Abstract
Fish farms are one of the principal food industries located in peri-urban and rural communities that use available resources to ensure the quality of their products. However, trout can suffer from bacterial infections affecting the sector and being a key component of human health risk. We aimed to identify and characterize Enterobacteriaceae in 46 trout (Oncorhynchus mykiss) in two fish farms in Lima, Peru. Adult trouts older than seven weeks (> 200 grams weight) were included. Cultures were performed in duplicate (n=192 trials) with swabs from the squamous surface and visceral cavity. The isolates were identified with the Vitek® 2 Compact system, and the minimum inhibitory concentrations (MIC) were interpreted with the CLSI VET 03-A guide. At El Molino and El Angelito fish farms, 66 (68.8%) and 57 (59.4%) isolates were obtained. The most frequently isolated species were Escherichia coli (56.8%), Proteus sp. (4.2%) and Klebsiella pneumoniae (2.6%). E. coli was present in all sampling areas, and Aeromonas hydrophila was only present in one open viscera sample at El Angelito fish farm. A. hydrophila showed antibiotic resistance to Ampicillin/Sulbactam (≥32 MIC), Oxytetracycline (>8 MIC), Imipenem (8 MIC), Levofloxacin (>8 MIC), and Ceftazidime (≥64 MIC). Our results suggest the presence of multi-resistant A. hydrophila in O. mykiss. Further studies are needed to understand the developmental context of A. hydrophila, which is crucial to the food industry, aquaculture and public health.
Keywords: Aeromonas hydrophila, rainbow trout, Oncorhynchus mykiss, fish, lake, Peru
Resumo
As pisciculturas são uma das principais indústrias alimentícias localizadas em comunidades peri-urbanas e rurais que utilizam os recursos disponíveis para garantir a qualidade de seus produtos. No entanto, a truta pode sofrer de infecções bacterianas que afetam o setor e são um componente chave do risco para a saúde humana. Nosso objetivo foi identificar e caracterizar Enterobacteriaceae em 46 trutas (Oncorhynchus mykiss) em duas pisciculturas em Lima, Peru. Foram incluídas trutas adultas com mais de sete semanas (> 200 gramas de peso). As culturas foram realizadas em duplicata (n=192 tentativas) com swabs da superfície escamosa e da cavidade visceral. Os isolados foram identificados com o sistema Vitek® 2 Compact, e as concentrações inibitórias mínimas (CIM) foram interpretadas com o guia CLSI VET 03-A. Nas pisciculturas El Molino e El Angelito foram obtidos 66 (68,8%) e 57 (59,4%) isolados. As espécies mais frequentemente isoladas foram Escherichia coli (56,8%), Proteus sp. (4,2%) e Klebsiella pneumoniae (2,6%). E. coli estava presente em todas as áreas de amostragem, e Aeromonas hydrophila estava presente apenas em uma amostra de vísceras abertas na piscicultura El Angelito. A. hydrophila mostrou resistência a antibióticos para Ampicilina/Sulbactam (≥32 MIC), Oxitetraciclina (>8 MIC), Imipenem (8 MIC), Levofloxacina (>8 MIC) e Ceftazidima (≥64 MIC). Nossos resultados sugerem a presença de A. hydrophila multirresistente em O. mykiss. Mais estudos são necessários para entender o contexto de desenvolvimento de A. hydrophila, que é crucial para a indústria de alimentos, aquicultura e saúde pública.
Palavras-chave: Aeromonas hydrophila, truta arco-íris, Oncorhynchus mykiss, peixe, lago, Peru
Introduction
Food quality assurance is one of the principal global components of the United Nations Sustainable Development Goals for 2030 (United Nations, 2016). This goal seeks to preserve human health as one of the essential elements for the progress of communities, and its task is to ensure the quality of food produced, stored, and transported for human and animal consumption (Sachs, 2012).
However, not all communities have access to safe food derived from their production. These infectious pathogens are responsible for many infections per year, with communities with poor water quality being the most affected (Food and Agriculture Organization, 2018a). As water contamination is one of the main aspects of food safety, it is necessary to monitor its microbiological quality, estimate its impact on human health and the environment, and promote the mandatory responses to prevent its contamination and mitigate its risks (Cabral, 2010; Zúniga-Estrada et al., 2006).
Globally, there is widespread interest in freshwater fish farms. Low- and-middle income countries mainly allocate a large part of their domestic economies to aquaculture production (Food and Agriculture Organization, 2018b; Instituto del Mar del Perú, 2015; Ministerio de la Producción, 2016). In Peru, the production of rainbow trout (Oncorhynchus mykiss and Salmo trutta) in fish farms is a cosmopolitan activity, focused in peri-urban and rural communities such as Cajamarca, Junin, and Ancash, which use these resources to generate economic income. Trout are prone to bacterial infections (by Vibrio and Aeromonas species), leading to infectious outbreaks that threaten mariculture and aquaculture with high morbidity and detrimental economic losses (Fuentes & Pérez, 1998; Senderovich et al., 2010; Topić Popovic et al., 2000;).
As well as Vibriosis (Abdelaziz et al., 2017), a global threaten bacterial disease caused mainly by V. anguillarum in rainbow trout, Aeromonas species are a usual health problem in Peru as they cause everything from ulceration to multi-organ haemorrhage. Several Peruvian studies (Baca, 2012; Instituto del Mar del Perú, 2008) have isolated A. salmonicida and A. hydrophila in sick rainbow trout in various Peruvian municipalities. However, microbiological evaluations of healthy trout, without signs of infection and in the absence of bacterial outbreaks (carrier species), have not yet been carried out.
We aimed to identify and characterize Enterobacteriaceae in trout (O. mykiss), evaluating mainly Vibrio sp. and Aeromonas sp. species from two fish farms in Peru.
Materials and methods
Study design and location
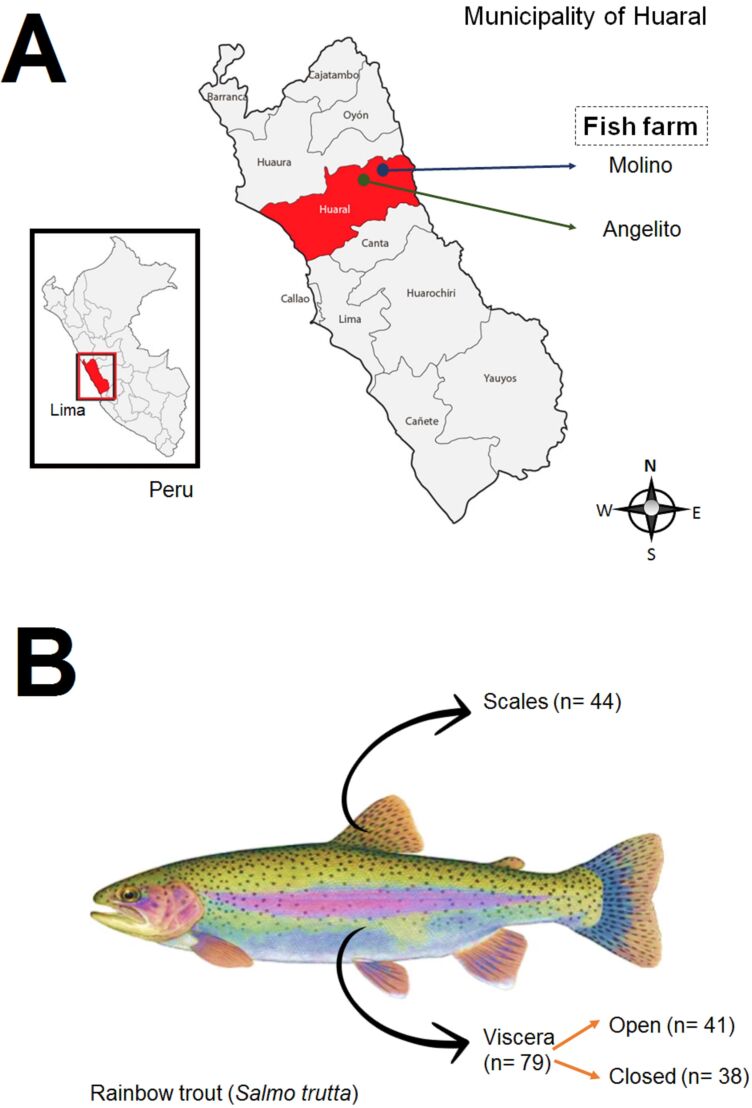
This study was a quantitative cross-sectional study in two fish farms in Huaral Municipality (11° 30′ 03′′ S, 77° 12′ 33′′ W), a city located north of Lima. Fish farms “El Angelito” and “El Molino” were included, after approval by the owners of each center (Figure 1). In these centers, trout farming is legal, and the quality of the products depends on each.
Figure 1. Study site (A) and sampling distribution in trout (B).
Population analysis and inclusion criteria
Forty-six trout from two fish farms in Huaral were included, following probability and double sampling. Inclusion criteria were adult trout older than seven weeks of age, > 200 grams in weight, and adult fish in fattening stage II. Young trout (fry) and sick trout were excluded. Sampling was performed with the following formula for an infinite population, considering a confidence level of 95% and α=0.05:
| (1) |
Microbiological evaluation
Following the guidance of the Instituto del Mar del Perú (2015) trout management guide, 48 trout per fish farm were sampled in duplicate, resulting in 192 microbiological tests. In situ sampling followed the methods of Sierralta Chichizola et al. (2016). Swabs of the squamous surface and visceral cavity of trout (open and closed) were taken in duplicate (Figure 2).
Figure 2. Sampling was performed on fish farms included in the study. Sampling has been carried out on the scales (A) and viscera (B) of Oncorhynchus mykiss. The fish farm delivers natural water (B and D) to an artisanal hatchery applied in the Huaral Valley.
All swabs will be transported with the Merck Brain-Heart Infusion based sample transport system (Darmstadt, Germany), were transported under refrigeration to the Laboratory of the Infectious Unit of NESH Hubbs in Lima. The swab samples were cultured on McConkey agar and selective medium thiosulphate citrate bile sucrose agar (TCBS), both from Merck (Darmstadt, Germany). These were incubated at 37±2°C under aerobic conditions for 24 hours. The positivity threshold was considered as ≥ 100 000 CFU/ml. The isolates were identified with the Vitek® 2 Compact system (BioMérieux, Marcy-l'Étoile, France), and the susceptibility test was interpreted with CLSI thresholds interpreted as minimum inhibitory concentrations (MIC) (Clinical and Laboratory Standard Institute, 2006).
Data collection and analysis
Data were collected directly from the Vitek analysis systems into a data matrix in SPPS v22.0 (IBM, Armonk, USA) for iOS and independently verified by two authors. Descriptive statistical analysis (frequency, percentage and means) were tabulated for categorical and continuous variables. The Chi-Square test was conducted to find differences between isolates from each fish farm, with a p-value of 0.05 and a 95 per cent confidence interval (CI) considered significant.
Ethical aspects
According to the National Institute of Health's Office of Animal Care and Use, this study fulfilled worldwide guidelines for conducting scientific research with experimental animals (Institute of Laboratory Animal Resources, 2005). Also, the study was approved by the institutional review board of Norbert Wiener University.
Results
A total of 192 cultures were performed, 96 for each farm. El Molino farm had 66 (68.8%) positive isolates, and El Angelito farm had 57 (59.4%) isolates. The most frequently isolated species were Escherichia coli with 109 (56.8%) isolates, followed by Proteus sp. and Klebsiella pneumoniae with 8 (4.2%) and 5 (2.6%) isolates, respectively (Table 1).
Table 1. Baseline of isolates in rainbow trout (Oncorhynchus mykiss) in two fish farms (N=192). Data in N(%).
| Isolates | El Molino | El Angelito | |||||
|---|---|---|---|---|---|---|---|
| OV | CV | SC | OV | CV | SC | ||
| Escherichia coli | 15 (15.6) | 18 (18.8) | 24 (24) | 17 (17.7) | 17 (17.7) | 18 (18.8) | |
| Klebsiella pneumoniae | 1 (1) | 2 (2.1) | 2 (2.1) | ||||
| Proteus sp. | 5 (5.2) | 3 (3.1) | |||||
| Aeromonas hydrophila | 1 (1) | ||||||
| TOTAL | 21 (21.9) | 21 (21.9) | 24 (25) | 20 (20.8) | 17 (17.7) | 20 (20.8) | |
Abbreviations: OV: open viscera, CV: closed viscera, SC: Scales.
According to the sampling zone, more bacteria were isolated from scales of O. mykiss with 44 (22.9%) isolates, followed by 41 (21.4%) isolates from open viscera and 38 (19.4%) from closed viscera. E. coli was present in all sampling areas, and A. hydrophila was only present in one open viscera sample at El Angelito fish farm.
The antibiotic susceptibility result of A. hydrophila is shown in Table 2. This isolate showed antibiotic resistance to Ampicillin/Sulbactam (≥32 MIC), Piperacillin/Tazobactam (≥128 MIC), Cefazolin (≥64 MIC), Cefazolin (≥64 MIC), Imipenem (8 MIC), Levofloxacin (>8 MIC), Oxytetracycline (>8 MIC) and Ceftazidime (≥64 MIC). None of the isolates was positive for Vibrio sp., and there was a significant difference between sampling sites (p=0.001).
Table 2. Antibiotic susceptibility resulted in Aeromonas hydrophila isolated from rainbow trout (Oncorhynchus mykiss). Susceptibility (green box) and resistance (red box) results to the tested antibiotics are shown.
| Antibiotics | MIC | Results |
|---|---|---|
| Ampicillin/sulbactam | >32 | |
| Amikacina | <2 | |
| Piperazine/Tazobactam | >128 | |
| Cefazoline | >64 | |
| Ceftazidima | >64 | |
| Cefepime | <1 | |
| Florfenicol | 5 | |
| Imipenem | 8 | |
| Gentamicin | <2 | |
| Ciprofloxacin | 0.5 | |
| Levofloxacin | >8 | |
| Oxytetracycline | >8 | |
| Trimethoprim-sulfamethoxazole | 160 |
Abbreviation: MIC: Minimum inhibitory concentration.
Discussion
This study in asymptomatic trout (O. mykiss) identified enterobacteria with a high frequency of antibiotic susceptibility, except for A. hydrophila, which is isolated from open viscera and with multi-resistant characteristics to 2/3 of the antibiotics tested.
The present study aimed to characterize enterobacteria of human interest in O. mykiss, using conventional bacteriological techniques, contributing to a better understanding of resistance patterns in aquatic animal carriers. Although it is demanding to find Aeromonas spp. in technified fish farms, it is also hard to find Aeromonas spp. (Katharios, 2019). Our results report multi-resistant bacteria in asymptomatic trout in peri-urban Peruvian fish farms.
The main complication of human infection in aquatic animals is cholera, a diarrhoeal disease with high annual mortality and a latent threat due to its pandemic nature (Senderovich et al., 2010). However, other species can cause large-scale infections in humans. These species are also associated not only with saltwater fish but also with freshwater species such as trout (fish of the Salmonidae subfamily), which are widely consumed by various populations such as the Peruvian population (Adlei, 2014; Drakeford & Pascoe, 2008). Aeromonas are pathogens linked to human diseases that present antibiotic resistance due to the abuse of antibiotics as prophylactics (González Salas et al., 2021).
For this, the National Fisheries Health Agency (SANIPES) of Peru included aeromoniasis within the Aquatic Animal Disease Control Programme a few years ago to exercise national-wide surveillance of these bacteria (Organismo Nacional de Sanidad Pesquera, 2016). There is a need to improve understanding of the causes and effects of contamination of agricultural water, crops and food animals. As well as effective means to prevent and remediate the problem (Food and Agriculture Organization, 2018a) because communities consume contaminated food.
Aeromonas spp. are ubiquitous aquatic organisms that affect humans and animals. Aeromonas species have been identified as causing disease in fish, such as A. veronii causing acute infection in diseased crucian carp (Carassius auratus gibelio) and Aeromonas sp. in O. mykiss (Chen et al., 2019). However, Aeromonas species have also been found in healthy trout or without an apparent sign of infection. A previous study in Croatia found 26 species of A. hydrophila in 336 wild freshwater fish, where only 3.5% showed signs of acute infection (Topić Popovic et al., 2000). A study in Oman has reported A. salmonicida in 9/417 fish with no clinical signs of the disease (Alghabshi et al., 2018). These results support our findings as we report A. hydrophila in trout without signs of clinical infection, contradicting the widely held view that Aeromonas spp. is an obligate fish pathogen.
The economic defeats caused by infection induced by loss of muscle and the creation of granulomas define the necessity of isolating these bacteria for the aquaculture business (Beaz-Hidalgo & Figueras, 2013). On the other hand, the presence of these Aeromonas is influenced by environmental conditions such as poor water quality, overcrowding, unhygienic handling, contaminated feed, and poor nutrition (Hossain & Heo, 2021).
As in Peru, many peri-urban and rural communities are responsible for trout farms where the weather and type of water used can lead to the growth Aeromonas in fish. The study by Zdanowicz et al. (2020) in Northern Poland has shown that in ponds filled with river water, a high abundance of Aeromonas has been reported for both adult fish (2.89 x 102 CFU/ml) and fry (4.22 x 102 CFU/ml) at inflow and outflow sites. Also, summer conditions may be related to the development of Aeromonas. For example, A. hydrophila has not been reported during the summer months, and seasonality is evident (Topić Popovic et al., 2000). We tested Aeromonas in late spring when there had been minimal prior rainfall and in trout raised on river water, both of which might be variables in Aeromonas development. Although these were not evaluated, future studies are required to include these conditions that may explain the presence of these pathogens in carrier trout.
Our findings demonstrated a high frequency of antibiotic resistance of A. hydrophila isolated from O. mykiss, whereas in previous research, β-lactam antibiotics were most affected (Dias et al., 2012; Topić Popovic et al., 2000; Zdanowicz et al., 2020). It appears that the antibiotic resistance profile may vary between species, thus the study by Chen et al. (2019) has shown that A. veronii causing infection in crucian carp was susceptible to β-lactams (imipenem), tetracyclines, nitrofurans (furazolidone), and polymyxin B. Topić Popovic et al. (2000) have shown that all A. hydrophila isolated from wild freshwater fish were resistant to penicillin G and novobiocin, but resistant to pipemidinic acid, tetracycline, trimethoprim, chloramphenicol, erythromycin, nitrofurantoin, and trimethoprim-sulfomethoxazole. Dias et al. (2012) have demonstrated in 299 Aeromonas isolates [including A. veronii (36.8%), A. hydrophilica (35.5%), and A. aquariorum (14.4%)] in ornamental fish in aquaria a high incidence of resistance to β-lactams (around 95% for amoxicillin, carbenicillin and ampicillin).
Zdanowicz et al., (2020) reported that only 5-6% of Aeromonas tested were resistant to chloramphenicol and ciprofloxacin, coinciding with our results where we did not evidence resistance to Florfenicol or ciprofloxacin. However, we describe an oxytetracycline resistance profile that is consistent with prior research and evidence of the effects of widespread antibiotic usage, both prophylactically and for the treatment of A. furunculosis (Adams et al., 1998). The evaluation of this antibiotic, including those not frequently used in aquaculture, is important for microbiological surveillance and food safety in the framework of One Health.
This study had limitations. First, as the study was cross-sectional, trout were assessed at a single point in time, with no subsequent follow-up. Second, Aeromonas species were identified by conventional bacteriological and biochemical methods that have proven effective in previous studies (Topić Popovic et al., 2000). However, molecular identification of both Aeromonas species and antibiotic resistance profiles is relevant.
Conclusions
This study isolated multi-antibiotic resistant A. hydrophila in asymptomatic trout (O. mykiss) from a peri-urban river-supplied fish farm in Peru. β-lactams, quinolones and oxytetracycline were all affected by antibiotic resistance. As a result, it is necessary to address the surveillance of these microorganisms that are important to the food business, food safety, and public health.
Footnotes
How to cite: Moya-Salazar, J., Díaz, C. S., Cañari, B., Badillo, R. X., Verano-Zelada, M., Chicoma-Flores, K., & Contreras-Pulache, H. (2022). Detection of pathogenic Aeromonas hydrophila from two rainbow trout (Oncorhynchus mykiss) farms in Peru. Brazilian Journal of Veterinary Medicine, 44, e000922. https://doi.org/10.29374/2527-2179.bjvm000922
Ethics statement: This study has been approved at the Ethics Committee of the Universidad Norbert Wiener with protocol number UNW-05301-2018-1.
Financial support: None.
Availability of complementary results: The authors must identify where readers can access any complementary information available, such as in an online repository or from the authors on request.
The work was carried out at Microbiology Laboratory, Unidad de Infecciosas of NESH hubbs Incs, Lima, Peru.
References
- Abdelaziz M., Ibrahem M. D., Ibrahim M. A., Abu-Elala N. M., Abdel-moneam D. A. Monitoring of different vibrio species affecting marine fishes in Lake Qarun and Gulf of Suez: Phenotypic and molecular characterization. Egyptian Journal of Aquatic Research. 2017;43(2):141–146. doi: 10.1016/j.ejar.2017.06.002. [DOI] [Google Scholar]
- Adams C. A., Austin B., Meaden P. G., McIntosh D. Molecular characterization of plasmid-mediated oxytetracycline resistance in Aeromonas salmonicida. Applied and Environmental Microbiology. 1998;64(11):4194–4201. doi: 10.1128/AEM.64.11.4194-4201.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlei A. Analysis of The Rainbow Trout, Oncorhynchus mykiss market in the world and Iran. Journal of Fisheries. 2014;8(2):81–88. [Google Scholar]
- Alghabshi A., Austin B., Crumlish M. Aeromonas salmonicida isolated from wild and farmed fish and invertebrates in Oman. International Aquatic Research. 2018;10(2):145–152. doi: 10.1007/s40071-018-0195-4. [DOI] [Google Scholar]
- Baca R. F. Aislamiento e identificación bioquímica de Aeromonas salmonicida en truchas arcoíris (Oncorhynchus mykiss) en fase juvenil en una piscigranja de la región Junín. Universidad Nacional Mayor de San Marcos, Facultad de Veterinaria; 2012. Tesis. [Google Scholar]
- Beaz-Hidalgo R., Figueras M. J. Aeromonas spp. whole genomes and virulence factors implicated in fish disease. Journal of Fish Diseases. 2013;36(4):371–388. doi: 10.1111/jfd.12025. [DOI] [PubMed] [Google Scholar]
- Cabral J. P. S. Water microbiology. Bacterial pathogens and water. International Journal of Environmental Research and Public Health. 2010;7(10):3657–3703. doi: 10.3390/ijerph7103657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Sun J., Han Z., Yang X., Xian J. A., Lv A., Hu X., Shi H. Isolation, Identification and Characteristics of Aeromonas veronii From Diseased Crucian Carp (Carassius auratus gibelio) Frontiers in Microbiology. 2019;10:2742. doi: 10.3389/fmicb.2019.02742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Standard Institute . Methods for antimicrobial disk susceptibility testing of bacteria isolated from aquatic animals: Approved guideline. CLSI; 2006. [Google Scholar]
- Dias C., Mota V., Martinez-Murcia A., Saavedra M. J. Antimicrobial resistance patterns of Aeromonas spp. isolated from ornamental fish. Journal of Aquaculture Research & Development. 2012;3:131. [Google Scholar]
- Drakeford B., Pascoe S. Substitutability of fishmeal and fish oil in diets for salmon and trout: A meta-analysis. Aquaculture Economics & Management. 2008;12(3):155–175. doi: 10.1080/13657300802306079. [DOI] [Google Scholar]
- Food and Agriculture Organization . The State of World Fisheries and Aquaculture 2018. Meeting the sustainable development goals. FAO; 2018. a. [Google Scholar]
- Food and Agriculture Organization . Water, Water, Land and Ecosystems (WLE) Program of the CGIAR, International Water Management Institute (IWMI). FAO; 2018. b. [Google Scholar]
- Fuentes R. J., Pérez H. J. Aislamiento de Aeromona hydrophila en trucha Arcoiris (Oncorhynchus mykiss) / Isolation of Aeromona hydrophila in rainbow trowt (Oncorhynchus mykiss). Case report. Veterinaria (México) 1998;29(1):117–119. [Google Scholar]
- González Salas R., Vidal del Río M., Pimienta Concepción I. Uso intensivo de antibióticos profilácticos en la acuicultura: Un problema creciente para la salud humana y animal. Revista Universidad & Sociedad. 2021;13(S2):204–210. [Google Scholar]
- Hossain S., Heo G. J. Ornamental fish: A potential source of pathogenic and multidrug-resistant motile Aeromonas spp. Letters in Applied Microbiology. 2021;72(1):2–12. doi: 10.1111/lam.13373. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources . Office of Animal Care and Use of National Institute of Health. Animal Welfare Commision on Life Sciences, US Department of Agriculture; 2005. [Google Scholar]
- Instituto del Mar del Perú . Anuario Científico Tecnológico. Vol. 8. IMARPE; 2008. [Google Scholar]
- Instituto del Mar del Perú . Guía para la Incubación y Alevinaje de Truchas Arcoíris. IMARPE; 2015. [Google Scholar]
- Katharios P. Disease prevention in farmed fish: New developments and research needs. Standing Committee on Agricultural Research; 2019. [Google Scholar]
- Ministerio de la Producción . Anuario Estadístico Pesquero y Acuícola 2016: La actividad productiva del sector en némeros. PRODUCE; 2016. [Google Scholar]
- Organismo Nacional de Sanidad Pesquera Programa Oficial de vigilancia y control de enfermedades en animales acuáticos: P01-SDSNA-SANIPES. 2016. http://www.sanipes.gob.pe
- Sachs J. D. From millennium development goals to sustainable development goals. Lancet. 2012;379(9832):2206–2211. doi: 10.1016/S0140-6736(12)60685-0. [DOI] [PubMed] [Google Scholar]
- Senderovich Y., Izhaki I., Halpern M. Fish as reservoirs and vectors of vibrio cholerae. PLoS One. 2010;5(1):e8607. doi: 10.1371/journal.pone.0008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierralta Chichizola V., Mayta Huatuco E., León Quispe J. Primer Registro de Plesiomonas shigelloides como Patógeno Oportunista de Tilapia Oreochromis niloticus (Linnaeus, 1758) enunaPiscigranja de Lima, Perú. Revista de Investigaciones Veterinarias del Perú. 2016;27(3):565–572. doi: 10.15381/rivep.v27i3.11996. [DOI] [Google Scholar]
- Topić Popovic N., Teskeredžić E., Strunjak-Perovic´ I., Čož-Rakovac R. Aeromonas hydrophila isolated from wild freshwater fish in Croatia. Veterinary Research Communications. 2000;24(6):371–377. doi: 10.1023/A:1006418116155. [DOI] [PubMed] [Google Scholar]
- United Nations . The Sustainable Development Goals Report. United Nations Publications; 2016. [Google Scholar]
- Zdanowicz M., Mudryk Z. J., Perliński P. Abundance and antibiotic resistance of Aeromonas isolated from the water of three carp ponds. Veterinary Research Communications. 2020;44(1):9–18. doi: 10.1007/s11259-020-09768-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zúniga-Estrada A., Tejeda-Trujillo F., Concha-Valdéz F., Heredia-Rojas N. Sanitary microbiology. Revista Latinoamericana de Microbiologia. 2006;48(2):226–230. [PubMed] [Google Scholar]