Abstract
Ciliated ependymal cells line the ventricular system of the brain and the cerebral aqueducts. This study characterizes the relative roles of pneumolysin and hydrogen peroxide (H2O2) in pneumococcal meningitis, using the in vitro ependymal ciliary beat frequency (CBF) as an indicator of toxicity. We have developed an ex vivo model to examine the ependymal surface of the brain slices cut from the fourth ventricle. The ependymal cells had cilia beating at a frequency of between 38 and 44Hz. D39 (wild-type) and PLN-A (pneumolysin-negative) pneumococci at 108 CFU/ml both caused ciliary slowing. Catalase protected against PLN-A-induced ciliary slowing but afforded little protection from D39. Lysed PLN-A did not reduce CBF, whereas lysed D39 caused rapid ciliary stasis. There was no effect of catalase, penicillin, or catalase plus penicillin on the CBF. H2O2 at a concentration as low as 100 μM caused ciliary stasis, and this effect was abolished by coincubation with catalase. An additive inhibition of CBF was demonstrated using a combination of both toxins. A significant inhibition of CBF at between 30 and 120 min was demonstrated with both toxins compared with either H2O2 (10 μM) or pneumolysin (1 HU/ml) alone. D39 released equivalent levels of H2O2 to those released by PLN-A, and these concentrations were sufficient to cause ciliary stasis. The brain slices did not produce H2O2, and in the presence of 108 CFU of D39 or PLN-A per ml there was no detectable bacterially induced increase of H2O2 release from the brain slice. Coincubation with catalase converted the H2O2 produced by the pneumococci to H2O. Penicillin-induced lysis of bacteria dramatically reduced H2O2 production. The hemolytic activity released from D39 was sufficient to cause rapid ciliary stasis, and there was no detectable release of hemolytic activity from the pneumolysin-negative PLN-A. These data demonstrate that D39 bacteria released pneumolysin, which caused rapid ciliary stasis. D39 also released H2O2, which contributed to the toxicity, but this was masked by the more severe effects of pneumolysin. H2O2 released from intact PLN-A was sufficient to cause rapid ciliary stasis, and catalase protected against H2O2-induced cell toxicity, indicating a role for H2O2 in the response. There is also a slight additive effect of pneumolysin and H2O2 on ependymal toxicity; however, the precise mechanism of action and the role of these toxins in pathogenesis remain unclear.
The introduction of antibiotics has dramatically improved the survival of patients with pneumococcal meningitis. However, despite modern intensive care, there is still a high morbidity and mortality associated with this disease (3, 23).
The use of animal models has increased our understanding of the disease process and has identified relevant pneumococcal virulence factors (27, 28). However, to understand the effects of virulence factors on individual cells and to perform rapid screening of potential bacterial toxins, the use of in vitro models holds obvious advantages. We have developed such an in vitro system whereby brain slices are prepared with an intact ciliated ependymal lining. The ciliary beat frequency (CBF) of ependymal cilia may be measured directly and continually to assess the function and integrity of ependymal cells. The ependyma is thought to act as a filter, relaying macromolecules to and from the cerebrospinal fluid (CSF), and to play a role in controlling CSF volume (7). A recent report has shown that ciliated ependymal cells may be neuronal stem cells from which other neuronal cell phenotypes originate (15).
Brain ependymal cells are exposed to the cytotoxins produced by pneumococci when the CSF is infected. The identity of pneumococcal virulence factors that inhibits brain ependymal ciliary function has not been fully investigated. One of the most important pneumococcal virulence factors is the pore-forming cytotoxin pneumolysin (21). This toxin causes ciliary stasis in the respiratory tract (24) and the ependyma (12, 20). However, this toxin is not the only pneumococcal cytotoxin. Duane et al. have shown that H2O2 released from pneumococci deficient in pneumolysin caused cytotoxic effects to rat alveolar epithelial cells (8) and concluded that H2O2 was important in pneumococcal pneumonia. However, it has been demonstrated that pneumolysin-negative pneumococci are much less virulent at causing pneumonia in mice than are wild-type bacteria (1). Therefore, there remains some debate about the overall role of H2O2 in pneumococcal disease processes. The pneumococcus utilizes pyruvate oxidase enzymes to produce H2O2 (25, 29). Upon its generation, H2O2 is catabolized by catalase (deficient in the pneumococcus). In addition, H2O2 is thought to diffuse from the bacterial membrane into host cells, where it causes oxidative damage (6).
Here we show that H2O2 is released from pneumolysin-negative pneumococci and is toxic to ependymal ciliary function. Pneumococcal H2O2 may be an additional virulence factor which should be considered when investigating the pathophysiology of pneumococcal meningitis.
MATERIALS AND METHODS
Chemicals.
All the chemicals used in this study were of analytical grade purity.
Brain slices.
Rats, 14 to 17 days old, were killed by cervical dislocation, and their brains were isolated. The cerebellum was removed and mounted on a vibrotome under ice-cold M199 medium (ICN Laboratories). The brain was sliced (250-μm slices) through the medulla oblongata and pons into the floor of the fourth ventricle so that the ciliated V-shaped floor was clear. The slices were mounted in 2 ml of prewarmed M199 medium prior to use.
CBF measurements.
The method used to measure CBF was identical to a previously described method (12, 20). Briefly, the brain slices were placed in a humidified (80 to 90% humidity) thermostatically controlled (37°C) incubation chamber surrounding a light microscope (Diphot; Nikon) and left to equilibrate for 30 min. Beating cilia were recorded (magnification, ×320) using a digital high-speed video camera (Kodak Ektapro motion analyzer, model 1012) at a rate of 400 frames per s with a shutter speed of 1 in 2,000. The camera allows video sequences to be recorded and played back at reduced frame rates or frame by frame. CBF may be determined by timing a given number of individual ciliary beat cycles. The basal CBF was measured at 30 min. Each time point represents the measurement of four individual cilia from each slice. Ciliated brain slices were then exposed for at least 60 min to cell culture medium containing 108 CFU of pneumococci per ml in the presence or absence of catalase (2,000 EU/ml). To determine the effect of penicillin, lysed bacteria (108 CFU/ml) were suspended in M199 medium containing 1 mg of penicillin per ml, incubated for 2 h at 37°C, and frozen at −70°C. This preparation was added to ependymal tissue in the presence or absence of catalase for at least 60 min. CBF measurements were made over 60 to 120 min to record any change in ciliary function.
Pneumococci.
The strains used were a type 2 wild-type strain (D39) and a pneumolysin-negative version made by insertion duplication mutagenesis (PLN-A) (1). Bacteria were grown in brain heart infusion broth (containing 0.5 mg of erythromycin per ml for growth of PLN-A to late log phase). The bacteria were not washed prior to experimental use. The pneumococci were exposed to 10 μg of penicillin per ml for 3 h and then frozen at −70°C in 10% fetal calf serum–M199 medium containing penicillin. Organism death was determined by colony counting, and lysis was determined by microscopy. Both bacteria (PLN-A and D39) were equally susceptible to penicillin. Overnight plate cultures were grown in an oxygen-free environment on 10% blood agar in the presence (PLN-A) or absence (D39) of erythromycin (1 mg/ml).
Purified pneumolysin.
Pneumolysin was purified as previously described (21). Briefly, recombinant toxin was overexpressed in Escherichia coli strain JM109. The bacteria were lysed by sonication, and the pneumolysin was purified by hydrophobic and ion-exchange chromatography. Toxin purity was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by Coomassie blue staining, which showed a single 52-kDa band accounting for 95% of the protein. A 1-ml volume of pneumolysin diluted in M199 was added to the tissue with a further 1 ml of medium. Repeated gentle pipetting was performed to mix the medium, and the tissues were incubated for up to 120 min prior to final measurement of CBF.
Viable-colony counting.
Stock suspensions of bacteria were serially (1 in 10) diluted to 10−6 in nanopure water. Prewarmed blood agar plates were marked into four sectors, and in each sector 60 μl of 10−3, 10−4, 10−5, or 10−6 bacterial dilution was pipetted. When dry, the plates were grown overnight in an oxygen-free jar, and the resulting colonies were counted from a sector containing 100 to 200 CFU. The number of CFU per milliliter in the original bacterial stock solution was calculated by multiplying the CFU counted by 16.6 (correcting for volume) and the dilution factor for that sector.
Hemolytic assay.
In a round-bottom 96-well plate, 50 μl of haemolytic fraction was serially diluted (1:1) into 50 μl of phosphate-buffered saline (8 mM NaHPO4, 1.5 mM KH2PO4, 2.5 mM KCl, 240 mM NaCl [pH 7.4]). Then 50 μl of a 2% suspension of compacted (4,000 × g for 2 min) sheep red blood cells was added to each well. The plate was then incubated for 30 min at 37°C. The hemolytic units (HU) were calculated from the well at which 50% hemolysis had occurred, with this well being the inverse of the number of dilutions made from the original hemolytic fraction.
Hydrogen peroxide release and assay.
Pneumococci (D39 and PLN-A) were grown to late log phase and harvested when the optical density at 500 nm was between 0.5 and 0.7. Following centrifugation, the pellet was resuspended in 3 ml of Hanks-HEPES (MgSO4, 0.1 g/liter; KCl, 0.4 g/liter; KHPO4, 0.06 g/liter; NaCl, 8 g/liter; NaHPO4, 0.05 g/liter; d-glucose, 1 g/liter; HEPES, 20 mM [pH 7.4]). The bacterial suspension was incubated at 37°C for 60 min. Then 100 μl of the suspension was sampled at 0, 5, 15, 30, 60 min. This sample was centrifuged at 5,000 × g for 2 min in a microcentrifuge. H2O2 was measured using a fluorometric assay (13) based on the oxidation of p-hydroxyphenylacetic acid (Sigma Chemicals, Poole, United Kingdom). An 800-μl volume of HEPES-buffered saline solution (20 mM HEPES, 250 mM NaCl [pH 7.4]) was added to each tube; to this was added 50 μl of p-hydroxyphenylacetic acid (7.4 mg/ml) and 100 μl of sample or standard H2O2 solution. The reaction was started by the addition of horseradish peroxidase (10 EU/ml) (Sigma Chemicals), and the reaction mixture was incubated for 30 min at 37°C in the dark. The reaction was terminated by the addition of 2 ml of 100 mM ice-cold borate buffer (pH 10.4). Fluorescence was measured at an excitation wavelength of 313nm and an absorption wavelength of 414nm (Shimadzu RF-1501). H2O2 concentrations were extrapolated from a standard curve of H2O2 (0 to 20 μM).
Electron microscopy.
For scanning electron microscopy, the tissues were fixed in Sorensen's phosphate-buffered (pH 7.4) gluteraldehyde (4%, wt/vol) (Sigma Chemicals). After postfixation in 1% (wt/vol) osmium tetroxide, samples were dehydrated through graded ethanol dilutions and immersed in hexamethyldisilazane (HMDS). The HMDS evaporated, leaving dry tissue with no phase boundary damage.
Statistics.
All data presented are mean and standard error of the mean for 4 to 13 independent experiments. Statistical analysis was performed where appropriate; individual curves were analysed by analysis of variance. If the data were significantly different by analysis of variance, individual data points were compared using a paired or unpaired Student t test, with Bonferroni correction for repeated measures.
RESULTS
Effects of intact pneumococci on ependymal CBF.
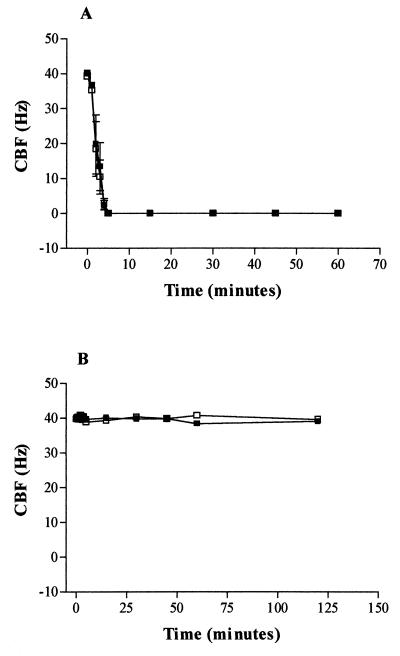
Rat brain slices cut from the fourth ventricle had cilia beating at a frequency of between 38 and 44 Hz. Both D39 and PLN-A pneumococci, at 108 CFU/ml, caused ciliary slowing (Fig. 1). The rate of inhibition was slightly increased in the presence of D39 compared with PLN-A (Fig. 1). To investigate any potential role of pneumococcal production of H2O2 in this inhibition, brain slices were coincubated with catalase (2,000 EU/ml). There was a small but significant (P < 0.05) decrease in the CBF of D39-treated slices at 30 and 60 min compared with that of D39- plus catalase-treated slices (Fig. 1A). This indicates that only a small component of the inhibition of CBF by D39 was mediated by H2O2. However, the addition of catalase to PLN-A (Fig. 1B) prevented the reduction in CBF seen on exposure to PLN-A alone (Fig. 1B). Indeed, from 5 min onward, all the CBF measurements for PLN-A plus catalase were significantly (P < 0.05) increased compared to those for PLN-A alone.
FIG. 1.
(A) Effect of D39 (108 CFU/ml) on ependymal CBF in the absence (■) or presence (□) of catalase (2,000 EU/ml). ∗, statistically (P < 0.05; paired t test) increased compared with D39. (B) Protective effect of catalase (2,000 EU/ml) (□) on PLN-A induced inhibition (■) of ependymal CBF. ∗, statistically (P < 0.05; paired t test) increased compared with PLN-A. All data are mean and standard error of the mean of four independent experiments.
Effects of lysed pneumococci on ependymal CBF.
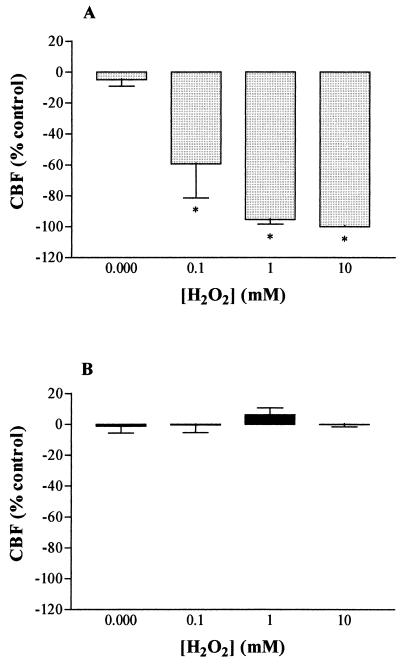
To examine whether an intact bacterial cell wall was a prerequisite for the inhibition of CBF, the pneumococci were lysed using penicillin (1 mg/ml). Lysed D39 bacteria caused rapid ciliary stasis, an effect which was not reversed by catalase (Fig. 2A). Lysed PLN-A did not reduce CBF, and catalase had no effect on CBF (Fig. 2B). To find the levels of H2O2 which were required to cause inhibition of CBF and to be sure that bacterial numbers were sufficient to cause this toxicity, brain slices were incubated in 100 μM, 1 mM and 10 mM H2O2. In the presence of all these concentrations, there was a statistically significant inhibition of the CBF at 15 min compared with control (Fig. 3A). The inhibitory effect of each concentration of H2O2 on CBF was reversed by coincubation with 2,000 EU of catalase per ml (Fig. 3B).
FIG. 2.
Effect of penicillin (1 mg/ml)-lysed pneumococci (108 CFU/ml) in the presence (□) or absence (■) of catalase (2,000 EU/ml). (A) D39; (B) PLN-A. There were no statistical differences in the data (mean and standard error of the mean of four experiments).
FIG. 3.
(A) Dose-dependent hydrogen peroxide inhibition of ependymal CBF. (B) Abolition of H2O2 inhibition by coincubation with catalase (2,000 EU/ml). All data are mean and standard error of the mean of five or six individual experiments. ∗, statistically (P < 0.05; paired t test) inhibited compared with no H2O2.
Pneumococcal H2O2 release.
Analysis of H2O2 levels at 0, 5, 15, and 30 min from a 108-CFU/ml stock suspension of pneumococci showed that maximal levels of H2O2 were present in the supernatant by 15 min. After this time, the net production and the net loss of H2O2 were equal and the concentrations remained at a steady state out to 120 min. The H2O2 levels in Table 1 are from the 60-min time point. The release of H2O2 from D39 and PLN-A was 81.4 ± 18 and 127 ± 58 μM, respectively (Table 1). There was a similar release of H2O2 from D39 and PLN-A in the presence of brain slices, indicating that pneumococcus-induced brain slice damage does not stimulate the release of H2O2 from the slice (Table 1). The brain slice alone did not release measurable levels of H2O2 (Table 1). Coincubation with catalase converted the H2O2 produced by the pneumococci (D39 and PLN-A) to H2O (Table 1). In addition, not surprisingly, penicillin-lysed D39 and PLN-A did not synthesize H2O2 (Table 1). Figure 4A shows that D39 but not PLN-A at 108 CFU/ml caused a significant (P < 0.05) time-dependent increase in hemolytic activity measured from the supernatant, consistent with the genetic modifications to the bacteria. H2O2 at 100 μM caused no hemolysis of sheep red blood cells. Catalase (40.1 ± 0.6 Hz), penicillin (40.3 ± 2.7 Hz), or catalase in the presence of penicillin (38.2 ± 5.2 Hz) did not affect CBF at 1 h.
TABLE 1.
Hydrogen peroxide release from 108 CFU/ml of pneumococci after 60 min at 37°C
| Strain and tissue | CFU/ml | Mean H2O2 concn (μM) at 60 min ± SEMa |
|---|---|---|
| D39 | 108 | 81.4 ± 18 |
| D39 + brain slice | 108 | 73.6 ± 23 |
| D39 + catalase (2,000 EU/ml) | 108 | 3.3 ± 0.5b |
| D39 + penicillin (1 mg/ml) | 108 lysed | 4 ± 2.2b |
| Brain slice | NAd | 0 ± 0 |
| PLN-A | 108 | 127 ± 58 |
| PLN-A + brain slice | 108 | 78 ± 8 |
| PLN-A + catalase (2,000 EU/ml) | 108 | 0 ± 0c |
| PLN-A + penicillin (1 mg/ml) | 108 lysed | 2.8 ± 1.7c |
Data are mean and standard error of the mean of 4 to 13 individual experiments.
∗ significantly (P < 0.05; unpaired t test) reduced compared with D39.
# Significantly (P < 0.05) reduced compared with PLN-A.
NA, not applicable.
FIG. 4.
(A) The supernatant from D39 (■) at 108 CFU/ml shows a time-dependent increase hemolytic activity, whereas PLN-A (□) at 108 CFU/ml shows no hemolytic activity in the supernatant over 60 min at 37°C. ∗, statistically (P < 0.05; paired t test) increased compared with 0 min. (B) Additive action of pneumolysin and H2O2 on ependymal CBF (■, control; □, 10 μM H2O2; ●, 1 HU of pneumolysin per ml; ○, 10 μM H2O2 plus 1 HU of pneumolysin per ml). ∗, statistically (P < 0.05, paired t test) inhibited compared with pneumolysin alone. All data are mean and standard error of the mean of five individual experiments.
Additive action of pneumolysin and H2O2.
To examine whether there is an additive action of pneumolysin (1 HU/ml) and H2O2 (10 μM) on the inhibition of ependymal CBF, an experiment was performed with low doses of both toxins alone and in combination (Fig. 4B). When the toxins were added in combination, there was a small but significant increase in the inhibition of CBF at 5, 30, 60, and 120 min compared with that induced by 1 HU of pneumolysin per ml (Fig. 4B). This indicates that there is some additive activity between the two toxins at these time points.
Ultrastructural changes of ependyma due to H2O2.
The scanning electron micrograph shows normal cilia (Fig. 5A) evenly distributed on the ependyma. In the presence of H2O2 (10 and 100 μM [Fig. 5B and C]), widespread morphological alterations from the normal epithelium were observed; sparse and disrupted cilia were found, and unciliated ependymal cell debris remained in the place of heavily ciliated cells. Coincubation of the brain slice with H2O2 (100 μM) and catalase (2,000 EU/ml) (Fig. 5D) protected the ependymal cilia from the toxic effects of the H2O2.
FIG. 5.
Scanning electron micrographs of ependymal cilia on a brain slice cut into the floor of the fourth ventricle. (A) Healthy cilia. (B and C) The cilia show an increasing amount of morphological alterations from normal at 60 min with higher concentrations of H2O2 (100 μM [B] and 1 mM [C]). (D) Catalase (100 μM H2O2 plus 2,000 EU of catalase per ml) protects the cilia from this disruption by H2O2. Bar, 4 μm (A) and 3.5 μm (B to D).
DISCUSSION
It has been shown previously that H2O2 disrupts the respiratory epithelium, causing ciliary stasis (4, 14, 17). H2O2 also depletes epithelial ATP levels, and because ciliary beating is heavily ATP dependent (5), this was suggested to be the mechanism for H2O2-induced epithelial ciliary stasis (30). This study demonstrates that H2O2 can inhibit ependymal CBF and that pneumococci release sufficient amounts of H2O2 to cause damage to ependymal cilia at concentrations of pneumococci that are commonly observed in patients with pneumococcal meningitis (2). At high (108 CFU/ml) concentrations of bacteria, there is a high degree of bacterial autolysis and toxin levels in the CSF will increase. Indeed, when incubated in the test tube at 108 CFU/ml, D39 increased the soluble hemolytic activity (Fig. 4A), which strongly suggests a high level of bacterial autolysis. From the additive experiments, it is clear that D39 pneumococci can induce ciliary stasis by a mechanism which probably involves both pneumolysin and H2O2. Pneumolysin is released from D39 as the pneumococci undergo autolysis; the levels of pneumolysin liberated are sufficient to cause rapid ciliary stasis, and thus the toxic effects of D39 H2O2 are masked. The precise mechanism(s) of action of these two toxins in combination on ependymal CBF and pathogenesis is unclear and deserves further study. Ependymal ciliary stasis caused by pneumolysin-negative pneumococci (PLN-A) was caused predominantly by H2O2 release, which was sufficient to cause maximal inhibition of CBF. The subtle effects of bacterial H2O2 cannot be determined from our experiments due to the high levels of bacterial H2O2 which were exposed to the ependymal cells. However, the mechanisms underlying H2O2-induced brain ciliary inhibition remains unclear; it is conceivable that ATP depletion, Ca2+, protein kinase C, or even membrane perturbation may be involved. H2O2 produced by bacteria readily diffuses across plasma membranes, where it can react rapidly to form other reactive species (16). These reactive species cause a wide array of biochemical changes in host cellular organelles, including stimulation of diacylglycerol production and subsequent activation of protein kinase C and the inhibition of Ca2+ homeostasis, all of which have been suggested as the cause of H2O2-induced ciliary stasis (17, 26).
Leib et al. showed that reactive oxygen species were produced in the CSF of rats with bacterial meningitis (18). Antioxidants (22) and reactive species scavenging compounds (9) attenuate pathophysiological responses associated with experimental pneumococcal meningitis, and therefore the released bacterial reactive species probably play a role in overall virulence.
The majority of pneumococcal H2O2 is a by-product of the carbohydrate-metabolizing enzyme pyruvate oxidase. A study has shown that when this enzyme was deleted by mutagenesis, the resulting strain of pneumococci had massively reduced virulence in vivo (25). One of the explanations for this observed lack of virulence was the reduced production of H2O2, and that would fit with the observations above. However, disruption of pyruvate oxidase has multiple effects on the bacterial phenotype, making interpretation of data difficult (25).
In addition to pyruvate oxidase, H2O2 can be synthesized by NADH oxidase (11). Two isoforms of bacterial NADH oxidase have been identified from two distinct genes (nox-1 and nox-2) (10). Both enzymes are expressed in Streptococcus mutans (10). nox has recently been identified in S. pneumoniae, and mutations of this gene reduced the overall virulence of the organism (D. Ogunniyi, R. Palman, S. Larpin, J. C. Paton, and M. C. Trombe, Proc. 4th Eur. Meet. Mol. Biol. Pneumococcus, abstr. A2, 1997). It will be interesting to investigate the relative contribution of each of the enzymes to pneumococcal virulence on the ependyma. In summary, these studies show that virulence of S. pneumoniae is multifactorial and that in order to develop therapeutic interventions, we must take into account all potential bacterial virulence factors. These findings suggest that the role(s) of both pneumolysin and H2O2 in the pathophysiology of pneumococcal meningitis requires further investigation.
ACKNOWLEDGMENT
This work was supported by a BUPA grant.
REFERENCES
- 1.Berry A M, Yother J, Briles D E, Paton J C. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect Immun. 1989;57:2037–2042. doi: 10.1128/iai.57.7.2037-2042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bingen E, Lambert-Zechovsky N, Mariani-Kurkdjian P, Doit C, Aujard Y, Fournerie F, Mathieu H. Bacterial counts in cerebrospinal fluid of children with meningitis. Eur J Clin Microbiol Infect Dis. 1990;9:278–281. doi: 10.1007/BF01968060. [DOI] [PubMed] [Google Scholar]
- 3.Bohr V, Paulson O, Rasmussen N. Pneumococcal meningitis: late neurological sequelae and features of prognostic impact. Arch Neurol. 1984;41:1045–1049. doi: 10.1001/archneur.1984.04050210043012. [DOI] [PubMed] [Google Scholar]
- 4.Burman W J, Martin W J. Oxidant-mediated ciliary dysfunction. Possible role in airway disease. Chest. 1986;89:410–413. doi: 10.1378/chest.89.3.410. [DOI] [PubMed] [Google Scholar]
- 5.Carson J L, Collier A M. Ciliary defects: cell biology and clinical perspectives. Adv Pediatr. 1988;35:139–165. [PubMed] [Google Scholar]
- 6.Cochrane C G. Cellular injury by oxidants. Am J Med. 1991;91:23S–30S. doi: 10.1016/0002-9343(91)90280-b. [DOI] [PubMed] [Google Scholar]
- 7.Del Bigio M R. The ependyma: a protective barrier between brain and cerebrospinal fluid. Glia. 1995;14:1–13. doi: 10.1002/glia.440140102. [DOI] [PubMed] [Google Scholar]
- 8.Duane P G, Rubins J B, Weisel H R, Janoff E N. Identification of hydrogen peroxide as a Streptococcus pneumoniae toxin for rat alveolar epithelial cells. Infect Immun. 1993;61:4392–4397. doi: 10.1128/iai.61.10.4392-4397.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.French J F, Craig T E, Downs T R, Ohkweiler D F, Carr A A, Dage R C. Protective effects of a cyclic nitrone antioxidant in animal models of endotoxic shock and chronic bacteraemia. Circ Shock. 1994;43:130–136. [PubMed] [Google Scholar]
- 10.Higuchi M, Shimada M, Matsumoto J, Yamamoto Y, Rhaman A, Kamio Y. Molecular cloning and sequence analysis of the gene encoding the H2O2-forming NADH oxidase from Streptococcus mutans. Biosci Biotechnol Biochem. 1994;58:1603–1607. doi: 10.1271/bbb.58.1603. [DOI] [PubMed] [Google Scholar]
- 11.Higuchi M, Shimada M, Yamamoto Y, Hayashi T, Koga T, Kamio Y. Identification of 2 distinct NADH oxidases corresponding to H2O2-forming and H2O-forming oxidase induced in Streptococcus mutans. J Gen Microbiol. 1993;139:2343–2351. doi: 10.1099/00221287-139-10-2343. [DOI] [PubMed] [Google Scholar]
- 12.Hirst, R. A., A. Rutman, K. S. Sikand, P. W. Andrew, T. J. Mitchell, and C. O'Callaghan. Effect of pneumolysin on rat brain ciliary function: comparison of brain slices with cultured ependymal cells. Pediatr. Res., in press. [DOI] [PubMed]
- 13.Jackett P S, Andrew P W, Aber V R, Lowrie D B. Hydrogen peroxide and superoxide release by alveolar macrophages from normal and BCG-vaccinated guinea pigs after intravenous challenge with mycobacterium tuberculosis. Br J Exp Pathol. 1981;62:419–428. [PMC free article] [PubMed] [Google Scholar]
- 14.Jackowski J T, Szepfalusi Z S, Wanner D A, Seybold Z S, Sielczak M W, Lauredo I T, Adams T, Abraham W M, Wanner A. Effects of P. aeruginosa-derived bacterial products on tracheal ciliary function: the role of O2 radicals. Am J Physiol. 1991;260:L61–L67. doi: 10.1152/ajplung.1991.260.2.L61. [DOI] [PubMed] [Google Scholar]
- 15.Johansson C B, Momma S, Clarke D L, Reisling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 16.Kantar A, Oggiano N, Giorgi P L, Braga P C, Fiorini C. Polymorphonuclear leukocyte-generated oxygen metabolites decrease beat frequency of human respiratory cilia. Lung. 1994;172:215–222. doi: 10.1007/BF00164438. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi K, Salathe M, Pratt M M, Cartagena N J, Soloni F, Seybold Z U, Wanner A. Mechanism of hydrogen peroxide-induced inhibition of sheep airway cilia. Am J Respir Cell Mol Biol. 1992;6:667–673. doi: 10.1165/ajrcmb/6.6.667. [DOI] [PubMed] [Google Scholar]
- 18.Leib S L, Kim Y S, Chow L L, Sheldon A R, Tauber M G. Reactive oxygen intermediates contribute to necrotic and apoptotic neuronal injury in an infant rat model of bacterial meningitis due to group B streptococci. J Clin Investig. 1996;98:2632–2639. doi: 10.1172/JCI119084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell T J, Walker J E, Saunders F K, Andrew P W, Boulnois G J. Expression of the pneumolysin gene in Escherichia coli: rapid purification and biological properties. Biochem Biophys Acta. 1989;1007:67–72. doi: 10.1016/0167-4781(89)90131-0. [DOI] [PubMed] [Google Scholar]
- 20.Mohamed B J, Mitchell T J, Andrew P W, Hirst R A, O'Callaghan C. The effect of the pneumococcal toxin, pneumolysin on brain ependymal cilia. Microb Pathog. 1999;27:303–309. doi: 10.1006/mpat.1999.0306. [DOI] [PubMed] [Google Scholar]
- 21.Paton J C, Andrew P W, Boulnois G C, Mitchell T J. Molecular analysis of the pathogenicity of Streptococcus pneumoniae: the role of pneumococcal proteins. Annu Rev Microbiol. 1993;47:89–115. doi: 10.1146/annurev.mi.47.100193.000513. [DOI] [PubMed] [Google Scholar]
- 22.Pfister H W, Koedel U, Lorenzl S, Tomaz A. Antioxidants attenuate microvascular changes in the early phase of experimental meningitis in rats. Stroke. 1992;23:1798–1804. doi: 10.1161/01.str.23.12.1798. [DOI] [PubMed] [Google Scholar]
- 23.Quagliarello V, Scheld M W. Bacterial meningitis: pathogenesis, pathophysiology and progress. N Engl J Med. 1992;327:864–872. doi: 10.1056/NEJM199209173271208. [DOI] [PubMed] [Google Scholar]
- 24.Rayner C F, Jackson A D, Rutman A, Dewar A, Mitchell T J, Andrew P W, Wilson R. Interaction of pneumolysin-sufficient and deficient isogenic variants of Streptococcus pneumoniae with human respiratory mucosa. Infect Immun. 1995;63:442–447. doi: 10.1128/iai.63.2.442-447.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spellerberg B, Cundell D R, Sandros J, Pearce B J, Idanpaan-Heikkila I, Rosenow C, Masure R. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol Microbiol. 1996;19:803–813. doi: 10.1046/j.1365-2958.1996.425954.x. [DOI] [PubMed] [Google Scholar]
- 26.Stommel E W, Stephens R E. Cyclic AMP and calcium in the differential control of mytillus gill cilia. J Comp Physiol. 1985;157:451–459. doi: 10.1007/BF00615145. [DOI] [PubMed] [Google Scholar]
- 27.Tauber M G, Khayam-Bashi H, Sande M A. Effects of ampicillin and corticosteroids on brain water content, CSF pressure and CSF lactate in experimental pneumococcal meningitis. J Infect Dis. 1985;151:528–534. doi: 10.1093/infdis/151.3.528. [DOI] [PubMed] [Google Scholar]
- 28.Tuomanen E I, Austrian R, Masure H R. Pathogenesis of pneumococcal infection. N Engl J Med. 1995;332:1280–1284. doi: 10.1056/NEJM199505113321907. [DOI] [PubMed] [Google Scholar]
- 29.Ukada S, Koukol J, Vennesland B. Lactic oxidase of the pneumococcus. J Bacteriol. 1959;78:714–725. doi: 10.1128/jb.78.5.714-725.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward P A. Mechanisms of endothelial cell killing by H2O2 or products of activated neutrophils. Am J Med. 1991;91:89S–94S. doi: 10.1016/0002-9343(91)90290-e. [DOI] [PubMed] [Google Scholar]