Abstract
Background
Photoplethysmography (PPG) is a low-cost and easy-to-implement method to measure vital signs, including heart rate (HR) and pulse rate variability (PRV) which widely used as a substitute of heart rate variability (HRV). The method is used in various wearable devices. For example, Samsung smartwatches are PPG-based open-source wristbands used in remote well-being monitoring and fitness applications. However, PPG is highly susceptible to motion artifacts and environmental noise. A validation study is required to investigate the accuracy of PPG-based wearable devices in free-living conditions.
Objective
We evaluate the accuracy of PPG signals—collected by the Samsung Gear Sport smartwatch in free-living conditions—in terms of HR and time-domain and frequency-domain HRV parameters against a medical-grade chest electrocardiogram (ECG) monitor.
Methods
We conducted 24-hours monitoring using a Samsung Gear Sport smartwatch and a Shimmer3 ECG device. The monitoring included 28 participants (14 male and 14 female), where they engaged in their daily routines. We evaluated HR and HRV parameters during the sleep and awake time. The parameters extracted from the smartwatch were compared against the ECG reference. For the comparison, we employed the Pearson correlation coefficient, Bland-Altman plot, and linear regression methods.
Results
We found a significantly high positive correlation between the smartwatch’s and Shimmer ECG’s HR, time-domain HRV, LF, and HF and a significant moderate positive correlation between the smartwatch’s and shimmer ECG’s LF/HF during sleep time. The mean biases of HR, time-domain HRV, and LF/HF were low, while the biases of LF and HF were moderate during sleep. The regression analysis showed low error variances of HR, AVNN, and pNN50, moderate error variances of SDNN, RMSSD, LF, and HF, and high error variances of LF/HF during sleep. During the awake time, there was a significantly high positive correlation of AVNN and a moderate positive correlation of HR, while the other parameters indicated significantly low positive correlations. RMSSD and SDNN showed low mean biases, and the other parameters had moderate mean biases. In addition, AVNN had moderate error variance while the other parameters indicated high error variances.
Conclusion
The Samsung smartwatch provides acceptable HR, time-domain HRV, LF, and HF parameters during sleep time. In contrast, during the awake time, AVNN and HR show satisfactory accuracy, and the other HRV parameters have high errors.
Introduction
Heart rate (HR) and heart rate variability (HRV) are physiological parameters reflecting autonomous nervous system regulations and general well-being. HR shows the number of heartbeats per minute, and HRV indicates the variation of time between two consecutive heartbeats or interbeat intervals (IBIs) [1]. Various HRV parameters can be extracted from IBIs, such as average normal IBIs (AVNN), standard deviation of normal IBIs (SDNN), and root mean square of the successive difference (RMSSD). HR and HRV parameters can provide insight into cardiovascular and autonomic nerve dysfunction [2]. Studies in the literature show the relationship between HRV parameters and different health issues such as diabetes [3], hypertension [4], depression [5], and autonomic imbalance [6]. Moreover, HRV parameters are associated with mental and physiological stress [7, 8], and sleep quality [9].
HR and HRV can be monitored using noninvasive methods such as Electrocardiography (ECG) and Photoplethysmography (PPG). ECG is the golden standard for HR and HRV parameters monitoring used in clinical trials. The method measures the electrical activity of the cardiovascular system using electrodes connected to the skins. However, it cannot be employed in home-based and/or long-term monitoring when people are engaged in different activities. Alternatively, PPG is a noninvasive optical method for HR and HRV monitoring. PPG—enabled by a light emitter and a photodetector—measures the volumetric variations of blood flow [10]. The method collects pulse rate variability (PRV), which widely used as a surrogate of HRV [11–19]. Although some studies report the difference between PRV and HRV, for example, in response to cold exposure [11, 20], they indicated that HRV is the major determinant of PRV. The authors in [13] also showed that HRV parameters could reliably be estimated by PPG signals.
PPG is a low-cost and convenient method implemented in many clinical and commercial wearable devices [21–23]. Recently, several PPG-based wearable devices have been proposed for health parameters monitoring in everyday life settings. Several studies leveraged different wearable devices, such as Samsung Gear Sport, Apple Watch, Fitbit, and Garmin Vivosmart, for health monitoring in different population-based groups [22–24]. With advancements in technology, it is expected that the use of such wearable devices will grow further as they become smaller and lighter with longer battery life.
However, PPG-based wearable devices are prone to environmental noises and motion artifacts (when users engage in various physical activities). These noises are inevitable in everyday life settings and affect the signal quality, resulting in poor/invalid health parameters extraction [25]. Therefore, using commercial PPG-based wearable devices for HR and HRV monitoring necessitates accuracy assessment, especially if the devices are used for health monitoring applications.
Several studies investigated the validation of HR measurements using wristbands in various situations across different population groups. In [26], the authors evaluated the HR of several wristbands—including Apple Watch, Basis Peak, Fitbit Surge, Microsoft Band, Mio Alpha 2, PulseOn, and Samsung Gear S2—during different physical activities. Other studies validated the HR extracted from Garmin Forerunner [27], the everlast smartwatch [28], Fitbit Charge HR [29], Empatica E4 [30], and Basis peak [31]. These studies showed the high accuracy of PPG-based wristbands for HR monitoring during resting and low-intensity activity in laboratory settings. The results also showed a decrease in the accuracy of HR when the intensity of activity increased. However, these studies are restricted to certain physical activities in laboratory settings. They are also limited to HR measurements. The accuracy of HR and HRV parameters is affected by different factors. For example, the accuracy of RMSSD can be affected by a distortion in a small part of the signals. However, SDNN accuracy can be impacted by outliers affecting the IBIs variations [32]. These characteristics of HRV parameters indicate the need to validate the accuracy of HRV parameters individually.
Studies evaluated the accuracy of HRV parameters extracted from wristbands and smartwatches including Apple Watch [14], Empatica E4 [15, 16], Microsoft band 2 [33], and the Wavelet wristband [34] against medical-grade ECG device. These studies indicated high accuracy of the smartwatches and wristbands in terms of HR and HRV parameters while the participants were resting. They also showed that motion artifacts highly affect the reliability of HRV parameters. However, these studies were limited to short-term data collection –less than one hour– in laboratory settings [16, 30, 33]. In addition, the majority of the previous works collected data only in seated positions [14, 15, 34].
We believe that there is a need to evaluate the accuracy of the smartwatch in everyday life settings where participants can engage in different activities and conditions. Such evaluation should also comprehensively assess the accuracy of time-domain and frequency-domain HRV parameters extracted from the raw PPG signals.
In this paper, we assess the validity of the Samsung Gear Sport smartwatch in terms of HR and several HRV parameters. The evaluation is performed against a medical-grade chest ECG monitor in a 24-hours continuous free-live setting monitoring. The data from 28 individuals are included in the evaluation. We use PPG and ECG signals collected from the Samsung smartwatch and ECG monitor to extract HR, AVNN, RMSSD, SDNN, pNN50, LF, HF, and LF/HF ratio in five-minute windows. We evaluate the parameters during sleep time and awake time. The evaluation is performed using a linear regression method, the Pearson correlation coefficient, and the Bland–Altman plot. Finally, We discuss the validity of the parameters based on the obtained results in everyday settings. In summary, the main contributions of this paper are as follows:
We investigate the validity of PPG signals acquired by the Samsung Gear Sport smartwatch in terms of HR and HRV parameters compared with a medical-grade chest ECG monitor
We conduct a 24-hours study in which 28 healthy participants are monitored remotely and continuously.
We analyze the HR and HRV parameters in five-minute windows during sleep-time and awake-time using the linear regression method, the Pearson correlation coefficient, and the Bland–Altman plot.
Method
Study design
An observational study was conducted to assess the validity of HR and HRV parameters collected under free-living conditions via Samsung Gear Sport smartwatches. The assessment was performed in comparison with an ECG monitor as the golden standard. The study included a convenience sample of healthy individuals recruited in Southwest Finland in July-August 2019.
Participants and recruitment
Forty-six healthy individuals between the age of 18 and 55 were recruited to participate in this study. The inclusion criteria were individuals who 1) were able to use wearable devices for 24 hours, 2) had no diagnosed cardiovascular disease, 3) had no symptoms of illness during the recruitment time, and 4) had no restrictions in physical activities.
In a face-to-face meeting with researchers, the eligible participants were informed about the purposes of the study, the procedure, and the instructions to use the devices. After the written informed consent, the devices—a Samsung Gear sport smartwatch [35] and a shimmer3 ECG device [36]—were delivered to the participants. The participants were asked to wear the wearable devices for 24 hours continuously while engaging in their daily routines and log their sleep and awake time.
After the data collection, we excluded the data of 18 participants due to technical and practical issues during the monitoring, for example, ECG electrodes were not adequately attached to the skin, and participants forgot to log their sleep and awake time. Therefore, the data of 28 participants (i.e., 14 female and 14 male) were included in the analysis. Table 1 summarizes the background information of the participants.
Table 1. Participants background information n = 27 (one participants didn’t fill the background questionnaire.
| Parameters | Values |
|---|---|
| Age, years, mean (SD) | 32.5 (6.6) |
| BMI, mean (SD) | 25.4 (5.2) |
| Exercise | |
| Almost daily | 8 |
| A few times a week | 13 |
| Once a week or fewer | 6 |
| Education | |
| Primary school | 1 |
| High school | 6 |
| College | 6 |
| University | 14 |
| Employment status | |
| Working | 22 |
| Student | 1 |
| Unemployed | 3 |
| Other | 1 |
Research ethics
The study was conducted according to the ethical principles based on the Declaration of Helsinki and the Finnish Medical Research Act (No 488/1999). The study protocol received a favorable statement from the ethics committee (University of Turku, Ethics committee for Human Sciences, Statement no: 44/2019). The participants were informed about the study, both orally and in writing, before the written informed consent was obtained. Participation was voluntary, and all the participants had the right to withdraw from the study at any time and without giving any reason. To compensate for the time used for the study, each participant got a gift card to the grocery store (20 euro) at the end of the monitoring period when returning the devices.
Data collection
The data collection included two wearable devices and self-report and background questionnaires. The participants were asked to wear a Samsung Gear Sport smartwatch on the wrist of their non-dominant hand. Moreover, they were asked to wear a Shimmer3 ECG device using a chest strap. The ECG was collected via four limb electrodes placed on the torso (i.e., left arm, right arm, left leg, and right leg). More details can be found in [37]. We also used self-report questionnaires by which the individuals logged their sleep and non-wear time. In our analysis, the self-report data were used to extract the sleep and awake time.
The Shimmer3 ECG device was selected to measure ECG as the gold standard method in our assessment. The Shimmer ECG is a compact and lightweight device that can be configured to measure ECG, accelerometer/gyroscope data, and skin temperature continuously [36]. The device also has sufficient internal memory and battery life for 24 hours of continuous data collection. We configured the Shimmer device to collect data with the sampling frequency of 512HZ, used in clinical trials to extract HR and HRV parameters accurately [38]. The data were stored on the device during the monitoring and were transferred to our cloud server after the monitoring for the analysis. In this study, Lead II ECG (right arm—left leg) was selected to extract the cardiac rhythm accurately.
The Samsung Gear Sport watch is a commercial open-source smartwatch that enables remote health monitoring [35]. The smartwatch provides PPG signals and gyroscope/accelerometer data at the sampling frequency of 20Hz. The watch runs an open-source Tizen operating system and has a built-in inertial measurement unit (IMU) to extract physical activity and sleep data. We developed a customized data collection application for the watch to collect 16 minutes of PPG signals every 30 minutes continuously. In the analysis, we removed the first minute of each PPG record, as it was unreliable due to sensor calibration. With this setup, the smartwatch’s battery life was more than 24 hours, so no battery charging was needed during the monitoring. The data was stored in the watch’s internal storage during the monitoring. Similar to the Shimmer device data, we transferred the watch’s data to the cloud after the monitoring.
Data analysis
In this section, we describe HR and HRV extraction from the collected PPG and ECG signals. We used short-term HRV analysis, which considers five-minute windows of signals for the analysis [32, 39]. The short-term HRV analysis was selected based on the duration of PPG recordings (16 minutes per 30 minutes). We then outline the statistical analysis methods used in this study.
HR and HRV extraction pipeline
The HR and HRV extraction pipeline consists of four steps: filtering, peak detection, abnormal peak removal, and feature extraction (see Fig 1). The raw PPG and ECG signals are divided into five-minute segments. In the following, we provide a detailed description of each step:
Fig 1. HR and HRV extraction pipeline.
1) Filtering: In this step, we remove frequencies that are out of the human heart rate range. We used a 5-order high-pass Butterworth filter with a cutoff frequency of 0.5 Hz for PPG signals and a bandpass Butterworth filter with 0.5 and 100 Hz cutoff frequencies for ECG signals. The cutoff frequencies were selected based on valid HR range and input signals’ frequencies.
2) Peak detection: This step finds the peaks corresponding to the heartbeat in PPG and ECG signals.
PPG peak detection—We used a deep-learning-based method introduced by Kazemi et al. [40] for PPG peak detection. The method outperforms other state-of-the-art methods [41, 42], particularly when the signal is noisy. The method is enabled by a dilated Convolutional Neural Networks (CNN) architecture. The dilated convolutions provide a large receptive field, enhancing the efficiency of time-series processing with CNNs. The model outputs the probability of a signal point being a systolic peak. A peak finder function then detects the peaks’ locations in the signal. The peak finder function first makes a list of all points in the signals with a probability value higher than a pre-defined threshold (selected experimentally). Then, this function extracts the peaks’ locations using a local maximum finder.
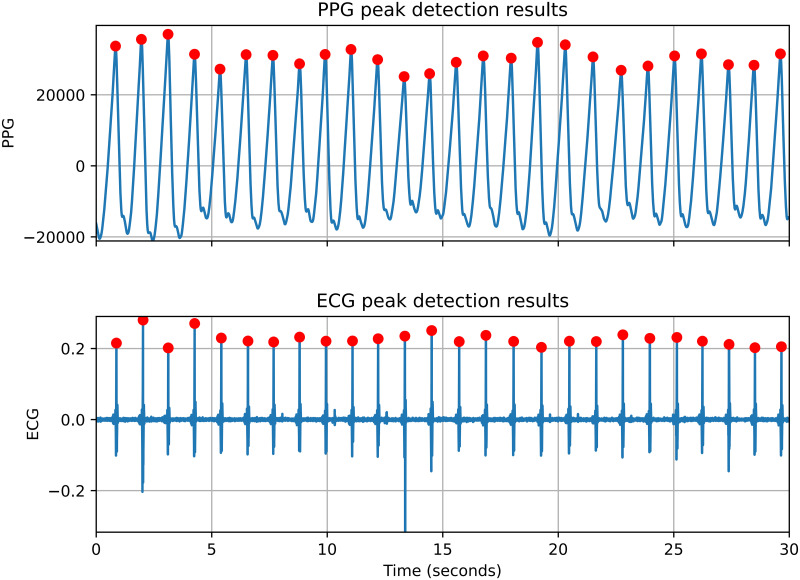
ECG peak detection—We developed a two-round peak detection algorithm to locate peaks in ECG signals. In the first round, the algorithm computes the average value of filtered ECG signals in a 5-minute window. Then, it uses this average value as a threshold to detect all possible peaks, including the real and false peaks. In the second round, the algorithm computes the average value of all detected peaks from the first round as a new threshold. By using the new threshold value and the heartbeat range (i.e., 20-200 beats per minute), the undetected R peaks are added, and false peaks are removed. Our peak detection method obtains higher accuracy in comparison with Pan-Tompkins [43] and Hamilton [44] algorithms. Fig 2 shows a sample of the peak detection results in a 30 seconds segment of PPG and corresponding ECG signals.
-
3) Abnormal peak removal: We used a rule-based method to remove invalid peaks extracted in the previous step. The invalid peaks removal rules are as follows:
We assume that the minimum and maximum heart rates are 20 and 200 beats per minute. Accordingly, the minimum and maximum peak-to-peak distances are 3000 and 300 milliseconds.
If the variation in NN intervals exceeds 20% of the average NN intervals, the exceeding part is removed. Accordingly, if more than 50% of the total NN intervals were removed, then the result of the entire 5-minute segment is not considered.
4) Feature extraction: In this step, we extracted HR and HRV parameters from normal interbeat intervals (NN intervals). The extracted time-domain HRV parameters are AVNN, SDNN, RMSSD, Percentage of successive NN intervals that differ by more than 50 ms (PNN50), and the frequency-domain parameters are low-frequency power (LF), high-frequency power (HF), and LF to HF ratio (LF/HF). Table 2 indicates the HRV parameters used in this study.
Fig 2. The peak detection results for a 30 seconds segment of PPG and ECG signals.
Table 2. Time domain and frequency domain HRV features and the descriptions.
| Feature | Units | Description |
|---|---|---|
| Time domain features | ||
| AVNN | ms | Mean of NN intervals |
| SDNN | ms | Standard deviation of NN intervals |
| RMSSD | ms | Root mean square of successive NN interval differences |
| pnn50 | % | Percentage of successive NN intervals that differ by more than 50 ms |
| Frequency domain features | ||
| LF power | s 2 | Power in low frequency band (0.04–0.15) |
| HF power | s 2 | Power in high frequency band (0.15–0.4) |
| LF/HF | % | LF to HF ratio |
Statistical analysis
We investigated the linear relationship between the smartwatch and Shimmer3 by performing the Pearson Correlation coefficient test on extracted parameters from two devices. We also applied a linear regression analysis method to assess the accuracy of the smartwatch’s HR and HRV parameters. We used the Samsung Watch’s data points (HR and HRV parameters) to fit the linear regression line. Then, we computed the R-squared value (r2) using the regression line and corresponding ECG data points to evaluate the closeness of baseline data to the smartwatch’s fitted regression line. We also computed the mean absolute error (MAE) using PPG data points and corresponding ECG data points to investigate the mean error.
Moreover, the Bland-Altman analysis was utilized to illustrate and estimate the agreement between the PPG and ECG results. The Bland-Altman analysis provides mean bias, standard deviation, and ±95% confidence intervals (CI) based on the differences between the Samsung Watch and Shimmer3. We leveraged python libraries including Scipy [45], sklearn [46], and Statsmodels [47] to implement the statistical analysis.
Results
We validated the PPG data collected via the Samsung smartwatch against the ECG data of the Shimmer device in free-living conditions. The analysis includes the data collected from 28 participants (i.e., 14 females and 14 males). We first assess the HR and HRV parameters derived from five-minute segments collected during sleep. Then, the five-minute PPG segments collected during the awake time are evaluated.
Comparisons of HR and HRV parameters of Samsung smartwatch and Shimmer3 in 5-minute time windows during sleep time
The sleep duration was acquired from the self-report questionnaires collected during the monitoring. We obtained the correlation between the HR and HRV parameters of the smartwatch and Shimmer3 in 5-minute segments. Table 3 indicates the Pearson correlation coefficient with the corresponding P-values, 95% Confidence Interval, mean biases, r2, and MAE of the HR and HRV parameters. As shown in Table 3, the HR, AVNN, SDNN, and PNN50 between the Samsung smartwatch and Shimmer3 are highly correlated. The correlation values of the RMSSD, LF, and HF are still high (positive) but slightly lower. The LF/HF ratio value shows a moderately positive relationship.
Table 3. Pearson correlation coefficient, P-values, 95% confidence interval, mean difference, r2 and mean absolute error between smartwatch and Shimmer3 HR and HRV parameters in 5-minute window during sleep.
| Parameter | Pearson correlation coefficient | P-value | Confidence Interval | Mean Bias | r 2 | Mean Absolute Error |
|---|---|---|---|---|---|---|
| HR | 0.941 | < 0.001 | [−7.53, 6.77] | −0.38 | 0.882 | 1.06 |
| AVNN | 0.960 | < 0.001 | [−83.87, 108.59] | 12.36 ms | 0.909 | 19.79 |
| RMSSD | 0.778 | < 0.001 | [−68.49, 32.01] | −18.24 ms | 0.405 | 22.44 |
| SDNN | 0.802 | < 0.001 | [−72.66, 28.29] | −22.19 ms | 0.246 | 23.31 |
| PNN50 | 0.964 | < 0.001 | [−13.21, 11.58] | −0.81 | 0.926 | 4.31 |
| LF | 0.784 | < 0.001 | [−1763.66, 834.77] | −464.45 ms2 | 0.206 | 476.13 |
| HF | 0.782 | < 0.001 | [−1188.67, 693.23] | −247.72 ms2 | 0.462 | 299.77 |
| LF/HF ratio | 0.622 | < 0.001 | [−2.24, 1.72] | −0.26 | 0.216 | 0.693 |
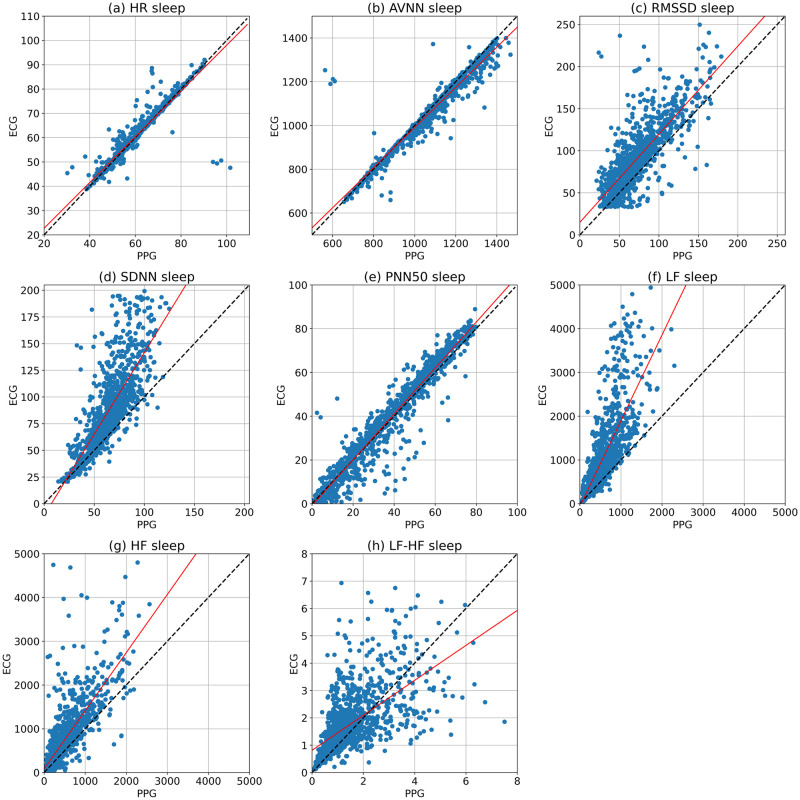
The regression analysis was used to compare the accuracy of the extracted parameters from the Samsung smartwatch against the reference ECG. Fig 3 illustrates the HR and HRV parameters collected by the Samsung smartwatch (PPG) and Shimmer3 (ECG). The regression analysis was performed for the five-minute segments, and the regression lines (in red) are indicated. There are also y = x lines (in black), representing the best outcome if the PPG and ECG values are equal. The r2 values are shown in Table 3, indicating the scatter of the data around the regression lines. As shown in Fig 3, the fitted lines of the HR, AVNN, and pNN50 closely follow ideal lines, and their r2 values are considerably high. However, the regression lines of other HRV parameters, including RMSSD, SDNN, LF, HF, and LF/HF relatively diverge, and their corresponding r2 values are moderate. In addition, MAE shows low errors for HR, AVNN, and PNN50, moderate errors for RMSSD, SDNN, LF, and HF, and relatively high errors for the LF/HF ratio.
Fig 3. The scatter plots and regression analysis of the HR, AVNN, RMSSD, SDNN, PNN50, LF, HF, and LF/HF collected from the Samsung smartwatch and Shimmer ECG in 5-minute segments during the sleep time.
The regression lines and ideal lines are indicated in red and black colors, respectively.
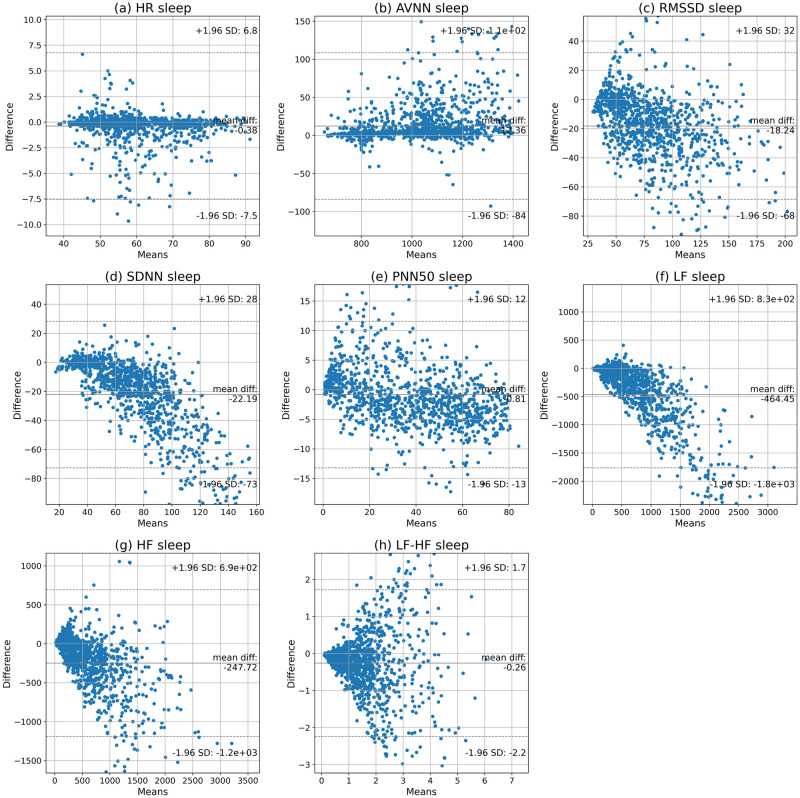
In addition, the Bland-Altman analysis was carried out to determine the agreement of the parameters extracted from the Samsung smartwatch and the reference ECG. The 95% confidence intervals and the mean biases are given in Fig 4 and Table 3. The results show that the smartwatch underestimates AVNN values (on average) but overestimates other parameters. In addition, there is a narrow 95% confidence interval for HR, RMSSD, SDNN, and PNN50; however, AVNN, LF, HF, and LF/HF ratio have relatively wider confidence intervals.
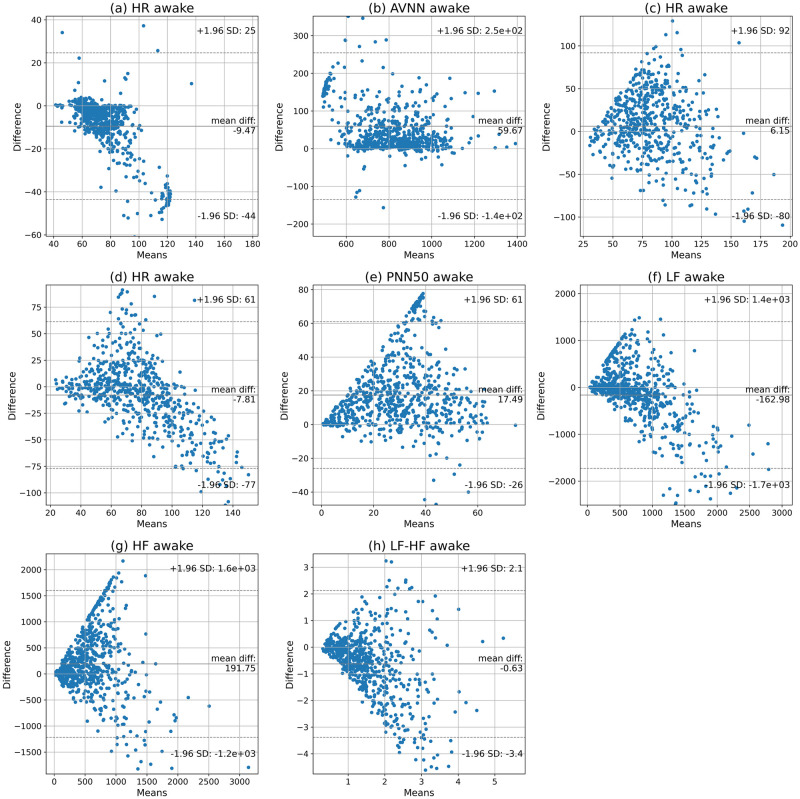
Fig 4. Bland-Altman plots of the HR, AVNN, RMSSD, SDNN, PNN50, LF, HF, and LF/HF in 5-minute segments obtained by smartwatch and Shimmer3 during sleep.
Comparisons of HR and HRV parameters of the Samsung smartwatch and Shimmer3 in 5-minute time windows during awake time
This section describes the comparison of the Samsung smartwatch and the reference ECG during awake time. The awake time was obtained by excluding the sleep time from 24-hours. The assessment was performed by comparing the HR and HRV parameters in 5-minute segments. Table 4 represents the Pearson correlation coefficients along with the corresponding P-values, 95% confidence interval, and mean biases of HR and HRV parameters collected during awake time. As shown in Table 4, the results show a high positive correlation between AVNN values, a moderate positive correlation between HR values, and low positive correlations of the other HRV parameters (i.e., RMSSD, SDNN, LF, HF, and LF/HF ratio) during awake time.
Table 4. The calculated Pearson correlation coefficient, P-values, 95% confidence interval, and mean difference between the smartwatch and Shimmer3 HR and HRV parameters in 5-minute window slots in awake time.
| Parameter | Pearson correlation coefficient | P-value | Confidence Interval | Mean Bias | r 2 | Mean Absolute Error |
|---|---|---|---|---|---|---|
| HR | 0.675 | <0.001 | [-43.58, 24.65] | -9.47 | 0.293 | 11.10 |
| AVNN | 0.833 | <0.001 | [-135.12, 254.44] | 59.66 ms | 0.582 | 63.62 |
| RMSSD | 0.251 | <0.001 | [-79.59, 91.89] | 6.15 ms | -0.191 | 32.11 |
| SDNN | 0.404 | <0.001 | [-76.99, 61.36] | -7.81 ms | 0.099 | 24.94 |
| PNN50 | 0.277 | <0.001 | [-26.0, 60.99] | 17.5 | -1.62 | 20.35 |
| LF | 0.350 | <0.001 | [-1727.72, 1402.18] | -162.77 ms2 | 0.075 | 484.98 |
| HF | 0.130 | <0.001 | [-1215.82, 1599.31] | 191.75 ms2 | -0.493 | 504.18 |
| LF/HF ratio | 0.211 | <0.001 | [-3.38, 2.12] | -0.63 | -0.453 | 1.04 |
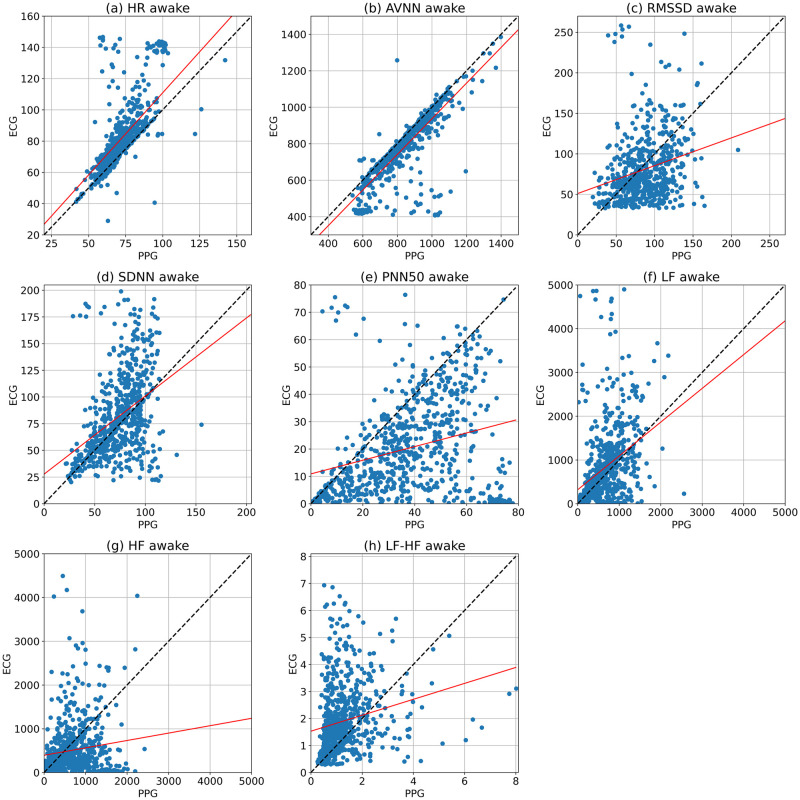
We used regression analysis to compare the accuracy of the Samsung smartwatch with the ECG device. Fig 5 illustrates the regression line (in red) of the HR and HRV parameters of the five-minute segments. In addition, the y = x line is shown in these plots, which indicate the highest accuracy when the watch’s parameters are equal to the golden standard values. The r2 values and MAE values are also shown in Table 4. The fitted lines of AVNN and HR are close to the ideal line. The r2 value for AVNN is relatively high and HR has moderate r2 value. However, the data points of the other HRV parameters, including RMSSD, SDNN, pnn50, LF, HF, and LF/HF ratio, are dispersed, and their r2 values are low. Moreover, MAE values are low for AVNN, moderate for HR, RMSSD, and SDNN, and high for the other HRV parameters.
Fig 5. The scatter plots and regression analysis of the HR, AVNN, RMSSD, SDNN, PNN50, LF, HF, and LF/HF ratio collected from the Samsung smartwatch and Shimmer ECG in 5-minute segments during awake time.
The regression and ideal lines are indicated in red and black, respectively.
We also utilized the Bland-Altman analysis to examine the agreement between the HR and HRV values during awake time. The mean biases and 95% confidence intervals are indicated in Fig 6 and Table 4. The results show that, on average, the Samsung smartwatch overestimates AVNN, RMSSD, PNN50, and HF, while it underestimates HR, SDNN, LF, and LF/HF ratio during awake time. Moreover, the 95% confidence intervals of the HR and HRV parameters are relatively wide.
Fig 6. Bland-Altman plots of the HR, AVNN, RMSSD, SDNN, PNN50, LF, HF, and LF/HF in 5-minute segments obtained by the smartwatch and Shimmer3 during awake time.
Discussion
Principle results
In this paper, we validated the accuracy of HR and HRV parameters extracted from PPG signals collected by the Samsung smartwatch during sleep and awake time. We used short-term HRV analysis, in which HRV parameters are obtained from five-minute PPG signals [32]. Our findings during sleep time show very low mean biases of HR and AVNN, relatively low mean biases of RMSSD, SDNN, pNN50, and LF/HF ratio, and moderate mean biases of LF and HF. During the awake time, the mean biases of RMSSD and SDNN are relatively low, while the biases of HR and other HRV parameters are moderate.
Moreover, HR, AVNN, RMSSD, SDNN, pNN50, LF, and HF extracted from the Samsung watch indicated high positive correlations, while LF/HF ratio showed a moderate positive correlation with the baseline during sleep time. However, during awake time, AVNN has a high positive correlation, HR has a moderate positive correlation, and the other HRV parameters have low positive correlations with the baseline. The Samsung smartwatch underestimates HR, SDNN, LF, and LF/HF ratio but overestimates AVNN during sleep time and awake time. Moreover, the watch underestimates RMSSD, pNN50, and HF during sleep time, although it overestimates these parameters during awake time.
The error variance of the parameters is higher during awake time compared with sleep time. During sleep time, the HR, AVNN, and pNN50 have relatively low error rates, RMSSD, SDNN, LF, and HF have moderate error rates, and LF/HF ratio has a high error rate. However, during awake time, AVNN has a moderate error rate, and HR and other HRV parameters have high error rates.
In conclusion, our findings show high accuracy of HR, AVNN, and pNN50 during sleep. RMSSD, SDNN, LF, and HF have satisfactory accuracy during sleep. However, during awake time, only AVNN and HR have acceptable accuracy. HRV collection—using the Samsung smartwatch during daily activities—requires 1) noise cancellation techniques [48] to improve the signal quality and/or 2) signal quality assessment techniques [49, 50] to ensure the collected signal is not distorted and subsequently the parameters’ accuracy is acceptable.
Comparison with previous studies
To the best of our knowledge, this is the first paper validating the HR and HRV parameters extracted from PPG signals of a smartwatch in free-living conditions. Previous studies showed high accuracy and low bias in HR extracted from wristbands including Empatica E4, Apple watch, Microsoft band, Fitbit, Garmin, PulseOn, Basis Peak, and the Wavelet wristband compared to ECG results [15, 16, 26–31, 34]. Our results also show high correlation and low bias in HR results during sleep. However, in comparison with the previous works, our results show a lower correlation of HR during the awake time when participants are involved in different activities.
Other studies validated HRV parameters during rest and specific activities. They showed the high accuracy of HRV parameters extracted from the Empatica E4 wristband for different population groups during rest and non-movement conditions [15, 16, 30]. The authors in [34] showed high correlations in SDNN and RMSSD extracted from the Wavelet wristband compared with the golden standard while resting in seated positions. Our results show a slightly lower correlation during sleep compared with previous results.
Moreover, previous studies indicated poor agreement of HRV parameters during activities for Empatica E4 [33, 51]. These studies also showed that the reliability of HRV parameters decreases with an increase in the intensity of activity. Their results follow our findings, showing higher accuracy during sleep time and lower accuracy during awake time.
Previous studies also indicated that time-domain HRV parameters have higher accuracy compared with the frequency-domain parameters. For example, the results in [14] showed high agreements of time-domain parameters during rest and mental stress but lower agreements of frequency-domain parameters for the apple watch. Microsoft Band 2 also had a higher error rate in LF/HF compared to time-domain parameters during rest and activity [33]. The results are in accordance with our results showing higher accuracy in time-domain parameters compared with frequency-domain parameters during sleep and awake time. In addition, the results in [14, 30] indicated that LF has higher accuracy than HF during the activity, which is in accordance with our results.
PPG signals also provide opportunities to assess the parameters of blood pressure (BP) and peripheral blood flow. [52–54]. Almarshad et al. showed that PPG and BP signals have the same frequency components as sequences of RR intervals in the LF band [52]. With the unique association of LF oscillations in RR intervals with autonomic processes, LF oscillations in PPG, as well as local regulation mechanisms, reflect the mechanism of autonomic control of blood circulation. According to the high coherence between BP and PPG signals, it is possible to study autonomic control of blood pressure using the PPG signal instead of BP. Moreover, other studies have demonstrated the functional autonomy of the LF oscillations of the heart rhythm and peripheral blood flow by PPG signal analysis [53, 54]. Extracting accurate HRV parameters and their oscillation is also desired for other health applications such as blood pressure measurement.
Limitations
This study is limited to 24-hours data collection in everyday life settings. In the future, we will consider validating the HR and HRV parameters extracted from the smartwatch in a longer data collection period (e.g., several days or weeks). Therefore, the assessment will provide a higher confidence level on the validity results of HR and HRV parameters.
Moreover, the generalizability of the results is limited to healthy populations as we only included healthy individuals in this study. Previous works showed that the accuracy of wearable devices can vary for different population group [55, 56]. Cardiovascular disorders, such as atrial fibrillation, may cause irregular heartbeats in PPG, which will affect the HRV parameters [1]. Our future work will consider validating PPG-based HRV parameters for different age groups and various health conditions.
Conclusion
In this paper, we comprehensively assessed the validity of HR and HRV parameters extracted from PPG signals collected for 24-hours by the Samsung Gear Sport smartwatch. The data from 28 participants were included in the study. The smartwatch was compared with an ECG device placed on the user’s chest. Our results showed low mean biases of HR, time-domain HRV, and LF/HF while moderate mean biases of LF and HF during sleep. The findings also indicated low error variances of HR, AVNN, and pNN50, moderate error variances of RMSSD, SDNN, LF, and HF, and a high error variance of LF/HF ratio during sleep. Moreover, there were high positive correlations for HR, time-domain HRV parameters, LF and HF, and a moderate positive correlation of LF/HF compared with the baseline parameters during sleep.
During the awake time, RMSSD and SDNN had low mean biases, while the other parameters showed moderate mean biases. Our findings indicated a low error variance of AVNN and a moderate error variance of HR, while the other parameters had high error variances. In addition, AVNN had a high positive correlation with the baseline, and HR had a moderate positive correlation. However, the other parameters had low positive correlations with the baseline parameters.
The smartwatch can accurately measure HR, AVNN, and pNN50 during sleep and AVNN during awake time. Moreover, the smartwatch can provide acceptable RMSSD, SDNN, LF, and HF during sleep and HR during awake time. Future work should include the assessment of the Smartwatch’s HR and HRV parameters of various population groups with different health conditions.
Acknowledgments
The authors would like to thank Elisa Lankinen, Mohsen Saei Dehghan, Bushra Zafar, and Henrika Merenlehto for contributing to the data collection.
Data Availability
The data used in this study include sensitive health information, and the informed consent signed by the participants does not allow the data to be made publicly available due to ethical restriction. According to the current approval by the Ethics Committee of University of Turku, the participants gave permission to use the collected data only for the purpose described in the consent. Data requests may be subject to individual consent and/or ethics committee approval. Researchers wishing to use the data should contact the Ethics Committee of University of Turku, contact details: FI-20014 Turun yliopisto, Finland, e-mail: eettinen@utu.fi. We recommend first to contact the PI of the research project, associate professor Anna Axelin, contact details: University of Turku, Department of Nursing Science, 20014 University of Turku, Finland, e-mail: anmaax@utu.fi.
Funding Statement
This research was supported by the Academy of Finland https://www.aka.fi/en/ (Awards 316810 (AMR), and 316811 (AA)) and U.S. National Science Foundation https://www.nsf.gov/ (Awards CNS-1831918 and FW-HTF CNS-2026614) (AMR). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. McCraty R, Shaffer F. Heart rate variability: new perspectives on physiological mechanisms, assessment of self-regulatory capacity, and health risk. Global advances in health and medicine. 2015;4(1):46–61. doi: 10.7453/gahmj.2014.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stein P, Barzilay J, Domitrovich P, Chaves P, Gottdiener J, Heckbert S, et al. The relationship of heart rate and heart rate variability to non-diabetic fasting glucose levels and the metabolic syndrome: The Cardiovascular Health Study. Diabetic medicine. 2007;24(8):855–863. doi: 10.1111/j.1464-5491.2007.02163.x [DOI] [PubMed] [Google Scholar]
- 3. Benichou T, Pereira B, Mermillod M, Tauveron I, Pfabigan D, Maqdasy S, et al. Heart rate variability in type 2 diabetes mellitus: A systematic review and meta–analysis. PloS one. 2018;13(4):e0195166. doi: 10.1371/journal.pone.0195166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Terathongkum S, Pickler RH. Relationships among heart rate variability, hypertension, and relaxation techniques. Journal of Vascular Nursing. 2004;22(3):78–82. doi: 10.1016/j.jvn.2004.06.003 [DOI] [PubMed] [Google Scholar]
- 5. Hartmann R, Schmidt FM, Sander C, Hegerl U. Heart rate variability as indicator of clinical state in depression. Frontiers in psychiatry. 2019;9:735. doi: 10.3389/fpsyt.2018.00735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. International journal of cardiology. 2010;141(2):122–131. doi: 10.1016/j.ijcard.2009.09.543 [DOI] [PubMed] [Google Scholar]
- 7.Taelman J, Vandeput S, Spaepen A, Huffel SV. Influence of mental stress on heart rate and heart rate variability. In: 4th European conference of the international federation for medical and biological engineering. Springer; 2009. p. 1366–1369.
- 8. Han HJ, Labbaf S, Borelli JL, Dutt N, Rahmani AM. Objective stress monitoring based on wearable sensors in everyday settings. Journal of Medical Engineering & Technology. 2020;44(4):177–189. doi: 10.1080/03091902.2020.1759707 [DOI] [PubMed] [Google Scholar]
- 9. Burton A, Rahman K, Kadota Y, Lloyd A, Vollmer-Conna U. Reduced heart rate variability predicts poor sleep quality in a case–control study of chronic fatigue syndrome. Experimental brain research. 2010;204(1):71–78. doi: 10.1007/s00221-010-2296-1 [DOI] [PubMed] [Google Scholar]
- 10. Castaneda D, Esparza A, Ghamari M, Soltanpur C, Nazeran H. A review on wearable photoplethysmography sensors and their potential future applications in health care. International journal of biosensors & bioelectronics. 2018;4(4):195. doi: 10.15406/ijbsbe.2018.04.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yuda E, Shibata M, Ogata Y, Ueda N, Yambe T, Yoshizawa M, et al. Pulse rate variability: a new biomarker, not a surrogate for heart rate variability. Journal of physiological anthropology. 2020;39(1):1–4. doi: 10.1186/s40101-020-00233-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gil E, Orini M, Bailon R, Vergara JM, Mainardi L, Laguna P. Photoplethysmography pulse rate variability as a surrogate measurement of heart rate variability during non-stationary conditions. Physiological measurement. 2010;31(9):1271. doi: 10.1088/0967-3334/31/9/015 [DOI] [PubMed] [Google Scholar]
- 13. Choi A, Shin H. Photoplethysmography sampling frequency: pilot assessment of how low can we go to analyze pulse rate variability with reliability? Physiological measurement. 2017;38(3):586. doi: 10.1088/1361-6579/aa5efa [DOI] [PubMed] [Google Scholar]
- 14. Hernando D, Roca S, Sancho J, Alesanco Á, Bailón R. Validation of the apple watch for heart rate variability measurements during relax and mental stress in healthy subjects. Sensors. 2018;18(8):2619. doi: 10.3390/s18082619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schuurmans AA, de Looff P, Nijhof KS, Rosada C, Scholte RH, Popma A, et al. Validity of the Empatica E4 wristband to measure heart rate variability (HRV) parameters: A comparison to electrocardiography (ECG). Journal of medical systems. 2020;44(11):1–11. doi: 10.1007/s10916-020-01648-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Menghini L, Gianfranchi E, Cellini N, Patron E, Tagliabue M, Sarlo M. Stressing the accuracy: Wrist-worn wearable sensor validation over different conditions. Psychophysiology. 2019;56(11):e13441. doi: 10.1111/psyp.13441 [DOI] [PubMed] [Google Scholar]
- 17.Bhowmik T, Dey J, Tiwari VN. A novel method for accurate estimation of HRV from smartwatch PPG signals. In: 2017 39th annual international conference of the IEEE engineering in medicine and biology society (EMBC). IEEE; 2017. p. 109–112. [DOI] [PubMed]
- 18. Kinnunen H, Rantanen A, Kenttä T, Koskimäki H. Feasible assessment of recovery and cardiovascular health: accuracy of nocturnal HR and HRV assessed via ring PPG in comparison to medical grade ECG. Physiological measurement. 2020;41(4):04NT01. doi: 10.1088/1361-6579/ab840a [DOI] [PubMed] [Google Scholar]
- 19.Jeyhani V, Mahdiani S, Peltokangas M, Vehkaoja A. Comparison of HRV parameters derived from photoplethysmography and electrocardiography signals. In: 2015 37th annual international conference of the ieee engineering in medicine and biology society (EMBC). IEEE; 2015. p. 5952–5955. [DOI] [PubMed]
- 20. Mejía-Mejía E, Budidha K, Abay TY, May JM, Kyriacou PA. Heart rate variability (HRV) and pulse rate variability (PRV) for the assessment of autonomic responses. Frontiers in physiology. 2020;11:779. doi: 10.3389/fphys.2020.00779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elgendi M. On the analysis of fingertip photoplethysmogram signals. Current cardiology reviews. 2012;8(1):14–25. doi: 10.2174/157340312801215782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saarikko J, Niela-Vilen H, Ekholm E, Hamari L, Azimi I, Liljeberg P, et al. Continuous 7-Month Internet of Things–Based Monitoring of Health Parameters of Pregnant and Postpartum Women: Prospective Observational Feasibility Study. JMIR formative research. 2020;4(7):e12417. doi: 10.2196/12417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laccetti AL, Slack Tidwell R, Sheth NP, Logothetis C, VanAlstine M. Remote patient monitoring using smart phone derived patient reported outcomes and Fitbit data to enable longitudinal predictive modeling in prostate cancer: Feasibility results and lessons on platform development.; 2019.
- 24. Sarhaddi F, Azimi I, Labbaf S, Niela-Vilén H, Dutt N, Axelin A, et al. Long-Term IoT-Based Maternal Monitoring: System Design and Evaluation. Sensors. 2021;21(7):2281. doi: 10.3390/s21072281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han H, Kim MJ, Kim J. Development of real-time motion artifact reduction algorithm for a wearable photoplethysmography. In: 2007 29th Annual international conference of the IEEE engineering in medicine and biology society. IEEE; 2007. p. 1538–1541. [DOI] [PubMed]
- 26. Shcherbina A, Mattsson CM, Waggott D, Salisbury H, Christle JW, Hastie T, et al. Accuracy in wrist-worn, sensor-based measurements of heart rate and energy expenditure in a diverse cohort. Journal of personalized medicine. 2017;7(2):3. doi: 10.3390/jpm7020003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Støve MP, Haucke E, Nymann ML, Sigurdsson T, Larsen BT. Accuracy of the wearable activity tracker Garmin Forerunner 235 for the assessment of heart rate during rest and activity. Journal of Sports Sciences. 2019;37(8):895–901. doi: 10.1080/02640414.2018.1535563 [DOI] [PubMed] [Google Scholar]
- 28. Hahnen C, Freeman CG, Haldar N, Hamati JN, Bard DM, Murali V, et al. Accuracy of Vital Signs Measurements by a Smartwatch and a Portable Health Device: Validation Study. JMIR mHealth and uHealth. 2020;8(2):e16811. doi: 10.2196/16811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiler DT, Villajuan SO, Edkins L, Cleary S, Saleem JJ. Wearable heart rate monitor technology accuracy in research: a comparative study between PPG and ECG technology. In: Proceedings of the Human Factors and Ergonomics Society Annual Meeting. vol. 61. SAGE Publications Sage CA: Los Angeles, CA; 2017. p. 1292–1296.
- 30.Barrios L, Oldrati P, Santini S, Lutterotti A. Evaluating the accuracy of heart rate sensors based on photoplethysmography for in-the-wild analysis. In: Proceedings of the 13th EAI international conference on pervasive computing technologies for healthcare; 2019. p. 251–261.
- 31. Hwang S, Seo J, Jebelli H, Lee S. Feasibility analysis of heart rate monitoring of construction workers using a photoplethysmography (PPG) sensor embedded in a wristband-type activity tracker. Automation in Construction. 2016;71:372–381. doi: 10.1016/j.autcon.2016.08.029 [DOI] [Google Scholar]
- 32. Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Frontiers in public health. 2017; p. 258. doi: 10.3389/fpubh.2017.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morelli D, Bartoloni L, Colombo M, Plans D, Clifton DA. Profiling the propagation of error from PPG to HRV features in a wearable physiological-monitoring device. Healthcare technology letters. 2018;5(2):59–64. doi: 10.1049/htl.2017.0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dur O, Rhoades C, Ng MS, Elsayed R, van Mourik R, Majmudar MD, et al. Design rationale and performance evaluation of the wavelet health wristband: benchtop validation of a wrist-worn physiological signal recorder. JMIR mHealth and uHealth. 2018;6(10):e11040. doi: 10.2196/11040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samsung. Samsung Gear Sport Smartwatch; Retrieved on January 2022.
- 36.Shimmer. Shimmer device specification; 2021. Available from: https://www.shimmersensing.com/products/shimmer3-ecg-sensor#applications-tab [cited December 2021].
- 37. Burns A, Greene BR, McGrath MJ, O’Shea TJ, Kuris B, Ayer SM, et al. SHIMMER™–A wireless sensor platform for noninvasive biomedical research. IEEE Sensors Journal. 2010;10(9):1527–1534. doi: 10.1109/JSEN.2010.2045498 [DOI] [Google Scholar]
- 38. Peltola M. Role of editing of RR intervals in the analysis of heart rate variability. Frontiers in physiology. 2012;3:148. doi: 10.3389/fphys.2012.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Electrophysiology TFotESoCtNASoP. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93(5):1043–1065. doi: 10.1161/01.CIR.93.5.1043 [DOI] [PubMed] [Google Scholar]
- 40. Kazemi K, Laitala J, Azimi I, Liljeberg P, Rahmani AM. Robust PPG Peak Detection Using Dilated Convolutional Neural Networks. Sensors. 2022;22(16):6054. doi: 10.3390/s22166054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Elgendi M, Norton I, Brearley M, Abbott D, Schuurmans D. Systolic peak detection in acceleration photoplethysmograms measured from emergency responders in tropical conditions. PloS one. 2013;8(10):e76585. doi: 10.1371/journal.pone.0076585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Gent P, Farah H, van Nes N, van Arem B. Analysing noisy driver physiology real-time using off-the-shelf sensors: Heart rate analysis software from the taking the fast lane project. Journal of Open Research Software. 2019;7(1). doi: 10.5334/jors.241 [DOI] [Google Scholar]
- 43. Pan J, Tompkins WJ. A real-time QRS detection algorithm. IEEE transactions on biomedical engineering. 1985; p. 230–236. doi: 10.1109/TBME.1985.325532 [DOI] [PubMed] [Google Scholar]
- 44.Hamilton P. Open source ECG analysis. In: Computers in cardiology. IEEE; 2002. p. 101–104.
- 45. Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nature Methods. 2020;17:261–272. doi: 10.1038/s41592-019-0686-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, et al. Scikit-learn: Machine Learning in Python. Journal of Machine Learning Research. 2011;12:2825–2830. [Google Scholar]
- 47.Seabold S, Perktold J. statsmodels: Econometric and statistical modeling with python. In: 9th Python in Science Conference; 2010. p. 1–10.
- 48. Chowdhury SS, Hyder R, Hafiz MSB, Haque MA. Real-time robust heart rate estimation from wrist-type PPG signals using multiple reference adaptive noise cancellation. IEEE journal of biomedical and health informatics. 2016;22(2):450–459. doi: 10.1109/JBHI.2016.2632201 [DOI] [PubMed] [Google Scholar]
- 49. Elgendi M. Optimal signal quality index for photoplethysmogram signals. Bioengineering. 2016;3(4):21. doi: 10.3390/bioengineering3040021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mahmoudzadeh A, Azimi I, Rahmani AM, Liljeberg P. Lightweight photoplethysmography quality assessment for real-time IoT-based health monitoring using unsupervised anomaly detection. Procedia Computer Science. 2021;184:140–147. doi: 10.1016/j.procs.2021.03.025 [DOI] [Google Scholar]
- 51. Michael S, Graham KS, Davis GM. Cardiac autonomic responses during exercise and post-exercise recovery using heart rate variability and systolic time intervals—a review. Frontiers in physiology. 2017;8:301. doi: 10.3389/fphys.2017.00301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Almarshad MA, Islam MS, Al-Ahmadi S, BaHammam AS. Diagnostic Features and Potential Applications of PPG Signal in Healthcare: A Systematic Review. In: Healthcare. vol. 10. MDPI; 2022. p. 547. [DOI] [PMC free article] [PubMed]
- 53. Karavaev AS, Borovik AS, Borovkova EI, Orlova EA, Simonyan MA, Ponomarenko VI, et al. Low-frequency component of photoplethysmogram reflects the autonomic control of blood pressure. Biophysical Journal. 2021;120(13):2657–2664. doi: 10.1016/j.bpj.2021.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Simonyan M, Borovkova EI, Skazkina V, Karavaev A, Shvartz V, Kiselev A, et al. Gender-related specificities of photoplethysmogram spectral assessment dynamics in healthy subjects during the passive tilt test. Russian Open Medical Journal. 2021;10(1):115. doi: 10.15275/rusomj.2021.0115 [DOI] [Google Scholar]
- 55. Cook JD, Prairie ML, Plante DT. Utility of the Fitbit Flex to evaluate sleep in major depressive disorder: a comparison against polysomnography and wrist-worn actigraphy. Journal of affective disorders. 2017;217:299–305. doi: 10.1016/j.jad.2017.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cook JD, Prairie ML, Plante DT. Ability of the multisensory jawbone UP3 to quantify and classify sleep in patients with suspected central disorders of hypersomnolence: a comparison against polysomnography and actigraphy. Journal of Clinical Sleep Medicine. 2018;14(5):841–848. doi: 10.5664/jcsm.7120 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study include sensitive health information, and the informed consent signed by the participants does not allow the data to be made publicly available due to ethical restriction. According to the current approval by the Ethics Committee of University of Turku, the participants gave permission to use the collected data only for the purpose described in the consent. Data requests may be subject to individual consent and/or ethics committee approval. Researchers wishing to use the data should contact the Ethics Committee of University of Turku, contact details: FI-20014 Turun yliopisto, Finland, e-mail: eettinen@utu.fi. We recommend first to contact the PI of the research project, associate professor Anna Axelin, contact details: University of Turku, Department of Nursing Science, 20014 University of Turku, Finland, e-mail: anmaax@utu.fi.