Abstract
The Flinders sensitive line (FSL) genetic animal model of depression exhibits marked immobility during forced swimming, an accepted index of depressivelike behavior in rodent depression models. The present experiment tested the hypothesis that swim test behavior in the FSL rats is influenced in part by early experience, specifically maternal environment. Male FSL and control Flinders resistant line (FRL) pups were cross fostered onto dams of the same or complementary strain. Nest quality and dam behavior during pup retrieval were measured on PN5 and PN8, and swim test behavior assessed in the adult males on PN60. FSL rats reared by foster FRL dams were significantly less immobile than FSL rats raised by FSL dams, but still significantly more immobile that the two FRL groups, which did not differ from each other. FSL dams took significantly longer to retrieve their pups and dropped them more often than the FRL control dams. Moreover, strain differences in maternal retrieval behavior significantly predicted later swim test immobility in the FSL animals. These findings suggest that swim test immobility in the FSL rats is modified by maternal environment. In contrast, the FRL control rats were relatively insensitive to the influence of maternal environment. The FSL model offers promise for understanding the interactions of genetic vulnerabilities and environmental influences in the etiology of clinical depression.
Keywords: FSL rat model of depression, FRL control rats, swim test immobility, cross-fostering, maternal behavior, pup retrieval, USVs
INTRODUCTION
Clinical depression is thought to result from the interaction of genetic vulnerability and psychological perturbation, particularly early in life (Heim, Plotsky, & Nemeroff, 2004). Animal models of depression produced from selective breeding may thus be particularly useful in illuminating gene-environment interactions that contribute to depression. One prominent animal model of depression, the Flinders sensitive line (FSL), was selectively breeding for cholinergic hypersensitivity, and has been shown to meet face, construct, and predictive validity criteria for a depression model (Overstreet, 1993, 2002). In studies of depressivelike behavior, for example, FSL animals reliably exhibit significantly greater immobility than Flinders resistant line (FRL) or outbred Sprague–Dawley (SD) control animals during forced swimming (Overstreet, 1993, 2002), a measure commonly used in the evaluation of depression like behavior in animal modes (Cryan, Markou, & Lucki, 2002). Importantly, and consistent with clinical literature on the latency of antidepressant effects in depressed patients, chronic but not acute administration of either the tricyclic antidepressant desipramine (Zangen, Overstreet, & Yadid, 1999) or the selective serotonin reuptake inhibitor paroxetine (Zangen, Overstreet, & Yadid, 1997) significantly reduces swim test immobility in the FSL rats to levels observed in both FRL and SD controls (see review by Overstreet, 2002). While the behavioral characteristics of FSL rats are hypothesized to result from selective breeding, the extent to which early experience contributes to these characteristics is unknown. The present study tested the hypothesis that swim test immobility in the FSL rats is the product of both genetic and early environmental influences rather than genes alone.
To test this hypothesis, FSL and control FRL pups were cross fostered to dams of the same or opposite strain. Cross fostering has been used in previous research to examine the contribution of early experience to adult phenotypes in other genetic models. Spontaneously hypertensive (SHR) rat pups cross-fostered onto normotensive Wistar–Kyoto or Sprague-Dawley dams, for example, show significant reductions in mean arterial blood pressure as adults compared to SHR rats raised by their biological dams or by nonbiological dams of the same strain (Cierpial & McCarty, 1987; McCarty & Lee, 1996), an effect that is thought to involve differences in early nutrition (Cierpial, Shasby, & McCarty, 1987; McCarty, Cierpial, Murphy, Lee, & Fields-Okotcha, 1992). Our primary research hypothesis was that adult swim test behavior in cross fostered animals would be modified by early environment in both rat strains. Specifically, FSL pups raised by FRL dams would become less immobile than FSL raised by FSL dams, while FRL pups raised by FSL dams would become more immobile than those raised by FRL dams. An alternative hypothesis was that changes in adult behavior would be limited to the FSL rats. This result would suggest that the FSL genotype alone confers a vulnerability to some aspects of the maternal environment that can influence adult swim test behavior. Previous studies examining the effects of chronic mild stress in FSL and FRL control rats, for example, suggest the FSL rats may be more sensitive to environmental challenges (Ayensu et al., 1995; Pucilowski, Overstreet, Rezvani, & Janowsky, 1993).
We used two established measures of maternal behavior—pup retrieval and nest quality—to examine potential correlates of adult swim test behavior. During pup retrieval pups are removed from the nest and placed on the opposite side of the cage, and dams are observed and timed as they return the pups to the nest (Stern, 1996). Dam behavior during pup retrieval is sensitive to a variety of influences, including genetic ones: female rats that were selectively bred for low avoidance in a passive avoidance task showed greater latencies to retrieve their pups and failed to retrieve more pups during the test session than dams bred for high avoidance (Ohta, Shirota, Tohei, & Taya, 2002). Nest quality is determined after the existing nest is disrupted and the dams given a fixed amount of time during which to rebuild it. Previous work has shown, for example, that lactating female rats treated with lipopolysaccharide, a model of depression like sickness behavior (Dantzer et al., 1998), build nests of lower quality than control females (Aubert, Goodall, Dantzer, & Gheusi, 1997). Both of these measures involved disruptions of the maternal environment, and therefore constituted mild stressors for the dams. Earlier work showing that saccharin intake is significantly lower in FSL rats undergoing chronic mild stress than in FRL controls suggests that FSL animals may be more sensitive to stressors than FRL rats (Pucilowski et al., 1993), and we hypothesized that pup retrieval efficiency and nest quality would both be reduced in the FSL dams compared to FRL controls. Moreover, we hypothesized that these differences in maternal behavior would be significantly related to the swim test behavior of the pups once they reached adulthood.
Finally, we assessed the emotional status of the pups by recording ultrasonic vocalizations (USVs) during brief social isolation. USVs with frequencies in the range of 30–90 kHz are emitted by neonatal rat pups between the ages of 5 and 20 days of age and typically elicit caregiving behaviors from the dam (Hofer, 1996). The rate of vocalization is affected by myriad physical and social factors, and increased calling is associated with higher levels of anxiety in the pup (Branchi, Santucci, & Alleva, 2001; Hofer, 1996). We considered pup emotional status relevant both as an index of the emotional impact of maternal environment (and therefore as a possible predictor of adult swim test behavior) as well as a potential influence on strain differences in maternal behavior. Again because of their increased sensitivity to stress (Ayensu et al., 1995; Pucilowski et al., 1993), we hypothesized that brief social isolation would elicit more intense calling by FSL pups compared to FRL controls.
MATERIALS AND METHODS
Animals
The subjects for this study were 54 male FSL (n = 26) and FRL (n = 28) rats and 14 FSL (n = 7) and FRL (n = 7) dems bred and in the Williams College Animal Facility. Pups were weaned on PN28 and placed into pairs or triplets in pathogen-free conditions. The colony room was maintained at 22°C with a 12:12 light:dark cycle with lights on at 0600 and off at 1800. Food and water were available ad libitum. All adult rats were handled for 3–4 min daily for at least 1 week before swim testing to reduce stress associated with human contact. During handling, experimenters removed the rats from their cages individually, placed them on an arm or lap, and allowed them to explore freely. The experimenters remained blind to adult animal strain and rearing condition until all testing was complete.
Cross-Fostering Procedure
In order to insure that any observed strain differences in maternal behavior were not influenced by the novelty of a first pregnancy, all dams in this study were multiparous. Fourteen male-female pairs—seven FSL pairs and seven FRL pairs—were placed into novel cages on the same day and housed together for 2 weeks, at which time all breeding males were removed from the cages. No later than 3 days after birth (all dams gave birth within 3 days of one another), rat litters were randomly assigned to one of two rearing conditions: with a dam of the same strain or with a dam of the opposite strain. Analyses indicated that the postnatal day on which pups were cross fostered did not influence dam or pup behavior in any test setting. This procedure generated a total of 14 litters with the following distribution (male pups only): FSL pups reared by FSL dams (n = 10), FSL pups reared by FRL dams (n = 16), FRL pups raised by FSL dams (n = 16), and FRL pups reared by FRL dams (n = 12). To control for any psychological or physiological effects of the cross-fostering procedure, all pups were cross-fostered—no pups were reared by their biological dams. In addition, all litters were culled to four males and two females at the time of cross-fostering to insure consistent litter size and composition. In two instances (both FSL pups with an FSL dam), original litters contained only three males, and the final cross-fostered litters contained three males and three females. Potential differences between these and other litters were controlled in the statistical analyses.
Nest Construction and Pup Retrieval
Ratings of nest quality and pup retrieval tests took place on PN5 and PN8. The timing of the test days was intended to reduce the potential confounding influences of both cross fostering and prior behavioral testing while permitting measurement of maternal behavior while the pups were still in early infancy. Before parturition dams were provided with finely sliced strips of newspaper and given the opportunity to construct nests. On the day of testing nest quality was rated on a 4-point scale (Quinones-Jenab, Batel, Schlussman, Ho, & Kreek, 1997): (1) no nest; (2) saucer-shaped nest; (3) nest with raised sides; (4) fully enclosed nest. After the nest was rated, the dam was removed from the home cage and placed in a holding cage. Pups were weighed and then lined up along the side of the home cage opposite the nest. The dam was then returned to the home cage and the following behaviors were recorded: latency to retrieve first pup, number of times pups were dropped, and total time to return all pups to the nest. Test sessions lasted 15 min, and dams that had not returned all pups to the nest after 15 min were given a score of 600 s for total retrieval time. After the retrieval evaluation on PN5, nests were disturbed and reevaluated on PN8. Other than nest disruption, cages were left undisturbed through PN8.
USV Recordings
To examine strain differences in vocal distress responses to brief social isolation, a subset of FSL and FRL pups (n = 31) were removed from their home cages on PN15 and placed on a heating pad (maintained at 32°C) for a period of 20 min. Then pups were placed individually into glass containers and a bat detector (Ultra Sound Advice, London, UK) was secured 12 cm above each container to register USVs. The bat detector was set between 55 and 60 kHz. Previous work has shown that this frequency range is appropriate for pups of this age (Brudzynski, Kehoe, & Callahan, 1999). The number of USVs emitted in a 2-minute isolation session was then tabulated by computer. Testing was conducted on PN15 to avoid potential bi-directional influences between USV recordings and evaluation of maternal behaviors.
Forced Swim Test
All rats were tested in a modified version of Porsolt’s forced swim test (Porsolt, Anton, Blavet, & Jalfre, 1978) at approximately PN60. Initially rats were placed individually into an opaque plastic cylinder filled approximately 30 cm high with 25°C water for 15 min. Rats were removed from the cylinder, dried, and placed in a warming cage for no less than 5 min before being returned to their home cages. Forty-eight hours after the initial test, rats were placed individually in an identical cylinder for 5 min. This session was recorded to videotape by way of a closed-circuit camera mounted on the ceiling directly above the swim cylinder, and videotapes were later scored for the following behaviors: swimming (horizontal movement in the water), climbing (any upward movement of the forepaws on the walls of the cylinder), and immobility (no movements beyond those needed to keep the head above water). Data are expressed as percent of the total 5 min spent swimming, climbing, or immobile. The number of feces excreted into the water was also counted as an indirect measure of anxiety.
Statistical Analyses
Data were analyzed by analysis of variance (ANOVA), and main and interaction effects were considered significant at p < .05. Differences between and among specific group means were examined with one-way ANOVA or Tukey HSD post hoc tests as appropriate. Relationships between maternal and swim test behaviors were examined using a multivariate linear regression analysis. Litter effects are a concern in any study of this kind (Zorrilla, 1997), and because of the limited sample size, we controlled for litter effects by including litter as a covariate in ANOVAs and the regression analysis. We also used robust standard errors in the analyses. Robust standard errors are used for more conservative inferences about the significance of variables in light of potential model misspecification (Huber, 1967; White, 1982). The Huber/White procedure in no way eliminates the problem of unobserved heterogeneity, such as litter effects, but it does lead to more conservative errors in light of model misspecification (of which failing to account for unobserved heterogeneity is one example).
RESULTS
Forced Swim Test
A two-way ANOVA revealed significant main effects for both pup strain [F(1, 50) = 90.54, p < .01] and dam strain [F(1, 50) = 5.55, p < .05] on immobility scores, but there was also a significant interaction effect [F(1, 50) = 5.04, p < .05]. Consequently, one-way ANOVAs were carried out. FSL rats that were cross fostered onto FRL dams by PN3 were significantly less immobile than FSL pups raised by FSL dams, F(1, 24) = 10.12, p < .01, but significantly more immobile than all of the FRL rats, F(1, 42) = 39.97, p < .01. In contrast, there was no impact of maternal strain on swim test immobility among FRL rats, F(1, 26) = 0.007, p > .05 (Fig. 1). As expected, the percentage of time spent actively swimming showed the opposite pattern of results (Fig. 1), and although FRL rats spent more time climbing than FSL rats, this difference was not statistically significant, F(1, 50) = 1.72, p > .05 (Fig. 1). Finally, FRL rats on average produced more feces during the 5 min swim test than FSL rats, F(1, 49) = 6.63, p < .05, and animals reared by FRL dams produced significantly more feces than those reared by FSL dams, F(1, 49) = 6.12, p < .05. Means comparisons showed that both effects were largely mediated by statistically significant differences between FRL rats reared by FRL dams and FSL rats raised by FSL dams (data not shown).
FIGURE 1.

Swim test behaviors of adult male FSL (n = 26) and FRL control rats (n = 28). All rats were cross-fostered onto FSL or FRL dams within 3 days of birth. (A) Mean ± SEM percent time immobile for FSL and FRL rats in a modified version of the Porsolt swim test. (B) Mean ± SEM percent time spent swimming for FSL and FRL control rats. (C) Mean ± SEM time spent climbing for FSL and FRL control rats. (a) significantly different from FRL rats (p < .001); (b) significantly different from FSL rats raised by FSL dams (p < .05); (c) significantly different from FRL rats (p < .01).
Maternal Behavior
The swim test data suggested that strain differences in immobility were susceptible to modulation by maternal environment. To examine potential associations with specific aspects of maternal behavior we assessed quality of nest construction and various aspects of dam behavior during pup retrieval. Nest quality was typically high—rated 3–4 on the scale—and there were no statistically significant differences among any of the rearing groups. During pup retrieval, however, FSL dams generally performed less efficiently than FRL dams, although strain differences were more pronounced on PN5 than PN8. There were no statistically significant strain differences in the latency to begin retrieving pups, although on PN5 FSL dams took longer to begin retrieval, a marginally significant effect, F(1, 10) = 3.14, p = 0.10. Total retrieval time was significantly greater for FSL dams than for FRL dams on PN5, F(1, 10) = 14.11, p < .01 and marginally greater on PN8, F(1, 10) = 3.15, p = 0.10, and dams were marginally more likely to take longer retrieving FSL pups than FRL pups, F(1, 10) = 3.66, p < .10. Finally, FSL dams dropped their pups significantly more often on PN5 than FRL dams, F(1, 10) = 8.42, p < .05; there were no statistically significant group differences on PN8. Pup retrieval data are shown in Figure 2.
FIGURE 2.
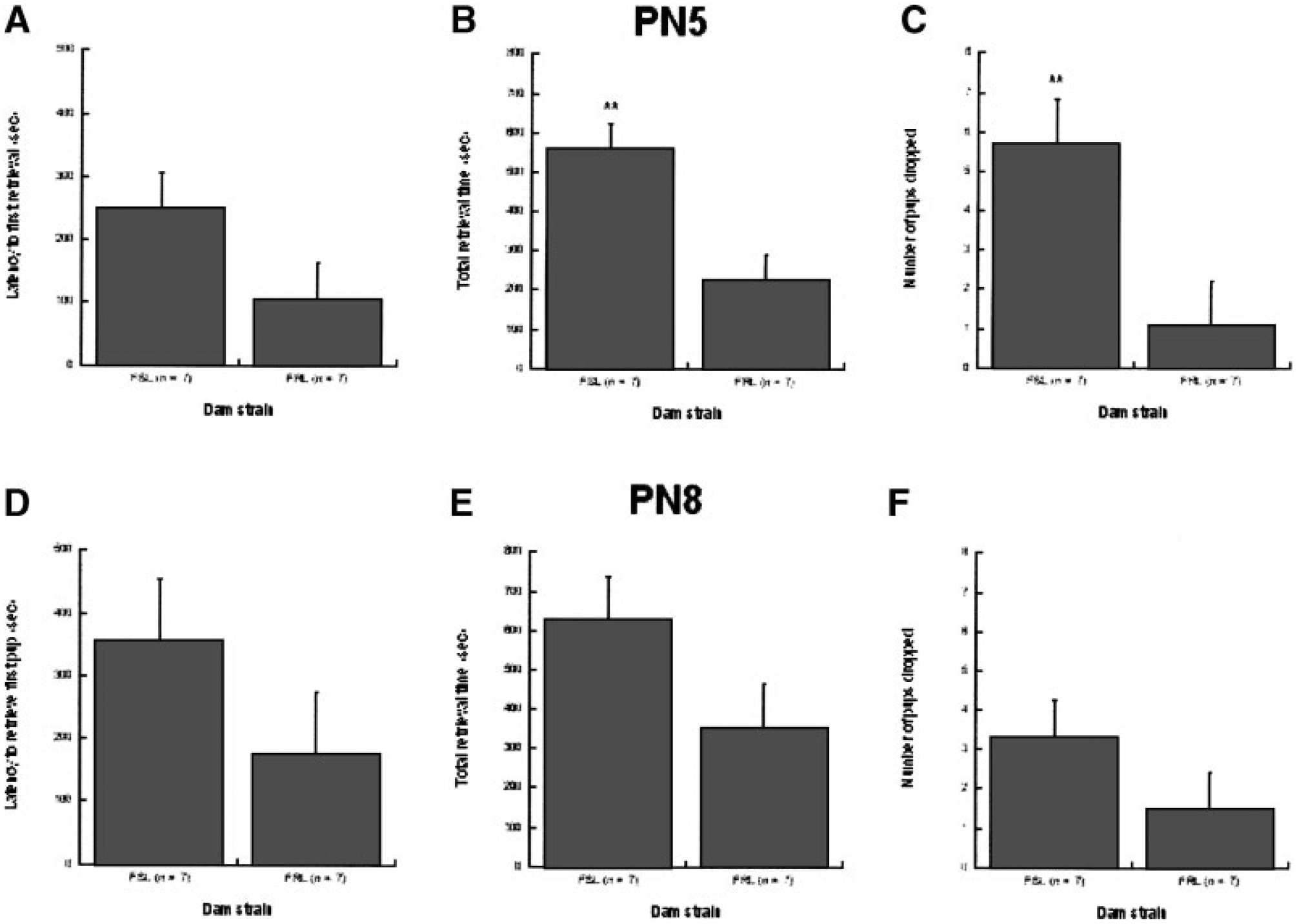
Maternal behaviors of FSL (n = 7) and FRL (n = 7) dams during pup retrieval task. All pups were cross-fostered to same-strain or opposite-strain dams. (A–C) PN5: Mean ± SEM latency to begin pup retrieval, total retrieval time, and total number of pup drops. FSL dams took significantly longer to retrieve their pups (p < .01) and dropped them more frequently (p < .05) than FRL dams. (D–F) PN8: Mean ± SEM latency to begin pup retrieval, total retrieval time, and total number of pup drops. *p < .05; **p < .01.
Regression Analysis
The foregoing analyses revealed similar patterns of maternal behavior on PN5 and PN8, although strain differences in behavior were only statistically significant on PN5. Multivariate linear regression analyses were then used to determine the extent to which maternal behavior during pup retrieval predicted swim test immobility in the pups once they reached PN60. The regression analyses were designed to test the two competing hypotheses outlined earlier. First, to test the hypothesis that maternal behavior would affect all pups equally, total retrieval time, latency to first pup retrieval, and number of pups dropped on both PN5 and PN8 were entered into a regression model. Due to significant collinearity among predictor variables, data for PN5 and PN8 were analyzed separately. Only total retrieval time on PN5 significantly predicted swim test immobility, β = .41, t(51) = 3.26, p < .01. To test the alternative hypothesis that the FSL rats would be particularly sensitive to cross fostering, pup strain was dummy coded, and a variable for the interaction between pup strain and PN5 total retrieval time created by multiplying the two variables together (Aiken & West, 1996). These variables were then entered into the model at the second step. This analysis showed that the interaction between pup strain and PN5 total retrieval time significantly predicted swim test immobility, β = .97, t(51) = 8.2, p < .001. To determine the nature of this interaction we examined the association between maternal behavior and swim test immobility in each pup strain independently. The results showed that PN5 total retrieval time significantly predicted swim test immobility in the FSL rats (β = .59, t(25) = 3.6, p = .001), but not in the FSL controls (β = −.04, t(27) = −.20, P >.05; Fig. 3).
FIGURE 3.
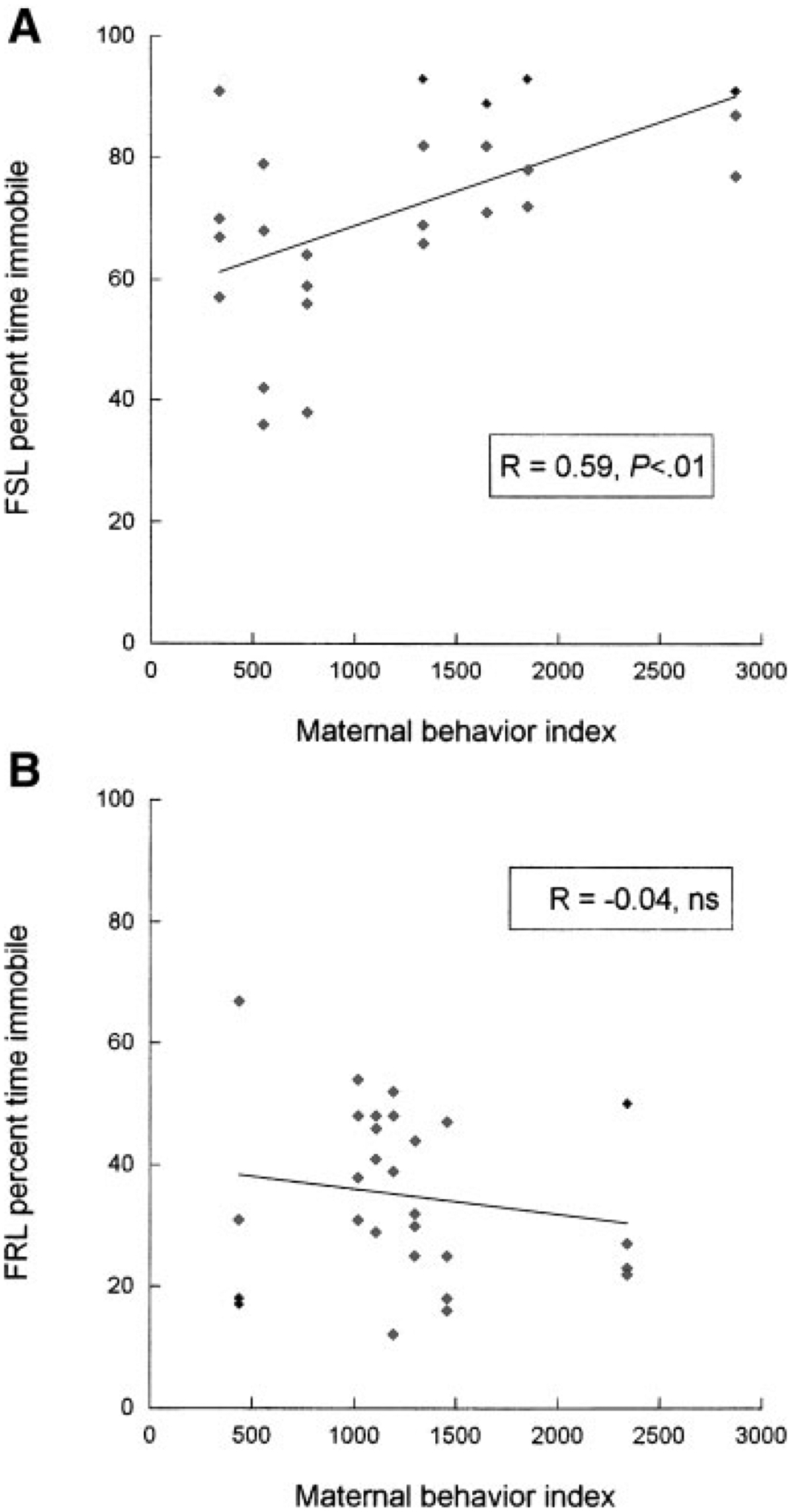
Correlation between PN5 total retrieval time and adult swim test immobility. A multivariate linear regression analysis indicated a significant interaction between pup strain and PN5 retrieval time (p < .05), and independent regressions on FSL and FRL rats showed that this index significantly predicted adult swim test immobility in adult male FSL rats (top; p < .001) but not FRL rats (bottom).
USV Recordings
USVs emitted during acute social isolation were measured on PN15 in a subset of the pups. During the 2 min testing period, pups that were cross fostered onto FSL dams called significantly more than pups being reared by FRL dams, F(1, 27) = 8.07, p < .01. Contrary to our expectations, FSL (M = 20.89) and FRL (M = 20.5) pups emitted almost identical numbers of calls, and there was no dam strain x pup strain interaction, F(1, 27) = 0.18, p > .05 (Fig. 4). Interestingly, the inclusion of female pups into the statistical analyses generated a significant gender effect, with the females calling significantly more than males, F(1, 38) = 9.89, p < .01, and a marginally significant strain difference, with FSL pups emitting more calls than FRL pups, F(1, 38) = 3.41, p < .07 (data not shown). Regression analyses showed that USVs emitted on PN15 did not predict later swim test behavior in any of the rearing conditions and was unrelated to strain differences in immobility.
FIGURE 4.
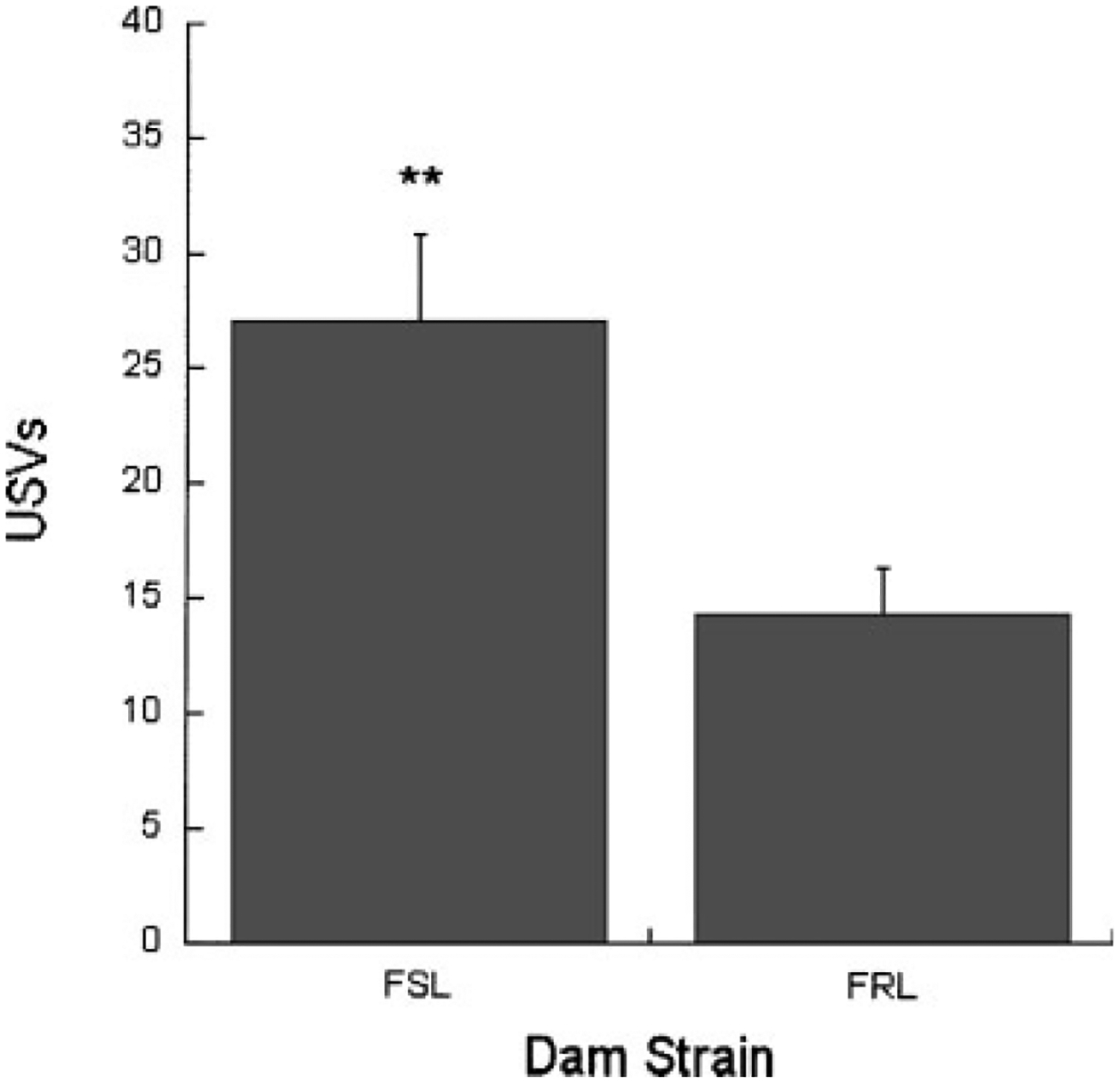
Mean ± SEM number of USVs emitted by FSL (n = 15) and FRL (n = 16) pups during brief (2 min) social isolation. Pups reared by FSL dams called significantly more than pups reared by FRL dams (p < .01).
DISCUSSION
This study tested the hypothesis that swim test immobility in the FSL rat model of depression would be modulated by early experience, and the results supported this hypothesis. Consistent with our predictions, FSL rats cross fostered as pups onto control FRL dams were significantly less immobile during forced swimming as adults than FSL rats raised by FSL dams. In contrast, FRL rats raised by FSL dams did not differ from FRL rats raised by FRL dams in swim test behavior. Collectively, these results suggest that adult behavior in the FSL rats is affected by maternal influences, but FRL rats are relatively insensitive to these influences. These results are also the first to show that a hallmark behavior of the FSL rats has a developmental component.
To examine potential maternal behavioral correlates of swim test behavior in the adult cross fostered rats, we assessed nest quality and pup retrieval on PN5 and PN8. Although there were no strain differences in nest quality, FSL dams took longer to complete pup retrieval and dropped more of the pups than FRL dams. This was true on both test days, but the strain differences were only statistically significant on PN5. Anecdotally, the FSL dams did not appear lethargic or uninterested in the pups during this task; rather, their behavior was disorganized and somewhat frenetic. Regression analyses showed that PN5 total retrieval time significantly predicted later swim test immobility in the FSL pups as adults. FRL swim test behavior was unrelated to maternal behaviors.
We did not measure other aspects of maternal behavior, such as grooming or nursing, that have been implicated in the normal development of pup behavior and brain function (Caldji et al., 1998; Liu et al., 1997; Meaney, Brake, & Gratton, 2002). The relationships between these behaviors and pup retrieval behavior measured in this study are not clear, although there is reason to believe that they may be related. Mostly, it is unlikely that two exposures to the pup retrieval task would have had a lasting impact on pup behavior. Nevertheless, dam behavior during pup retrieval significantly predicted swim test immobility in the FSL pups, and the most parsimonious explanation for these results is that pup retrieval behavior was associated with other maternal behaviors that more directly influenced swim test immobility. This possibility is supported by a recent study in which FSL dams showed decreased licking and nonnutritive contact with their biological pups as well as greater stress-induced latencies in retrieving their pups during a pup retrieval task compared to control dams (Lavi-Avnon, Yadid, Overstreet, & Weller, 2005). Interestingly, and consistent with the results of the present study, FSL dams did not differ from SD dams in their nest building behavior either under resting conditions or after a swim stress (Lavi-Avnon et al., 2005). Coupled with results of the present study, these observations converge on the hypothesis that abnormal maternal behavior in the FSL dams, indexed by reduced licking, contact, and efficiency of pup retrieval, contributes to the high levels of immobility typically seen in adult FSL rats. Future studies in which a broad range of maternal behaviors are examined in the context of cross fostering will be required to fully test this hypothesis.
Another potential influence on dam behavior, other than dam strain, was the behavior of the strain of the pups they were raising. The reciprocal influences of dam and pup behavior have been well documented (Fleming, O’Day, & Kraemer, 1999; Magnusson & Fleming, 1995), and previous studies of cross-fostered SHR and normotensive rats, for example, showed that dam behavior shifted to resemble the strain of the pups they were rearing (Cierpial, Murphy, & McCarty, 1990). The results of the present study, however, do not support a prominent influence of pup behavior on dam behavior. With the exception of a marginally significant difference in pup retrieval time on PN5, behavior during pup retrieval was more consistent between dams of the same strain than between dams rearing the same strain of pups. In addition, USV data showed that pups being raised by FSL dams called more than pups being raised by FRL dams while pup strain had no significant effect on calling. As we noted above, these are incomplete measures of potential reciprocal influences of pup and dam behavior, and it is possible that other measures of home cage maternal behavior may reveal a greater influence of pup behavior. Nevertheless, these results suggest that the dam behaviors we measured were influenced more by dam strain than pup strain.
In contrast to the FSL strain, swim test behavior in the FRL rats appeared to be governed more by their genetic background than by their early environment. Swim test behaviors in the FRL rats raised by FSL dams were virtually indistinguishable from those in FRL pups raised by FRL dams. In addition, the association between PN5 maternal retrieval behavior and swim test immobility was only significant for FSL rats; there was no relationship for FRL animals. Some aspects of FRL pup behavior were influenced by dam strain; FRL pups being raised by FSL dams called more on PN15 than those being raised by FRL dams. However, this influence did not appear to last into adulthood. These data suggest that while the FSL strain is a valuable model for the etiology of clinical depression, the FRL strain may be a valuable model of resilience.
The results of this study echo the findings of a parallel line of research focused on neurochemical abnormalities in the FSL rats. Recent work on gene-environment interactions in clinical depression showed that a heritable form of the serotonin transporter gene conferred increased risk of depression in response to life stress (Caspi et al., 2003). Previous studies involving the FSL animals have shown that levels of serotonin (Zangen et al., 1997) and dopamine (Friedman et al., 2005; Zangen, Nakash, Overstreet, & Yadid, 2001) in limbic areas of the brain, particularly the nucleus accumbens, are abnormal in the FSL rats compared to SD controls, and that neurochemical abnormalities underlie strain differences in swim test immobility (Zangen et al., 1997, 2001). These previous studies are interesting in light of the present results, given the well-established role of the mesolimbic dopamine system and the nucleus accumbens in maintaining a range of maternal behaviors, including pup retrieval (Champagne et al., 2004; Keer & Stern, 1999; Numan et al., 2005; Wilkins, Logan, & Kehoe, 1997). Studies focused on pre- and postnatal influences on the developmental relationships between specific neurochemical systems and depressivelike behavior in the FSL rats thus have the potential to contribute to our understanding of the etiology of clinical depression.
In sum, full expression of strain-typical swim test immobility in the FSL genetic animal model of depression requires both genetic and early environmental influences, and certain aspects of dam behavior predict FSL swim test behavior. Future work examining maternal behavior under resting conditions are needed in order to determine whether specific aspects of routine maternal behavior, such as suckling or grooming, are linked to adult pup behavior. These preliminary results support the value of the FSL model for examining the relative contributions of genotype and environmental experience, as well as their interactions, to physiological and behavioral abnormalities that may be pertinent to our understanding of human depression.
Acknowledgments
The authors are grateful to Betty Zimmerberg for the use of her USV recording equipment and to three anonymous reviewers for their invaluable comments on an earlier version of this study. We also thank Jackie Dinzey, Sarah Oboyski, and Abby Davidson for their assistance with experimental procedures. This study was supported by grant 1R15AI052336-01 to E.F.
REFERENCES
- Aiken LS, & West SG (1996). Multiple regression: Testing and interpreting interactions. London: Sage Publishers. [Google Scholar]
- Aubert A, Goodall G, Dantzer R, & Gheusi G (1997). Differential effects of lipopolysaccharide on pup retrieving and nest building in lactating mice. Brain, Behavior, and Immunity, 11, 107–118. [DOI] [PubMed] [Google Scholar]
- Ayensu WK, Pucilowski O, Mason GA, Overstreet DH, Rezvani AH, & Janowsky DS (1995). Effects of chronic mild stress on serum complement activity, saccharin preference, and corticosterone levels in Flinders lines of rats. Physiology and Behavior, 57, 165–169. [DOI] [PubMed] [Google Scholar]
- Branchi I, Santucci D, & Alleva E (2001). Ultrasonic vocalisation emitted by infant rodents: A tool for assessment of neurobehavioural development. Behavioural Brain Research, 125, 49–56. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Kehoe P, & Callahan M (1999). Sonographic structure of isolation-induced ultrasonic calls of rat pups. Developmental Psychobiology, 34, 195–204. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, & Meaney MJ (1998). Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proceeding of the National Academy of Science of the United States of America, 95, 5335–5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, & Poulton R (2003). Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science, 301, 386–389. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, & Meaney MJ (2004). Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. Journal of Neuroscience, 24, 4113–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cierpial MA, & McCarty R (1987). Hypertension in SHR rats: Contribution of maternal environment. American Journal of Physiology, 253, H980–H984. [DOI] [PubMed] [Google Scholar]
- Cierpial MA, Murphy CA, & McCarty R (1990). Maternal behavior of spontaneously hypertensive and Wistar-Kyoto normotensive rats: Effects of reciprocal cross-fostering of litters. Behavioral Neural Biology, 54, 90–96. [DOI] [PubMed] [Google Scholar]
- Cierpial MA, Shasby DE, & McCarty R (1987). Patterns of maternal behavior in the spontaneously hypertensive rat. Physiology and Behavior, 39, 633–637. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, & Lucki I (2002). Assessing antidepressant activity in rodents: Recent developments and future needs. Trends in Pharmacological Sciences, 23, 238–245. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Laye S, Bret-Dibat JL, Parnet P, & Kelley KW (1998). Cytokines and sickness behavior. Annals of the New York Academy of Sciences, 840, 586–590. [DOI] [PubMed] [Google Scholar]
- Fleming AS, O’Day DH, & Kraemer GW (1999). Neurobiology of mother-infant interactions: Experience and central nervous system plasticity across development and generations. Neuroscience and Biobehaviorial Reviews, 23, 673–685. [DOI] [PubMed] [Google Scholar]
- Friedman A, Dremencov E, Crown H, Levy D, Mintz M, Overstreet DH, & Yadid G (2005). Variability of the mesolimbic neuronal activity in a rat model of depression. Neuroreport,16, 513–516. [DOI] [PubMed] [Google Scholar]
- Heim C, Plotsky PM, & Nemeroff CB (2004). Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology, 29, 641–648. [DOI] [PubMed] [Google Scholar]
- Hofer MA (1996). Multiple regulators of ultrasonic vocalization in the infant rat. Psychoneuroendocrinology, 21, 203–217. [DOI] [PubMed] [Google Scholar]
- Huber PJ (1967). The behavior of maximum likelihood estimates under nonstandard conditions. Proceedings of the Fifth Berkeley Symposium on Mathematics, Statistics, and Probability, 1, 223. [Google Scholar]
- Keer SE, & Stern JM (1999). Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiology and Behavior, 67, 659–669. [DOI] [PubMed] [Google Scholar]
- Lavi-Avnon Y, Yadid G, Overstreet DH, & Weller A (2005). Abnormal patterns of maternal behavior in a genetic animal model of depression. Physiology and Behavior, 84, 607–615. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, & Meaney MJ (1997). Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science, 277, 1659–1662. [DOI] [PubMed] [Google Scholar]
- Magnusson JE, & Fleming AS (1995). Rat pups are reinforcing to the maternal rat: Role of sensory cues. Psychobiology, 23, 69–75. [Google Scholar]
- McCarty R, Cierpial MA, Murphy CA, Lee JH, & Fields-Okotcha C (1992). Maternal involvement in the development of cardiovascular phenotype. Experientia, 48, 315–322. [DOI] [PubMed] [Google Scholar]
- McCarty R, & Lee JH (1996). Maternal influences on adult blood pressure of SHRs: A single pup cross-fostering study. Physiology and Behavior, 59, 71–75. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Brake W, & Gratton A (2002). Environmental regulation of the development of mesolimbic dopamine systems: A neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology, 27, 127–138. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Schwarz JM, Neuner CM, Flood TF, & Smith CD (2005). Medial preoptic area interactions with the nucleus accumbens-ventral pallidum circuit and maternal behavior in rats. Behavioural Brain Research, 158, 53–68. [DOI] [PubMed] [Google Scholar]
- Ohta R, Shirota M, Tohei A, & Taya K (2002). Maternal behavior, milk ejection, and plasma hormones in Hatano high- and low-avoidance rats. Hormones and Behavior, 42, 116–125. [DOI] [PubMed] [Google Scholar]
- Overstreet DH (1993). The Flinders sensitive line rats: A genetic animal model of depression. Neuroscience and Biobehavioral Reviews, 17, 51–68. [DOI] [PubMed] [Google Scholar]
- Overstreet DH (2002). Behavioral characteristics of rat lines selected for differential hypothermic responses to cholinergic or serotonergic agonists. Behavior Genetics, 32, 335–348. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, & Jalfre M (1978). Behavioural despair in rats: A new model sensitive to antidepressant treatments. European Journal of Pharmacology, 47, 379–391. [DOI] [PubMed] [Google Scholar]
- Pucilowski O, Overstreet DH, Rezvani AH, & Janowsky DS (1993). Chronic mild stress-induced anhedonia: Greater effect in a genetic rat model of depression. Physiology and Behavior, 54, 1215–1220. [DOI] [PubMed] [Google Scholar]
- Quinones-Jenab V, Batel P, Schlussman SD, Ho A, & Kreek MJ (1997). Cocaine impairs maternal nest building in pregnant rats. Pharmacology and Biochemical Behavior, 58, 1009–1013. [DOI] [PubMed] [Google Scholar]
- Stern JM (1996). Somatosensation and maternal care in Norway rats. In Rosenblatt JS & Snowdon CT (Eds.), Parental care: Evolution, mechanisms, and adaptive significance (pp. 243–294). San Diego: Academic Press. [Google Scholar]
- White H (1982). Maximum-likelihood estimation of misspecified models. Econometrica, 50, 1–25. [Google Scholar]
- Wilkins AS, Logan M, & Kehoe P (1997). Postnatal pup brain dopamine depletion inhibits maternal behavior. Pharmacology and Biochemical Behavior, 58, 867–873. [DOI] [PubMed] [Google Scholar]
- Zangen A, Nakash R, Overstreet DH, & Yadid G (2001). Association between depressive behavior and absence of serotonin-dopamine interaction in the nucleus accumbens. Psychopharmacology (Berl), 155, 434–439. [DOI] [PubMed] [Google Scholar]
- Zangen A, Overstreet DH, & Yadid G (1997). High serotonin and 5-hydroxyindoleacetic acid levels in limbic brain regions in a rat model of depression: Normalization by chronic antidepressant treatment. Journal of Neurochemistry, 69, 2477–2483. [DOI] [PubMed] [Google Scholar]
- Zangen A, Overstreet DH, & Yadid G (1999). Increased catecholamine levels in specific brain regions of a rat model of depression: Normalization by chronic antidepressant treatment. Brain Research, 824, 243–250. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP (1997). Multiparous species present problems (and possibilities) to developmentalists. Development Psychobiology, 30, 141–150. [DOI] [PubMed] [Google Scholar]


