnante C, Valcamonica G, et al. Hereditary hemorrhagic telangiectasia associated with cortical development malformation due to a start loss mutation in ENG. BMC Neurol 2020;20:316 10.1186/s12883-020-01890-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leventer RJ, Jansen A, Pilz DT, et al. Clinical and imaging heterogeneity of polymicrogyria: a study of 328 patients. Brain 2010;133:1415–27 10.1093/brain/awq078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curry CJ, Lammer EJ, Nelson V, et al. Schizencephaly: heterogeneous etiologies in a population of 4 million California births. Am J Med Genet A 2005;137:181–89 10.1002/ajmg.a.30862 [DOI] [PubMed] [Google Scholar]
- 15.Barkovich AJ, Rowley H, Bollen A. Correlation of prenatal events with the development of polymicrogyria. AJNR Am J Neuroradiol 1995;16:822–27 [PMC free article] [PubMed] [Google Scholar]
- 16.Griffiths PD. Schizencephaly revisited. Neuroradiology 2018;60:945–60 10.1007/s00234-018-2056-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yakovlev PI, Wadsworth RC. Schizencephalies; a study of the congenital clefts in the cerebral mantle; clefts with hydrocephalus and lips separated. J Neuropathol Exp Neurol 1946;5:169–206 10.1097/00005072-194607000-00001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.